Abstract
Background
The breast cancer incidence in Asia is rising. To explore whether the etiology of breast cancer is different from the known risk factors from studies in Western countries, we conducted a nested case-control study using data from the Taiwan National Health Insurance Research Database (NHIRD).
Methods
All medical conditions based on the first three digits of the ICD-9 and a list of medical conditions based on literature review were retrieved for each case and control. The odds ratios (OR) and 95% confidence intervals (CI) of the associations between medical conditions and breast cancer risks were estimated using conditional logistic regression and adjusted for occupation, number of breast cancer screening, and the average number of outpatient visits prior the diagnosis. The associations were also estimated for younger (<50 years old) and older subjects separately.
Results
The analyses included 4,884 breast cancer cases and 19,536 age-matched controls. Prior breast diseases (OR, 95% CI: 2.47, 2.26–2.71), obesity (1.43, 1.04–1.96), endometriosis (1.44, 1.15–1.80), uterine leiomyoma (1.20, 1.03–1.40), hypertensive diseases (1.14, 1.05–1.25), and disorders in lipid metabolism (1.13, 1.04–1.24) were associated with increased breast cancer risk. No heterogeneity was observed between age groups (<50 and ≥50 years old).
Conclusions
In addition to benign breast diseases, obesity, endometriosis, uterine leiomyoma, hypertensive diseases, and disorders of lipid metabolism were associated with a subsequent breast cancer risk.
Impacts
Our results suggest that estrogen related factors may play an important role in breast cancer risks in the Taiwanese female population.
Introduction
Worldwide, breast cancer was the most common cancer and the most important cause of deaths from cancer in women in 2012 [1], and the incidence is still rising [2]. Particularly in Asia, the annual increase in breast cancer incidences have been reported to be doubled or tripled in the past two decades [3].
Although breast cancer incidence is increasing globally, the pattern differs between Asia and Western countries. The peak age at diagnosis was around 60–70 years in the Western countries, whereas the peak age at diagnosis was about 40–50 years in the Asian populations [2]. It is generally accepted that the etiology of breast cancer is similar worldwide [4], i.e. the reproductive and hormonal factors. The increase in Asia has shown strong birth-cohort effects and the increase may simply reflect the changes in prevalence and composition of risk factors of the breast cancer in the younger generation [5]. It has been noted that the breast cancers in young women are more likely to be “triple-negative” carcinomas (i.e. ER-, PR-, and HER2-) and to be more aggressive than that in the elderly, thus results in higher risks of recurrence or death [6]. However, unlike what is reported in the western countries, young breast cancer patients in Taiwan seem to have higher prevalence of ER+ or PR+ tumors than the older patients [7,8]. These results suggested that westernization may not be the only explanation to the increase in breast cancer incidence.
It has been suggested that cluster of diseases may imply similar etiologies between diseases, common risk factors, or effects of treatment for the prior disease [9]. A prior study had suggested that, in addition to breast cancer, the age-adjusted incidence rates of uterine and ovarian cancers also increased from 1979 to 2007 in Taiwan, and these cancers showed high prevalence of hormone receptor expressions [7]. Other medical conditions, which have been related to breast cancer, were hypothesized to be via estrogen modulation [10], metabolic disruption [11], impaired immune functions or inflammation [12], and medical radiation [13]. Most previous studies on medical conditions and breast cancer risk are based on case-control studies, which may suffer from recall bias, or based on a pre-designed medical list. The Taiwan National Health Insurance (NHI) program covers over 99% Taiwanese population and provides extensive medical information. A National Health Insurance Research Database (NHIRD) was created by National Health Research Institutes (NHRI) for academic research.
The aim of the study was to investigate the etiology of breast cancer by exploring the associations between prior chronic diseases and breast cancer risks using NHIRD.
Material and Methods
Source Population
The NHI program has been described previously [14]. Briefly, the program covered over 22 million people in Taiwan, which represented over 99% of the entire population. The Longitudinal Health Insurance Database (LHID)– 2000, 2005, and 2010 is part of the NHIRD and contains claim data of a one-million random sample that covers the beneficiaries registered during 1996–2000, in 2005, and in 2010, respectively. The basic demographic characteristics, e.g. birthday, sex, insured company and area, dates of visits, diagnoses, procedures, and/or prescriptions since 1996 onward were retrieved for each sampled individuals. The ID numbers of the sampled individuals were scrambled. Major diagnoses, such as stroke and acute coronary syndrome, had been validated in previous studies [15,16,17]. To assure the data quality, we set the observation period 2000–2010. The current analyses were based on LHID2000 and LHID2005. The study has been approved by the ethical review board of the NHRI, Taiwan.
Study Population
Disease and breast cancer status (yes or no) were defined as if a subject ever received care for that specific disease or cancer from any outpatient clinic at least twice or ever been hospitalized due to the specific disease or cancer (based on the major diagnostic code). The earlier date of the visits of the outpatient clinic or the hospitalization was assigned as the date of diagnosis.
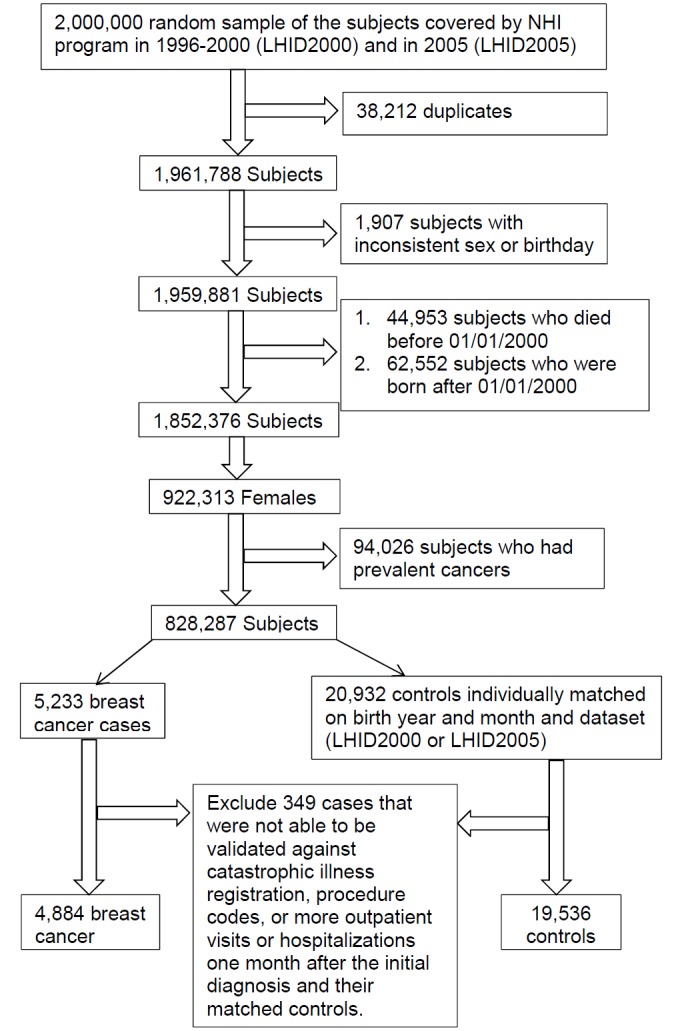
Fig 1 shows the procedure of the selection of the study population. 38,212 duplicates were excluded from the combined sample. After carefully screening for the basic insured information, inpatient, and outpatient data files, we also excluded 1,907 subjects with inconsistent sex or birthday. 44,953 subjects who were dead before January 1, 2000 were excluded from the LHID2000 and 62,552 subjects who were born after January 1, 2000 were excluded from both the LHID2000 and LHID2005 because they were not at risk of developing cancer during the entire observation period. Among the remaining 922,313 women, we further excluded those with diagnosis of benign or malignant neoplasms (ICD9: 140–239 or A-code: A08-A17) in 2000 and 2001 (considered as prevalent cases, N = 94,026). Cancers diagnosed after 2002 were defined as newly developed. Among the remaining subjects, we identified 5,233 newly diagnosed breast cancer cases between year 2002 and 2010. Four controls were selected and individually matched to the index case by the birth year and month, and the dataset (LHID2000 or LHID2005). The index dates were defined as the date of the breast cancer diagnosis for the case and the index case’s breast cancer diagnosis date for the controls. The cases were then validated by the catastrophic illness registration (HV), procedure codes, or more outpatient visits or hospitalization one month after the initial diagnosis.
Fig 1. Selection of study population.
Statistical Analysis
The breast cancer incidence was calculated from the whole LHID2000 and LHID2005, respectively. Medical histories based on the first three digits of the ICD-9 and the corresponding A-code and aggregated medical conditions based on literature review were retrieved for each case and control (S1 Table). The pre-designed medical condition list includes breast diseases, diseases in hormone modulation, diseases in endocrine system, metabolism related diagnosis, and diseases attributed to common risk factors, e.g., diet and alcohol. Only the disease status prior to the index date was considered. Conditional logistic regression models were used to calculated the odds ratios (OR) and 95% confidence intervals (CI) of the associations between medical conditions and breast cancer risk. The confounding factors included were occupation, number of screening test before the index date (0, 1 and ≥2 times), and the average outpatient visit 6 months prior to the index date. The occupation at the index date was used as a surrogate for socioeconomic status. Occupation was categorized into groups: civil servants, teachers, employees of governmental or private businesses, professionals, and technicians; farmers or fishermen; and low income family supported by social welfare or veterans, people without specific employer, or self-employed people, and the others [18]. The screening variable was retrieved from the outpatient data file with a diagnosed code of V761. The heterogeneity between age groups was estimated by using the I 2 statistics which represents the percentage of total variation contributed by between-study variance [19]. Sensitivity analyses were performed by excluding the casesets that the cases were not validated.
Analyses were performed using SAS 9.3. All tests were two sided and statistical significance was assessed at the level of 0.05.
Results
Among the 5,233 newly diagnosed breast cancer cases, 2,526 were identified from the LHID2000 and the other 2,707 were identified from the LHID2005 (Table 1). Among these subjects, 4,884 cases can be validated. The calculated age-standardized incidence rates were 42/100,000 in LHID2000 and 44/100,000 in LHID2005 (data not shown). Cases tended to receive one or more screening tests, but there were still more than 90% cases who did not receive any screening before the breast cancer diagnosis. Cases also had higher average outpatient visits than the controls (Table 1).
Table 1. Characteristic distribution of the study population.
| LHID 2005 | LHID 2000 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Case | Control | Case | Control | |||||||
| N | % | N | % | P 1 | N | % | N | % | p 1 | |
| Total | 2514 | 10056 | 2370 | 9480 | ||||||
| Age | ||||||||||
| <40 | 325 | 12.9 | 1300 | 12.9 | Matched | 292 | 12.3 | 1,165 | 12.3 | Matched |
| 40–45 | 342 | 13.6 | 1367 | 13.6 | 316 | 13.3 | 1,271 | 13.4 | ||
| 45–50 | 463 | 18.4 | 1849 | 18.4 | 440 | 18.6 | 1,764 | 18.6 | ||
| 50–55 | 410 | 16.3 | 1643 | 16.3 | 386 | 16.3 | 1,535 | 16.2 | ||
| 55–60 | 289 | 11.5 | 1154 | 11.5 | 308 | 13.0 | 1,232 | 13.0 | ||
| > = 60 | 685 | 27.2 | 2743 | 27.3 | 628 | 26.5 | 2,513 | 26.5 | ||
| Index year | ||||||||||
| 2002–2004 | 634 | 25.2 | 2536 | 25.2 | Matched | 649 | 27.4 | 2,596 | 27.4 | Matched |
| 2005–2007 | 883 | 35.1 | 3532 | 35.1 | 780 | 32.9 | 3,120 | 32.9 | ||
| 2008–2010 | 997 | 39.7 | 3988 | 39.7 | 941 | 39.7 | 3,764 | 39.7 | ||
| Occupation | ||||||||||
| Civil servants, teachers, governmental or private business employees, professionals, and technicians | 1,452 | 57.8 | 5,593 | 55.6 | <0.01 | 1,346 | 56.8 | 5,407 | 57.0 | <0.01 |
| Farmers or fishermen | 277 | 11.0 | 1,608 | 16.0 | 284 | 12.0 | 1,414 | 14.9 | ||
| Low income families supported by social welfare or veterans, or supported by another family member | 785 | 31.2 | 2,855 | 28.4 | 740 | 31.2 | 2,659 | 28.0 | ||
| Number of screen tests | ||||||||||
| 0 | 9,663 | 384.4 | 2,286 | 22.7 | <0.01 | 9,128 | 385.1 | 2,175 | 22.9 | <0.01 |
| 1 | 338 | 13.4 | 196 | 1.9 | 290 | 12.2 | 162 | 1.7 | ||
| > = 2 | 55 | 2.2 | 32 | 0.3 | 62 | 2.6 | 33 | 0.3 | ||
| Average ambulatory visit per year | ||||||||||
| ≤6 | 371 | 14.8 | 1,755 | 17.5 | <0.01 | 359 | 15.1 | 1,907 | 20.1 | <0.01 |
| 6–12 | 585 | 23.3 | 2,434 | 24.2 | 574 | 24.2 | 2,267 | 23.9 | ||
| 12–24 | 878 | 34.9 | 3,450 | 34.3 | 835 | 35.2 | 3,138 | 33.1 | ||
| >24 | 680 | 27.0 | 2,417 | 24.0 | 602 | 25.4 | 2,168 | 22.9 | ||
1 Chi-square test or Fisher’s exact test if any number in a cell is less than five.
Medical conditions that presented in at least five cases and were statistically significant associated with breast cancer (p<0.05) in LHID2005 were validated in the LHID2000 and the dataset that combining LHID2000 and LHID2005. Table 2 presents the significant associations in all three datasets. As expected, various forms of breast disease were statistically significant associated with breast cancer. Conversely, general medical examinations (V70) were inversely associated with breast cancer (OR = 0.87, 95% CI = 0.81–0.94).
Table 2. Associations that are significant 1 between prior medical conditions and subsequent breast cancer risk.
| ICD9 | Disease | LHID2005 | LHID2000 | LHID2005+LHID2000 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Case | OR 2 | 95% CI | Control | Case | OR 2 | 95% CI | Control | Case | OR 2 | 95% CI | ||
| 217 | Benign neoplasm of breast | 181 | 136 | 2.95 | (2.33, 3.73) | 172 | 127 | 2.86 | (2.26, 3.64) | 353 | 263 | 2.90 | (2.46, 3.43) |
| 611 | Other disorders of breast | 613 | 327 | 2.20 | (1.90, 2.55) | 604 | 352 | 2.45 | (2.11, 2.83) | 1,217 | 679 | 2.32 | (2.09, 2.57) |
| 239 | Neoplasms of unspecified nature | 80 | 36 | 1.68 | (1.13, 2.50) | 80 | 40 | 2.01 | (1.36, 2.97) | 160 | 76 | 1.85 | (1.40, 2.44) |
| 523 | Gingival and periodontal diseases | 5,708 | 1,568 | 1.24 | (1.12, 1.37) | 5,193 | 1,420 | 1.18 | (1.07, 1.31) | 10,901 | 2,988 | 1.21 | (1.13, 1.30) |
| 521 | Diseases of hard tissues of teeth | 5,397 | 1,477 | 1.20 | (1.09, 1.32) | 4,927 | 1,359 | 1.21 | (1.09, 1.33) | 10,324 | 2,836 | 1.20 | (1.12, 1.29) |
| 272 | Disorders of lipoid metabolism | 1,828 | 535 | 1.15 | (1.02, 1.30) | 1,560 | 457 | 1.12 | (0.98, 1.27) | 3,388 | 992 | 1.13 | (1.04, 1.24) |
| 789 | Other symptoms involving abdomen and pelvis | 3,143 | 774 | 0.89 | (0.80, 0.99) | 2,776 | 682 | 0.88 | (0.79, 0.98) | 5,919 | 1,456 | 0.88 | (0.82, 0.95) |
| V70 | General medical examination | 3,248 | 795 | 0.86 | (0.78, 0.96) | 2,904 | 722 | 0.88 | (0.79, 0.98) | 6,152 | 1,517 | 0.87 | (0.81, 0.94) |
| 724 | Other and unspecified disorders of back | 3,951 | 945 | 0.83 | (0.75, 0.92) | 3,594 | 868 | 0.83 | (0.74, 0.92) | 7,545 | 1,813 | 0.83 | (0.77, 0.89) |
| 784 | Symptoms involving head and neck | 3,395 | 821 | 0.86 | (0.77, 0.95) | 3,006 | 695 | 0.78 | (0.70, 0.87) | 6,401 | 1,516 | 0.82 | (0.76, 0.88) |
1 P<0.05 with at least 5 exposed cases.
2 Conditional logistic regression based on the criteria that the disease must be 0.5 years prior to the index date. Models were adjusted for occupation, screen tests (never, once, and twice or above), and average ambulatory visit per year
Diseases may be presented in aggregated forms and the codings may depend on the physicians’ practice. Table 3 shows the associations between the selected medical conditions and breast cancer risks. Of note, breast cancer cases had 18.0 (95% CI = 16.4–19.8) times more likely to have breast diseases prior to the breast cancer diagnosis than their matched controls. The risk was greatly reduced if we re-defined a one-year lag time between the breast disease and the breast cancer diagnosis (OR = 2.67, 95% CI = 2.45–2.92). However, the new definition did not change the associations between other medical conditions and breast cancer risks significantly. Further exclude cases who had other newly diagnosed malignant cancers prior their breast cancer diagnosis did not affect the association materially.
Table 3. The association between selected medical conditions and breast cancer risks.
| Conditional Logistic Regression | Define disease ≥1 year prior index date | Further exclude cases who had prior cancer history | Further adjust for occupation, screening, and the average outpatient visit per year | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Case | OR | 95% CI | Control | Case | OR | 95% CI | Control | Case | OR | 95% CI | OR | 95% CI | |
| Breast disease | 3,131 | 1,897 | 18.0 | (16.4, 19.8) | 1,705 | 967 | 2.67 | (2.45, 2.92) | 1,668 | 943 | 2.66 | (2.43, 2.91) | 2.47 | (2.26, 2.71) |
| Benign neoplasm of breast | 411 | 1,104 | 13.5 | (11.9, 15.3) | 353 | 263 | 3.12 | (2.65, 3.68) | 339 | 253 | 3.11 | (2.63, 3.68) | 2.90 | (2.46, 3.43) |
| Disorders of breast | 1,602 | 2,402 | 11.6 | (10.1, 12.7) | 1,447 | 798 | 2.50 | (2.28, 2.75) | 1,417 | 780 | 2.50 | (2.27, 2.75) | 2.31 | (2.10, 2.55) |
| Alcohol-related diagnosis | 119 | 37 | 1.10 | (0.67, 1.80) | 109 | 33 | 1.15 | (0.69, 1.92) | 108 | 33 | 1.22 | (0.83, 1.81) | 1.12 | (0.67, 1.87) |
| Metabolic disorders (any 3 of the following) | 1,258 | 365 | 1.19 | (1.05, 1.35) | 1,080 | 312 | 1.18 | (1.03, 1.35) | 1,043 | 300 | 1.17 | (1.02, 1.35) | 1.09 | (0.95, 1.26) |
| Hypertensive diseases | 5,471 | 1,545 | 1.26 | (1.17, 1.37) | 5,065 | 1,409 | 1.22 | (1.12, 1.32) | 4,904 | 1,357 | 1.20 | (1.11, 1.31) | 1.14 | (1.05, 1.25) |
| Diabetes mellitus | 2,658 | 758 | 1.19 | (1.08, 1.30) | 2,433 | 685 | 1.16 | (1.06, 1.28) | 2,357 | 663 | 1.16 | (1.05, 1.28) | 1.09 | (0.98, 1.20) |
| Disorders of lipoid metabolism | 3,766 | 1,101 | 1.26 | (1.16, 1.36) | 3,388 | 992 | 1.25 | (1.15, 1.36) | 3,273 | 958 | 1.25 | (1.15, 1.36) | 1.13 | (1.04, 1.24) |
| Overweight and obesity | 170 | 59 | 1.39 | (1.03, 1.88) | 143 | 55 | 1.55 | (1.13, 2.11) | 139 | 53 | 1.53 | (1.12, 2.11) | 1.43 | (1.04, 1.96) |
| Cholelithiasis or disorders of the gallbladder | 717 | 203 | 1.14 | (0.97, 1.34) | 623 | 176 | 1.14 | (0.96, 1.35) | 607 | 165 | 1.09 | (0.92, 1.30) | 1.05 | (0.88, 1.25) |
| Benign neoplasm of ovary | 324 | 105 | 1.31 | (1.05, 1.64) | 269 | 88 | 1.32 | (1.03, 1.69) | 263 | 81 | 1.24 | (0.96, 1.60) | 1.21 | (0.95, 1.56) |
| Ovarian dysfunction | 204 | 58 | 1.14 | (0.85, 1.53) | 178 | 55 | 1.24 | (0.91, 1.68) | 178 | 53 | 1.19 | (0.88, 1.63) | 1.17 | (0.86, 1.59) |
| Noninflammatory disorders of ovary, fallopian tube, and broad ligament | 260 | 73 | 1.13 | (0.87, 1.47) | 238 | 66 | 1.11 | (0.84, 1.46) | 233 | 63 | 1.08 | (0.82, 1.44) | 1.05 | (0.79, 1.39) |
| Disorders of thyroid gland | 1,449 | 431 | 1.21 | (1.08, 1.35) | 1,298 | 379 | 1.18 | (1.05, 1.33) | 1,263 | 360 | 1.15 | (1.02, 1.30) | 1.11 | (0.98, 1.25) |
| Endometriosis | 370 | 137 | 1.51 | (1.24, 1.85) | 303 | 111 | 1.49 | (1.19, 1.86) | 297 | 108 | 1.48 | (1.18, 1.85) | 1.44 | (1.15, 1.80) |
| Uterine leiomyoma | 903 | 311 | 1.42 | (1.24, 1.63) | 758 | 242 | 1.30 | (1.12, 1.52) | 743 | 236 | 1.30 | (1.11, 1.51) | 1.20 | (1.03, 1.40) |
| Disorders of parathyroid gland | 35 | 14 | 1.60 | (0.86, 2.98) | 30 | 9 | 1.20 | (0.57, 2.53) | 29 | 9 | 1.24 | (0.59, 2.62) | 1.14 | (0.54, 2.43) |
| Benign neoplasma of rectum and anal canal | 37 | 6 | 0.65 | (0.27, 1.54) | 32 | 5 | 0.63 | (0.24, 1.61) | 32 | 3 | 0.38 | (0.12, 1.23) | 0.62 | (0.24, 1.61) |
Table 4 displays the associations between the selected medical conditions and breast cancer risk by age group. Prior breast diseases were associated with breast cancer risk in both age groups. Obesity, leiomyoma, hypertensive diseases and disorders of lipid metabolism were associated with breast cancer risk only in the older group, but there were no differences in the strength of the associations (pheterogeneity = 0.47 for obesity, pheterogeneity = 0.20 leiomyoma, pheterogeneity = 0.54 for hypertensive diseases and pheterogeneity = 0.39 for disorders of lipid metabolism).
Table 4. The association between selected medical conditions and breast cancer risks by age at breast cancer diagnosis.
| <50 | ≥50 | I 2 | p for heterogeneity between the two age groups | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Name | Control | Case | OR | 95% CI | Control | Case | OR | 95% CI | ||
| Breast disease | 849 | 498 | 2.76 | (2.42, 3.14) | 856 | 469 | 2.24 | (1.97, 2.55) | 79.5% | 0.027 |
| Benign neoplasm of breast | 180 | 139 | 3.24 | (2.57, 4.10) | 173 | 124 | 2.65 | (2.08, 3.36) | 27.2% | 0.241 |
| Disorders of breast | 715 | 418 | 2.64 | (2.30, 3.03) | 732 | 380 | 2.04 | (1.78, 2.35) | 84.4% | 0.011 |
| Alcohol-related diagnosis | 27 | 11 | 1.59 | (0.79, 3.23) | 39 | 8 | 0.81 | (0.37, 1.75) | 16.8% | 0.273 |
| Metabolic disorders (any 3 of the following) | 78 | 22 | 1.00 | (0.61, 1.62) | 1002 | 290 | 1.09 | (0.95, 1.27) | 0.0% | 0.719 |
| Hypertensive diseases | 640 | 180 | 1.09 | (0.91, 1.30) | 4425 | 1229 | 1.16 | (1.05, 1.28) | 0.0% | 0.535 |
| Diabetes mellitus | 292 | 86 | 1.10 | (0.85, 1.41) | 2141 | 599 | 1.08 | (0.97, 1.21) | 0.0% | 0.918 |
| Disorders of lipoid metabolism | 483 | 136 | 1.05 | (0.85, 1.28) | 2905 | 856 | 1.16 | (1.05, 1.28) | 0.0% | 0.386 |
| Overweight and obesity | 64 | 21 | 1.24 | (0.75, 2.04) | 79 | 34 | 1.59 | (1.05, 2.40) | 0.0% | 0.465 |
| Cholelithiasis or disorders of the gallbladder | 154 | 39 | 1.00 | (0.70, 1.42) | 469 | 137 | 1.07 | (0.88, 1.31) | 0.0% | 0.734 |
| Benign neoplasm of ovary | 200 | 58 | 1.08 | (0.79, 1.46) | 69 | 30 | 1.56 | (1.00, 2.41) | 31.6% | 0.227 |
| Ovarian dysfunction | 139 | 40 | 1.11 | (0.77, 1.58) | 39 | 15 | 1.43 | (0.78, 2.62) | 0.0% | 0.526 |
| Noninflammatory disorders of ovary, fallopian tube, and broad ligament | 176 | 41 | 0.87 | (0.62, 1.23) | 62 | 25 | 1.58 | (0.98, 2.55) | 63.1% | 0.100 |
| Disorders of thyroid gland | 558 | 164 | 1.14 | (0.95, 1.38) | 740 | 215 | 1.09 | (0.93, 1.28) | 0.0% | 0.708 |
| Endometriosis | 227 | 82 | 1.42 | (1.10, 1.85) | 76 | 29 | 1.50 | (0.97, 2.33) | 0.0% | 0.844 |
| Uterine leiomyoma | 460 | 130 | 1.11 | (0.90, 1.37) | 298 | 112 | 1.37 | (1.09, 1.73) | 40.5% | 0.195 |
| Disorders of parathyroid gland | 9 | 7 | 3.15 | (1.16, 8.51) | 21 | 2 | 0.34 | (0.08, 1.49) | 53.5% | 0.142 |
| Benign neoplasma of rectum and anal canal | 6 | 2 | 1.33 | (0.27, 6.60) | 26 | 3 | 0.47 | (0.14, 1.56) | 0.0% | 0.603 |
Discussion
In addition to benign breast diseases, which have been recognized as a marker of enhanced risk or a precursor of breast cancer [20], endometriosis and uterine leiomyoma have yield strong association with a subsequent breast cancer. Although the association between diseases and breast cancer risks did not differ by age group, young patients had more uterine leiomyoma (6%) and endometriosis (4%) than the older patients (4% and 1%, respectively).
Estrogen has been implicated in numerous diseases, including cancers of the breast, ovary, colorectal, and endometrial cancers, cardiovascular diseases, osteoporosis, neurodegenerative diseases, insulin resistance, endometriosis, uterine leiomyoma, and obesity [21,22]. A prior study on estrogen-related cancer described increased incidences of breast, uterine, and ovarian cancers and a similar bell-shaped age-specific incidence curve for breast and uterine cancers in Taiwan [7]. Consistent with these results, we also observed strong associations of endometriosis and uterine leiomyoma with breast cancer. On the other hand, estrogen reduces risks of cardiovascular diseases, osteoporosis, and neurodegenerative diseases [23,24]. We observed null association between these diseases and breast cancer (data not shown). Both osteoporosis and neurodegenerative diseases mainly affect the elderly. In our study, we only consider medical conditions prior breast cancer diagnosis. With the improvement of breast cancer survival, it may be interesting to explore the impact of osteoporosis and neurodegenerative disease on breast cancer prognosis.
The association between endometriosis and breast cancer had been reported previously, but the results were inconsistent [25]. However, most of previous studies were conducted in Western countries. A summary result based on a recent review [25] yield a marginal association. A recent publication using the same dataset as ours reported null-association between newly diagnosed endometriosis and future breast cancer risks (HR = 1.15, 95% CI = 0.61–2.15) [26]. The study included 2266 endometriosis cases and 9064 age-matched controls selected from the outpatient dataset. The median age of the study population was 31–40 years old. This study accumulated 69 breast cancer cases in five years (2003–2008). In our study, we examined the association between breast cancer and pre-existing medical conditions; endometriosis is one of the selected medical conditions. The median age of breast cancer diagnosis was 51 years old and it was 46 years old among those who had history of endometriosis. The differences in age distribution and the length of observation period could be the reasons for the discrepancy. Furthermore, our controls were selected from the beneficiaries’ registration data file, which included healthy women who never used the service. That is, the background risk of breast cancer may be different among the two control groups.
We identified three publications [10,27,28] on uterine leiomyoma and breast cancer, but none of these studies reported an association.
Although ovarian diseases share risk factors with breast cancer in endogenous estrogen and reproductive characteristics, most studies failed to observe an association, except one study [29]. However, this study [29] included endometriosis in the ovarian diseases, which may explain the observed associations. Of noted, previous studies suggested that inclusion of women who received oophorectomy may be the reason for the null association between endometriosis or ovarian disease and breast cancer risks [30,31].
Metabolic disorders have been associated with several types of cancer, including the breast cancer [11,32]. Metabolic syndrome and its individual components (except for glucose) were negatively associated with premenopausal breast cancer [11], but positively associated with postmenopausal breast cancer [32]. An earlier NHIRD study, comparing diabetes patients to the age-matched controls, resulted in higher hazard ratio for the older group (≥65 years old, HR = 1.61, 95% CI = 1.45–1.78) [33]. Although diabetes was associated with breast cancer, it loses its association when stratified by age. Nevertheless, the ORs were similar. The null associations could be due to small sample size in subgroups. Adjusting for the average number of outpatient visit decreased the risk estimate, which may suggest the presence of detection bias in diabetic patients. Mutual adjusted for the other metabolic disorders, i.e. hypertensive diseases, diabetes mellitus, disorders of lipoid metabolism, and overweight and obesity, did not change the associations materially (data not shown).
We did not observe an association between thyroid diseases and breast cancer risk. An elevated breast cancer risk is well documented in thyroid cancer patients [34,35,36], particularly in young women [34,35]. A meta-analysis study has reported an association between thyroid diseases and breast cancer [37] although the conclusion is not definitely [38]. It has been suggested that the increased breast cancer risk could be attributed to the iodine 131I treatment [27]; however, the evidence was not conclusive, either [39]. Recent evidence of urinary estrogen DNA-adducts suggested that estrogen may act like chemicals to activate the carcinogenic metabolites and initiates both thyroid and breast cancers [40,41].
Most studies on polyps or cholelithiasis and breast cancer were based on the common risk factor theory, e.g. diet. However, except for a few studies [42,43], most studies reported null association, as well as ours. In 2010, the International Agency for Research on Cancer pronounced that “the occurrence of malignant tumors of the oral cavity… and female breast is causally related to consumption of alcoholic beverage” [44]. We examined the association between alcohol-related disorders and breast cancer risk to explore the role of alcohol on breast cancer. However, we did not observe an association between the diseases. This could be because that Taiwanese woman had lower prevalence of heavy episodic drinking (defined as ≥60g pure alcohol at one occasion during the past month, 12% in America, 13% in Europe, and 1.6% in Taiwan) [45,46], which might suggest that alcohol play a minor role in breast cancer in Taiwan.
Other associations, such as gingival and periodontal diseases or unspecified disorders of back are difficult to explain. We cannot rule out the possibility of false findings, although the strengths of associations were similar in both LHID2000 and LHID2005. Nevertheless, it is reasonable that general medical examination (ICD9: V70) was inversely associated with breast cancer risks. It is likely that the use of general medical examination results in early detection of pre-malignant lesions, thus the patient could receive proper treatments before the lesion progresses to a malignant tumor. On the other hand, increased medical surveillance may increase the possibility of detecting a cancer.
Other limitations of the present analyses include that we did not consider the treatment, duration, and the severity of the preceding diseases, as well as the behavior risk factors, in the current analyses. The increased or decreased breast cancer risk could be a result of the treatment of the previous disease or the shared risk factors; therefore, the associations do not imply causality in etiology. Secondly, chance findings could be resulted from multiple comparisons; these results have to be interpreted with causation. Thirdly, having some diseases, e.g. metabolic syndrome, might imply an increased medical surveillance, thus may increase the probability of detecting a breast cancer, and vice versa. These were evident in higher number of screening tests and average ambulatory visit per year in Table 1. This limitation could result in another problem—misclassification—in our study, e.g. the strong association between breast disease and breast cancer. To deal with the problem, we set several lag time to evaluate the impact of misclassification (S2 Table). The association dropped to a reasonable range after a three-month lag time. Another source of misclassification could be due to disease definition. We cannot rule out the possibility of misclassification with our disease definition. In general, increasing the number of claim records is a common strategy to improve accuracy [47,48]. In our dataset, most subjects with medical conditions still had outpatient visits or hospitalization records one month after the initial diagnosis, e.g. >95% for hypertension, diabetes, and disorders of lipid metabolism and >80% for breast diseases, leiomyoma, and endometriosis. However, polyps or benign neoplasm of rectum and anal canal, obesity and overweight and noninflammatory disorders of ovary, fallopian rube, and broad ligament had relatively low validation rate by more outpatient visits or hospitalization records (60%~70%). Because we dealt with cases and controls in the same way, this type of misclassification is usually non-differential between cases and controls. We expected the bias would be toward the null. Fifthly, family history is also a risk factor for breast cancer, particularly among the young patients. It is possible that there may be related subjects in the study population. However, due to the anonymous nature of the database, it is impossible for us to identify the related subjects. Finally, we do not have menopausal status in the dataset; however, matching on age enables us to partially control for this factor.
In general, the use of claim data reduces the possibility of recall bias and assures a relatively completed medical history. Both hypotheses- and data-driven analyses reached similar conclusions. The results from the data-driven analyses may worth for further investigation.
In conclusion, our results suggest that estrogen-related factors may play an important role in breast cancer risks in the Taiwanese female population. It may be worthy of an investigation on the effects of endocrine-disrupting chemicals on estrogen-related diseases, such as endometriosis, uterine leiomyoma, and the related cancers.
Supporting Information
(DOCX)
(DOCX)
Acknowledgments
The study is supported by the National Health Research Institutes PH-104-PP-15. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations
- CI
confidence interval
- ICD9
International Classification of Diseases version 9
- LHID
Longitudinal Health Insurance Database
- NHI
National Health Insurance
- NHIRD
National Health Insurance Research Database
- OR
odds ratio
- NHRI
National Health Research Institutes
Data Availability
All data in the study are available upon request to the National Health Research Institute. Due to legal restrictions imposed by the government of Taiwan in relation to the “Personal Information Protection Act”, data cannot be made publicly available. Requests for data can be sent as a formal proposal to the NHIRD (http://nhird.nhri.org.tw/index1.php)
Funding Statement
The study is supported by the National Health Research Institutes (PH-104-PP-15). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Bray F, Ren JS, Masuyer E, Ferlay J. Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int. J. Cancer. 2013;132:1133–1145. 10.1002/ijc.27711 [DOI] [PubMed] [Google Scholar]
- 2. Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J. Clin. Oncol. 2006;24:2137–2150. [DOI] [PubMed] [Google Scholar]
- 3. Youlden DR, Cramb SM, Dunn NA, Muller JM, Pyke CM, Baade PD. The descriptive epidemiology of female breast cancer: an international comparison of screening, incidence, survival and mortality. Cancer Epidemiol. 2012;36:237–248. 10.1016/j.canep.2012.02.007 [DOI] [PubMed] [Google Scholar]
- 4. Bhoo-Pathy N, Yip CH, Hartman M, Uiterwaal CS, Devi BC, Peeters PH et al. Breast cancer research in Asia: adopt or adapt Western knowledge? Eur. J. Cancer. 2013;49:703–709. 10.1016/j.ejca.2012.09.014 [DOI] [PubMed] [Google Scholar]
- 5. Shen YC, Chang CJ, Hsu C, Cheng CC, Chiu CF, Cheng AL. Significant difference in the trends of female breast cancer incidence between Taiwanese and Caucasian Americans: implications from age-period-cohort analysis. Cancer Epidemiol. Biomarkers Prev. 2005;14:1986–1990. [DOI] [PubMed] [Google Scholar]
- 6. Axelrod D, Smith J, Kornreich D, Grinstead E, Singh B, Cangiarella J et al. Breast cancer in young women. J. Am. Coll. Surg. 2008;206:1193–1203. 10.1016/j.jamcollsurg.2007.12.026 [DOI] [PubMed] [Google Scholar]
- 7. Lin CH, Chen YC, Chiang CJ, Lu YS, Kuo KT, Huang CS et al. The emerging epidemic of estrogen-related cancers in young women in a developing Asian country. Int. J. Cancer. 2012;130:2629–2637. 10.1002/ijc.26249 [DOI] [PubMed] [Google Scholar]
- 8. Lin CH, Liau JY, Lu YS, Huang CS, Lee WC, Kuo KT et al. Molecular subtypes of breast cancer emerging in young women in Taiwan: evidence for more than just westernization as a reason for the disease in Asia. Cancer Epidemiol. Biomarkers Prev. 2009;18:1807–1814. 10.1158/1055-9965.EPI-09-0096 [DOI] [PubMed] [Google Scholar]
- 9. Veli EM Schatzkin A. Common Environmental risk factors. 1999;213–223. [Google Scholar]
- 10. Franceschi S, la VC, Negri E, Parazzini F, Boyle P. Breast cancer risk and history of selected medical conditions linked with female hormones. Eur. J. Cancer. 1990;26:781–785. [DOI] [PubMed] [Google Scholar]
- 11. Bjorge T, Lukanova A, Jonsson H, Tretli S, Ulmer H, Manjer J et al. Metabolic syndrome and breast cancer in the me-can (metabolic syndrome and cancer) project. Cancer Epidemiol. Biomarkers Prev. 2010;19:1737–1745. 10.1158/1055-9965.EPI-10-0230 [DOI] [PubMed] [Google Scholar]
- 12. Gong Z, Quan L, Yao S, Zirpoli G, Bandera EV, Roberts M et al. Innate immunity pathways and breast cancer Risk in African American and European-American women in the Women's Circle of Health Study (WCHS). PLoS. One. 2013;8:e72619 10.1371/journal.pone.0072619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hill DA, Preston-Martin S, Ross RK, Bernstein L. Medical radiation, family history of cancer, and benign breast disease in relation to breast cancer risk in young women, USA. Cancer Causes Control. 2002;13:711–718. [DOI] [PubMed] [Google Scholar]
- 14. Wu CY, Chen YJ, Ho HJ, Hsu YC, Kuo KN, Wu MS et al. Association between nucleoside analogues and risk of hepatitis B virus-related hepatocellular carcinoma recurrence following liver resection. JAMA. 2012;308:1906–1914. [DOI] [PubMed] [Google Scholar]
- 15. Wu CY, Chan FK, Wu MS, Kuo KN, Wang CB, Tsao CR et al. Histamine2-receptor antagonists are an alternative to proton pump inhibitor in patients receiving clopidogrel. Gastroenterology. 2010;139:1165–1171. 10.1053/j.gastro.2010.06.067 [DOI] [PubMed] [Google Scholar]
- 16. Lin CC, Lai MS, Syu CY, Chang SC, Tseng FY. Accuracy of diabetes diagnosis in health insurance claims data in Taiwan. J. Formos. Med. Assoc. 2005;104:157–163. [PubMed] [Google Scholar]
- 17. Cheng CL, Kao YH, Lin SJ, Lee CH, Lai ML. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol. Drug Saf. 2011;20:236–242. 10.1002/pds.2087 [DOI] [PubMed] [Google Scholar]
- 18. Tseng CH Oral cancer in Taiwan: is diabetes a risk factor? Clin. Oral Investig. 2013;17:1357–1364. 10.1007/s00784-012-0820-3 [DOI] [PubMed] [Google Scholar]
- 19. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Degnim AC, Visscher DW, Berman HK, Frost MH, Sellers TA, Vierkant RA et al. Stratification of breast cancer risk in women with atypia: a Mayo cohort study. J. Clin. Oncol. 2007;25:2671–2677. [DOI] [PubMed] [Google Scholar]
- 21. Deroo BJ Korach KS. Estrogen receptors and human disease. J. Clin. Invest. 2006;116:561–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Burns KA Korach KS. Estrogen receptors and human disease: an update. Arch. Toxicol. 2012;86:1491–1504. 10.1007/s00204-012-0868-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Deroo BJ Korach KS. Estrogen receptors and human disease. J. Clin. Invest. 2006;116:561–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Burns KA Korach KS. Estrogen receptors and human disease: an update. Arch. Toxicol. 2012;86:1491–1504. 10.1007/s00204-012-0868-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Munksgaard PS Blaakaer J. The association between endometriosis and gynecological cancers and breast cancer: a review of epidemiological data. Gynecol. Oncol. 2011;123:157–163. 10.1016/j.ygyno.2011.06.017 [DOI] [PubMed] [Google Scholar]
- 26. Kok VC, Tsai HJ, Su CF, Lee CK. The Risks for Ovarian, Endometrial, Breast, Colorectal, and Other Cancers in Women With Newly Diagnosed Endometriosis or Adenomyosis: A Population-Based Study. Int. J. Gynecol. Cancer. 2015;25:968–976. 10.1097/IGC.0000000000000454 [DOI] [PubMed] [Google Scholar]
- 27. Moseson M, Koenig KL, Shore RE, Pasternack BS. The influence of medical conditions associated with hormones on the risk of breast cancer. Int. J. Epidemiol. 1993;22:1000–1009. [DOI] [PubMed] [Google Scholar]
- 28. Talamini R, Franceschi S, Favero A, Negri E, Parazzini F, la VC. Selected medical conditions and risk of breast cancer. Br. J. Cancer. 1997;75:1699–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jung SJ, Song M, Choi JY, Song N, Park SK, Yoo KY et al. Association of selected medical conditions with breast cancer risk in Korea. J. Prev. Med. Public Health. 2013;46:346–352. 10.3961/jpmph.2013.46.6.346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schairer C, Persson I, Falkeborn M, Naessen T, Troisi R, Brinton LA. Breast cancer risk associated with gynecologic surgery and indications for such surgery. Int. J. Cancer. 1997;70:150–154. [DOI] [PubMed] [Google Scholar]
- 31. Weiss HA, Brinton LA, Potischman NA, Brogan D, Coates RJ, Gammon MD et al. Breast cancer risk in young women and history of selected medical conditions. Int. J. Epidemiol. 1999;28:816–823. [DOI] [PubMed] [Google Scholar]
- 32. Esposito K, Chiodini P, Colao A, Lenzi A, Giugliano D. Metabolic syndrome and risk of cancer: a systematic review and meta-analysis. Diabetes Care. 2012;35:2402–2411. 10.2337/dc12-0336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen HF, Liu MD, Chen P, Chen LH, Chang YH, Wen PC et al. Risks of Breast and Endometrial Cancer in Women with Diabetes: A Population-Based Cohort Study. PLoS. One. 2013;8:e67420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vassilopoulou-Sellin R, Palmer L, Taylor S, Cooksley CS. Incidence of breast carcinoma in women with thyroid carcinoma. Cancer. 1999;85:696–705. [DOI] [PubMed] [Google Scholar]
- 35. Chen AY, Levy L, Goepfert H, Brown BW, Spitz MR, Vassilopoulou-Sellin R. The development of breast carcinoma in women with thyroid carcinoma. Cancer. 2001;92:225–231. [DOI] [PubMed] [Google Scholar]
- 36. Chuang SC, Hashibe M, Yu GP, Le AD, Cao W, Hurwitz EL et al. Radiotherapy for primary thyroid cancer as a risk factor for second primary cancers. Cancer Lett. 2006;238:42–52. [DOI] [PubMed] [Google Scholar]
- 37. Hardefeldt PJ, Eslick GD, Edirimanne S. Benign thyroid disease is associated with breast cancer: a meta-analysis. Breast Cancer Res. Treat. 2012;133:1169–1177. 10.1007/s10549-012-2019-3 [DOI] [PubMed] [Google Scholar]
- 38. Simon MS, Tang MT, Bernstein L, Norman SA, Weiss L, Burkman RT et al. Do thyroid disorders increase the risk of breast cancer? Cancer Epidemiol. Biomarkers Prev. 2002;11:1574–1578. [PubMed] [Google Scholar]
- 39. Hieu TT, Russell AW, Cuneo R, Clark J, Kron T, Hall P et al. Cancer risk after medical exposure to radioactive iodine in benign thyroid diseases: a meta-analysis. Endocr. Relat Cancer. 2012;19:645–655. 10.1530/ERC-12-0176 [DOI] [PubMed] [Google Scholar]
- 40. Gaikwad NW, Yang L, Pruthi S, Ingle JN, Sandhu N, Rogan EG et al. Urine biomarkers of risk in the molecular etiology of breast cancer. Breast Cancer (Auckl.). 2009;3:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zahid M, Goldner W, Beseler CL, Rogan EG, Cavalieri EL. Unbalanced estrogen metabolism in thyroid cancer. Int. J. Cancer. 2013;133:2642–2649. 10.1002/ijc.28275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lowenfels AB, Domellof L, Lindstrom CG, Bergman F, Monk MA, Sternby NH. Cholelithiasis, cholecystectomy, and cancer: a case-control study in Sweden. Gastroenterology. 1982;83:672–676. [PubMed] [Google Scholar]
- 43. Johansen C, Chow WH, Jorgensen T, Mellemkjaer L, Engholm G, Olsen JH. Risk of colorectal cancer and other cancers in patients with gall stones. Gut. 1996;39:439–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.IARC Working Group Alcohol Consumption and Ethyl Carbamate (Urethane). 2010; [PMC free article] [PubMed]
- 45.Global status report on alcohol and health 2014. 2014;
- 46.National Health Interview Survey 2009 Report. 2012;
- 47. Lin CC, Lai MS, Syu CY, Chang SC, Tseng FY. Accuracy of diabetes diagnosis in health insurance claims data in Taiwan. J. Formos. Med. Assoc. 2005;104:157–163. [PubMed] [Google Scholar]
- 48. Wu CS, Lai MS, Gau SS, Wang SC, Tsai HJ. Concordance between patient self-reports and claims data on clinical diagnoses, medication use, and health system utilization in Taiwan. PLoS. One. 2014;9:e112257 10.1371/journal.pone.0112257 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
All data in the study are available upon request to the National Health Research Institute. Due to legal restrictions imposed by the government of Taiwan in relation to the “Personal Information Protection Act”, data cannot be made publicly available. Requests for data can be sent as a formal proposal to the NHIRD (http://nhird.nhri.org.tw/index1.php)


