Abstract
Context
Exposure to ozone has acute respiratory effects, but few human clinical studies have evaluated cardiovascular effects.
Objective
We hypothesized that ozone exposure alters pulmonary and systemic vascular function, and cardiac function, with more pronounced effects in subjects with impaired antioxidant defense from deletion of the glutathione S-transferase M1 gene (GSTM1 null).
Methods
24 young, healthy never-smoker subjects (12 GSTM1 null) inhaled filtered air, 100 ppb ozone, and 200 ppb ozone for 3 hours, with intermittent exercise, in a double-blind, randomized, crossover fashion. Exposures were separated by at least 2 weeks. Vital signs, spirometry, arterial and venous blood nitrite levels, impedance cardiography, peripheral arterial tonometry, estimation of pulmonary capillary blood volume (Vc), and blood microparticles and platelet activation were measured at baseline and during 4 hours after each exposure.
Results
Ozone inhalation decreased lung function immediately after exposure (mean±SE change in FEV1, air: −0.03±0.04 L; 200 ppb ozone: −0.30±0.07 L; p<0.001). The immediate post-exposure increase in blood pressure, caused by the final 15-minute exercise period, was blunted by 200 ppb ozone exposure (mean±SE change for air: 16.7±2.6 mm Hg; 100 ppb ozone: 14.5±2.4 mm Hg; 200 ppb ozone: 8.5±2.5 mm Hg; p=0.02). We found no consistent effects of ozone on any other measure of cardiac or vascular function. All results were independent of the GSTM1 genotype.
Conclusions
We did not find convincing evidence for early acute adverse cardiovascular consequences of ozone exposure in young healthy adults. The ozone-associated blunting of the blood-pressure response to exercise is of unclear clinical significance.
Keywords: Air pollution, ozone, vascular, human, cardiac
INTRODUCTION
Exposure to ambient air pollution increases the risk for acute cardiovascular events. Increases in ambient particulate matter (PM) are associated with cardiovascular mortality, acute myocardial infarction, congestive heart failure, cardiac arrhythmias, and stroke (Frampton and Utell, 2006, U.S. EPA, 2009, Brook et al., 2010), and it is now generally accepted that the relationship is causal.
Ozone is a widespread ground-level air pollutant with well-documented acute respiratory effects (Frampton, 2011). However, it remains unclear whether ozone exposure has acute cardiovascular effects. A few recent time-series studies have found significant relationships between ozone levels and cardiovascular mortality (Bell et al., 2004, Gryparis et al., 2004, Bell et al., 2005). These associations were independent of PM exposures (Bell et al., 2007), and were seen at ozone concentrations well below the U.S. National Ambient Air Quality Standard (NAAQS), which was recently reduced from 80 to 75 ppb averaged over 8 hours. Deaths increased 0 to 3 days after increases in ozone levels. In Houston, Texas, ozone and PM exposure were significantly associated with out-of-hospital cardiac arrest (Ensor et al., 2013).
There is also evidence for long-term cardiovascular effects of ozone exposure. In an analysis using cohort data from the American Cancer Society (Jerrett et al., 2009), ozone exposure was associated with long-term respiratory and cardiovascular mortality, but cardiovascular mortality was no longer significant when PM2.5 concentrations were considered in two-pollutant models. However, in a subsequent analysis using improved individual exposure assessment (Jerrett et al., 2013), ozone exposure was significantly associated with deaths from ischemic heart disease. Few human clinical studies have examined the acute cardiovascular effects of ozone exposure, and those findings are inconsistent. Thus, uncertainty remains regarding ozone cardiovascular effects.
Oxidative stress is a fundamental mechanism proposed for air pollutant cardiovascular effects (Romieu et al., 2008). Oxidative stress from pollutant exposure is linked experimentally with vascular endothelial dysfunction (Nurkiewicz et al., 2006), in part due to reduced bioavailability of nitric oxide (NO). We hypothesized that the reactive products of ozone exposure injure pulmonary vascular endothelium as well as the airway epithelium, reducing NO bioavailability and transport, thereby impairing both pulmonary and systemic vascular function, with indirect effects on cardiac function.
If the oxidative stress hypothesis for effects of air pollution is correct, then people with impaired antioxidant defenses should be at increased risk. Prior studies have linked deletion of the glutathione S-transferase M1 gene (GSTM1 null) with increased susceptibility to PM effects on heart rate variability (Schwartz et al., 2005) and respiratory function (Romieu et al., 2004). We further hypothesized that GSTM1 null subjects would show enhanced effects of ozone on cardiovascular function.
METHODS
Study Population
The study was approved by the Research Subjects Review Board of the University of Rochester Medical Center, and informed, written consent was obtained from all subjects. We recruited 24 healthy never-smoker subjects 18 to 40 years of age, 12 GSTM1 null and 12 wild-type (GSTM1+). Subjects were without pulmonary or cardiovascular disease and had normal spirometry, a normal electrocardiogram, and a negative urine pregnancy test (females). Subjects were instructed to exclude anti-inflammatory drugs for the duration of the study and to exclude caffeine, large fatty meals, and vigorous exercise during and 24 hours before study days. Subjects were not studied within 6 weeks of a respiratory infection.
Protocol
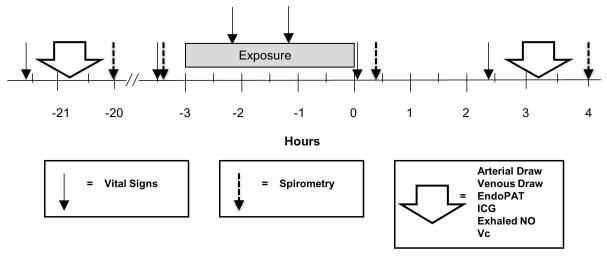
The study used a double-blinded, randomized (blocked by order of presentation and genotype), crossover design. The protocol is summarized in Figure 1. The exposure concentrations, duration, and exercise level were chosen to be sufficient to induce pulmonary function changes in some subjects, but still be relevant to ambient outdoor exposure scenarios. Subjects followed a low nitrate diet beginning with the evening meal 2 days prior to each exposure session, to minimize dietary effects on plasma nitrate measurements. They attended the Clinical Research Center the day before exposure and vital signs and baseline study measurements were obtained. Subjects were then transported to a nearby hotel where they ate a standardized meal and spent the night. After a standardized breakfast subjects were transported to the Medical Center for the experimental exposure. Subjects underwent 3-hour exposures to filtered air, 100 ppb ozone, or 200 ppb ozone, with alternating 15-minute periods of rest and exercise on a bicycle ergometer (target minute ventilation 25 L/min/m2 body surface area). Our facility for controlled human clinical studies, and methods for ozone generation and monitoring, have been described previously (Frampton et al., 1997). Temperature and relative humidity were maintained at approximately 21°C and 40%, respectively. The three exposure sessions were separated by at least 2 weeks.
Figure 1.
Protocol. Timing of outcome measures are indicated by the vertical arrows. Measurements and procedures were completed in the same order over a period of approximately 1.5 hours. ICG: Impedance cardiography; NO: Nitric oxide; EndoPAT: peripheral arterial tonometry; Vc: estimation of pulmonary capillary blood volume.
Health Measurements
Determination of GSTM1 status utilized polymerase chain reaction (PCR) amplification of exons 4 and 5 of the GSTM1 allele, using PCR primers and conditions described by Schwartz et al (Schwartz et al., 2005) with appropriate controls. Vital signs were measured immediately before, twice during, immediately after, and 2.5 hours after each exposure. Subjects scored 17 symptoms on a questionnaire, using a continuous scale, 30 min before and 5 min after each exposure. Spirometry (Model CPF-S, Medical Graphics, St. Paul, MN) was performed according to standards established by the American Thoracic Society, the day before, immediately after, and 4 hours after exposure.
The following outcomes were measured in the same order, over a period of 1.5 hours the day before and beginning approximately 2.5 hours after exposure, at approximately the same time of day. 1. The pulmonary capillary blood volume (Vc) was estimated using measurements of the diffusing capacities for carbon monoxide and nitric oxide, and the subject’s hemoglobin level (Guenard et al., 1987). 2. NO bioavailability and consumption were estimated by simultaneously measuring arterial and venous whole blood nitrite levels, as previously described (Morgan et al., 2010). 3. Changes in cardiac function, including cardiac index, stroke-volume index, and left ventricular ejection time were estimated noninvasively using impedance cardiography (Yancy and Abraham, 2003). 4. Changes in circulating blood microparticles and platelet activation, as indicators of potential for thrombosis, were measured using flow cytometry, as previously described (Stewart et al., 2010). 5. Airway NO production was measured using multiple exhalation flow rates and a 2-compartment model, as a noninvasive measure of airway inflammation (Pietropaoli et al., 1999). 6. Systemic vascular endothelial function was measured by determining the reactive hyperemia index (RHI) using peripheral artery tonometry (EndoPAT 2000, Itamar Medical Ltd., Cesare, Israel).
Statistics
The design of the study was a 3 by 3 crossover trial with 24 subjects exposed to clean air and ozone concentrations of 100 and 200 ppb. The wash out period was 2 weeks between exposures. The timing of the outcome measures is shown in Figure 1. We used two linear mixed models with random subject effects, adjusting for baseline by including the baseline measurement in the response vector (Jones and Kenward, 2003). In the primary analysis model, the independent variables were order, time, exposure, and exposure by time interaction. The secondary analysis examined ozone response by genotype, and the independent variables were order, time, exposure, exposure by time interaction, exposure by gene interaction, time by gene interaction, and time by exposure by gene (three-way) interaction.
Symptom scores were compared using the Wilcoxon sign-rank test.
RESULTS
Subjects and Exposures
Twenty-four subjects (12 GSTM1 null) completed the study. Table 1 shows the subject characteristics, according to genotype.
Table 1.
Subject characteristics*.
| GSTM1 null | GSTM1+ | |
|---|---|---|
| Age, yr | 27.3 (4.2) | 25.4 (2.8) |
| Sex, M/F | 7/5 | 8/4 |
| FEV1, % predicted | 98.3 (11.0) | 99.0 (12.6) |
| FEV1/FVC, % | 82.3 (4.9) | 86.3 (4.3) |
| BSA, m2 | 1.87 (0.18) | 1.91 (0.23) |
Data are means (SD). FEV1, forced expiratory volume in 1 sec; FVC, forced vital capacity; BSA, body surface area.
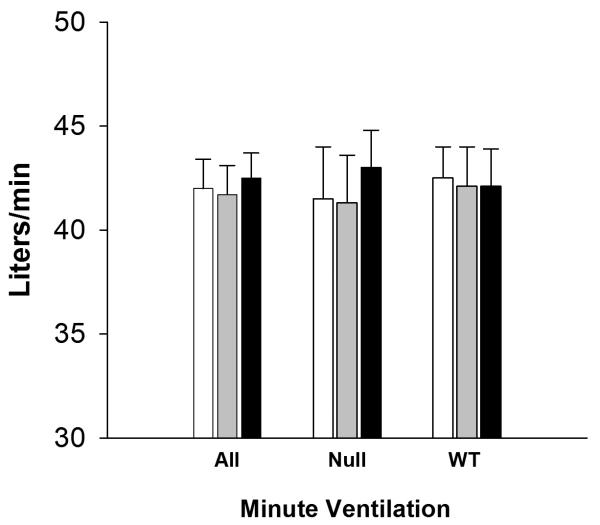
Mean±SD ozone concentrations were near the target values for air, 100 ppb, and 200 ppb ozone, respectively: 4±1, 104±12, and 202±14 ppb. The average minute ventilation during exercise did not differ by exposure, or by genotype (Figure 2).
Figure 2.
Minute ventilation during exercise (means (SD)) for exposures to clean air (open bars), 100 ppb ozone (shaded bars), 200 ppb ozone (black bars).
Symptoms
Ozone exposure increased the total symptom score (increase following air: 2.75; 100 ppb: 3.25; 200 ppb: 4.71; p<0.005 for air vs 200 ppb), chest tightness (increase following air: 0.04; 100 ppb: 0.04; 200 ppb: 0.79; p<0.005 for air vs 200 ppb), and throat irritation (increase following air: 0.00; 100 ppb: 0.00; 200 ppb: 0.13; p<0.01 for air vs 200 ppb). There were no significant changes in other symptoms, including fast heartbeat, irregular heartbeat, or shortness of breath.
Lung Function
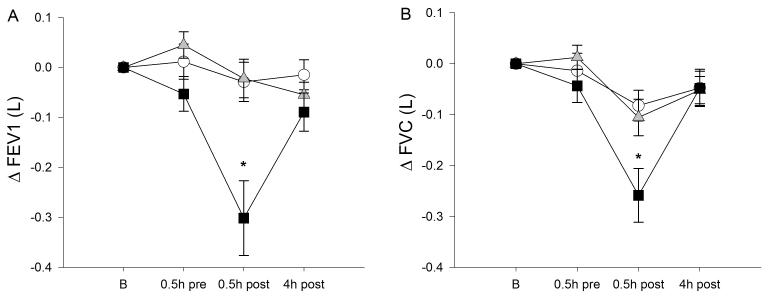
Table 2 and Figure 3 show the effects of ozone exposure on lung function. As expected, the forced expiratory volume at 1 sec (FEV1) and forced vital capacity (FVC) declined immediately following ozone exposure in a concentration-dependent fashion, and largely recovered by 4 hours after exposure. FEV1/FVC and the forced expiratory flow at 25 to 75% of FVC (FEF25-75) also decreased following ozone exposure. These findings indicate both restrictive and obstructive effects. There were no significant differences in ozone effects on lung function by genotype. There were no ozone effects on airway production of NO.
Table 2.
Spirometry*.
| Air | Ozone 100 ppb |
Ozone 200 ppb |
Effect, p | |
|---|---|---|---|---|
|
|
||||
| FEV1 (L) | E, p=0.02 T×E, p<0.001 |
|||
| Before | 3.89 (0.19) | 3.88 (0.18) | 3.92 (0.19) | |
| 20 min after | 3.87 (0.20) | 3.86 (0.19) | 3.62 (0.19) | |
| 4 h after | 3.87 (0.19) | 3.83 (0.19) | 3.83 (0.18) | |
| Max change (%) | −0.59 (1.08) | −0.58 (1.03) | −7.85 (1.80) | |
| FVC (L) | E, p=0.08 T×E, p=0.0015 |
|||
| Before | 4.71 (0.22) | 4.72 (0.22) | 4.72 (0.22) | |
| 20 min after | 4.65 (0.23) | 4.62 (0.22) | 4.46 (0.22) | |
| 4 h after | 4.66 (0.22) | 4.67 (0.22) | 4.67 (0.21) | |
| Max change (%) | −1.81 (0.69) | −2.26 (0.86) | −5.56 (1.09) | |
| FEV1/FVC (%) | E, p=0.046 T×E, p<0.001 |
|||
| Before | 82.5 (1.1) | 82.3 (1.2) | 83.0 (1.1) | |
| 20 min after | 83.2 (1.4) | 83.7 (1.2) | 80.9 (1.4) | |
| 4 h after | 83.3 (1.4) | 82.0 (1.2) | 81.9 (1.1) | |
| Max change (%) | 1.19 (0.88) | 1.79 (0.71) | −2.55 (1.09) | |
Data are means (SE). % change data are shown for comparison with other studies; statistical analyses were performed using the actual measurements. FEV1, forced expiratory volume in 1 sec; FVC, forced vital capacity; FEF25-75, forced expiratory flow at 25 to 75% of FVC; E, exposure; T×E, interaction between time of measurement and exposure in the primary mixed models analysis.
Figure 3.
Change in FEV1 (A) and FVC (B) following exposure to clean air (open circles), 100 ppb ozone (shaded triangles), and 200 ppb ozone (solid squares). FEV1 and FVC decreased immediately after ozone exposure (asterisk: time × exposure, p<0.001). Data are means (SE).
Vital Signs
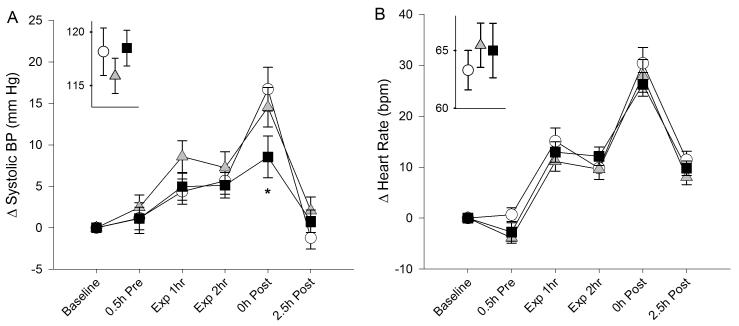
As shown in Table 3 and Figure 4, both blood pressure and heart rate increased immediately after exposure, relative to baseline measurements, reflecting the effects of exercise during the last 15 minutes of exposure. Analysis revealed a significant exposure-time interaction (p=0.02) with a blunting of the increase in systolic blood pressure at the end of the 200 ppb ozone exposure, compared with air. The mean blood pressure was similarly affected, but changes in diastolic blood pressure were not significant. There were no significant differences in heart rate or oxygen saturation, and no influence of GSTM1 genotype on the ozone effects.
Table 3.
Blood pressure (BP)*.
| Air | Ozone 100 ppb | Ozone 200 ppb | Effect, p | |
|---|---|---|---|---|
|
|
||||
| Systolic BP | T×E, p=0.02 | |||
| Before | 118.2 (2.2) | 115.9 (1.7) | 118.5 (1.7) | |
| Exposure 1 hr | 122.5 (1.7) | 124.5 (1.8) | 123.5 (1.4) | |
| Exposure 2 hr | 123.8 (1.6) | 123.2 (2.0) | 123.6 (1.6) | |
| 0 h after | 134.9 (2.1) | 130.5 (1.9) | 127.0 (2.2) | |
| 2.5 h after | 117.0 (2.2) | 118.0 (2.0) | 119.3 (2.2) | |
| Diastolic BP | NS | |||
| Before | 64.1 (1.6) | 63.6 (1.4) | 64.7 (1.7) | |
| Exposure 1 h | 77.3 (1.3) | 76.3 (1.6) | 77.1 (1.3) | |
| Exposure 2 h | 76.9 (1.2) | 77.6 (1.2) | 78.9 (1.2) | |
| 0 h after | 78.5 (1.9) | 76.5 (1.5) | 74.8 (1.4) | |
| 2.5 h after | 63.5 (1.3) | 64.6 (1.4) | 63.3 (1.3) | |
Data are means (SE). E, exposure; T×E, interaction between time of measurement and exposure in the primary mixed models analysis; NS, not significant.
Figure 4.
Change in systolic blood pressure (A) and heart rate (B) following exposure to clean air (open circles), 100 ppb ozone (shaded triangles), and 200 ppb ozone (solid squares). Baseline measurements are shown in the insets. “Exp” denotes measurements obtained at rest during exposure. “0h Post” measurements were obtained immediately after the final exercise period. The exercise-related increase in blood pressure immediately after air exposure was blunted after 120 ppb ozone exposure (asterisk: time-exposure interaction p=0.02). Data are means (SE).
Measures of Cardiac and Vascular Function
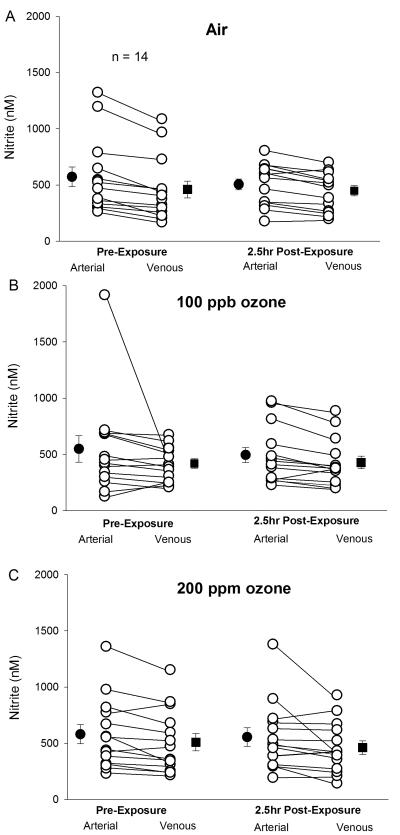
There were no significant effects of ozone exposure on Vc, any parameter of impedance cardiography, reactive hyperemia measured with peripheral arterial tonometry, blood microparticles or platelet activation, or arterial and venous measurements of NO. There were missing data for measurement of blood NO parameters, because of difficulty obtaining arterial blood from some subjects. Figure 5 shows arterial and venous measurements of whole blood nitrite before and 2.5 hours after exposure for the 14 subjects with complete data for all exposures. Of these, 3 were female and 7 were GSTM1 null. As expected, nitrite levels were lower in the venous than the arterial circulation, consistent with nitrite consumption in the peripheral circulation (Morgan et al., 2010). However, there were no effects of ozone exposure on this arterial-venous difference.
Figure 5.
Plasma nitrite in arterial and venous blood obtained simultaneously from the same arm, before and 2.5 hours after each exposure, for the 14 subjects with complete data. Filled symbols are means (SE).
DISCUSSION
We exposed 24 healthy subjects to two concentrations of ozone and clean air, using a combination of ozone concentration, exposure time, and exercise intensity designed to cause an acute decrement in lung function in some subjects. The primary objective was to determine whether such ozone exposures can alter vascular or cardiac function in a way that might be considered adverse for people with critical cardiovascular disease. Our primary focus was on changes in endothelial function and in NO bioavailability, both of which play fundamental rolls in the early genesis and manifestation of atherosclerotic cardiovascular disease.
We did see the expected concentration-related reductions in lung function immediately after exposure to ozone. The 200 ppb concentration of ozone would also be expected to increase airway inflammation. We saw no increase in airway NO production. Although it is likely that insufficient time had elapsed for the maximum inflammatory response to develop, other clinical studies with later measurements (Barath et al., 2013b) have also failed to detect ozone-induced increases in exhaled NO, suggesting that ozone-induced airway inflammation may not increase airway NO production.
In this study, 200 ppb ozone appeared to blunt the exercise-induced increase in blood pressure immediately after exposure. This was an unexpected finding, and could represent a chance finding. However, other studies describe findings consistent with ours. In a panel study of 70 subjects with type 2 diabetes (Hoffmann et al., 2012), an increase of 13.3 ppb in the 5-day average ozone concentration was associated with a decrease in the systolic blood pressure of 5.2 mmHg. Another human clinical study (Barath et al., 2013a) found that exposure to 300 ppb ozone for 75 min, with intermittent exercise, enhanced, rather than suppressed, brachial artery vasodilator responses to intra-arterial infusions of acetylcholine and nitroprusside. Increased responsiveness of vascular smooth muscle to vasodilators is consistent with our observation of blunted increases in blood pressure following exercise. The potential mechanisms could involve alterations in autonomic nervous system output or vascular tone, leading to decreases in systemic vascular resistance. The clinical significance of these observations are not clear at this time.
Panel and other epidemiological studies have shown associations between ozone levels and increases in blood pressure and heart rate (Cakmak et al., 2011), changes in the T wave of the ECG (Bhaskaran et al., 2011), increases in ischemic cardiovascular events (Bogdanov and Osterud), bradycardia in high risk infants (Peel et al., 2011), and ventricular tachycardia (Bartell et al., 2013). However, in panel and epidemiology studies where multiple ambient pollutants are involved, it is difficult to disentangle the effects of ozone from other pollutants including PM.
We did not find ozone effects on Vc, systemic vascular function, platelet activation, circulating microparticles, NO bioavailability, or noninvasively-measured cardiac function. We prospectively stratified subjects by GSTM1 genotype, but found no interactions between genotype and any of the study outcomes. This study did not find evidence for acute adverse cardiovascular effects of ozone exposure.
There have been few previous human clinical studies primarily focusing on the cardiovascular effects of ozone. Drechsler-Parks (Drechsler-Parks, 1995) found that 2-h exposures of 8 healthy subjects to 0.6 ppm NO2 plus 0.45 ppm ozone, with intermittent exercise, reduced the cardiac output (measured noninvasively by impedance cardiography) relative to air exposure (neither pollutant alone caused a significant effect). This could have been the result of increased pulmonary vascular resistance and reduced left ventricular filling. Gong et al. (Gong Jr. et al., 1998), using right heart catheterization to directly assess cardiac function, studied 10 hypertensive and six healthy males exposed to air or 300 ppb ozone for 3 h, with intermittent exercise. Ozone exposure increased the heart rate and the rate-pressure product, suggesting that ozone exposure may increase myocardial oxygen demand and worsen ischemia in patients with coronary artery disease. There were no effects on the cardiac index, pulmonary artery pressure, or pulmonary capillary wedge pressure. This study was limited by a nonrandomized exposure sequence (air always first), air and ozone exposures on consecutive days, and no delayed measurements of hemodynamic parameters. These design issues were necessitated by the use of an invasive procedure to assess cardiovascular function, and may have biased the study toward a negative result.
Devlin et al. (Devlin et al., 2012) examined the cardiovascular and inflammatory effects of exposure to 300 ppb ozone vs. air for 2 hours, with intermittent exercise in healthy subjects. High-frequency heart rate variability decreased significantly immediately after exposure, and there was elevation of some circulating markers of inflammation, including interleukin-8, interleukin-1, and C-reactive protein. There was a statistically significant but very small (1.2%) increase in the QTc interval, a measure of cardiac repolarization. These exposures also caused airway inflammation and reductions in lung function, as expected, and the observed acute changes in heart rate variability may have been caused in part by the respiratory effects. In contrast, Barath et al. (Barath et al., 2013a) did not find ozone effects on heart rate variability, while Fakhri et al. (Fakhri et al., 2009) found that ozone exposure increased HF heart rate variability (p=0.051).
Inhalation of both concentrated ambient air particles and ozone for 2 hours at rest was reported to cause acute vascular and cardiac effects (Brook et al., 2002, Urch et al., 2005), which were not reproduced by exposure to ozone alone (Fakhri et al., 2009, Urch et al., 2010, Sivagangabalan et al., 2011, Kusha et al., 2012).
The effects of ozone exposure on sympathetic nerve activity was studied recently (Tank et al., 2011). 14 healthy subjects inhaled clean air and 250 ppb ozone for 3 hours with intermittent exercise, and cardiovascular measures were performed approximately 20 hours after exposure. There were no effects of ozone exposure on heart rate, blood pressure, cardiac output measured by the inert gas re-breathing method, plasma norepinephrine levels, or muscle sympathetic nerve activity. Overall, these essentially negative studies are consistent with our observed absence of major cardiovascular effects of ozone.
Strengths of our study included controlled exposures to a single pollutant under consistent conditions, two concentrations of ozone that were relevant to ambient levels in polluted cities, clean air control exposures, the well-characterized group of healthy subjects, and the inclusion of a genetically susceptible group. Among the weaknesses were a relatively small number of subjects, and limitation of the study to healthy subjects who are less likely to be susceptible to clinically important effects of air pollution. In addition, some of our pulmonary and systemic vascular markers were measured at a single post-exposure interval, and we cannot exclude the possibility that effects on these markers occurred either before or after our measurements.
The relative absence of cardiovascular effects observed in our study, when considered with the relative paucity of observed cardiovascular effects in other clinical and panel studies, suggests that ozone exposure in healthy subjects, at concentrations sufficient to evoke airway responses, may not have significant acute adverse cardiovascular effects, beyond those that represent physiologic responses to the known pulmonary effects. However, further clinical studies are needed to confirm this finding.
Acknowledgments
This research has been funded by grants from the National Institutes of Health (RC1 ES018519, R01 ES017428, P30 ES01247, UL1 RR024160, T32 007271), ExxonMobil, and CONCAWE (Conservation of Air and Water in Europe).
Footnotes
DECLARATION OF INTEREST
The authors report no declarations of interest.
REFERENCES
- Barath S, Langrish JP, Lundbäck M, Bosson JA, Goudie C, Newby DE, Sandström T, Mills NL, Blomberg A. Ozone exposure does not impair vascular function or affect heart rate variability in humans. Toxicol Sci. 2013a;135:292–299. doi: 10.1093/toxsci/kft157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barath S, Mills N, Adelroth E, Olin A-C, Blomberg A. Diesel exhaust but not ozone increases fraction of exhaled nitric oxide in a randomized controlled experimental exposure study of healthy human subjects. Environmental Health. 2013b;12:36. doi: 10.1186/1476-069X-12-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartell SM, Longhurst J, Tjoa T, Sioutas C, Delfino RJ. Particulate air pollution, ambulatory heart rate variability, and cardiac arrhythmia in retirement community residents with coronary artery disease. Environ Health Perspect. 2013;121:1135–1141. doi: 10.1289/ehp.1205914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell ML, Dominici F, Samet JM. A meta-analysis of time-series studies of ozone and mortality with comparison to the national morbidity, mortality, and air pollution study. Epidemiology. 2005;16:436–445. doi: 10.1097/01.ede.0000165817.40152.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell ML, Kim JY, Dominici F. Potential confounding of particulate matter on the short-term association between ozone and mortality in multisite time-series studies. Environ Health Perspect. 2007;115:1591–1595. doi: 10.1289/ehp.10108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell ML, McDermott A, Zeger SL, Samet JM, Dominici F. Ozone and short-term mortality in 95 US urban communities, 1987-2000. JAMA. 2004;292:2372–2378. doi: 10.1001/jama.292.19.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskaran K, Hajat S, Armstrong B, Haines A, Herrett E, Wilkinson P, Smeeth L. The effects of hourly differences in air pollution on the risk of myocardial infarction: case crossover analysis of the MINAP database. BMJ. 2011;343:d5531. doi: 10.1136/bmj.d5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanov VY, Osterud B. Cardiovascular complications of diabetes mellitus: The Tissue Factor perspective. Thromb Res. 2010;125:112–118. doi: 10.1016/j.thromres.2009.06.033. [DOI] [PubMed] [Google Scholar]
- Brook RD, Brook JR, Urch B, Vincent R, Rajagopalan S, Silverman F. Inhalation of fine particulate air pollution and ozone causes acute arterial vasoconstriction in healthy adults. Circulation. 2002;105:1534–1536. doi: 10.1161/01.cir.0000013838.94747.64. [DOI] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S, Pope CA, 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC, Jr., Whitsel L, Kaufman JD. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- Cakmak S, Dales R, Leech J, Liu L. The influence of air pollution on cardiovascular and pulmonary function and exercise capacity: Canadian Health Measures Survey (CHMS) Environ Res. 2011;111:1309–1312. doi: 10.1016/j.envres.2011.09.016. [DOI] [PubMed] [Google Scholar]
- Devlin RB, Duncan KE, Jardim M, Schmitt MT, Rappold AG, Diaz-Sanchez D. Controlled exposure of healthy young volunteers to ozone causes cardiovascular effects. Circulation. 2012;126:104–111. doi: 10.1161/CIRCULATIONAHA.112.094359. [DOI] [PubMed] [Google Scholar]
- Drechsler-Parks DM. Cardiac output effects of O3 and NO2 exposure in healthy older adults. Toxicol Ind Health. 1995;11:99–109. doi: 10.1177/074823379501100109. [DOI] [PubMed] [Google Scholar]
- Ensor KB, Raun LH, Persse D. A Case-Crossover Analysis of Out-of-Hospital Cardiac Arrest and Air Pollution. Circulation. 2013;127:1192–1199. doi: 10.1161/CIRCULATIONAHA.113.000027. [DOI] [PubMed] [Google Scholar]
- Fakhri AA, Ilic LM, Wellenius GA, Urch B, Silverman F, Gold DR, Mittleman MA. Autonomic effects of controlled fine particulate exposure in young healthy adults: effect modification by ozone. Environ Health Perspect. 2009;117:1287–1292. doi: 10.1289/ehp.0900541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frampton MW. Ozone air pollution: how low can you go? Am J Respir Crit Care Med. 2011;184:150–151. doi: 10.1164/rccm.201104-0606ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frampton MW, Morrow PE, Torres A, Cox C, Voter KZ, Utell MJ. Ozone responsiveness in smokers and nonsmokers. Am J Respir Crit Care Med. 1997;155:116–121. doi: 10.1164/ajrccm.155.1.9001299. [DOI] [PubMed] [Google Scholar]
- Frampton MW, Utell MJ. Exposure to airborne particles: health effects and mechanisms. Clin Occup Environ Med. 2006;5:747–898. [Google Scholar]
- Gong H, Jr., Wong R, Sarma RJ, Linn WS, Sullivan ED, Shamoo DA, Anderson KR, Prasad SB. Cardiovascular effects of ozone exposure in human volunteers. Am J Respir Crit Care Med. 1998;158:538–546. doi: 10.1164/ajrccm.158.2.9709034. [DOI] [PubMed] [Google Scholar]
- Gryparis A, Forsberg B, Katsouyanni K, Analitis A, Touloumi G, Schwartz J, Samoli E, Medina S, Anderson HR, Niciu EM, Wichmann HE, Kriz B, Kosnik M, Skorkovsky J, Vonk JM, Dortbudak Z. Acute Effects of Ozone on Mortality from the “Air Pollution and Health: A European Approach” Project. Am J Respir Crit Care Med. 2004;170:1080–1087. doi: 10.1164/rccm.200403-333OC. [DOI] [PubMed] [Google Scholar]
- Guenard H, Varene N, Vaida P. Determination of lung capillary blood volume and membrane diffusing capacity in man by the measurements of NO and CO transfer. Respir Physiol. 1987;70:113–120. doi: 10.1016/s0034-5687(87)80036-1. [DOI] [PubMed] [Google Scholar]
- Hoffmann B, Luttmann-Gibson H, Cohen A, Zanobetti A, De Souza C, Foley C, Suh HH, Coull BA, Schwartz J, Mittleman M, Stone P, Horton E, Gold DR. Opposing effects of particle pollution, ozone, and ambient temperature on arterial blood pressure. Environ Health Perspect. 2012;120:241–246. doi: 10.1289/ehp.1103647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrett M, Burnett RT, Beckerman BS, Turner MC, Krewski D, Thurston G, Martin RV, Van Donkelaar A, Hughes E, Shi Y, Gapstur SM, Thun MJ, Pope CA. Spatial analysis of air pollution and mortality in California. Am J Respir Crit Care Med. 2013;188:593–599. doi: 10.1164/rccm.201303-0609OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrett M, Burnett RT, Pope CA, 3rd, Ito K, Thurston G, Krewski D, Shi Y, Calle E, Thun M. Long-term ozone exposure and mortality. N Engl J Med. 2009;360:1085–1095. doi: 10.1056/NEJMoa0803894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B, Kenward MG. Design and analysis of cross-over trials. Chapman and Hall; New York, NY: 2003. [Google Scholar]
- Kusha M, Masse S, Farid T, Urch B, Silverman F, Brook RD, Gold DR, Mangat I, Speck M, Nair K, Poku K, Meyer C, Mittleman MA, Wellenius GA, Nanthakumar K. Controlled Exposure Study of Air Pollution and T-Wave Alternans in Volunteers without Cardiovascular Disease. Environ Health Perspect. 2012;120:1157–1161. doi: 10.1289/ehp.1104171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MA, Frasier LM, Stewart JC, Mack CM, Gough MS, Graves BT, Apostolakos MJ, Doolin KP, Darling DC, Frampton MW, Pietropaoli AP. Artery-to-vein differences in nitric oxide metabolites are diminished in sepsis. Crit Care Med. 2010;38:1069–1077. doi: 10.1097/CCM.0b013e3181d16a3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurkiewicz TR, Porter DW, Barger M, Millecchia L, Rao KM, Marvar PJ, Hubbs AF, Castranova V, Boegehold MA. Systemic microvascular dysfunction and inflammation after pulmonary particulate matter exposure. Environ Health Perspect. 2006;114:412–419. doi: 10.1289/ehp.8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peel JL, Klein M, Flanders WD, Mulholland JA, Freed G, Tolbert PE. Ambient air pollution and apnea and bradycardia in high-risk infants on home monitors. Environ Health Perspect. 2011;119:1321–1327. doi: 10.1289/ehp.1002739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietropaoli AP, Perillo IB, Torres A, Perkins PT, Frasier LM, Utell MJ, Frampton MW, Hyde RW. Simultaneous measurement of nitric oxide production by conducting and alveolar airways of humans. J Appl Physiol. 1999;87:1532–1542. doi: 10.1152/jappl.1999.87.4.1532. [DOI] [PubMed] [Google Scholar]
- Romieu I, Castro-Giner F, Kunzli N, Sunyer J. Air pollution, oxidative stress and dietary supplementation: a review. Eur Respir J. 2008;31:179–197. doi: 10.1183/09031936.00128106. [DOI] [PubMed] [Google Scholar]
- Romieu I, Sienra-Monge JJ, Ramirez-Aguilar M, Moreno-Macias H, Reyes R, N. I, Estela Del Rio-Navarro B. Genetic polymorphism of GSTM1 and antioxidant supplementation influence lung function in relation to ozone exposure in asthmatic children in Mexico City. Thorax. 2004;59:8–10. [PMC free article] [PubMed] [Google Scholar]
- Schwartz J, Park SK, O'neill MS, Vokonas PS, Sparrow D, Weiss S, Kelsey K. Glutathione-S-transferase M1, obesity, statins, and autonomic effects of particles: gene-by-drug-by-environment interaction. Am J Respir Crit Care Med. 2005;172:1529–1533. doi: 10.1164/rccm.200412-1698OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivagangabalan G, Spears D, Masse S, Urch B, Brook RD, Silverman F, Gold DR, Lukic KZ, Speck M, Kusha M, Farid T, Poku K, Shi E, Floras J, Nanthakumar K. The effect of air pollution on spatial dispersion of myocardial repolarization in healthy human volunteers. J Am Coll Cardiol. 2011;57:198–206. doi: 10.1016/j.jacc.2010.08.625. [DOI] [PubMed] [Google Scholar]
- Stewart JC, Chalupa DC, Devlin RB, Frasier LM, Huang L-S, Little EL, Lee SM, Phipps RP, Pietropaoli AP, Taubman MB, Utell MJ, Frampton MW. Vascular effects of ultrafine particles in persons with type 2 diabetes. Environ Health Perspect. 2010;118:1692–1698. doi: 10.1289/ehp.1002237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tank J, Biller H, Heusser K, Holz O, Diedrich A, Framke T, Koch A, Grosshennig A, Koch W, Krug N, Jordan J, Hohlfeld JM. Effect of acute ozone induced airway inflammation on human sympathetic nerve traffic: a randomized, placebo controlled, crossover study. PLoS One. 2011;6:e18737. doi: 10.1371/journal.pone.0018737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. EPA . Integrated Science Assessment for Particulate Matter (Final Report) U.S. Environmental Protection Agency; Washington, DC: 2009. EPA/600/R-08/139F. [PubMed] [Google Scholar]
- Urch B, Silverman F, Corey P, Brook JR, Lukic KZ, Rajagopalan S, Brook RD. Acute blood pressure responses in healthy adults during controlled air pollution exposures. Environ Health Perspect. 2005;113:1052–1055. doi: 10.1289/ehp.7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urch B, Speck M, Corey P, Wasserstein D, Manno M, Lukic KZ, Brook JR, Liu L, Coull B, Schwartz J, Gold DR, Silverman F. Concentrated ambient fine particles and not ozone induce a systemic interleukin-6 response in humans. Inhal Toxicol. 2010;22:210–218. doi: 10.3109/08958370903173666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancy C, Abraham W. Noninvasive hemodynamic monitoring in heart failure: utilization of impedance cardiography. Congestive Heart Failure. 2003;9:241–250. doi: 10.1111/j.1751-7133.2003.tb00021.x. [DOI] [PubMed] [Google Scholar]