Abstract
Dental biofilm development involves initial colonization of the tooth’s surface by pioneer colonizers, followed by cell-cell coaggregation between the pioneer and later colonizers. Streptococcus gordonii is one of the pioneer colonizers. In addition to its role in oral biofilm development, S. gordonii also is a pathogen in infective endocarditis in susceptible humans. A surface adhesin, Hsa, has been shown to play a critical role in colonization of S. gordonii on the heart tissue; however, its role in oral biofilm development has not been reported. In this study we demonstrate that Hsa is essential for coaggregation between S. gordonii and Veillonella sp., which are bridging species connecting the pioneer colonizers to the late colonizers. Interestingly, the same domains shown to be required for Hsa binding to sialic acid on the human cell surface are also required for coaggregation with Veillonella sp. However, sialic acid appeared not to be required for this intergeneric coaggregation. This result suggests that although the same domains of Hsa are involved in binding to eukaryotic as well as Veillonella cells, the binding mechanism is different. The gene expression pattern of hsa was also studied and shown not to be induced by coaggregation with Veillonella sp.
Introduction
It is believed that more than 700 bacterial species colonize the human oral cavity, and each human mouth may harbor as many as 120 species [1, 2]. The formation of this multispecies community involves a sequential process, with pioneer colonizers adhering to the tooth’s surface, followed by early/middle colonizers adhering to the pioneer colonizers, and finally by the late colonizers adhering to the early/middle colonizers [3, 4]. The mitis streptococci (S. gordonii, S. oralis and S. sanguinis etc.) are considered pioneer colonizers, which can comprise as much as 80% of the early dental biofilm population [5]. The Veillonella species are one of early/middle colonizers. They are closely associated with the streptococci via cell-cell coaggregation as well as metabolic complementation—lactic acid produced by the streptococci serves as major carbon source for veillonellae, and the consequential lactate removal raises local pH, thus relieving the streptococci from toxicity of their own metabolic waste. This mutualistic relationship helps Veillonella sp to colonize and grow in the early biofilm community, which then provides attachment sites and possibly different kinds of metabolic complementation for the late colonizers such as the periodontopathogen Porphyromonas gingivalis [6, 7]. Given the importance of early/middle colonizers such as veillonellae in biofilm development and dysbiosis, understanding the mechanism of coaggregation between Veillonella species and streptococci would provide an important knowledge base for developing strategies of disease prevention.
Our previous studies have identified a surface protein named Hag1 in V. atypica strain OK5, which is responsible for Veillonella binding to S. gordonii [8]. We further demonstrated that the binding partner for Hag1 is likely a protein, as proteinase treatment of S. gordonii completely abolished coaggregation [8]. In this study, we report the identification of the corresponding adhesin from S. gordonii. We show that a previously characterized sialic acid binding adhesin, Hsa, is required for coaggregation with V. atypica as well as other Veillonella species. We further demonstrate that the same functional domains of Hsa responsible for binding to sialic acid on mammalian cells also are required for coaggregation with V. atypica; however, a different mechanism seems to be utilized in Hsa binding to mammalian cells rather than to veillonellae cells. Finally, reporter expression analyses showed that hsa gene expression is not induced or enhanced by coaggregation with V. atypica.
Materials and Methods
Bacterial strains and culture conditions
Bacterial strains and plasmids used or constructed in this study are listed in Table 1. Streptococcus gordonii DL1 was grown in brain heart infusion broth (BHI; Difco), or on BHI agar plates. For transformant selection, cells were grown in Todd–Hewitt broth (Difco) with 0.3% yeast extract (Difco) (THYE) plus erythromycin (12.5 μg ml-1) or spectinomycin (500 μg ml-1). For selection of genetically complemented mutant strains, THYE broth supplemented with 250 μg ml-1 spectinomycin was utilized. Veillonella atypica OK5 and other veillonella strains were grown in brain heart infusion broth (BHI; Difco) supplemented with 0.6% sodium lactate (BHIL), or on BHIL agar plates. All bacterial strains were grown anaerobically (85% N2, 10% CO2, 5% H2) at 37°C. Escherichia coli DH5α cells (Thermo Fisher Scientific) were grown in Luria-Bertani (LB; Difco) medium with aeration at 37°C. E. coli strains carrying plasmids were grown in LB medium containing spectinomycin (250 μg/ml) or tetracycline (10 μg/ml).
Table 1. Bacterial strains and plasmids used in this study.
| Strains and plasmids | Characteristics | Reference |
|---|---|---|
| Strains | ||
| E. coli DH5a | Cloning strain | |
| V. atypica OK5 | Wild type | [9] |
| V. parvula PK1910 | Wild type | This work |
| V. parvula OK1 | Wild type | This work |
| V. atypica OK2 | Wild type | This work |
| V. rogosae OK3 | Wild type | This work |
| V. dispar OK4 | Wild type | This work |
| V. atypica OK6 | Wild type | This work |
| V. atypica OK7-1 | Wild type | This work |
| V. dispar OK7-2 | Wild type | This work |
| V. dispar OK8 | Wild type | This work |
| V. dispar OK9 | Wild type | This work |
| V. dispar OK10 | Wild type | This work |
| V. rogosae OK11 | Wild type | This work |
| S. gordonii DL1 | Wild type | [10] |
| Δhsa | DL1 hsa deletion mutant | This work |
| ΔsrtA | DL1 srtA deletion mutant | [11] |
| ΔsspA/B | DL1 sspA/B deletion mutant | This work |
| S. gordonii-hsa::luc | DL1 hsa promoter luciferase reporter | This work |
| Δhsa-hsa::luc | hsa promoter luciferase reporter in hsa mutant | This work |
| Plasmids | ||
| pFW5-hsa p-luc | Suicide vector pFW5::luc of S. gordonii with hsa promoter region; Specr | This work |
| pAS8741 | Plasmid containing the complete hsa gene | [12] |
| pAS8748 | Deletion of SR1 and NR2 regions in Hsa | [12] |
| pAS8744 | Deletion of SR1 region in Hsa | [12] |
| pAS8746 | Deletion of NR2 region in Hsa | [12] |
| pAS8749 | Deletion of part of SR2 region in Hsa | [12] |
| pAS8375 | Deletion of part of SR2 region in Hsa | [12] |
| pAS8165 | Deletion of SR2 region in Hsa | [12] |
| pAS8164 | Deletion of SR1 and SR2 regions in Hsa | [12] |
Construction of S. gordonii surface protein mutants
PCR primers used in this study are listed in Table 2. The hsa mutant, srtA mutant, and sspA/B double mutant were constructed by overlapping PCR. Briefly, the upstream and downstream fragments of the target genes were generated by PCR using the specific primer pairs (Table 2). The ermAM fragment was generated by PCR using primer pair ermAM-F/ermAM-R. The three fragments were mixed and used as template for overlapping PCR without primers. The final amplicons were transformed into S. gordonii DL1 and mutants were selected for erythromycin resistance.
Table 2. Primers used in this study.
| Primer | Sequence (5’ to 3’) | Purpose |
|---|---|---|
| hsa-up-F | TAACAAGCTTAGTACACGGG | hsa deletion |
| hsa-up-R | CAAGAATAAACTGCCAAAGCGCGTAACACGTTCAACTTC | hsa deletion |
| hsa-dn-F | TATACTACTGACAGCTTCATAGGAGCTTTGGGATTGGC | hsa deletion |
| hsa-dn-R | GCATCCCCAAGTTCTGTTTC | hsa deletion |
| sspAB-up-F | TCGGTGGTGGGATGACTTAC | sspA/B deletion |
| sspAB-up-R | CAAGAATAAACTGCCAAAGTGCCCCTAAGACAGCTCCAC | sspA/B deletion |
| sspAB-dn-F | TATACTACTGACAGCTTCATGCCTTACCTAGGCTTGGC | sspA/B deletion |
| sspAB-dn-R | CCTACTGTCGGAACTGTACC | sspA/B deletion |
| srtA-up-F | CATGGCCTGTAGCTCAATC | srtA deletion |
| srtA-up-R | CAAGAATAAACTGCCAAAGGGAAGGAAGCATAAGTTTAATGC | srtA deletion |
| srtA-dn-F | TATACTACTGACAGCTTCCCTTCTCGTCTTGCAACTC | srtA deletion |
| srtA-dn-R | ACCTAAGAGACGGTGACCAG | srtA deletion |
| ermAM-F | CTTTGGCAGTTTATTCTTGAC | gene deletion |
| ermAM-R | GAAGCTGTCAGTAGTATACC | gene deletion |
| hsa p-luc-F | ACGCGTCGACACGAAAAGGAGTCCTAGTAGCTACA | Luciferase reportor construction |
| hsa p-luc-R | CGCGGATCCGTAATCCCCTCTACTTAATTTAATA | Luciferase reportor construction |
Coaggregation assay
Co-aggregation assays were performed as previously described [13] with minor modifications: Mid-log cells were harvested and washed twice with coaggregation buffer (1 mM Tris buffer [pH 8.0], 0.1 mM CaC12, 0.1 mM MgCl2, 150 mM NaCl) at room temperature. Then, cells were resuspended in coaggregation buffer and normalized to OD600 = 1.2. Equal volumes (0.1ml) of each cell suspension were mixed in a micro-centrifuge tube and vortexed briefly. The tubes were left standing at room temperature until aggregates appeared, usually 15–30 min. Tubes containing each cell suspension alone (0.2 ml) were included as controls. All assays were repeated at least 3 times.
Construction of hsa reporter strain
The hsa-luc reporter was constructed as follows: A ~ 600 bp fragment of the hsa promoter region was PCR amplified using primer pairs hsa p-luc-F/hsa p-luc-R (Table 2). The product was digested with SalI and BamHI and cloned into the suicide vector pFW5-luc digested with the same restriction enzymes to create the fusion plasmid pFW5-hsa p-luc. The confirmed plasmid was transformed into S. gordonii DL1 and transformants were selected on BHI plates supplemented with spectinomycin (500 μg mL-1). Transformants were further confirmed by PCR.
Luciferase assays
Overnight culture of S. gordonii hsa-luc reporter strains were spun down and re-suspended with fresh BHI media to OD600 ~1.0. Suspended cultures then were 1:20 diluted into fresh BHI media. For co-culture test, overnight culture of V. atypica OK5 was centrifuged and re-suspended with fresh BHI to OD600 ~1.0, and then was diluted into BHI media with S. gordonii/V. atypica ratio 5:1. All cultures were grown in anaerobic condition for 24 hours, and the data were measured every 2 hours. Luciferase assays were performed by adding 25 μL of 1 mM D-luciferin (Sigma) solution (suspended in 0.1 M citrate buffer, pH 6.0) into 100 μL samples, and luciferase activities were measured using a TD 20/20 luminometer (Turner Biosystems, Sunnyvale, CA). For single species cultures, the optical densities at 600 nm were measured with a spectrophotometer (Bio-Rad, smartspec 3000). For mixed species cultures, colony-forming units (CFU/ml) were obtained by plate counting. Luciferase activity was expressed either as relative light unit (RLU)/OD600 (for single-species culture), or as RLU/CFUx1000 (for mixed species culture).
Results
Coaggregation between S. gordonii DL1 and veillonellae
Coaggregation between oral streptococci especially S. gordonii and veillonellae has been studied previously [8, 14]. To gain a general picture of the coaggregation pattern between our model strain S. gordonii DL1 and our clinical veillonellae isolates, we used the modified in vitro coaggregation assay [13] to qualitatively assess the coaggregation ability between S. gordonii DL1 and the veillonellae strains. As shown in Table 3, S. gordonii DL1 coaggregated with two of four V. atypica strains, all two V. parvula strains, and one of two V. rogosae strains. Interestingly, none of the five V. dispar strains coaggregated with S. gordonii DL1.
Table 3. Coaggregation of S. gordonii wild-type and Δhsa strain with various Veilloenlla species/ strains.
| Strain name | S. gordonii DL1 | Δhsa |
|---|---|---|
| V. atypica OK2 | + | − |
| V. atypica OK5 | + | − |
| V. atypica OK6 | − | NT |
| V. atypica OK7-1 | − | NT |
| V. dispar OK7-2 | − | NT |
| V. dispar OK8 | − | NT |
| V. dispar OK9 | − | NT |
| V. dispar OK10 | − | NT |
| V. dispar OK4 | − | NT |
| V. parvula PK1910 | + | + |
| V. parvula OK1 | + | − |
| V. rogosae OK3 | + | − |
| V. rogosae OK11 | − | NT |
+: coaggregation; -: no coaggregation; NT: Not Tested
Identification of the adhesin in S. gordonii DL1 responsible for coaggregation with veillonellae
In our previous study, we demonstrated that proteinase K treatments of S. gordonii abolished co-aggregation with V. atypica, suggesting that a surface protein/adhesin is probably involved [8]. Four types of surface adhesins have been identified in S. gordonii: cell-surface fibrillar proteins (CshA and CshB), sialic-acid binding protein (Hsa/GspB), amylase-binding proteins (AbpA and AbpB), and antigen I/II (AgI/II) family proteins (SspA and SspB) [15–19]. To have a quick survey of the known surface adhesins in S. gordonii, we initially constructed mutant strains with deletion of surface adhesin genes sspA/B, hsa, and srtA. As both Hsa and SspA/B belong to LPXTG-motif-containing surface proteins, SrtA was used as a positive control because its deletion would eliminate all surface proteins with a LPXTG-containing cell wall anchoring domain [11]. In vitro coaggregation assay was used to assess the effect of these mutations on coaggregation with V. atypica. As shown in Fig 1, the sspA/B double mutation did not have any effect on coaggregation with V. atypica, while the hsa and srtA mutations completely abolished coaggregation. This result suggested that Hsa is the protein responsible for coaggregation with V. atypica. To see if the hsa mutation had the same effect on other Veillonella strains that coaggregated with S. gordonii, the same coaggregation assay was performed. As shown in Table 3, the mutation abolished coaggregation with all but one (V. parvula PK1910) coaggregation partner. This result indicates that Hsa is the adhesin mediating coaggregation with V. atypica strains OK2 and OK5, V. parvula strain OK1, and V. rogosae OK3, and that coaggregation with V. parvula PK1910 (previously V. atypica PK1910) is probably mediated by a completely different mechanism.
Fig 1. Coaggregation of S. gordonii DL1 (wt) and surface protein mutants with V. atypica OK5.
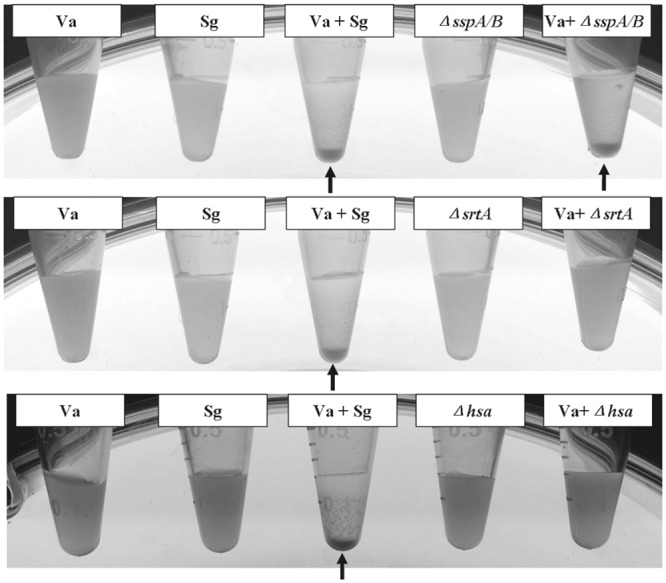
Coaggregation-positive pairs form aggregates that precipitate at the bottom of the tube (arrowhead), while cells of the single species cultures or mixed cultures with the hsa and srtA mutants remain in suspension. Sg: S. gordonii DL1; Va: V. atypica OK5.
Identification of Hsa domains that are responsible for coaggregation between S. gordonii DL1 and V. atypica OK5
The surface adhesin Hsa/GspB has been characterized extensively [16, 19]. The protein has been shown to bind to sialic acid on the surface of different types of mammalian cells [20–22]. In S. gordonii, the protein has 2178 amino acids, which can be divided into 5 domains: the N-terminal non-repeat region (NR1), followed by serine-rich repeat region 1 (SR1), non-repeat region 2 (NR2), serine-rich repeat region 2 (SR2), and the C-terminal wall-anchor domain (CWAD) [Fig 2, [12]]. A series of plasmids containing deletions of different domains has been previously constructed by Dr. Takahashi’s group, and all plasmids were shown by Western blot to express truncated Hsa proteins corresponding to the respective hsa deletions they carry in the mutant strains [12]. To determine which domain is responsible for binding to V. atypica, we used these plasmids to complement the hsa deletion mutation strain. A total of 8 complement strains were thus constructed and tested in an in vitro coaggregation assay with V. atypica. As shown in Fig 3, five strains containing plasmids pAS8748, pAS8744, pAS8746, pAS8165, and pAS8164 failed to restore coaggregation with V. atypica, suggesting that the deleted regions of Hsa encoded by the DNA fragments in these plasmids are required for coaggregation. As illustrated in Fig 2, plasmid pAS8165 contains a complete deletion of the SR2 region, suggesting that the SR2 region is required by coaggregation. However, since plasmids pAS8749 contains only the C-terminal part of SR2, and plasmid pAS8375 contains only the N-terminal part of SR2, and both restored coaggregation with V. atypica, it appears that the N- and C-terminal region of SR2 is redundant for Hsa function. In addition to the SR2 fragment, both SR1 and NR2 domains are also required for coaggregation, because plasmids containing SR1 (pAS8746) or NR2 (pAS8744) deletions did not complement the coaggregation deficiency of the hsa deletion. Taken together, we conclude that the intact domains of SR1 and NR2 plus either the N- or C-terminal domain of SR2 are required for Hsa binding to V. atypica.
Fig 2. Graphic illustration of the various regions deleted in the complementing plasmids.
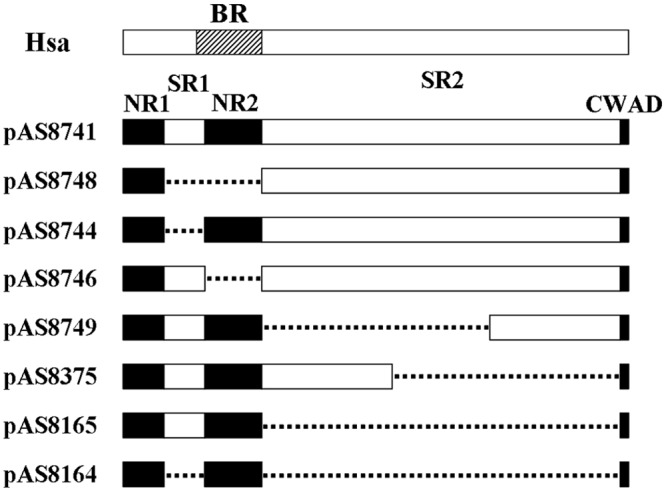
The basic region of Hsa was located on amino acid position 228–449. pAS8741 carries an intact hsa gene; pAS8748 encodes a Hsa protein lacking the SR1 and NR2 regions (HsaΔ105–446); pAS8744 encodes a Hsa protein lacking the SR1 region (HsaΔ105–245); pAS8746 encodes a Hsa protein lacking the NR2 region (HsaΔ246–446); pAS8749 encodes a Hsa protein lacking the N-terminal part of the SR2 region (HsaΔ448–1459); pAS8375 encodes a Hsa protein lacking the C-terminal part of the SR2 region (HsaΔ1092–2143); pAS8165 encodes a Hsa protein lacking the entire SR2 region (HsaΔ448–2143); and pAS8164 encodes a Hsa protein lacking both SR1 and SR2 regions (HsaΔ105–245, Δ448–2143). The dotted lines indicate the regions deleted. BR: basic region; NR: non-repeat region; SR: serine-rich repeat region; CWAD: cell wall anchoring domain.
Fig 3. Coaggregation of S. gordonii DL1 (wt), hsa mutant (Δhsa), and the eight complemented strains with V. atypica OK5.
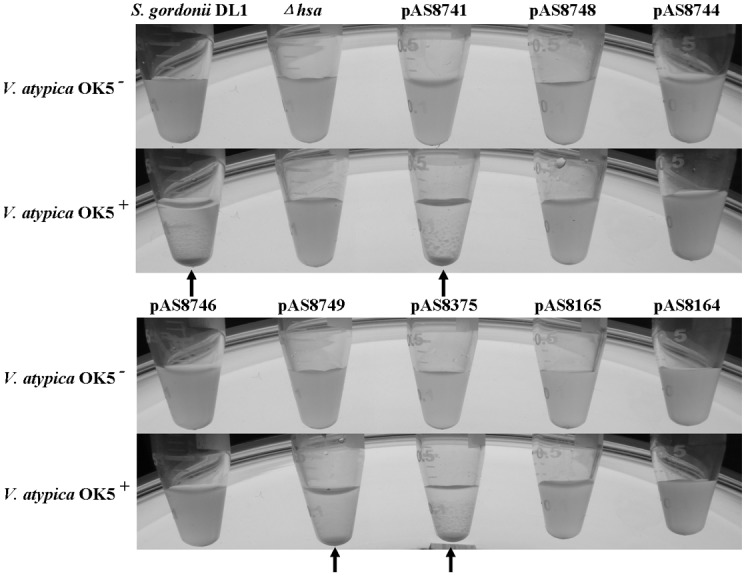
S. gordonii DL1 and complemented strains containing pAS8741, pAS8749 and pAS8375 formed aggregates with V. atypica OK5 and precipitated at the bottom of the tube (arrowhead), while cells of the single species cultures or mixed cultures with the Δhsa mutant or complemented strains containing pAS8748, pAS8744, pAS8746, pAS8165 and pAS8164 remained in solution.
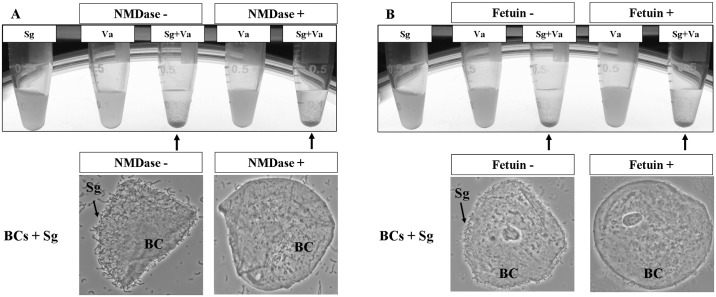
Sialic acid is not involved in Hsa binding to V. atypica OK5
It was well known that sialic acid is the binding partner for Hsa adhesin, and the basic region (BR) of Hsa mediates its adhesion to sialic acid[23, 24]. The BR of Hsa is located on amino acid position 228–449, encompassing the C-terminal portion of SR1 and the entire region of NR2 (Fig 2). Results presented above demonstrated that both SR1 and NR2 regions are also required for coaggregation with V. atypica OK5, suggesting that sialic acid or similar molecules may exist on the veillonellae cell surface[20, 25]. To see whether V. atypica could potentially synthesize sialic acids, we searched the genome sequence of V. atypica strains in the HOMD database for putative sialic acid biosynthesis genes, and found none. The draft sequence of strain V. atypica OK5 (P. Zhou and F. Qi, unpublished data) used in this study was also searched, and no sialic acid biosynthesis genes were found. To further confirm that no sialic acid from the V. atypica cell surface is involved in coaggregation with S. gordonii, we first treated V. atypica OK5 with neuraminidase prior to the coaggregation assay. This treatment did not affect coaggregation, although similar treatment of human buccal cells completely abolished attachment by S. gordonii DL1 (Fig 4A). Next, we added fetuin to the coaggregation assay. Fetuin is a group of molecules with sialylated carbohydrate structures that are similar to the sialoproteins on mammalian cell surface [23]. We reasoned that if a sialic acid-like structure on V. atypica cell surface were involved, then adding fetuin would inhibit coaggregation. It turned out that fetuin had no effect on coaggregation either (Fig 4B). Similarly, adding fetuin to the buccal cells nearly completely abolished attachment by S. gordonii DL1 cells (Fig 4). Taken together, we concluded that sialic acid is not involved in Hsa-mediated coaggregation with V. atypica OK5, although it is required for S. gordonii attachment to human buccal cells.
Fig 4. Coaggregation of S. gordonii DL1 with V. atypica OK5 treated with neuraminidase (A) or fetuin (B).
Note that none of the treatments affected coaggregation between the two species, and S. gordonii cannot adhere to buccal cells treated by neuraminidase or fetuin. The arrows indicate the aggregates at the bottom of the tube. Sg: S. gordonii DL1; Va: V. atypica OK5.
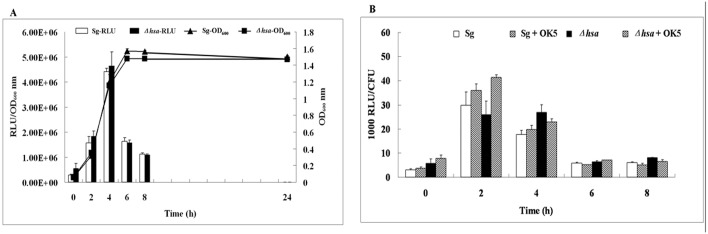
The expression of hsa gene is not regulated by coaggregation with V. atypica
To see whether the expression of hsa gene is regulated by coaggregation between S. gordonii and V. atypica, we constructed a firefly luciferase reporter in the S. gordonii DL1 wild type as well as in the Δhsa mutant strain. The expression of hsa-luc reporter was first measured over the growth curve in the wild type and the mutant single species cultures. As shown in Fig 5A, the hsa gene expression started at early log phase, peaked at mid-log phase, and rapidly turned down at early stationary phase. There is no difference in the level or the pattern of gene expression in the wild-type and the hsa mutant strains, suggesting no feedback regulation. Next, we measured hsa-luc expression of the two strains in mixed cultures with V. atypica OK5 (Fig 5B). No difference was observed between single and mixed species cultures, or between coaggregated (S. gordonii wild-type + OK5) and non-coaggregated (S. gordonii Δhsa + OK5) mixed cultures, suggesting that coaggregation does not affect gene expression of hsa.
Fig 5.
(A) Luciferase expression pattern and growth curve of S. gordonii DL1 and Δhsa mutant. The growth of 2 strains was measured every 2 hours and at OD600 nm, and luciferase expression (relative light unit, RLU) was normalized by growth curve (OD600). (B) Luciferase expression pattern of S. gordonii DL1 and Δhsa mutant mixed with V. atypica OK5. CFU (colony-forming unit) was measured every 2 hours and utilized to normalize RLU. 1000 RLU/CFU was shown in Y-axis. Sg: S. gordonii DL1; OK5: V. atypica OK5. The results are shown as the means ± SD of three independent experiments.
Discussion
In the oral biofilm, cell-cell coaggregation plays a critical role in biofilm development. Of particular importance is the coaggregation between pioneer colonizers and the early/middle colonizers. As the latter are bridging species for colonization of late colonizers many of which are periodontopathogens, understanding the mechanisms of coaggregation between the pioneer colonizers and the bridging species can lead to development of knowledge-based strategy for prevention of periodontal diseases.
The mitis streptococci are major pioneer colonizers, and species of the Veillonella genus are one of the most prevalent and numerically dominant bridging species. The two groups of bacteria physically coaggregate with each other especially amongst strains isolated from the same anatomical sites [14, 26]. This coaggregation enables the Veillonella species to join the early biofilm community, for the veillonellae bacteria on their own have very weak (if any) attachment ability to the salivary pellicles on the tooth’s surface [1, 27, 28].
A number of early studies attempted to identify surface adhesins mediating coaggregation between streptococci and veillonellae [29, 30]. Weerkamp and McBride analyzed the surface component that is responsible for Streptococcus salivarius HB binding to V. alcalescens V1 (now V. parvula V1), and they found that a fraction adhered to V. alcalescens V1 was released upon lysozyme treatment of the latter [29]. Using chemical mutagenesis and natural selection, Handley et al. selected four mutants of S. salivarius HB that abolished the ability to coaggregate with V. parvula V1. However, the nature of these mutants has not been determined [29, 30]. Thus, until now no adhesin has been definitively identified in streptococci that is responsible for coaggregation with veillonellae. In this study, we identified Hsa from S. gordonii as the adhesin mediating coaggregation with V. atypica strains OK2 and OK5, V. rogosae strain OK3, and V. parvula strain OK1, but not with V. parvula strain PK1910 (Table 3), Additionally, not all strains in V. atypica and V. rogosae coaggregated with S. gordonii, suggesting that this intergeneric coaggregation may be strain specific, which is also consistent with previous reports [26].
The finding that Hsa being the adhesin mediating coaggregation of S. gordonii with some Veillonella species/strains is very interesting, because Hsa has been well characterized as a sialic acid binding adhesin mediating attachment of S. gordonii to human cells including heart tissues [31]. Thus, Hsa has been considered an important virulence factor in infective endocarditis [32, 33]. Our finding demonstrates, for the first time, that Hsa is perhaps a multivalent adhesin mediating not only attachment to mammalian cells but also to prokaryotic cells. Interestingly, in our recent study, we identified a surface adhesin, Hag1, in V. atypica, which mediates coaggregation not only with several bacterial species including S. gordonii, but also with human buccal cells [8]. Whether it is common for oral microbial species to produce adhesins with binding capacity to multiple partners awaits further investigation.
Perhaps the most exciting finding from this study is that the same functional domains of Hsa required for binding to sialic acid on mammalian cells are also required for binding to Veillonella cells. However, genomic analysis, combined with neuraminidase treatment of V. atypica cells and fetuin inhibition assay, failed to provide evidence for the existence of sialic acids or similarly structured molecules on the Veillonella cell surface. Thus, there exists a possibility that subdomains may exist in the binding domain of Hsa, which could be responsible for binding to different target molecules on different cell surfaces. Detailed genetic mutagenesis and identification of the amino acid residues involved in binding are needed to answer this question.
Data Availability
All relevant data are within the paper.
Funding Statement
The work was supported by 2R15DE019940, 5R21DE024235, National Institutes of Health, http://www.nih.gov.
References
- 1. Huang R, Li M, Gregory RL. Bacterial interactions in dental biofilm. Virulence. 2011;2(5):435–44. 10.4161/viru.2.5.16140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hajishengallis G, Lamont RJ. Beyond the red complex and into more complexity: the polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Molecular oral microbiology. 2012;27(6):409–19. 10.1111/j.2041-1014.2012.00663.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kolenbrander PE, Palmer RJ Jr., Rickard AH, Jakubovics NS, Chalmers NI, Diaz PI. Bacterial interactions and successions during plaque development. Periodontology 2000. 2006;42:47–79. 10.1111/j.1600-0757.2006.00187.x . [DOI] [PubMed] [Google Scholar]
- 4. Kolenbrander PE, Palmer RJ Jr., Periasamy S, Jakubovics NS. Oral multispecies biofilm development and the key role of cell-cell distance. Nature reviews Microbiology. 2010;8(7):471–80. 10.1038/nrmicro2381 . [DOI] [PubMed] [Google Scholar]
- 5. Nyvad B, Kilian M. Microbiology of the early colonization of human enamel and root surfaces in vivo. Scandinavian journal of dental research. 1987;95(5):369–80. . [DOI] [PubMed] [Google Scholar]
- 6. Valm AM, Mark Welch JL, Rieken CW, Hasegawa Y, Sogin ML, Oldenbourg R, et al. Systems-level analysis of microbial community organization through combinatorial labeling and spectral imaging. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(10):4152–7. 10.1073/pnas.1101134108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kolenbrander PE. Multispecies communities: interspecies interactions influence growth on saliva as sole nutritional source. International journal of oral science. 2011;3(2):49–54. 10.4248/IJOS11025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhou P, Liu J, Merritt J, Qi F. A YadA-like autotransporter, Hag1 in Veillonella atypica is a multivalent hemagglutinin involved in adherence to oral streptococci, Porphyromonas gingivalis, and human oral buccal cells. Mol Oral Microbiol. 2014. Epub 2014/12/03. 10.1111/omi.12091 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu J, Xie Z, Merritt J, Qi F. Establishment of a tractable genetic transformation system in Veillonella spp. Applied and environmental microbiology. 2012;78(9):3488–91. 10.1128/AEM.00196-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pakula R, Walczak W. On the nature of competence of transformable streptococci. Journal of general microbiology. 1963;31:125–33. . [DOI] [PubMed] [Google Scholar]
- 11. Nobbs AH, Vajna RM, Johnson JR, Zhang Y, Erlandsen SL, Oli MW, et al. Consequences of a sortase A mutation in Streptococcus gordonii . Microbiology. 2007;153(Pt 12):4088–97. 10.1099/mic.0.2007/007252-0 . [DOI] [PubMed] [Google Scholar]
- 12. Takahashi Y, Yajima A, Cisar JO, Konishi K. Functional analysis of the Streptococcus gordonii DL1 sialic acid-binding adhesin and its essential role in bacterial binding to platelets. Infection and immunity. 2004;72(7):3876–82. 10.1128/IAI.72.7.3876-3882.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cisar JO, Kolenbrander PE, McIntire FC. Specificity of coaggregation reactions between human oral streptococci and strains of Actinomyces viscosus or Actinomyces naeslundii . Infection and immunity. 1979;24(3):742–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hughes CV, Andersen RN, Kolenbrander PE. Characterization of Veillonella atypica PK1910 adhesin-mediated coaggregation with oral Streptococcus spp. Infection and immunity. 1992;60(3):1178–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tanzer JM, Grant L, Thompson A, Li L, Rogers JD, Haase EM, et al. Amylase-binding proteins A (AbpA) and B (AbpB) differentially affect colonization of rats' teeth by Streptococcus gordonii . Microbiology. 2003;149(Pt 9):2653–60. . [DOI] [PubMed] [Google Scholar]
- 16. Takahashi Y, Konishi K, Cisar JO, Yoshikawa M. Identification and characterization of hsa, the gene encoding the sialic acid-binding adhesin of Streptococcus gordonii DL1. Infection and immunity. 2002;70(3):1209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McNab R, Forbes H, Handley PS, Loach DM, Tannock GW, Jenkinson HF. Cell wall-anchored CshA polypeptide (259 kilodaltons) in Streptococcus gordonii forms surface fibrils that confer hydrophobic and adhesive properties. Journal of bacteriology. 1999;181(10):3087–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jakubovics NS, Kerrigan SW, Nobbs AH, Stromberg N, van Dolleweerd CJ, Cox DM, et al. Functions of cell surface-anchored antigen I/II family and Hsa polypeptides in interactions of Streptococcus gordonii with host receptors. Infection and immunity. 2005;73(10):6629–38. 10.1128/IAI.73.10.6629-6638.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bensing BA, Sullam PM. An accessory sec locus of Streptococcus gordonii is required for export of the surface protein GspB and for normal levels of binding to human platelets. Molecular microbiology. 2002;44(4):1081–94. . [DOI] [PubMed] [Google Scholar]
- 20. Takahashi Y, Sandberg AL, Ruhl S, Muller J, Cisar JO. A specific cell surface antigen of Streptococcus gordonii is associated with bacterial hemagglutination and adhesion to alpha2-3-linked sialic acid-containing receptors. Infection and immunity. 1997;65(12):5042–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ruhl S, Cisar JO, Sandberg AL. Identification of polymorphonuclear leukocyte and HL-60 cell receptors for adhesins of Streptococcus gordonii and Actinomyces naeslundii . Infection and immunity. 2000;68(11):6346–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Herzberg MC, Brintzenhofe KL, Clawson CC. Aggregation of human platelets and adhesion of Streptococcus sanguis . Infection and immunity. 1983;39(3):1457–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Takamatsu D, Bensing BA, Prakobphol A, Fisher SJ, Sullam PM. Binding of the streptococcal surface glycoproteins GspB and Hsa to human salivary proteins. Infection and immunity. 2006;74(3):1933–40. 10.1128/IAI.74.3.1933-1940.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Takamatsu D, Bensing BA, Cheng H, Jarvis GA, Siboo IR, Lopez JA, et al. Binding of the Streptococcus gordonii surface glycoproteins GspB and Hsa to specific carbohydrate structures on platelet membrane glycoprotein Ibalpha. Molecular microbiology. 2005;58(2):380–92. 10.1111/j.1365-2958.2005.04830.x . [DOI] [PubMed] [Google Scholar]
- 25. Yajima A, Urano-Tashiro Y, Shimazu K, Takashima E, Takahashi Y, Konishi K. Hsa, an adhesin of Streptococcus gordonii DL1, binds to alpha2-3-linked sialic acid on glycophorin A of the erythrocyte membrane. Microbiology and immunology. 2008;52(2):69–77. 10.1111/j.1348-0421.2008.00015.x . [DOI] [PubMed] [Google Scholar]
- 26. Hughes CV, Kolenbrander PE, Andersen RN, Moore LV. Coaggregation properties of human oral Veillonella spp.: relationship to colonization site and oral ecology. Applied and environmental microbiology. 1988;54(8):1957–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kolenbrander PE. Oral microbial communities: biofilms, interactions, and genetic systems. Annual review of microbiology. 2000;54:413–37. 10.1146/annurev.micro.54.1.413 . [DOI] [PubMed] [Google Scholar]
- 28. Kolenbrander PE. Intergeneric coaggregation among human oral bacteria and ecology of dental plaque. Annual review of microbiology. 1988;42:627–56. 10.1146/annurev.mi.42.100188.003211 . [DOI] [PubMed] [Google Scholar]
- 29. Weerkamp AH, McBride BC. Identification of a Streptococcus salivarius cell wall component mediating coaggregation with Veillonella alcalescens V1. Infection and immunity. 1981;32(2):723–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Handley PS, Hargreaves J, Harty DW. Ruthenium red staining reveals surface fibrils and a layer external to the cell wall in Streptococcus salivarius HB and adhesion deficient mutants. Journal of general microbiology. 1988;134(12):3165–72. . [DOI] [PubMed] [Google Scholar]
- 31. Jung CJ, Yeh CY, Shun CT, Hsu RB, Cheng HW, Lin CS, et al. Platelets enhance biofilm formation and resistance of endocarditis-inducing streptococci on the injured heart valve. The Journal of infectious diseases. 2012;205(7):1066–75. 10.1093/infdis/jis021 . [DOI] [PubMed] [Google Scholar]
- 32. Urano-Tashiro Y, Yajima A, Takahashi Y, Konishi K. Streptococcus gordonii promotes rapid differentiation of monocytes into dendritic cells through interaction with the sialic acid-binding adhesin. Odontology / the Society of the Nippon Dental University. 2012;100(2):144–8. 10.1007/s10266-011-0044-z . [DOI] [PubMed] [Google Scholar]
- 33. Takahashi Y, Takashima E, Shimazu K, Yagishita H, Aoba T, Konishi K. Contribution of sialic acid-binding adhesin to pathogenesis of experimental endocarditis caused by Streptococcus gordonii DL1. Infection and immunity. 2006;74(1):740–3. 10.1128/IAI.74.1.740-743.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.