Abstract
Toxoplasmosis is the clinical and pathological consequence of acute infection with the obligate intracellular apicomplexan parasite Toxoplasma gondii. Symptoms result from tissue destruction that accompanies lytic parasite growth. This review updates current understanding of the host cell invasion, parasite replication and eventual egress that comprise the lytic cycle, as well as the ways T. gondii manipulates host cells to assure survival. Since the publication of a previous iteration of this review 15 years ago, important advances have been made in our molecular understanding of parasite growth and mechanisms of host cell egress, and knowledge of the parasite’s manipulation of the host has rapidly progressed. Here we cover molecular advances and current conceptual frameworks that include each of these topics, with an eye to what might be known 15 years from now.
Keywords: Apicomplexa, invasion, egress, endodyogeny, IRG, inflammasome
1. INTRODUCTION
A review of the Toxoplasma gondii lytic cycle first appeared under the same name as this review 15 years ago (17). At that time the lytic cycle was differentiated into five steps: attachment, invasion, vacuole formation, replication, and egress, with calcium signaling identified as a critical regulator. Although these basic steps have not changed, new findings facilitated by advances in technology and the improved experimental access of Toxoplasma have improved our understanding of their connections and details within them. First, as their many shared features have become apparent,, egress is now viewed as linked to and immediately preceding invasion. In addition, the understanding of the structure and function of the parasitophorous vacuole and its interface with the host cell has seen a tumultuous advance over the last fifteen years. Finally, molecular detail on signaling pathways has permeated into each step of the lytic cycle and discussion of them is integrated into each section.
1.1 Toxoplasma gondii
Toxoplasma is a member of the Apicomplexa, a phylum comprising 5000+ protozoa that are almost exclusively obligate intracellular pathogens of both vertebrate and invertebrate animals. Together with the ciliates and dinoflagellates, the Apicomplexa make up the super-phylum of Alveolata, which all share a peripheral alveolar membrane system, which in the Apicomplexa is known as the inner membrane complex (IMC). The parasitic life style of the Apicomplexa contrasts with the variety of life styles found among the ciliates and dinoflagellates. These evolutionary distinctions suggest that the Apicomplexa evolved from free-living, photosynthetic organisms into the diverse obligate intracellular parasites that are observed in extant members. Within the Apicomplexa Toxoplasma is a member of the cyst forming coccidia.
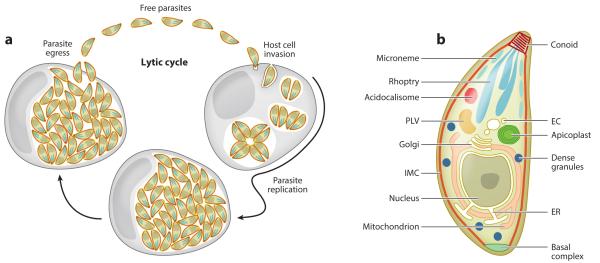
Since an excellent review chronicling the discovery of the Toxoplasma is available (44) we focus here on the key aspects of the parasite’s life cycle. The asexual or vegetative stages of Toxoplasma have been encountered in virtually any mammal and bird tested while the coccidian or sexual development stages are exclusive to cats of any sort (Felids). The typical route of infection for coccidian parasites like Toxoplasma is through sporozoites developing within the oocysts shed in the feces of the definitive host. When an intermediate host ingests sporulated oocyst the sporozoites will excyst in the small intestine and infect epithelial cells. Subsequently, these sporozoites will develop into tachyzoites that replicate inside the parasitophorous vacuole established upon invasion. Following replication they will actively egress from the host cell, which will be destroyed in the process. Freed tachyzoites will continue this cycle of invasion, replication and egress: the lytic cycle (Figure 1a). Tachyzoites spread throughout all tissues in the body until they are controlled by a potent host immune response, which triggers the parasite to differentiate into the bradyzoite stage. Bradyzoites comprise the persistent stage of Toxoplasma infection and are maintained for the life span of the host. Consumption of bradyzoite tissue cysts from infected animals also represents a second route of horizontal transmission and is the route of infection for the definitive (feline) host. Within a host, bradyzoites can differentiate back into tachyzoites upon loss of a potent immune response, which is a major cause of Toxoplasma-associated morbidity and mortality in immune compromised humans. Finally, unlike most coccidians, Toxoplasma is capable of vertical transmission during which tachyzoites cross the placenta and infect the developing fetus (congenital infection).
Figure 1.
The Toxoplasma lytic cycle and basic tachyzoite organization. (a) The lytic cycle of invasion, replication and egress. (b) Organelles in the secretory pathway are shown in white; the actual secretory organelles in three hues of blue. PLV, plant like vacuole; EC endosome compartment; IMC, inner membrane complex; ER, endoplasmic reticulum.
1.2 Toxoplasmosis
Clinical manifestations associated with Toxoplasma infection are very diverse and range from myocarditis, ocular toxoplasmosis (retinochoroiditis, choroiditis), encephalitis, and hydrocephalus to mental diseases (102). Although local rates vary tremendously, on a global scale 1/3 of the human population is infected with Toxoplasma. Clinical disease due to Toxoplasma is not common because it is predominantly the fast replicating tachyzoite stage that is responsible for tissue destruction resulting in clinical manifestations and this stage is typically well-controlled by the host immune response. Bradyzoite infection is latent and does not generate any clear clinical disease. Two conditions provide heightened risks for clinical disease: 1. immunosuppression, which typically results in bradyzoites converting back to the tachyzoites stage; 2. a primary infection during pregnancy which can result in congenital infection of the fetus, which lacks a mature immune system, thus facilitating uncurbed tachyzoite replication.
2. HOST CELL EGRESS AND INVASION
Intracellular and extracellular parasites represent two distinct biological states. Extracellular parasites do not divide, are highly motile, extrude the conoid and secrete the contents of their microneme organelles. Intracellular parasites divide, are non-motile and do not secrete micronemes or extrude their conoid (see Figure 1b for identity of the organelles). The switch between states is accompanied by many dramatic changes in gene expression (55, 84), mRNA availability for translation (87), and translocation of the glycolytic enzymes from the cytoplasm to the cortex (121). Motility, and therefore secretion of motility-associated motors and adhesins from the micronemes, is required for the portion of the parasite lifecycle that spans egress, movement to a new host cell and its subsequent invasion. Because of these similar requirements, egress is considered as the first step in invasion (67). However, our molecular understanding of invasion and egress have begun to indicate that, although gliding and secretion are required for both processes, several aspects are specifically tailored to only either egress or invasion. Host cell calpains, for example, disassemble the host cell’s cytoskeleton to forge a path out for the parasite and are only required for egress (32). The formation of the moving junction (described in detail below) is likewise required only for invasion.
2.1 Triggers and Signaling Toward Egress
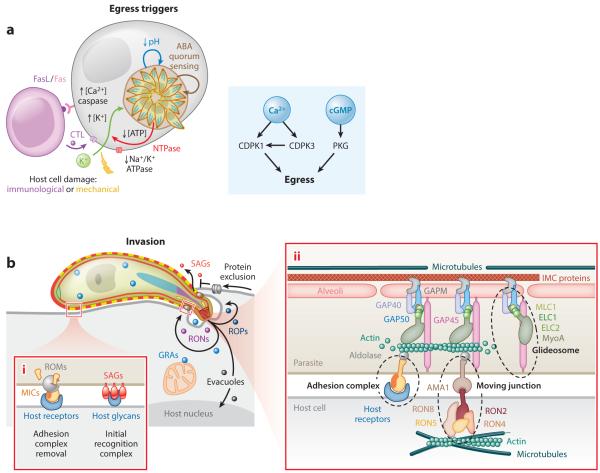
Activation toward the extracellular state can be considered as the first step of the lytic cycle. The proposed triggers can be divided into those that occur in the presence and absence of a host immune response (summarized in Figure 2a). Several arms of host immunity respond to Toxoplasma infection and some are paths toward egress (99, 116, 143). Best understood are perforin and death-receptor (Fas/FasL)-mediated damage to the host cell following a cytotoxic CD8+ T cell (CTL) response (117). Damage to the host cell leads to a drop in intracellular K+, which triggers egress (106).
Figure 2.
Activation of egress, gliding motility and host cell invasion. (a) Schematic overview of described egress stimuli. The cytotoxic T-cell response (CTL) releases perforin inserting in the plasma membrane. Fas receptor activation induces host cell necrosis rather than apoptosis. Triangular insert: summary of Ca2+ and cGMP secondary messenger signaling following triggers to egress. (b) Invasion gliding motility and glideosome composition (modified from (53)); panel 1. Initial attachment and adhesion release. Panel 2. Molecular composition and mechanism of adhesion, gliding motility, the glideosome, and the moving junction. Not depicted is the family of actin binding proteins.
In the absence of an immune response, parasites replicate to maturity and initiate egress through several different mechanisms. One such mechanism involves abscisic acid (ABA), which the parasite continuously produces during intracellular replication. When the concentration of ABA rises above a threshold (quorum sensing), the parasite egresses (109). It is unclear how ABA is sensed or its signal is transduced, but interestingly, pharmacological interference with ABA production leads to enhanced bradyzoite differentiation, indicating ABA may play multiple roles during the parasite’s lifecycle.
In addition, parasite replication leads to acidification of the parasitophorous vacuole. Egress is also triggerred when decreasing pH overcomes the suppression of microneme secretion and motility by K+ ions (124). This implicates K+ in egress, though its principal role appears to be during invasion when the parasite is in the intracellular environment, as will be discussed below. The pH change is especially relevant to perforin like protein 1 (PLP1), a microneme protein which inserts itself in membranes at low pH and is critical to the formation of pores in the vacuolar and host cell’s plasma membranes to forge a path out of the host cell (75). The mechanisms of vacuole acidification are currently unknown. Several parasite H+-ATPases have been described but their role in this process is poorly understood. The relative topologies of the pH and ABA pathways are also unknown and they may intersect or run parallel to one another (124).
Yet another player associated with egress is a family of nucleotide triphosphate degrading enzymes (NTPases) that the parasite secretes into the vacuole. It has been postulated that the NTPases increasingly deplete host cell ATP leading to the Na+/K+-ATPase pumps running dry, a drop in K+ and eventually to egress. It is unclear, however, whether the redox state within the vacuole activates the NTPases and thus it remains unclear whether the NTPases act as a primary sensory pathway or are secondary effectors (135).
Taken together, it is clear that egress of T. gondii from the host cell involves various mechanisms. Individual pathways are tuned to different environmental cues and multiple pathways may communicate and converge. Genetic and pharmacological manipulations can trigger dramatically inflated vacuole sizes that eventually physically rupture the host cell in absence of regulated egress (e.g. (32, 47, 75)). Hence, timing of egress is controlled by the parasite and is not due to a simple physical rupture of the host cell.
Ca2+ and cGMP
The signals triggering egress are transduced by two secondary messengers: cytoplasmic Ca2+ and cGMP. The essential role of Ca2+ in egress and invasion is well documented (for recent reviews see (110, 120)). Chelation of intracellular Ca2+ abrogates motility and microneme secretion, while increase of cytoplasmic Ca2+ concentration by ionophores triggers egress, enhances microneme secretion and motility, and increases invasion capacity (147). Calcium stored in the acidocalcisome and the endoplasmic reticulum can be released upon stimulation, whereas calcium stored in the mitochondrion plays a minor role (110, 147). Pharmacological evidence indicates the presence of 1,4,5-triphosphate inositol (IP3) responsive channels (28) and cADPR (produced by ADP-ribosyl cyclase) sensing ryanodine response channels in the endoplasmic reticulum (35), but molecular evidence for these channels is lacking in the genome (92). In addition, there is a putative role for Ca2+ from outside the parasite (113). The failure to bioinformatically identify these Ca2+-channels that are almost certainly present suggests that the primary structure of T. gondii Ca2+-channels deviates dramatically from the well-studied model organism channels. Despite this gap in knowledge, the identity of some other operators acting on Ca2+ in the parasite such as SERCA channels in the ER (108), a vacuolar Ca2+ dependent ATPase pump in the acidocalcisome (93), and a Na+/H+-exchanger which is involved in Ca2+-homeostasis (8) has been established.
Although the upstream regulators are again unknown, cGMP is produced by membrane bound guanylyl cyclases and degraded by cyclic nucleotide phosphodiesterases (PDEs). The only known effector of cGMP in apicomplexans is protein kinase G (PKG). A recent study in Plasmodium berghei identified that the production of lipids, notably the production of phophatydylinositol (4,5) biphosphate (PI(4,5)P2) by PIP5K (22) is controlled by PKG activity. In turn PI(4,5)P2 is the substrate for phospho-inositol protein lipase C (PI-PLC), which then produces the secondary messenger IP3, triggering the release of intracellular Ca2+ through IP3 responsive Ca2+ channels. These data provide an upstream connection between PKG and Ca2+-levels, which likely applies to Toxoplasma. This extrapolation is supported by inhibition of Toxoplasma P-glycoprotein transporters, which results in reduced or aberrant lipid synthesis and defects in Ca2+-signaling, although it cannot be excluded that this effect originates in the residence of the transporters in the acidocalcisome (19). PKG activation also leads to phosphorylation of IMC proteins, glideosome (motility) proteins, and vesicular trafficking proteins, any or all of which may be important for parasite egress.
Global phosphoproteomic studies (111, 144) and specific studies of two Ca2+-dependent protein kinases, CDPK1 (89) and CDPK3 (145) have generated insights into specific phosphorylation events associated with Ca2+-release. The global studies have identified hundreds of phosphoproteins involved in signal transduction cascades (kinases, phosphatases), the regulation of exocytosis, the cortical cytoskeleton (IMC), and the control of parasite motility, as well as many hypothetical proteins with as yet unknown functions. The identification of the IMC in both cGMP- and Ca2+-dependent processes is intriguing as this structure is the platform for motility.
CDPKs are kinases not found in the mammalian host and have therefore garnered significant interest as potential drug targets. CDPK1 is required for microneme secretion (78, 90), while CPDK3 is involved in the K+-mediated egress pathway (56, 91, 96). Target identification for CDPK1 did not immediately provide intuitive insights in its mechanism of action (89), whereas the CDPK3 target identification hinted at roles upstream of other Ca2+-dependent signaling pathways, in ion-homeostasis and metabolism (145).
2.2 Gliding Motility
Gliding motility is considered critical to Toxoplasma tachyzoite invasion of host cells. However, the role of gliding in invasion is not uniquely conserved across apicomplexan parasites, and the bigger common denominator is that the innovation of gliding permitted the crossing of biological barriers, greatly expanding host range and tissue tropism (60). While tachyzoites demonstrate three different forms of motility on slides—circular gliding (full circles), helical gliding (half circle followed by a flip), and twirling (parasite spins its apex when standing on its base) (64)—this translates into spiral movements in a 3D gel matrix (85). The concept of apicomplexan gliding motility is based on the following principles: 1. microneme secretion of adhesion molecules on the apical end which insert in the parasite’s plasma membrane; 2. anchoring of the adhesion molecules on the cytoplasmic side to short actin filaments; 3. apical to basal translocation of actin filaments by a myosin motor; 4. anchoring of the myosin motor in the IMC membrane; 5. release of microneme proteins on the basal end by intra-membrane cutting rhomboid proteases (Figure 2b-1) (recent reviews (97, 130)).
Microneme Secretion and MIC Proteins
The micronemes are located on the apical end of the parasite (Figure 1a) and are secreted in a Ca2+-dependent fashion. Although an attractive model was that the micronemes merge with the rhoptry neck to purge their contents into the outside milieu, genetic ablation of rhoptries does not impair microneme secretion, suggesting a direct fusion with the plasma membrane (11). Germane to motility there are several microneme (MIC) protein complexes that recognize a variety of substrates, notably glycosaminoglycans present in the extracellular matrix and on the surface of host cells (for a recent review see (29)).
The glideosome
The engine powering gliding motility is Myosin A (MyoA) (98), a class XIVa myosin contained in a protein complex known as the glideosome (Figure 2b-2). MyoA is in complex with glideosome associated proteins 45 and 50 (GAP45, GAP50) (72). GAP50 is the main anchor in the IMC whereas GAP45 spans the space between the plasma membrane and IMC outer membrane through lipid modifications anchoring it in both membranes (53), which likely secures the distance between the two membranes so that the motor can reach the actin filaments associated with the MIC proteins. Furthermore, the glideosome contains a myosin light chain (MLC1) and two essential light chains (ELC1 and ELC2) which regulate the activity of the motor: the MLC1 phosphorylation and Ca2+-binding by the ELCs changes their confirmations and modulates MyoA activity (111, 141). In addition, the phosphorylation state of MyoA itself is critical (141) and underscores the multiple levels of Ca2+-mediated control preventing premature motor activation, thereby curbing premature egress.
Actin Polymerization
The spatio-temporal control of actin polymerization is critical for activation of motility. Compared to other eukaryotes, Apicomplexa encode a limited set of a dozen actin interacting proteins (9). The cytoplasmic concentration of G-actin is relatively high (8-10 μM), whereas F-actin is not readily detectable (41). Actin only polymerizes in very short (100 nm) unstable filaments (129, 133). F-actin in normal parasites is observed in cables or bundles restricted to areas where there is contact between the parasite and host cell or substrate (148). Branched networks are not observed, which is consistent with the absence of ARP2/3 required to form actin branches. These observations fit a model wherein filaments are only formed transiently and where the spatiotemporal dynamics of filament formation likely controls the proper directionality and timing of motility. A key question relevant to the Apicomplexa is how the actin filaments are connected with the cytoplasmic domain of the MIC adhesion protein complexes. For several years the glycolytic enzyme aldolase was a strong candidate, but recently, genetic deletion of aldolase was demonstrated not to block invasion, disproving this model (132). Therefore, the connection of actin to the adhesive proteins secreted from the micronemes is still an open question.
Rhomboid Proteases
Transportation of MIC protein complexes to the basal end would lead to a pile up, unless the complexes are released from the parasite. This release is facilitated by several rhomboid proteases, which have the ability to cleave transmembrane domains inside the membrane spanning sequence (24, 43). Hence, when gliding, the parasites leave behind trails of MIC proteins.
2.3 Invasion
Attachment
Following egress, tachyzoites glide around in the extracellular environment. Compared to the time spent intracellularly this condition is of relatively short duration, but it provides the parasite opportunities to disseminate to new tissues. The first contact of the parasite with a potentially new host cell is through its surface protein coat. The parasite is covered in GPI-anchored surface antigens known as SAGs. The SAG1 structure recognizes sulfated proteoglycans on the host cell (66). This model is supported by the inhibition of host cell attachment in cells with a reduced amount of SAG1 on their surface (16), or upon addition of exogenous glycans (27).
Moving Junction
Following initial recognition the parasite is triggered to engage into a tighter interaction. This is mediated by the secreted microneme proteins and subsequently by the formation of the moving junction (MJ) (74). The MJ is a cooperative structure between the microneme protein AMA1 and proteins residing in the rhoptry neck (RON proteins). Proteins in the rhoptry neck are secreted before the proteins residing in the rhoptry bulb (ROPs) (3). The presence of rhoptry proteins in the host cell’s cytoplasm implies that a breach in the host’s plasma membrane is made (63), but the molecular details of this breach are as yet unclear. In contrast to the calcium-based regulation of micronemes, the signals for rhoptry release remain obscure. It has been shown that AMA1 (100), MIC8 (77) and RON5 (10) are required for rhoptry release but the underlying mechanisms are unknown. In the MJ, AMA1 is anchored in the parasite’s plasma membrane and interacts with the RON-complex anchored in the host cell’s plasma membrane (Figure 2b-2). Direct interaction between AMA1 and the transmembrane domain protein RON2 has been resolved at the molecular level. In addition, RON8 mediates the contact of the complex with the host cytoskeleton and is required for proper interaction (136), whereas RON4 and RON5 appear to have a more structural function in organizing the architecture of the complex (15).
Entry
After establishing the MJ the parasite lunges forward thereby invaginating the host cell plasma membrane, which becomes the parasitophorous vacuole membrane (PVM). Interestingly, the MJ functions as a sieve for proteins in the host cell’s plasma membrane, excluding all transmembrane proteins and proteins in lipid rafts (103). In effect, this results in a vacuolar compartment lacking receptors for the intracellular host cell machinery and yields a non-fusogenic membrane that is refractory to fusion with the host cell membrane trafficking machinery.
Motility
One of the veils surrounding host cell invasion is whether motility is a strict requirement. It has been shown that many of the glideosome components are required for efficient invasion, but that a fraction of the parasites is still able to invade and develop normally. This suggests a potential alternative mechanism powering the entry of the host cell (45) while plasticity between different myosins has also been demonstrated (52). Therefore, the mechanism of host cell invasion is currently still a dynamic area of research.
3. PARASITE REPLICATION
The parasite employs a unique mode of cell division known as endodyogeny. Endodyogeny unfolds through the formation of two daughter cells within the boundaries of a mature mother parasite, which is consumed at the end of the process (50, 131, 137) (Figure 3). As discussed in detail below, Toxoplasma’s cell division process differs at several levels from its mammalian host.
Figure 3.
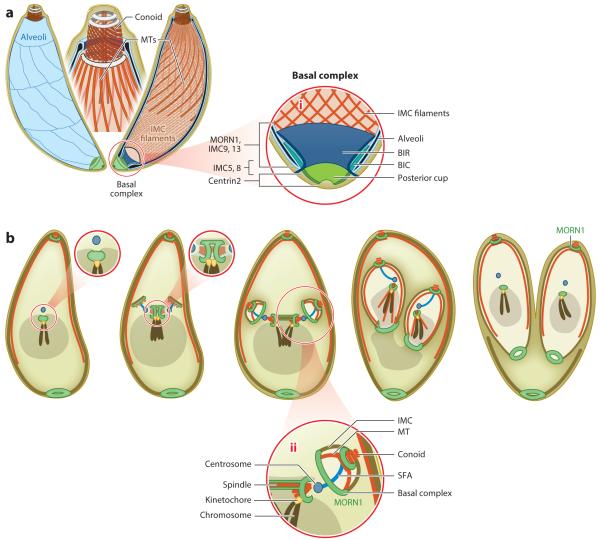
The cortical cytoskeleton and cell division. (a) A patchwork of alveoli (flattened vesicles) underlies the plasma membrane (left) which in turn are supported by a meshwork of intermediate filament-like IMC proteins (right). At the apical end (enlarged in center) the conoid composed of MT is an extrudable basket, whereas 22 cortical, subpellicular microtubules emanate from the apical end to about 2/3 the length of the parasite. At the basal end the cytoskeleton is capped by the basal complex and posterior cup, as magnified in panel 1. BIC, basal inner complex; BIR, basal inner ring. (b) Concurrent mitosis and cell division are coordinated by the centrosome. Mitosis is closed without chromosome condensation whereas the spindle resides eccentrically at the apical side of the nucleus. Throughout the cell cycle the 14 chromosomes are clustered at the centromeres and anchored at the spindle pole (centrocone) by the kinetochore. Daughter bud assembly starts before completion of mitosis and is driven by assembly of the cortical cytoskeleton in an apical to basal direction. The nucleus is anchored in the daughter scaffolds through the centrosome and SFA fiber. Constriction of the basal complex drives tapering of the daughter bud towards the basal end and is required to separate the daughter parasites. Magnification of black dotted regions next to relevant panel. Panel 2 magnifies the interaction between nucleus, centrosome and daughter cytoskeleton. SFA, striated fiber assemblin. Schematic modified from (62).
3.1 Cell Cycle Progression
The tachyzoite has a haploid genome (1N). During S-phase the DNA is replicated, but duplication pauses at a ~1.8N DNA content, at which time the mitotic spindle starts to appear. Therefore, a true G2 stage separating S phase and mitosis is missing in these parasites, but the slow down in S-phase upon reaching 1.8N might be the functional equivalent. Another distinct feature is that daughter budding, and as such cytokinesis, starts when reaching the 1.8N DNA content point.
The molecular machinery underlying cell cycle progression is, like in other systems, based on cyclins and cyclin dependent kinases (Cdks). Although cyclins and Cdks have been identified in Toxoplasma, it has not been established which pairs control which step of the cell cycle (82). Cell cycle progression is characterized by two different subtranscriptomes; one with a G1 signature (biosynthetic and metabolic genes turned on), and one with an S/M signature (DNA replication, cytoskeleton, and invasion organelle genes turned on). In fact, one third of genes are expressed in bi-modal patterns throughout the cell cycle controlled by a unique family of ApiAP2 transcription factors, many of which are active in sequential waves themselves (14).
The genome harbors candidates for the spindle assembly checkpoint controlled by the Anaphase Promoting Complex (APC/C), but these have not been studied in detail. In summary, we know the identity of the major cell cycle progression regulators, but the molecular mechanism is still largely undefined.
3.2 Centrosome as Organization Hub
Toxoplasma cell division has been approached by forward genetics (61). This has drawn the attention to a pivotal role for the centrosome in coordinating the mitotic, karyokinetic and cytokinetic events (34, 48, 49, 139). The centrosome resides near the spindle pole embedded in the nuclear envelope. Late in G1 the centrosome migrates to the basal end of the nucleus and divides (65). At this point the microtubules for the spindle are assembled, nucleating from the centrosome (33). Following migration back to the apical side the spindle is established to facilitate mitosis and the first daughter cytoskeleton elements start to accumulate on the duplicated centrosomes.
At the heart of the Toxoplasma centrosome resides a pair of atypical parallel centrioles (105). The protein composition has only recently started to become elucidated in more detail by identifying orthologs found in higher eukaryote centrosomes (139). The striking observation was that the Toxoplasma centrosome comprises two functionally distinct regions: a proximal region that appears to organize mitosis and a distant region that is associated with initiating cell division. This functional separation of the centrosome’s role in mitosis from its role in cell division permits the uncoupling of the mitotic cycle from the cell division cycle, which is used during merogony in the cat gut where not every mitosis is followed by daughter budding.
The centrosomal cycle is coordinated by a variety of kinases. Although the genome lacks the typical Polo like kinases, various Aurora and NIMA-related kinases are present. The NIMA-related kinase Nek1 controls centrosome splitting whereas the three Ark kinases are associated with various aspects of the centrosome though their exact role has not yet been pinpointed ((139) and Chen, Gubbels, Doerig, Daher, manuscript in preparation). Furthermore, two kinases not associated with mammalian centrosome biology have been associated with the Toxoplasma centrosome. The first is Ca2+-dependent kinase, CDPK7, required for centrosomal localization and integrity (104). The second is a Mitogen Activated Protein Kinase Like protein, MAPK-L1 (25), which in mammals works in the signal transduction cascade but in Toxoplasma is required for anchoring the daughter bud to the centrosome (139).
3.3 Mitosis
The Toxoplasma genome is maintained in 14 chromosomes totaling 65 Mb of DNA. Mitosis is closed, meaning that segregation of duplicated chromosomes occurs within the confines of the nuclear envelope (131). The spindle pole is embedded in the nuclear envelope in a structure known as the centrocone, which appears to be a modified or specialized nuclear pore (Francia & Striepen, personal communication) (Figure 3b). The centrocone, but not the spindle pole microtubules, is retained throughout the cell cycle. As in other eukaryotes, the chromosomes attach to the spindle microtubules via the kinetochore protein complex assembled on the centromere (48). The centromeres are always clustered together and anchored to the centrocone (23), which in interphase is independent of the presence of microtubules (48). The reason for this persistent clustering is not well understood but might be relevant for maintaining genome integrity.
3.4 Cortical Cytoskeleton
In the past 15 years much progress has been made in our understanding of the cortical cytoskeleton, which drives the assembly of the daughter parasites (see (4) for a detailed recent review). The cortical Toxoplasma cytoskeleton is a complex, layered structure comprised of an outer plasma membrane and underlying IMC (131). The IMC is composed of a double membrane system assembled from a series of alveolar vesicles (Figure 3a). On the cytoplasmic side a family of IMC Soluble Proteins (ISPs) differentiates the various alveolar subcompartments (12). In addition, a 10 nm filamentous network of intermediate filament-like IMC proteins lines the cytoplasmic side of the IMC membranes and endows the shape to the parasite (5, 95). The IMC rests on a final cytoskeletal layer composed of 22 subpellicular microtubules emanating from the apical end of the parasite and ending at 2/3 the length of the parasite. Specialized cytoskeletal structures are present at the extreme anterior and posterior ends of the parasite, which are known as the apical and basal complex, respectively. At the apical end the IMC is connected to a tubulin basket known as the conoid, which is extruded out of the parasite upon egress in a Ca2+-dependent fashion. The conoid is a complex structure bound by several apical rings (69, 76). At the basal end of the IMC lies the basal complex, a multi-layered structure containing MORN1 and a set of dedicated IMC proteins (4, 62, 88). At the extreme basal end of the parasite lies the posterior cup, which is composed of Centrin2 (68, 69, 95). As discussed below, the basal complex functions as the contractile ring in completing cell division.
3.5 Internal Budding
The division cycle starts with the duplication of the Golgi apparatus followed by centrosome duplication (65). The budding of daughters is driven by the assembly of the cortical cytoskeleton which forms the scaffold in which all subsequently formed or divided organelles will be anchored (50, 112). Several aspects of daughter budding are shared with the machinery assembling flagella, suggesting shared ancestry (see side bar). The first daughter elements recruited to the (outer) centrosome are IMC15 and Rab11B (5, 139), the latter of which traffics Golgi derived vesicles into the daughter alveoli (1). In addition, striated fiber assemblin (SFA) proteins are recruited to form an SFA fiber that anchors the centrosome in the developing cytoskeleton at the base of the conoid (Figure 3b-2) (49). The next critical cytoskeleton elements to be incorporated are MORN1 and the ISP proteins. MORN1 is associated with the early alveolar vesicles and then transitions to the apical and basal complexes (62, 68), whereas ISP2 is essential for early IMC development (12). From here on the cytoskeleton grows from the basal end (the basal complex) by deposition of various IMC proteins and growing subpellicular microtubules into a widening cone. Coinciding with the completion of karyokinesis, which also concludes division of the endoplasmic reticulum, the subpellicular microtubules stop growing when they reach the 2/3-point of the ultimate length of the daughter cell.
Halfway through the assembly of the daughter scaffold several notable changes occur. A subset of IMC proteins initially assembled in the daughter scaffold transitions to the basal complex where, at the same time, Centrin2 is recruited. Upon continuing growth of the bud the basal complex starts to contract resulting in the tapering of the parasite toward the basal end (68). Since unlike in its mammalian counterpart constriction is independent of actin/myosin, Centrin2 has been postulated to provide the constrictive power (68).
At this point, the two daughters are trapped within the mother and further growth is facilitated by the organized disassembly of the mother’s cytoskeleton (Figure 3b, far right panel). Disassembly progresses in an apical to basal direction. Where the mother’s IMC retracts, the plasma membrane is zippered onto the IMC by the deposition of GAP45 in a Rab11a dependent process (2, 53, 105). Interestingly, the basal complex does not completely separate the parasites and a cytoplasmic bridge remains present.
The apical secretory organelles (micronemes and rhoptries) assemble de novo in the daughter buds (112) in a process requiring the alveolate specific dynamin related protein DrpB (21). Protein trafficking during assembly to the secretory organelles, apicoplast and the IMC goes through the secretory pathway using a repurposed endosomal system to cover the wide variety of destinations (142). Vesicular targeting is mediated by the Rab family of GTPases (81). However, not for all organelles could a specific Rab be identified suggestion additional sorting mechanisms. An extended family of vacuolar sorting proteins (Vsps) could potentially function here, whereas organelle specific palmitoyltransfereases do also partake in organelle specific protein targeting (54).
The apicoplast divides by association with the centrosome and its scission concludes by the action of another dynamin related protein, DrpA (146). Right before basal complex constriction concludes, the mitochondrion enters the daughters (112). Taken together, the timing of daughter budding with respect to the cell cycle and the intimate relationship between daughter budding and mitosis are the hallmarks of tachyzoite cell division.
4. PARASITE-HOST INTERACTION (VACUOLE)
The original Black and Boothroyd review had limited details regarding the role that the host cell plays in Toxoplasma lytic growth largely because at that time few details were known. Over the past 15 years, this has changed and we now have a better (yet still incomplete) understanding of how the host cell contributes to the parasite’s life cycle. In the preceding sections, we highlighted how the host cell contributes to invasion and egress. Below, we will discuss how the parasite manipulates host cell processes to evade host cell defenses and to facilitate its replication.
4.1 Immune Evasion
Host Cell Autophagic Degradation of the Parasitophorous Vacuole
Innate and adaptive immune responses are required for resistance to Toxoplasma and IFNγ is critical for both (140). IFNγ kills Toxoplasma by upregulating the expression of anti-parasitic genes that utilize a variety of mechanisms to kill the parasite. These include autophagy-dependent degradation of the PVM by the p47 family of IFNγ-regulated GTPases (IRGs) (37) and degradation of essential nutrients such as tryptophan by indoleamine dioxygenase (118). Toxoplasma has developed two primary ways of evading these IFNγ effectors.
First, Toxoplasma secretes proteins into the host cell cytoplasm that directly inactivate some effectors. The best-studied example of this mechanism is the inactivation of the IFNγ-inducible GTPases by ROP5/ROP18/ROP17 (see Sibley Chapter in this issue for further details). Interestingly, ROP5/ROP18/ROP17 are polymorphic between parasite strains and ROP5 is the major virulence factor in mice (13, 123). Given that higher primates do not express functional IRGs it is tempting to speculate that Toxoplasma polymorphic virulence factors co-evolved with a specific clade of host species to ensure parasite survival (70).
Toxoplasma also avoids IFNγ-dependent killing by interfering with IFNγ regulated gene expression (80). IFNγ activates gene expression by stimulating the phosphorylation of the STAT1 transcription factor, which allows STAT1 to translocate to the nucleus and bind DNA (122). Toxoplasma does not inhibit STAT1 phosphorylation or nuclear localization. Rather, it blocks STAT1 from binding to the promoters of its target genes by disrupting chromatin remodeling and by preventing its recycling between promoters (83, 127). The STAT1-inhibiting parasite factor(s) is/are unknown but since all known Toxoplasma strains inhibit STAT1 this factor will likely be non-polymorphic (80).
4.2 CD40
The IRGs are not the only proteins that can degrade the PV via autophagy. CD40 is a ubiquitously expressed member of the TNF receptor superfamily and genetic disruption of this gene as well as its ligand, CD154, decreases murine resistance Toxoplasma (7). Upon CD154 binding, CD40 kills Toxoplasma by inducing the accumulation of autophagy-associated proteins on the PV surface and this is followed by degradation of the PV and the parasite (6). But, Toxoplasma can also evade CD40-triggered autophagy and does so by activating Epidermal Growth Factor receptor signaling that prevents autophagosome formation (107).
4.3 Host Cell Death Pathways (Apoptosis and Pyroptosis)
Killing of an infected host cell is an important innate defense strategy and inflammasome-dependent pyroptosis has emerged as a cell death pathway critical for resistance (30, 36). The inflammasome is a multiprotein complex that assembles after a sensor protein binds a microbial-derived molecule. The complex then activates caspase 1 whose activity leads to IL-1β and IL-18 secretion and also triggers the formation of large pores in the plasma membrane resulting in cell lysis and death. The inflammasome was demonstrated to be important for resistance to Toxoplasma since polymorphisms in human and rat inflammasome sensor gene, NLRP1, correlated with resistance to Toxoplasma (31, 150).
Although humans and rats use the inflammasome to control parasite growth, they do so by different mechanisms. In rats, inflammasome activation leads to rapid death of the infected host cells (30). But infected human host cells do not die suggesting a role for IL-1β/IL-18 signaling or involvement of a novel mechanism (59). Another key difference between humans and rats is that inflammasome activation in humans requires GRA15, which is a dense granule protein secreted into the host cell that regulates NF-κB (59). In mice, Toxoplasma also activates the inflammasome via both NLRP1 and NLRP3 and as with human they do not undergo pyroptosis following infection (46, 58).
Three critical questions remain regarding the interaction between Toxoplasma and the host inflammasome. First, how do polymorphisms in NLRP1 alter resistance to Toxoplasma? Second, which parasite factors are sensed by the inflammasome? Third why does inflammasome activation fail to activate pyroptosis in human and murine cells and what impact does this have on virulence. One possibility is that after caspase 1 is activated a rhoptry or dense granule factor is secreted into the host cell and this factor acts to prevent pyroptosis but has no effect on cytokine synthesis.
Apoptosis is a second cell death pathway that is activated by intrinsic (e.g. mitochondrial damage) and extrinsic (e.g. Fas ligand) stimuli that culminate in activation of caspase 3. Toxoplasma inhibits caspase 3 activation as well as several upstream caspases that regulate either the intrinsic or extrinsic pathways (57). It has been proposed that Toxoplasma inhibits host cell apoptosis via increased expression of anti-apoptotic proteins, activation of cell survival signaling pathways, and inhibition of pro-apoptotic proteins (26, 79, 101, 114). But unlike the genetic studies in humans and rodents linking pyroptosis to susceptibility to Toxoplasma infection, a similar link between toxoplasmosis and apoptosis is lacking. Thus, the importance for host cell apoptosis in host resistance remains to be established.
4.4 Host Cell Manipulation to Promote Replicative Niche Development
Membrane Trafficking
During parasite invasion, the PV is formed using lipids derived from the host plasma membrane and most GPI-linked and peripheral plasma membrane proteins but not transmembrane proteins (103, 138). These data supported a model that the PV was non-fusogenic state due to its ability to avoid host endosomes and lysosomes. This was challenged when it was shown that parasite to scavenge lipids such as cholesterol and sphingolipids by redirecting host endolysosomal- and Golgi-derived vesicles to the PV (38, 125). How these lipids cross the PV and are taken up by parasite is unknown. A dense granule protein, GRA7, may function in the former process (39) while receptor-mediated or bulk flow endocytic pathways may be used for the latter (42).
In contrast to endolysosomal trafficking, it has long been recognized that large host organelles such as mitochondria and endoplasmic reticulum are closely apposed to the PV (73). How host ER is recruited to the PV remains unknown. Host mitochondrial recruitment was recently discovered to only occur in cells infected with type I parasites and this is due to a dense granule protein, MAF1 that is secreted into the host cytosol and appears to act to modulate host cytokine expression (115). Important questions remain regarding MAF1 including how MAF1 (or any dense granule or rhoptry protein) enters the host cell or how it recruits mitochondria.
Transcription and Signaling
Early transcriptomic studies revealed that regardless of the host cell type used, infection induced large-scale changes to host cell transcription, which likely leads to global changes in the cell’s physiology (18). Not surprisingly, immune response genes (e.g. chemokines and cytokines) represented one clade of these genes. The others, including those involved in metabolism, cell growth, and signaling, provided a glimpse at host cell processes needed to support parasite growth. As an example, host mevalonate metabolic genes are upregulated most likely due to parasite scavenging of host isoprenoids (86).
The large numbers of host cell genes modulated by infection also suggested that infection modulated the activity of a diverse array of host cell transcription factors. Some of these (NF-κB, STAT3, STAT6, NFAT4, and STAT5) are regulated by polymorphic factors and appear to be important not for parasite replication but rather for shaping the resulting immune response (71, 94, 126, 128). However, other host cell transcriptions factors are activated (including Hypoxia-Inducible Factor 1 (HIF-1), c-myc, and the Early Growth Response factor (EGR)) and appear to do so in a non-polymorphic manner (119, 134, 149). The mechanisms by which these factors are activated appear to be diverse. EGR is dependent on p38 MAP kinase activation and this is mediated by GRA24, which is a dense granule protein that is injected into the host cell (20). c-myc is activated by host JNK MAP kinase signaling although it remains to be determined how Toxoplasma activates this kinase (51). Finally, HIF-1 is activated by signaling through members host serine/threonine kinase receptor family, ALK4,5,7 although the parasite factor that activates ALK4,5,7 is also unknown (149). Of the these host transcription factors, parasite growth is only dependent on HIF-1 although HIF-1 is primarily required at physiological oxygen levels (134).
5. SUMMARY POINT LIST
The lytic cycle is associated with the most severe pathology
The intracellular and extracellular parasites represent two distinct biological states
Ca2+ and cGMP are secondary messengers in transition between the two states
Internal budding and mitosis are coordinated by the centrosome
Parasite division concludes by constriction of the basal complex
A diverse array of proteins secreted by the parasite in some cases tailored to the specific mammalian host manipulates the host cell’s physiology and the immune response at numerous levels
6. FUTURE ISSUES LIST
How are the triggers for egress sensed by the parasite and how are these signals transduced to release Ca2+ and produce cGMP as the molecular identify of these players is largely unknown?
How is rhoptry secretion triggered and how are its contents released in the host cell’s cytoplasm?
What controls and mediates the specific disassembly of the mother parasite structures in the late stages of cell division?
What is the contractile mechanism driving basal complex constriction?
How is the wide variety in host tropism covered by the parasite on the level of specific parasite-host interaction following invasion?
Which parasite factors are sensed by the inflammasome sensor and which modulate the inflammasome?
How does Toxoplasma regulate host membrane trafficking?
Which host proteins are required for parasite growth?
Sidebar Highlights.
Toxoplasma cell division by internal budding is related to the flagella forming machinery
The composition of the division apparatus of Toxoplasma suggests its mechanistically derived from the flagellar machinery (see (50) for a recent review). Although tachyzoites do not carry flagella, the male microgametes have two and the most likely ancestor also carries two flagella (50). Indeed assembly of the tubulin containing conoid follows recruitment of the striated fiber assembling (SFA) fiber, which is also present in algal flagellates such as Chlamydomonas (49). Furthermore, the conoid harbors additional evidence that the cytoskeleton is derived from the flagellar apparatus in the presence of a SAS-6-like protein residing at its basis, next to a normal SAS6 in the centrosome; SAS6 proteins function in microtubule organization) (40). Finally, a centrin molecule, TgCentrin2 (68), and a Myosin VI (TgMyosinJ; Engelberg and Gubbels unpublished data) are present at both the apicomplexan basal complex and the flagellar transition zone.
Acknowledgements
Work in the Gubbels lab is sponsored by National Institutes of Health grants R01AI081924, R03AI107475, R21AI099658, and F32AI108251 (to BIC), and American Cancer Society grant RSG-12-175-01-MPC. IJB is supported by NIH Grants R01AI069986 and R21AI107257.
Acronyms/Terms
- IMC
Inner Membrane Complex; the peripheral alveolar membrane cytoskeleton system defining the super-phylum of Alveolata comprising the dinoflagellates, cillates and Apicomplexa
- CDPKs
Calcium Dependent Protein Kinases; an apicomplexan protein family that contains both calmodulin-like calcium binding and a Ser/Thr protein kinase domain
- Gliding motility
Adhesion dependent actinomyosin based motility underlying tissue migration and host cell invasion of Apicomplexa
- Moving Junction
The protein complex at the host-parasite interface of invasion composed of rhoptry neck (RON) proteins and microneme secreted AMA1
- Enodyogeny
Cell division through internal budding of two daughter parasites within the confinement of the mother parasite
- ApiAP2
Apicomplexan Apetala 2 family of transcription factors unique to the Apicomplexa that each contain 1-4 AP2 DNA binding domains
- IRGs
A family of IFNγ-regulated GTPases able to sense and destruct the intracellular parasite in mice.
- Inflammasome
A A multiprotein complex present that detects microbial-derived factors and promotes IL-1β and IL-18 synthesis and secretion and pyroptotic host cell death.
- NLRPs
Nucleotide-binding domain and leucine-rich repeat (NLR) containing, pyrin domain containing family of proteins which function in the inflammasome
References cited
- 1.Agop-Nersesian C, Egarter S, Langsley G, Foth BJ, Ferguson DJ, Meissner M. Biogenesis of the inner membrane complex is dependent on vesicular transport by the alveolate specific GTPase Rab11B. PLoS Pathog. 2010;6:e1001029. doi: 10.1371/journal.ppat.1001029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agop-Nersesian C, Naissant B, Ben Rached F, Rauch M, Kretzschmar A, et al. Rab11A-controlled assembly of the inner membrane complex is required for completion of apicomplexan cytokinesis. PLoS Pathog. 2009;5:e1000270. doi: 10.1371/journal.ppat.1000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexander DL, Mital J, Ward GE, Bradley P, Boothroyd JC. Identification of the Moving Junction Complex of Toxoplasma gondii: A Collaboration between Distinct Secretory Organelles. PLoS Pathog. 2005;1:e17. doi: 10.1371/journal.ppat.0010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson-White BR, Beck JR, Chen CT, Meissner M, Bradley PJ, Gubbels MJ. Cytoskeleton assembly in Toxoplasma gondii cell division. Int. Rev. Cell Mol. Biol. 2012;298:1–31. doi: 10.1016/B978-0-12-394309-5.00001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson-White BR, Ivey FD, Cheng K, Szatanek T, Lorestani A, et al. A family of intermediate filament-like proteins is sequentially assembled into the cytoskeleton of Toxoplasma gondii. Cell Microbiol. 2011;13:18–31. doi: 10.1111/j.1462-5822.2010.01514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andrade RM, Wessendarp M, Gubbels MJ, Striepen B, Subauste CS. CD40 induces macrophage anti-Toxoplasma gondii activity by triggering autophagy-dependent fusion of pathogen-containing vacuoles and lysosomes. J Clin Invest. 2006;116:2366–77. doi: 10.1172/JCI28796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andrade RM, Wessendarp M, Subauste CS. CD154 activates macrophage antimicrobial activity in the absence of IFN-gamma through a TNF-alpha-dependent mechanism. J Immunol. 2003;171:6750–6. doi: 10.4049/jimmunol.171.12.6750. [DOI] [PubMed] [Google Scholar]
- 8.Arrizabalaga G, Ruiz F, Moreno S, Boothroyd JC. Ionophore-resistant mutant of Toxoplasma gondii reveals involvement of a sodium/hydrogen exchanger in calcium regulation. J Cell Biol. 2004;165:653–62. doi: 10.1083/jcb.200309097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baum J, Papenfuss AT, Baum B, Speed TP, Cowman AF. Regulation of apicomplexan actin-based motility. Nat Rev Microbiol. 2006;4:621–8. doi: 10.1038/nrmicro1465. [DOI] [PubMed] [Google Scholar]
- 10.Beck JR, Chen AL, Kim EW, Bradley PJ. RON5 is critical for organization and function of the Toxoplasma moving junction complex. PLoS Pathog. 2014;10:e1004025. doi: 10.1371/journal.ppat.1004025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beck JR, Fung C, Straub KW, Coppens I, Vashisht AA, et al. A Toxoplasma palmitoyl acyl transferase and the palmitoylated Armadillo Repeat protein TgARO govern apical rhoptry tethering and reveal a critical role for the rhoptries in host cell invasion but not egress. PLoS Pathog. 2013;9:e1003162. doi: 10.1371/journal.ppat.1003162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beck JR, Rodriguez-Fernandez IA, Cruz de Leon J, Huynh MH, Carruthers VB, et al. A novel family of Toxoplasma IMC proteins displays a hierarchical organization and functions in coordinating parasite division. PLoS Pathog. 2010;6:e1001094. doi: 10.1371/journal.ppat.1001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Behnke MS, Khan A, Wootton JC, Dubey JP, Tang K, Sibley LD. Virulence differences in Toxoplasma mediated by amplification of a family of polymorphic pseudokinases. Proc Natl Acad Sci U S A. 2011;108:9631–6. doi: 10.1073/pnas.1015338108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Behnke MS, Wootton JC, Lehmann MM, Radke JB, Lucas O, et al. Coordinated Progression through Two Subtranscriptomes Underlies the Tachyzoite Cycle of Toxoplasma gondii. PLoS ONE. 2010;5:e12354. doi: 10.1371/journal.pone.0012354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Besteiro S, Dubremetz JF, Lebrun M. The moving junction of apicomplexan parasites: a key structure for invasion. Cellular Microbiology. 2011;13:797–805. doi: 10.1111/j.1462-5822.2011.01597.x. [DOI] [PubMed] [Google Scholar]
- 16.Bishop JR, Crawford BE, Esko JD. Cell surface heparan sulfate promotes replication of Toxoplasma gondii. Infect Immun. 2005;73:5395–401. doi: 10.1128/IAI.73.9.5395-5401.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Black MW, Boothroyd JC. Lytic cycle of Toxoplasma gondii. Microbiol Mol Biol Rev. 2000;64:607–23. doi: 10.1128/mmbr.64.3.607-623.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blader IJ, Manger ID, Boothroyd JC. Microarray Analysis Reveals Previously Unknown Changes in Toxoplasma gondii-infected Human Cells. J. Biol Chem. 2001;276:24223–31. doi: 10.1074/jbc.M100951200. [DOI] [PubMed] [Google Scholar]
- 19.Bottova I, Sauder U, Olivieri V, Hehl AB, Sonda S. The P-glycoprotein inhibitor GF120918 modulates Ca2+-dependent processes and lipid metabolism in Toxoplasma gondii. PLoS ONE. 2010;5:e10062. doi: 10.1371/journal.pone.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Braun L, Brenier-Pinchart MP, Yogavel M, Curt-Varesano A, Curt-Bertini RL, et al. A Toxoplasma dense granule protein, GRA24, modulates the early immune response to infection by promoting a direct and sustained host p38 MAPK activation. J Exp Med. 2013;210:2071–86. doi: 10.1084/jem.20130103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Breinich MS, Ferguson DJ, Foth BJ, van Dooren GG, Lebrun M, et al. A Dynamin Is Required for the Biogenesis of Secretory Organelles in Toxoplasma gondii. Curr Biol. 2009;19:277–86. doi: 10.1016/j.cub.2009.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brochet M, Collins MO, Smith TK, Thompson E, Sebastian S, et al. Phosphoinositide metabolism links cGMP-dependent protein kinase G to essential Ca(2)(+) signals at key decision points in the life cycle of malaria parasites. PLoS biology. 2014;12:e1001806. doi: 10.1371/journal.pbio.1001806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brooks CF, Francia ME, Gissot M, Croken MM, Kim K, Striepen B. Toxoplasma gondii sequesters centromeres to a specific nuclear region throughout the cell cycle. Proc Natl Acad Sci U S A. 2011;108:3767–72. doi: 10.1073/pnas.1006741108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brossier F, Jewett TJ, Sibley LD, Urban S. A spatially localized rhomboid protease cleaves cell surface adhesins essential for invasion by Toxoplasma. Proc Natl Acad Sci U S A. 2005;102:4146–51. doi: 10.1073/pnas.0407918102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown KM, Suvorova E, Farrell A, Wiley BB, Marth G, et al. Forward Genetic Screening Identifies a Small Molecule that Blocks Toxoplasma gondii Growth By Inhibiting Both Host- and Parasite-Encoded Kinases. PLoS Pathog. 2014;10:e1004180. doi: 10.1371/journal.ppat.1004180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carmen JC, Sinai AP. The Differential Effect of Toxoplasma Gondii Infection on the Stability of BCL2-Family Members Involves Multiple Activities. Front Microbiol. 2011;2:1. doi: 10.3389/fmicb.2011.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carruthers VB, Hakansson S, Giddings OK, Sibley LD. Toxoplasma gondii uses sulfated proteoglycans for substrate and host cell attachment. Infect Immun. 2000;68:4005–11. doi: 10.1128/iai.68.7.4005-4011.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carruthers VB, Moreno SN, Sibley LD. Ethanol and acetaldehyde elevate intracellular [Ca2+] and stimulate microneme discharge in Toxoplasma gondii. Biochem J. 1999;342(Pt 2):379–86. [PMC free article] [PubMed] [Google Scholar]
- 29.Carruthers VB, Tomley FM. Microneme proteins in apicomplexans. Subcell Biochem. 2008;47:33–45. doi: 10.1007/978-0-387-78267-6_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cavailles P, Flori P, Papapietro O, Bisanz C, Lagrange D, et al. A highly conserved Toxo1 haplotype directs resistance to toxoplasmosis and its associated caspase-1 dependent killing of parasite and host macrophage. PLoS Pathog. 2014;10:e1004005. doi: 10.1371/journal.ppat.1004005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cavailles P, Sergent V, Bisanz C, Papapietro O, Colacios C, et al. The rat Toxo1 locus directs toxoplasmosis outcome and controls parasite proliferation and spreading by macrophage-dependent mechanisms. Proc Natl Acad Sci U S A. 2006;103:744–9. doi: 10.1073/pnas.0506643103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chandramohanadas R, Davis PH, Beiting DP, Harbut MB, Darling C, et al. Apicomplexan Parasites Co-Opt Host Calpains to Facilitate Their Escape from Infected Cells. Science. 2009 doi: 10.1126/science.1171085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen C-T, Farrell M, de Leon J, Nwagbara B, Ebbert P, et al. Compartmentalized EB1 sequentially regulates 1 mitosis and cytokinesis during Toxoplasma replication. Submitted. 2015 [Google Scholar]
- 34.Chen CT, Gubbels MJ. The Toxoplasma gondii centrosome is the platform for internal daughter budding as revealed by a Nek1 kinase mutant. J Cell Sci. 2013;126:3344–55. doi: 10.1242/jcs.123364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chini EN, Nagamune K, Wetzel DM, Sibley LD. Evidence that the cADPR signalling pathway controls calcium-mediated microneme secretion in Toxoplasma gondii. Biochem J. 2005;389:269–77. doi: 10.1042/BJ20041971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cirelli KM, Gorfu G, Hassan MA, Printz M, Crown D, et al. Inflammasome sensor NLRP1 controls rat macrophage susceptibility to Toxoplasma gondii. PLoS Pathog. 2014;10:e1003927. doi: 10.1371/journal.ppat.1003927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Collazo CM, Yap GS, Sempowski GD, Lusby KC, Tessarollo L, et al. Inactivation of LRG-47 and IRG-47 reveals a family of interferon gamma-inducible genes with essential, pathogen-specific roles in resistance to infection. J Exp Med. 2001;194:181–8. doi: 10.1084/jem.194.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coppens I. Contribution of host lipids to Toxoplasma pathogenesis. Cell Microbiol. 2006;8:1–9. doi: 10.1111/j.1462-5822.2005.00647.x. [DOI] [PubMed] [Google Scholar]
- 39.Coppens I, Dunn JD, Romano JD, Pypaert M, Zhang H, et al. Toxoplasma gondii sequesters lysosomes from mammalian hosts in the vacuolar space. Cell. 2006;125:261–74. doi: 10.1016/j.cell.2006.01.056. [DOI] [PubMed] [Google Scholar]
- 40.de Leon JC, Scheumann N, Beatty W, Beck JR, Tran JQ, et al. A SAS-6-like protein suggests that the Toxoplasma conoid complex evolved from flagellar components. Eukaryot Cell. 2013;12:1009–19. doi: 10.1128/EC.00096-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dobrowolski JM, Niesman IR, Sibley LD. Actin in the parasite Toxoplasma gondii is encoded by a single copy gene, ACT1 and exists primarily in a globular form. Cell Motil Cytoskeleton. 1997;37:253–62. doi: 10.1002/(SICI)1097-0169(1997)37:3<253::AID-CM7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 42.Dou Z, McGovern OL, Di Cristina M, Carruthers VB. Toxoplasma gondii ingests and digests host cytosolic proteins. MBio. 2014;5:e01188–14. doi: 10.1128/mBio.01188-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dowse TJ, Pascall JC, Brown KD, Soldati D. Apicomplexan rhomboids have a potential role in microneme protein cleavage during host cell invasion. Int J Parasitol. 2005;35:747–56. doi: 10.1016/j.ijpara.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 44.Dubey JP. The history of Toxoplasma gondii--the first 100 years. J Eukaryot Microbiol. 2008;55:467–75. doi: 10.1111/j.1550-7408.2008.00345.x. [DOI] [PubMed] [Google Scholar]
- 45.Egarter S, Andenmatten N, Jackson AJ, Whitelaw JA, Pall G, et al. The toxoplasma Acto-MyoA motor complex is important but not essential for gliding motility and host cell invasion. PLoS ONE. 2014;9:e91819. doi: 10.1371/journal.pone.0091819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ewald SE, Chavarria-Smith J, Boothroyd JC. NLRP1 is an inflammasome sensor for Toxoplasma gondii. Infect Immun. 2014;82:460–8. doi: 10.1128/IAI.01170-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Farrell A, Thirugnanam S, Lorestani A, Dvorin JD, Eidell KP, et al. A DOC2 protein identified by mutational profiling is essential for apicomplexan parasite exocytosis. Science. 2012;335:218–21. doi: 10.1126/science.1210829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Farrell M, Gubbels MJ. The Toxoplasma gondii kinetochore is required for centrosome association with the centrocone (spindle pole) Cellular Microbiology. 2014;16:78–94. doi: 10.1111/cmi.12185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Francia ME, Jordan CN, Patel JD, Sheiner L, Demerly JL, et al. Cell division in Apicomplexan parasites is organized by a homolog of the striated rootlet fiber of algal flagella. PLoS biology. 2012;10:e1001444. doi: 10.1371/journal.pbio.1001444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Francia ME, Striepen B. Cell division in apicomplexan parasites. Nature reviews. Microbiology. 2014;12:125–36. doi: 10.1038/nrmicro3184. [DOI] [PubMed] [Google Scholar]
- 51.Franco M, Shastri AJ, Boothroyd JC. Infection by Toxoplasma gondii specifically induces host c-Myc and the genes this pivotal transcription factor regulates. Eukaryot Cell. 2014;13:483–93. doi: 10.1128/EC.00316-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frenal K, Marq JB, Jacot D, Polonais V, Soldati-Favre D. Plasticity between MyoC- and MyoA-glideosomes: an example of functional compensation in Toxoplasma gondii invasion. PLoS Pathog. 2014;10:e1004504. doi: 10.1371/journal.ppat.1004504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Frenal K, Polonais V, Marq JB, Stratmann R, Limenitakis J, Soldati-Favre D. Functional dissection of the apicomplexan glideosome molecular architecture. Cell Host Microbe. 2010;8:343–57. doi: 10.1016/j.chom.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 54.Frenal K, Tay CL, Mueller C, Bushell ES, Jia Y, et al. Global analysis of apicomplexan protein S-acyl transferases reveals an enzyme essential for invasion. Traffic. 2013;14:895–911. doi: 10.1111/tra.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gaji RY, Behnke MS, Lehmann MM, White MW, Carruthers VB. Cell cycle-dependent, intercellular transmission of Toxoplasma gondii is accompanied by marked changes in parasite gene expression. Mol Microbiol. 2011;79:192–204. doi: 10.1111/j.1365-2958.2010.07441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Garrison E, Treeck M, Ehret E, Butz H, Garbuz T, et al. A forward genetic screen reveals that calcium-dependent protein kinase 3 regulates egress in Toxoplasma. PLoS Pathog. 2012;8:e1003049. doi: 10.1371/journal.ppat.1003049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goebel S, Gross U, Luder CG. Inhibition of host cell apoptosis by Toxoplasma gondii is accompanied by reduced activation of the caspase cascade and alterations of poly(ADP-ribose) polymerase expression. J Cell Sci. 2001;114:3495–505. doi: 10.1242/jcs.114.19.3495. [DOI] [PubMed] [Google Scholar]
- 58.Gorfu G, Cirelli KM, Melo MB, Mayer-Barber K, Crown D, et al. Dual role for inflammasome sensors NLRP1 and NLRP3 in murine resistance to Toxoplasma gondii. mBio. 2014;5 doi: 10.1128/mBio.01117-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gov L, Karimzadeh A, Ueno N, Lodoen MB. Human innate immunity to Toxoplasma gondii is mediated by host caspase-1 and ASC and parasite GRA15. MBio. 2013;4 doi: 10.1128/mBio.00255-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gubbels MJ, Duraisingh MT. Evolution of apicomplexan secretory organelles. Int J Parasitol. 2012;42:1071–81. doi: 10.1016/j.ijpara.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gubbels MJ, Lehmann M, Muthalagi M, Jerome ME, Brooks CF, et al. Forward Genetic Analysis of the Apicomplexan Cell Division Cycle in Toxoplasma gondii. PLoS Pathog. 2008;4:e36. doi: 10.1371/journal.ppat.0040036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gubbels MJ, Vaishnava S, Boot N, Dubremetz JF, Striepen B. A MORN-repeat protein is a dynamic component of the Toxoplasma gondii cell division apparatus. J Cell Sci. 2006;119:2236–45. doi: 10.1242/jcs.02949. [DOI] [PubMed] [Google Scholar]
- 63.Hakansson S, Charron AJ, Sibley LD. Toxoplasma evacuoles: a two-step process of secretion and fusion forms the parasitophorous vacuole. Embo J. 2001;20:3132–44. doi: 10.1093/emboj/20.12.3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hakansson S, Morisaki H, Heuser J, Sibley LD. Time-lapse video microscopy of gliding motility in Toxoplasma gondii reveals a novel, biphasic mechanism of cell locomotion. Mol Biol Cell. 1999;10:3539–47. doi: 10.1091/mbc.10.11.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hartmann J, Hu K, He CY, Pelletier L, Roos DS, Warren G. Golgi and centrosome cycles in Toxoplasma gondii. Mol Biochem Parasitol. 2006;145:125–7. doi: 10.1016/j.molbiopara.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 66.He XL, Grigg ME, Boothroyd JC, Garcia KC. Structure of the immunodominant surface antigen from the Toxoplasma gondii SRS superfamily. Nat Struct Biol. 2002;9:606–11. doi: 10.1038/nsb819. [DOI] [PubMed] [Google Scholar]
- 67.Hoff EF, Carruthers VB. Is Toxoplasma egress the first step in invasion? Trends Parasitol. 2002;18:251–5. doi: 10.1016/s1471-4922(02)02240-7. [DOI] [PubMed] [Google Scholar]
- 68.Hu K. Organizational changes of the daughter basal complex during the parasite replication of Toxoplasma gondii. PLoS Pathog. 2008;4:e10. doi: 10.1371/journal.ppat.0040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hu K, Johnson J, Florens L, Fraunholz M, Suravajjala S, et al. Cytoskeletal components of an invasion machine--the apical complex of Toxoplasma gondii. PLoS Pathog. 2006;2:e13. doi: 10.1371/journal.ppat.0020013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hunn JP, Feng CG, Sher A, Howard JC. The immunity-related GTPases in mammals: a fast-evolving cell-autonomous resistance system against intracellular pathogens. Mamm Genome. 2011;22:43–54. doi: 10.1007/s00335-010-9293-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jensen KD, Hu K, Whitmarsh RJ, Hassan MA, Julien L, et al. Toxoplasma gondii rhoptry 16 kinase promotes host resistance to oral infection and intestinal inflammation only in the context of the dense granule protein GRA15. Infect Immun. 2013;81:2156–67. doi: 10.1128/IAI.01185-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Johnson TM, Rajfur Z, Jacobson K, Beckers CJ. Immobilization of the type XIV myosin complex in Toxoplasma gondii. Mol Biol Cell. 2007;18:3039–46. doi: 10.1091/mbc.E07-01-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jones TC, Hirsch JG. The interaction between Toxoplasma gondii and mammalian cells. II. The absence of lysosomal fusion with phagocytic vacuoles containing living parasites. J Exp Med. 1972;136:1173–94. doi: 10.1084/jem.136.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kafsack BF, Carruthers VB, Pineda FJ. Kinetic modeling of Toxoplasma gondii invasion. J Theor Biol. 2007;249:817–25. doi: 10.1016/j.jtbi.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kafsack BF, Pena JD, Coppens I, Ravindran S, Boothroyd JC, Carruthers VB. Rapid membrane disruption by a perforin-like protein facilitates parasite exit from host cells. Science. 2009;323:530–3. doi: 10.1126/science.1165740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Katris NJ, van Dooren GG, McMillan PJ, Hanssen E, Tilley L, Waller RF. The apical complex provides a regulated gateway for secretion of invasion factors in Toxoplasma. PLoS Pathog. 2014;10:e1004074. doi: 10.1371/journal.ppat.1004074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kessler H, Herm-Gotz A, Hegge S, Rauch M, Soldati-Favre D, et al. Microneme protein 8--a new essential invasion factor in Toxoplasma gondii. J Cell Sci. 2008;121:947–56. doi: 10.1242/jcs.022350. [DOI] [PubMed] [Google Scholar]
- 78.Kieschnick H, Wakefield T, Narducci CA, Beckers C. Toxoplasma gondii attachment to host cells is regulated by a calmodulin-like domain protein kinase. J Biol Chem. 2001;276:12369–77. doi: 10.1074/jbc.M011045200. [DOI] [PubMed] [Google Scholar]
- 79.Kim L, Denkers EY. Toxoplasma gondii triggers Gi-dependent PI 3-kinase signaling required for inhibition of host cell apoptosis. J Cell Sci. 2006;119:2119–26. doi: 10.1242/jcs.02934. [DOI] [PubMed] [Google Scholar]
- 80.Kim SK, Fouts AE, Boothroyd JC. Toxoplasma gondii dysregulates IFN-gamma-inducible gene expression in human fibroblasts: insights from a genome-wide transcriptional profiling. J Immunol. 2007;178:5154–65. doi: 10.4049/jimmunol.178.8.5154. [DOI] [PubMed] [Google Scholar]
- 81.Kremer K, Kamin D, Rittweger E, Wilkes J, Flammer H, et al. An Overexpression Screen of Toxoplasma gondii Rab-GTPases Reveals Distinct Transport Routes to the Micronemes. PLoS Pathog. 2013;9:e1003213. doi: 10.1371/journal.ppat.1003213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kvaal CA, Radke JR, Guerini MN, White MW. Isolation of a Toxoplasma gondii cyclin by yeast two-hybrid interactive screen. Mol Biochem Parasitol. 2002;120:187–94. doi: 10.1016/s0166-6851(01)00454-6. [DOI] [PubMed] [Google Scholar]
- 83.Lang C, Hildebrandt A, Brand F, Opitz L, Dihazi H, Luder CG. Impaired chromatin remodelling at STAT1-regulated promoters leads to global unresponsiveness of Toxoplasma gondii-Infected macrophages to IFN-gamma. PLoS Pathog. 2012;8:e1002483. doi: 10.1371/journal.ppat.1002483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lescault PJ, Thompson AB, Patil V, Lirussi D, Burton A, et al. Genomic data reveal Toxoplasma gondii differentiation mutants are also impaired with respect to switching into a novel extracellular tachyzoite state. PLoS ONE. 2010;5:e14463. doi: 10.1371/journal.pone.0014463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Leung JM, Rould MA, Konradt C, Hunter CA, Ward GE. Disruption of TgPHIL1 alters specific parameters of Toxoplasma gondii motility measured in a quantitative, three-dimensional live motility assay. PLoS ONE. 2014;9:e85763. doi: 10.1371/journal.pone.0085763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li ZH, Ramakrishnan S, Striepen B, Moreno SN. Toxoplasma gondii relies on both host and parasite isoprenoids and can be rendered sensitive to atorvastatin. PLoS Pathog. 2013;9:e1003665. doi: 10.1371/journal.ppat.1003665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lirussi D, Matrajt M. RNA granules present only in extracellular toxoplasma gondii increase parasite viability. International journal of biological sciences. 2011;7:960–7. doi: 10.7150/ijbs.7.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lorestani A, Sheiner L, Yang K, Robertson SD, Sahoo N, et al. A Toxoplasma MORN1 Null Mutant Undergoes Repeated Divisions but Is Defective in Basal Assembly, Apicoplast Division and Cytokinesis. PLoS ONE. 2010;5:e12302. doi: 10.1371/journal.pone.0012302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lourido S, Jeschke GR, Turk BE, Sibley LD. Exploiting the unique ATP-binding pocket of Toxoplasma calcium-dependent protein kinase 1 to identify its substrates. ACS chemical biology. 2013;8:1155–62. doi: 10.1021/cb400115y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lourido S, Shuman J, Zhang C, Shokat KM, Hui R, Sibley LD. Calcium-dependent protein kinase 1 is an essential regulator of exocytosis in Toxoplasma. Nature. 2010;465:359–62. doi: 10.1038/nature09022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lourido S, Tang K, Sibley LD. Distinct signalling pathways control Toxoplasma egress and host-cell invasion. Embo J. 2012;31:4524–34. doi: 10.1038/emboj.2012.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lovett JL, Marchesini N, Moreno SN, Sibley LD. Toxoplasma gondii microneme secretion involves intracellular Ca(2+) release from inositol 1,4,5-triphosphate (IP(3))/ryanodine-sensitive stores. J Biol Chem. 2002;277:25870–6. doi: 10.1074/jbc.M202553200. [DOI] [PubMed] [Google Scholar]
- 93.Luo S, Ruiz FA, Moreno SN. The acidocalcisome Ca2+-ATPase (TgA1) of Toxoplasma gondii is required for polyphosphate storage, intracellular calcium homeostasis and virulence. Mol Microbiol. 2005;55:1034–45. doi: 10.1111/j.1365-2958.2004.04464.x. [DOI] [PubMed] [Google Scholar]
- 94.Ma JS, Sasai M, Ohshima J, Lee Y, Bando H, et al. Selective and strain-specific NFAT4 activation by the Toxoplasma gondii polymorphic dense granule protein GRA6. J Exp Med. 2014;211:2013–32. doi: 10.1084/jem.20131272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mann T, Beckers C. Characterization of the subpellicular network, a filamentous membrane skeletal component in the parasite Toxoplasma gondii. Mol Biochem Parasitol. 2001;115:257–68. doi: 10.1016/s0166-6851(01)00289-4. [DOI] [PubMed] [Google Scholar]
- 96.McCoy JM, Whitehead L, van Dooren GG, Tonkin CJ. TgCDPK3 Regulates Calcium-Dependent Egress of Toxoplasma gondii from Host Cells. PLoS Pathog. 2012;8:e1003066. doi: 10.1371/journal.ppat.1003066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Meissner M, Ferguson DJ, Frischknecht F. Invasion factors of apicomplexan parasites: essential or redundant? Curr Opin Microbiol. 2013;16:438–44. doi: 10.1016/j.mib.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 98.Meissner M, Schluter D, Soldati D. Role of Toxoplasma gondii myosin A in powering parasite gliding and host cell invasion. Science. 2002;298:837–40. doi: 10.1126/science.1074553. [DOI] [PubMed] [Google Scholar]
- 99.Melzer T, Duffy A, Weiss LM, Halonen SK. The gamma interferon (IFN-gamma)-inducible GTP-binding protein IGTP is necessary for toxoplasma vacuolar disruption and induces parasite egression in IFN-gamma-stimulated astrocytes. Infect Immun. 2008;76:4883–94. doi: 10.1128/IAI.01288-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mital J, Meissner M, Soldati D, Ward GE. Conditional Expression of Toxoplasma gondii Apical Membrane Antigen-1 (TgAMA1) Demonstrates That TgAMA1 Plays a Critical Role in Host Cell Invasion. Mol Biol Cell. 2005;16:4341–9. doi: 10.1091/mbc.E05-04-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Molestina RE, Payne TM, Coppens I, Sinai AP. Activation of NF-{kappa}B by Toxoplasma gondii correlates with increased expression of antiapoptotic genes and localization of phosphorylated I{kappa}B to the parasitophorous vacuole membrane. J Cell Sci. 2003;116:4359–71. doi: 10.1242/jcs.00683. [DOI] [PubMed] [Google Scholar]
- 102.Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet. 2004;363:1965–76. doi: 10.1016/S0140-6736(04)16412-X. [DOI] [PubMed] [Google Scholar]
- 103.Mordue DG, Hakansson S, Niesman I, Sibley LD. Toxoplasma gondii resides in a vacuole that avoids fusion with host cell endocytic and exocytic vesicular trafficking pathways. Exp Parasitol. 1999;92:87–99. doi: 10.1006/expr.1999.4412. [DOI] [PubMed] [Google Scholar]
- 104.Morlon-Guyot J, Berry L, Chen CT, Gubbels MJ, Lebrun M, Daher W. The Toxoplasma gondii Calcium Dependent Protein Kinase 7 is involved in early steps of parasite division and is crucial for parasite survival. Cellular Microbiology. 2014;16:95–114. doi: 10.1111/cmi.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Morrissette NS, Sibley LD. Disruption of microtubules uncouples budding and nuclear division in Toxoplasma gondii. J Cell Sci. 2002;115:1017–25. doi: 10.1242/jcs.115.5.1017. [DOI] [PubMed] [Google Scholar]
- 106.Moudy R, Manning TJ, Beckers CJ. The loss of cytoplasmic potassium upon host cell breakdown triggers egress of Toxoplasma gondii. J Biol Chem. 2001;276:41492–501. doi: 10.1074/jbc.M106154200. [DOI] [PubMed] [Google Scholar]
- 107.Muniz-Feliciano L, Van Grol J, Portillo JA, Liew L, Liu B, et al. Toxoplasma gondii-induced activation of EGFR prevents autophagy protein-mediated killing of the parasite. PLoS Pathog. 2013;9:e1003809. doi: 10.1371/journal.ppat.1003809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nagamune K, Beatty WL, Sibley LD. Artemisinin induces calcium-dependent protein secretion in the protozoan parasite Toxoplasma gondii. Eukaryot Cell. 2007;6:2147–56. doi: 10.1128/EC.00262-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nagamune K, Hicks LM, Fux B, Brossier F, Chini EN, Sibley LD. Abscisic acid controls calcium-dependent egress and development in Toxoplasma gondii. Nature. 2008;451:207–10. doi: 10.1038/nature06478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nagamune K, Moreno SN, Chini EN, Sibley LD. Calcium regulation and signaling in apicomplexan parasites. Subcell Biochem. 2008;47:70–81. doi: 10.1007/978-0-387-78267-6_5. [DOI] [PubMed] [Google Scholar]
- 111.Nebl T, Prieto JH, Kapp E, Smith BJ, Williams MJ, et al. Quantitative in vivo Analyses Reveal Calcium-dependent Phosphorylation Sites and Identifies a Novel Component of the Toxoplasma Invasion Motor Complex. PLoS Pathog. 2011;7:e1002222. doi: 10.1371/journal.ppat.1002222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nishi M, Hu K, Murray JM, Roos DS. Organellar dynamics during the cell cycle of Toxoplasma gondii. J Cell Sci. 2008;121:1559–68. doi: 10.1242/jcs.021089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pace DA, McKnight CA, Liu J, Jimenez V, Moreno SN. Calcium Entry in Toxoplasma gondii and Its Enhancing Effect of Invasion-linked Traits. J Biol Chem. 2014;289:19637–47. doi: 10.1074/jbc.M114.565390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Payne TM, Molestina RE, Sinai AP. Inhibition of caspase activation and a requirement for NF-{kappa}B function in the Toxoplasma gondii-mediated blockade of host apoptosis. J Cell Sci. 2003;116:4345–58. doi: 10.1242/jcs.00756. [DOI] [PubMed] [Google Scholar]
- 115.Pernas L, Adomako-Ankomah Y, Shastri AJ, Ewald SE, Treeck M, et al. Toxoplasma effector MAF1 mediates recruitment of host mitochondria and impacts the host response. PLoS Biol. 2014;12:e1001845. doi: 10.1371/journal.pbio.1001845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Persson CM, Lambert H, Vutova PP, Dellacasa-Lindberg I, Nederby J, et al. Transmission of Toxoplasma gondii from infected dendritic cells to natural killer cells. Infect Immun. 2009;77:970–6. doi: 10.1128/IAI.00833-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Persson EK, Agnarson AM, Lambert H, Hitziger N, Yagita H, et al. Death receptor ligation or exposure to perforin trigger rapid egress of the intracellular parasite Toxoplasma gondii. J Immunol. 2007;179:8357–65. doi: 10.4049/jimmunol.179.12.8357. [DOI] [PubMed] [Google Scholar]
- 118.Pfefferkorn ER. Interferon gamma blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophan. Proc Natl Acad Sci U S A. 1984;81:908–12. doi: 10.1073/pnas.81.3.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Phelps ED, Sweeney KR, Blader IJ. Toxoplasma gondii rhoptry discharge correlates with activation of the early growth response 2 host cell transcription factor. Infect Immun. 2008;76:4703–12. doi: 10.1128/IAI.01447-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Plattner H, Sehring IM, Mohamed IK, Miranda K, De Souza W, et al. Calcium signaling in closely related protozoan groups (Alveolata): Non-parasitic ciliates (Paramecium, Tetrahymena) vs. parasitic Apicomplexa (Plasmodium, Toxoplasma) Cell calcium. 2012;51:351–82. doi: 10.1016/j.ceca.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 121.Pomel S, Luk FC, Beckers CJ. Host cell egress and invasion induce marked relocations of glycolytic enzymes in Toxoplasma gondii tachyzoites. PLoS Pathog. 2008;4:e1000188. doi: 10.1371/journal.ppat.1000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ramana CV, Gil MP, Schreiber RD, Stark GR. Stat1-dependent and -independent pathways in IFN-gamma-dependent signaling. Trends Immunol. 2002;23:96–101. doi: 10.1016/s1471-4906(01)02118-4. [DOI] [PubMed] [Google Scholar]
- 123.Reese ML, Zeiner GM, Saeij JP, Boothroyd JC, Boyle JP. Polymorphic family of injected pseudokinases is paramount in Toxoplasma virulence. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1015980108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Roiko MS, Svezhova N, Carruthers VB. Acidification Activates Toxoplasma gondii Motility and Egress by Enhancing Protein Secretion and Cytolytic Activity. PLoS Pathog. 2014;10:e1004488. doi: 10.1371/journal.ppat.1004488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Romano JD, Sonda S, Bergbower E, Smith ME, Coppens I. Toxoplasma gondii salvages sphingolipids from the host Golgi through the rerouting of selected Rab vesicles to the parasitophorous vacuole. Mol Biol Cell. 2013;24:1974–95. doi: 10.1091/mbc.E12-11-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rosowski EE, Lu D, Julien L, Rodda L, Gaiser RA, et al. Strain-specific activation of the NF-kappaB pathway by GRA15, a novel Toxoplasma gondii dense granule protein. J Exp Med. 2011;208:195–212. doi: 10.1084/jem.20100717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rosowski EE, Nguyen QP, Camejo A, Spooner E, Saeij JP. Toxoplasma gondii Inhibits gamma interferon (IFN-gamma)- and IFN-beta-induced host cell STAT1 transcriptional activity by increasing the association of STAT1 with DNA. Infect Immun. 2014;82:706–19. doi: 10.1128/IAI.01291-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Saeij JP, Coller S, Boyle JP, Jerome ME, White MW, Boothroyd JC. Toxoplasma co-opts host gene expression by injection of a polymorphic kinase homologue. Nature. 2007;445:324–7. doi: 10.1038/nature05395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sahoo N, Beatty W, Heuser J, Sept D, Sibley LD. Unusual kinetic and structural properties control rapid assembly and turnover of actin in the parasite Toxoplasma gondii. Mol Biol Cell. 2006;17:895–906. doi: 10.1091/mbc.E05-06-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sharma P, Chitnis CE. Key molecular events during host cell invasion by Apicomplexan pathogens. Curr Opin Microbiol. 2013;16:432–7. doi: 10.1016/j.mib.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 131.Sheffield HG, Melton ML. The fine structure and reproduction of Toxoplasma gondii. J Parasitol. 1968;54:209–26. [PubMed] [Google Scholar]
- 132.Shen B, Sibley LD. Toxoplasma aldolase is required for metabolism but dispensable for host-cell invasion. Proc Natl Acad Sci U S A. 2014;111:3567–72. doi: 10.1073/pnas.1315156111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Skillman KM, Diraviyam K, Khan A, Tang K, Sept D, Sibley LD. Evolutionarily divergent, unstable filamentous actin is essential for gliding motility in apicomplexan parasites. PLoS Pathog. 2011;7:e1002280. doi: 10.1371/journal.ppat.1002280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Spear W, Chan D, Coppens I, Johnson RS, Giaccia A, Blader IJ. The host cell transcription factor hypoxia-inducible factor 1 is required for Toxoplasma gondii growth and survival at physiological oxygen levels. Cell Microbiol. 2006;8:339–52. doi: 10.1111/j.1462-5822.2005.00628.x. [DOI] [PubMed] [Google Scholar]
- 135.Stommel EW, Ely KH, Schwartzman JD, Kasper LH. Toxoplasma gondii: dithiol-induced Ca2+ flux causes egress of parasites from the parasitophorous vacuole. Exp Parasitol. 1997;87:88–97. doi: 10.1006/expr.1997.4187. [DOI] [PubMed] [Google Scholar]
- 136.Straub KW, Peng ED, Hajagos BE, Tyler JS, Bradley PJ. The Moving Junction Protein RON8 Facilitates Firm Attachment and Host Cell Invasion in Toxoplasma gondii. PLoS Pathog. 2011;7:e1002007. doi: 10.1371/journal.ppat.1002007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Striepen B, Jordan CN, Reiff S, van Dooren GG. Building the perfect parasite: cell division in apicomplexa. Plos Pathogens. 2007;3:e78. doi: 10.1371/journal.ppat.0030078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Suss-Toby E, Zimmerberg J, Ward GE. Toxoplasma invasion: the parasitophorous vacuole is formed from host cell plasma membrane and pinches off via a fission pore. Proc Natl Acad Sci U S A. 1996;93:8413–8. doi: 10.1073/pnas.93.16.8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Suvorova ES, Francia M, Striepen B, White MW. PLoS Biol. 2015. A novel bipartite centrosome coordinates the apicomplexan cell cycle. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Suzuki Y, Orellana MA, Schreiber RD, Remington JS. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science. 1988;240:516–8. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- 141.Tang Q, Andenmatten N, Triana MA, Deng B, Meissner M, et al. Calcium-dependent phosphorylation alters Class XIVa myosin function in the protozoan parasite Toxoplasma gondii. Mol Biol Cell. 2014 doi: 10.1091/mbc.E13-11-0648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tomavo S, Slomianny C, Meissner M, Carruthers VB. Protein trafficking through the endosomal system prepares intracellular parasites for a home invasion. PLoS Pathog. 2013;9:e1003629. doi: 10.1371/journal.ppat.1003629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tomita T, Yamada T, Weiss LM, Orlofsky A. Externally triggered egress is the major fate of Toxoplasma gondii during acute infection. J Immunol. 2009;183:6667–80. doi: 10.4049/jimmunol.0900516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Treeck M, Sanders JL, Elias JE, Boothroyd JC. The phosphoproteomes of Plasmodium falciparum and Toxoplasma gondii reveal unusual adaptations within and beyond the parasites' boundaries. Cell Host Microbe. 2011;10:410–9. doi: 10.1016/j.chom.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Treeck M, Sanders JL, Gaji RY, LaFavers KA, Child MA, et al. The calcium-dependent protein kinase 3 of toxoplasma influences basal calcium levels and functions beyond egress as revealed by quantitative phosphoproteome analysis. PLoS Pathog. 2014;10:e1004197. doi: 10.1371/journal.ppat.1004197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.van Dooren GG, Reiff SB, Tomova C, Meissner M, Humbel BM, Striepen B. A Novel Dynamin-Related Protein Has Been Recruited for Apicoplast Fission in Toxoplasma gondii. Curr Biol. 2009;19:267–76. doi: 10.1016/j.cub.2008.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wetzel DM, Chen LA, Ruiz FA, Moreno SN, Sibley LD. Calcium-mediated protein secretion potentiates motility in Toxoplasma gondii. J Cell Sci. 2004;117:5739–48. doi: 10.1242/jcs.01495. [DOI] [PubMed] [Google Scholar]
- 148.Wetzel DM, Hakansson S, Hu K, Roos D, Sibley LD. Actin filament polymerization regulates gliding motility by apicomplexan parasites. Mol Biol Cell. 2003;14:396–406. doi: 10.1091/mbc.E02-08-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wiley M, Sweeney KR, Chan DA, Brown KM, McMurtrey C, et al. Toxoplasma gondii activates hypoxia-inducible factor (HIF) by stabilizing the HIF-1alpha subunit via type I activin-like receptor kinase receptor signaling. J Biol Chem. 2010;285:26852–60. doi: 10.1074/jbc.M110.147041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Witola WH, Mui E, Hargrave A, Liu S, Hypolite M, et al. NALP1 influences susceptibility to human congenital toxoplasmosis, proinflammatory cytokine response, and fate of Toxoplasma gondii-infected monocytic cells. Infect Immun. 2011;79:756–66. doi: 10.1128/IAI.00898-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Annotated key references
- Moudy R, Manning TJ, Beckers CJ. The loss of cytoplasmic potassium upon host cell breakdown triggers egress of Toxoplasma gondii. J Biol Chem. 2001;276:41492–501. doi: 10.1074/jbc.M106154200. First comphrehensive analysis of the egress mechanism by innovative assays. [DOI] [PubMed] [Google Scholar]
- Lourido S, Shuman J, Zhang C, Shokat KM, Hui R, Sibley LD. Calcium-dependent protein kinase 1 is an essential regulator of exocytosis in Toxoplasma. Nature. 2010;465:359–62. doi: 10.1038/nature09022. First proof of a Ca2+-dependent protein kinase, a protein family absent from mammals, with an essential role in invasion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner M, Schluter D, Soldati D. Role of Toxoplasma gondii myosin A in powering parasite gliding and host cell invasion. Science. 2002;298:837–40. doi: 10.1126/science.1074553. Shows that Myosin A, a class XIVa myosin, is the main motor driving motility and invasion. [DOI] [PubMed] [Google Scholar]
- Suvorova ES, Francia M, Striepen B, White MW. A novel bipartite centrosome coordinates the apicomplexan cell cycle. PLoS Biol. 2015 doi: 10.1371/journal.pbio.1002093. in press. In this paper the mechanical basis for how mitosis and daughter budding are coordinated by the centrome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander DL, Mital J, Ward GE, Bradley P, Boothroyd JC. Identification of the Moving Junction Complex of Toxoplasma gondii: A Collaboration between Distinct Secretory Organelles. PLoS Pathog. 2005;1:e17. doi: 10.1371/journal.ppat.0010017. Identification of the composition of the moving junction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francia ME, Jordan CN, Patel JD, Sheiner L, Demerly JL, Fellows JD, de Leon JC, Morrissette NS, Dubremetz JF, Striepen B. Cell division in Apicomplexan parasites is organized by a homolog of the striated rootlet fiber of algal flagella. PLoS biology. 2012;10:e1001444. doi: 10.1371/journal.pbio.1001444. First report on the shared ancesty between endodyogeny and the machinery underlying flagellum construction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorestani A, Sheiner L, Yang K, Robertson SD, Sahoo N, Brooks CF, Ferguson DJ, Striepen B, Gubbels MJ. A Toxoplasma MORN1 Null Mutant Undergoes Repeated Divisions but Is Defective in Basal Assembly, Apicoplast Division and Cytokinesis. PLoS ONE. 2010;5:e12302. doi: 10.1371/journal.pone.0012302. Demonstration that the basal complex is the functional ortholog of the contractile ring in cell division. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TC, Hirsch JG. The interaction between Toxoplasma gondii and mammalian cells. II. The absence of lysosomal fusion with phagocytic vacuoles containing living parasites. J Exp Med. 1972;136:1173–94. doi: 10.1084/jem.136.5.1173. This was the first report showing that host organelles interact with the PV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou Z, McGovern OL, Di Cristina M, Carruthers VB. Toxoplasma gondii ingests and digests host cytosolic proteins. MBio. 2014;5:e01188–14. doi: 10.1128/mBio.01188-14. This work discovered an endocytic pathway in Toxoplasma. [DOI] [PMC free article] [PubMed] [Google Scholar]