Abstract
Background
Apolipoprotein E (apoE) has been shown to play a pivotal role in the development of cardiovascular disease, attributable to its function in lipid trafficking and immune modulating properties; however, its role in modulating inflammation in the setting of acute lung injury (ALI) is unknown.
Objective
To determine whether apoE-deficient mice (apoE−/−) are more susceptible to ALI compared to wild-type (WT) animals.
Methods
Two independent models of ALI were employed. Firstly, WT and apoE−/− mice were randomized to acid aspiration (50 μl of 0.1 N hydrochloric acid) followed by 4 h of mechanical ventilation. Secondly, WT and apoE−/− mice were randomized to 72 h of hyperoxia exposure or room air. Thereafter, the intrinsic responses of WT and apoE−/− mice were assessed using the isolated perfused mouse lung (IPML) setup. Finally, based on elevated levels of oxidized low-density lipoprotein (oxLDL) in apoE−/−, the effect of oxLDL on lung endothelial permeability and inflammation was assessed.
Results
In both in vivo models, apoE−/− mice demonstrated greater increases in lung lavage protein levels, neutrophil counts, and cytokine expression (p < 0.05) compared to WT mice. Experiments utilizing the IPML setup demonstrated no differences in intrinsic lung responses to injury between apoE−/− and WT mice, suggesting the presence of a circulating factor as being responsible for the in vivo observations. Finally, the exposure of lung endothelial cells to oxLDL resulted in increased monolayer permeability and IL-6 release compared to native (nonoxidized) LDL.
Conclusions
Our findings demonstrate a susceptibility of apoE−/− animals to ALI that may occur, in part, due to elevated levels of oxLDL.
Keywords: Apolipoprotein E, Acute lung injury, Acid aspiration, Hyperoxia, Oxidized low-density lipoprotein, Gastric acid aspiration
Introduction
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) represent a spectrum of disease characterized by the acute onset of severe hypoxemia as a result of noncardiogenic pulmonary edema [1]. Pathologically, an intense neutrophilic inflammatory process is observed within the alveolar air spaces, accompanied by an influx of protein-rich edema fluid, compromise of the alveolar epithelial membrane, and inactivation of the pulmonary surfactant system [2, 3]. Despite years of investigation, the clinical outcomes associated with ALI remain poor [4] and thus the discovery of novel pathways that contribute to the underlying pathogenesis of ALI may be important in defining new and effective therapeutic paradigms [5]. The identification of mechanisms that have been innately associated with other nonpulmonary inflammatory disease states may offer one potential avenue whereby the lessons learned from these disease processes could be exploited in the setting of ALI.
In the context of cardiovascular disease, apolipoprotein E (apoE) has been shown to play a pivotal role in the development of premature atherosclerosis on the basis of its known function in lipid trafficking [6]. Furthermore, it has been demonstrated that the apoE-deficient mouse (apoE−/−) develops premature atherosclerosis on the basis of serum hypercholesterolemia [7, 8] and an aberrant inflammatory response [9–11]. For example, observed rises in an oxidized form of low-density lipoprotein (oxLDL) in the apoE−/− mouse have been shown to act as a potent proinflammatory stimulus critical to the development of arterial plaque formation [12–14]. Although recent studies have identified a potential role for apoE in the pathophysiology of certain pulmonary diseases such as emphysema [15] and asthma [16], its role in the pathogenesis of ALI remains uncharacterized.
In the current study, it was hypothesized that apoE−/− mice would be more susceptible to the development of ALI. In order to test this hypothesis, 2 independent models of ALI were employed, including: (i) acid aspiration with mechanical ventilation and (ii) hyperoxia-induced lung injury. Overall, in both models of ALI, apoE−/− animals demonstrated an enhanced response to injury compared to wild-type (WT) animals as determined by increases in lung lavage cytokines, neutrophils, and protein leak. Subsequently, further studies were performed in order to determine whether the lungs of apoE−/− mice are susceptible to ALI using the ex-vivo isolated perfused mouse lung (IPML) model or whether circulating factors such as oxLDL may contribute to the ALI pathogenesis through effects on lung vascular endothelial cells.
Materials and Methods
Animals: Ethics Statement
All animal experiments were performed in concordance with Canadian Council of Animal Care animal health rules and regulations and were approved by the University of Western Ontario (Animal Use Subcommittee; AUS), the Lawson Health Research Institute (protocol No. 2009-091, London, Ont., Canada), and the Animal Care and Use Committee of the National Institute of Environmental Health Sciences (Research Triangle Park, N.C., USA). Eight- to 15-week-old male WT C57BL/6 and apoE−/− (C57/BL6 background) (Jackson Labs, Bar Harbor, Me., USA) mice were used for all experiments and were permitted to acclimatize for 72 h prior to use in experiments. All animals were allowed unrestricted access to standard chow and water.
Acid Aspiration and Ventilation-Induced Lung Injury Model
WT and apoE−/− animals were sedated and anaesthetized using ketamine hydrochloride (100 mg/kg) and medetomidine (50 mg/kg). Each animal received buprenorphine (1.5 μg subcutaneously) for analgesia. Arterial and venous access was established and a mid-line tracheostomy was performed with an 18-gauge angiocatheter endotracheal tube placed and secured. Subsequently, animals were mechanically ventilated for 15 min [respiratory rate, 150 breaths/min; tidal volume (VT), 8 ml/kg; positive end-expiratory pressure (PEEP), 3 cm H2O, and fraction of inspired oxygen (FIO2), 100%] (Harvard Instruments, St. Laurent, Que., Canada), at which point an initial arterial blood gas was obtained. Animals with an initial arterial oxygenation (PaO2) >400 (inclusion criteria) were randomized to receive an intratracheal bolus of air or 50 μl of 0.1 N hydrochloric acid, followed by 4 h of mechanical ventilation. Separate nonventilated (Non-Vent) WT and apoE−/− animals were sacrificed and included as controls. Thus, there were a total of 6 experimental groups that included: 2 nonventilated control groups (WT Non-Vent and apoE−/− Non-Vent), 2 control, ventilated ‘air bolus’ groups with the above parameters (WT Air and apoE−/− Air), and 2 ventilated, intra-tracheal ‘acid administration’ groups (WT Acid and apoE−/− Acid) with similar ventilation parameters. The animals were monitored via continuous measurement of their peak inspiratory pressure (PIP) and mean arterial blood pressure through an indwelling arterial catheter and PaO2 was assessed at 0, 120, and 240 min. At the completion of the ventilation protocol, the animals were sacrificed (i.v. sodium pentobarbital, 110 mg/kg) and whole-lung lavage was performed and analyzed as previously described [17]. Serum was collected from all 6 experimental groups just prior to the end of the experiment and analyzed for 9 proinflammatory cytokines and chemokines (G-CSF, GM-CSF, IL-1β, IL-6, IP-10, KC, MIP-2, LIX, and TNF-α) using a Millipore Milliplex kit (Millipore, Billerica, Mass., USA) and the Luminex xMAP detection system on a Luminex 100 (Linco Research, St. Charles, Mo., USA) as per the manufacturer’s instructions.
Lung Lavage Analysis
Immediately upon retrieval, surfactant was obtained through high-speed centrifugation and analyzed as previously described [18]. The remaining lavage supernatant was analyzed for protein concentration using a Micro BCA protein assay kit (Pierce, Rockford, Ill., USA) following the manufacturer’s instructions, and lavage cell counts were performed as previously described [19]. Lavage cytokines were quantified using a Millipore Milliplex kit (Millipore) and the Luminex xMAP detection system on a Luminex 100 (Linco Research), both as per the manufacturer’s instructions.
Hyperoxia-Induced Lung Injury Model
As a second model of lung injury, the response of WT and apoE−/− animals to 72 h of hyperoxia exposure was evaluated. WT and apoE−/− animals were placed individually in a Hazleton M60 battery inside a Hazleton 1000 chamber (Lab Products, Maywood, N.J., USA). After acclimation, mice were exposed to >95% O2 (UHP grade, minimum purity 99.994% O2 tanks; National Welders, Durham, N.C., USA) for 24 h/day for 72 h. All animals were allowed unrestricted access to standard chow and water. The temperature (22 ± 1°C) and humidity (50 ± 15%) of the chambers were monitored. Control mice were placed in a Hazleton M60 battery with food and water provided ad lib and exposed to filtered conditioned air for the duration of the study. All chambers were opened for approximately 30 min at the same time each morning to allow health checks, water and feed checks, and excreta paper change. Immediately after the end of each exposure, the mice were sacrificed by sodium pentobarbital overdose (100–150 mg/kg). Whole-lung lavage and measurements of lavage protein, cell counts, and cytokine levels were performed as described earlier.
IPML Model
In order to determine whether the lungs themselves of apoE−/− mice are susceptible to the development of ALI, the IPML model was employed in the acid aspiration model to examine the responses of apoE−/− lungs ex vivo as previously described [17]. Briefly, WT and apoE−/− mice were randomized to air or acid aspiration (50 μl of 0.1 N hydrochloric acid) as described above, resulting in 4 experimental groups (WT Air, WT Acid, apoE−/− Air, and apoE−/− Acid). In this experiment, animals were allowed to recover for 4 h after air or acid delivery and were then sacrificed and intubated, and the thorax was incised to expose the heart and lungs. Thereafter, selective catheterization of the left ventricle and pulmonary artery was performed and the pulmonary circulation was cleared by ‘flushing’ the lungs with perfusate (RPMI 1640 lacking phenol red and supplemented with 2% low endotoxin grade bovine serum albumin; Sigma, St. Louis, Mo., USA) and subsequently continuously perfused while the isolated lungs were mechanically ventilated for 2 h (Harvard Instruments) with a respiratory rate of 150 breaths/min, a VT of 8 ml/kg, and a slightly higher PEEP value (5 cm H2O) than that in the vivo experiments described above to compensate for the absence of intrapleural pressure. Notably, the RPMI was not supplemented with ferric nitrite or ferrous sulfate, which may be anticipated to contribute to lipid peroxidation, particularly in the setting of elevated cholesterol levels. The PIP was monitored throughout the mechanical ventilation. Four nonventilated, nonperfused groups were included for comparison, comprising animals that were sedated and received an air or acid instillation, were allowed to recover as above, and were sacrificed 4 h later but did not undergo the IPML or mechanical ventilation procedure. At completion of the ventilation protocol, or at sacrifice of the controls, whole-lung lavage was performed and samples were collected as described above.
oxLDL and Pulmonary Vascular Endothelial Cell Culture
Baseline levels of total cholesterol and lipid peroxidation (malondialdehyde; MDA) of serum samples from WT and apoE−/− mice used in the in vivo acid aspiration model were analyzed using the London Health Sciences Centre Core Laboratory Facility (total cholesterol) and a commercially available kit through a controlled reaction with thiobarbituric acid (TBARS, lipid peroxidation) as per the manufacturer’s instructions (Cayman Chemical, Ann Arbor, Mich., USA). In order to assess the effects of oxLDL on pulmonary vascular endothelial cells (PMVEC) in vitro, cells were isolated from peripheral, subpleural pulmonary tissue of naïve mice as previously described [20]. All experiments were conducted with PMVEC at passages 3–8. PMVEC permeability was assessed by the flux of Evans Blue dye-labeled albumin (EB-albumin) across PMVEC monolayers, as previously described [21]. For this assay, 1 × 105 PMVEC were grown to confluence on gelatin-coated cell culture inserts (3.0-μm pore) in 24-well plates. PMVEC monolayers were treated with human native LDL or copper sulphate oxLDL (protein concentrations: 15, 50, and 150 μg/ml; Intracel, Frederick, Mass., USA) versus the buffer control for 8 h. At various time points (2, 4, and 8 h), 150 μl of a 4% solution of albumin was added to the lower compartment of the well, while 50 μl of a 0.67 mg/ml EB-albumin solution was added to the upper compartment. The amount of EB-albumin that leaked across the PMVEC monolayer into the lower compartment of the PMVEC monolayers over exactly 1 h was quantified by measuring the absorbance of the media in the lower compartment at 595 nm. The trans-PMVEC EB-albumin flux under experimental conditions is expressed as a percentage of the total EB-albumin added to the upper compartment, normalized to control conditions. Subsequently, confluent monolayers of PMVEC were exposed to media, native LDL, or oxLDL for 16 h. The supernatant was collected and the concentrations of IL-6 were determined using a commercially available ELISA kit as per the manufacturer’s instructions (Pharmingen, San Diego, Calif., USA).
Statistical Analysis
All data are expressed as means ± SEM. Statistical analysis was performed using the statistical software package GraphPad Prism (GraphPad Software, La Jolla, Calif., USA). Treatment effects were determined using a two-way analysis of variance (ANOVA) followed by Bonferroni’s post hoc test. p < 0.05 was considered statistically significant. A two-tailed t test was used to assess significant baseline differences between levels of lipid peroxidation products in WT and apoE−/− animals, with values <0.05 considered statistically significant.
Results
Response of apoE−/− Mice to Acid Aspiration and Mechanical Ventilation in vivo
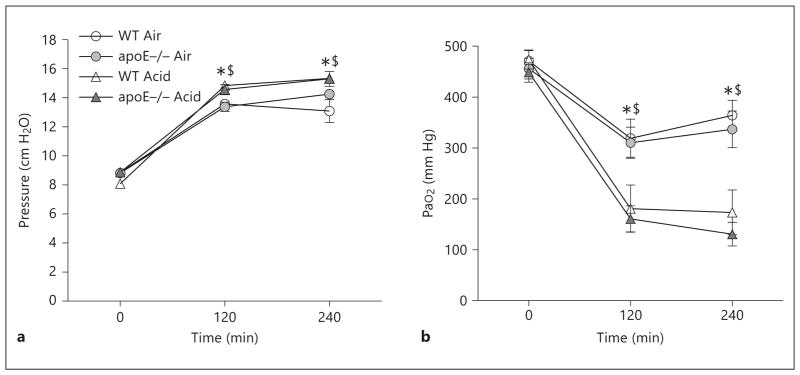
The pulmonary response of WT and apoE−/− mice to acid aspiration with mechanical ventilation, as a model of ALI, was evaluated. Overall, acid aspiration with mechanical ventilation was well tolerated, with no premature deaths observed in any group. PIP values and oxygenation values for the 4 groups of mechanically ventilated mice are shown in figure 1 a and b, respectively. In both air-instilled and acid-instilled animals, there was a significant increase in PIP and a reduction in PaO2 at both 120 and 240 min of mechanical ventilation compared to their respective time 0 values. As anticipated, in all animals receiving an intratracheal bolus of acid there was a significant rise in PIP and a reduction in PaO2 at 120 and 240 min of mechanical ventilation compared to animals receiving an intratracheal bolus of air. No differences were observed between genotypes in either air-instilled or acid-instilled animals at any time point during mechanical ventilation.
Fig. 1.
Lung physiology. PIP (a) and PaO2 (b) for WT and apoE−/− mice randomized to intratracheal air or acid aspiration and mechanical ventilation (n = 6–7 animals/group). * p < 0.05 for 120 and 240 min vs. baseline for the respective genotype; $ p < 0.05 for Acid vs. Air instillation.
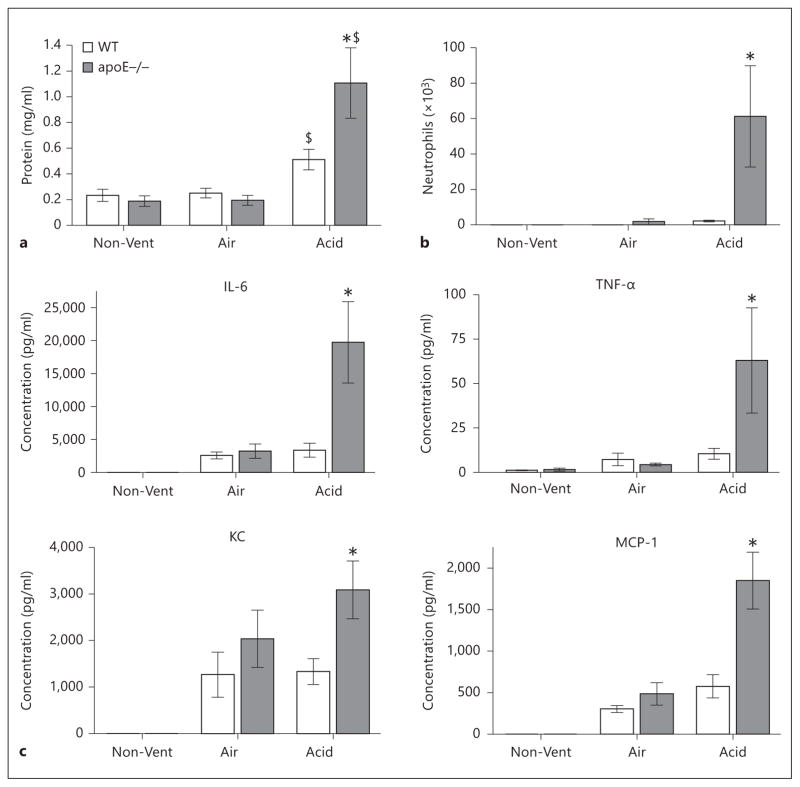
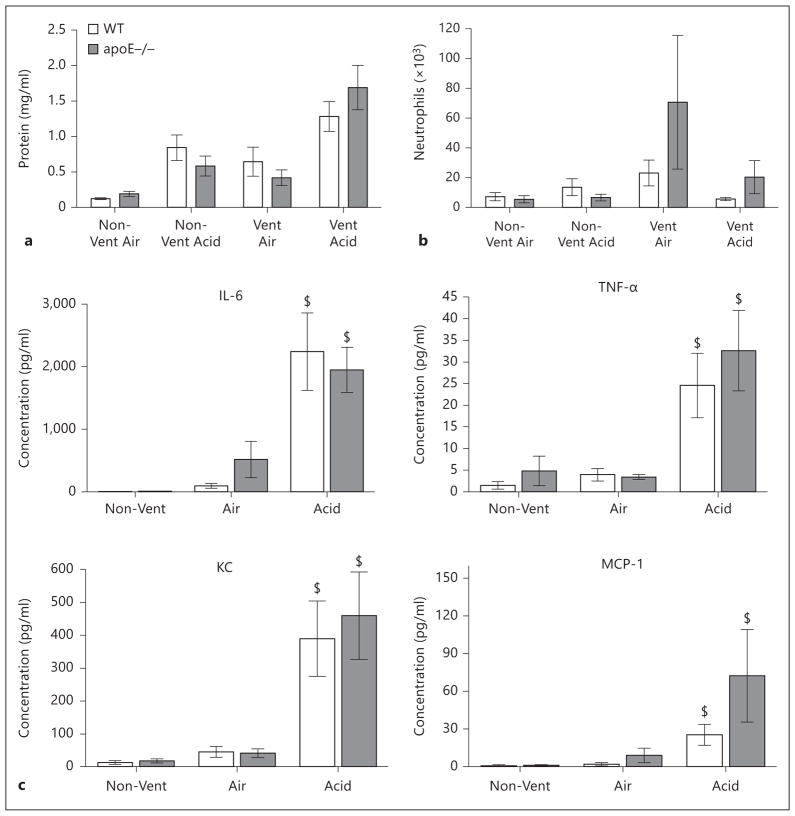
At the completion of ventilation, lung lavage samples were analyzed for protein concentration, neutrophil counts, and cytokine levels (fig. 2a–c). Firstly, a baseline comparison of the two uninjured, nonventilated groups demonstrated no significant difference between genotypes with respect to lung lavage levels of protein, cytokines, or neutrophil counts. Similarly, in animals randomized to the control air bolus followed by mechanical ventilation, there were also no differences with respect to lavage protein or neutrophils compared to genotyped-matched, nonventilated controls and similarly no differences were observed between genotypes. In contrast, in animals randomized to acid instillation followed by mechanical ventilation, there was a significant increase in lavage protein levels compared to genotype-matched animals treated with air instillation and mechanical ventilation (1.11 ± 0.27 mg/ml vs. 0.51 ± 0.08 mg/ml). Of relevance to our hypothesis, acid-treated apoE−/− mice demonstrated significantly greater levels of lavage protein, neutrophils, and cytokines (IL-6, TNF-α, KC, and MCP-1) compared to acid-treated WT animals.
Fig. 2.
Lavage analysis of WT and apoE−/− mice in acid aspiration with mechanical ventilation-induced lung injury. a Lavage protein. b Lavage neutrophil counts. c Lavage cytokines (IL-6, TNF-α, KC, and MCP-1). n = 6–7 animals/group. $ p < 0.05 vs. Air (matched genotype); * p < 0.05 vs. WT Acid.
Additionally, baseline analysis of the serum levels of a wide panel of proinflammatory chemokines and cytokines demonstrated no significant differences between genotypes with respect to any analyte within the systemic circulation (table 1). The values of these mediators were significantly increased in all 4 ventilated groups. Interestingly, and in contrast to the lavage levels of the inflammatory mediators, there were no consistent statistical differences between genotypes in the levels of inflammatory mediators in serum (table 1). Furthermore there was no significant effect of genotype on the amount of surfactant or the percentage of functional large aggregate component of surfactant in any of the experimental groups (data not shown).
Table 1.
Concentrations of cytokines/chemokines in the serum of WT and apoE−/− animals
| Serum analyte | WT | apoE−/− | WT | apoE−/− | WT | apoE−/− |
|---|---|---|---|---|---|---|
|
| ||||||
| Non-Vent | Non-Vent | Air Vent | Air Vent | Acid Vent | Acid Vent | |
| G-CSF | 249±72 | 307±71 | 49,550±375a | 44,353±1,690a | 48,287±685a | 48,579±247a |
| GM-CSF | 18±10 | 0±0 | 59±10a | 35±17a, d | 11±8a | 21±19a, c |
| IL-1β | 7±6 | 0±0 | 86±17 | 59±9 | 46±8 | 46±6 |
| IL-6 | 3±1 | 6±3 | 25,006±536a | 22,513±1,560a | 24,085±621a | 25,104±61a |
| KC | 333±83 | 65±17 | 28,235±2,369a | 13,511±3,744a, d | 22,738±4,482a | 17,057±3,403a |
| MIP-2 | 0±0 | 0±0 | 8,732±2,327a | 3,203±1,011 | 4,644±1,826 | 3,868±963 |
| IP-10 | 107±11 | 138.8±10.6 | 27,325±3,033a | 14,337±1,746a, d | 16,802±184a,b | 13,697±1,903a |
| LIX | 2,896±499 | 5,281±1,748 | 5,482±766 | 3,678±1,021 | 3,288±751 | 5,664±1,980 |
| TNF-α | 3±2 | 0.5±0.5 | 310±41a | 350±90 | 163±35a | 491±63a, e |
Values are presented as means ± SEM (pg/ml). n = 6–7 animals/group.
p < 0.05 vs. Non-Vent.
p < 0.05 for WT Air vs. WT Acid.
p < 0.05 for apoE−/− Air vs. apoE−/− Acid.
p < 0.05 for WT Air vs. apoE−/− Air.
p < 0.05 for WT Acid vs. apoE−/− Acid.
Response of apoE−/− Mice to Hyperoxia-Induced Lung Injury in vivo
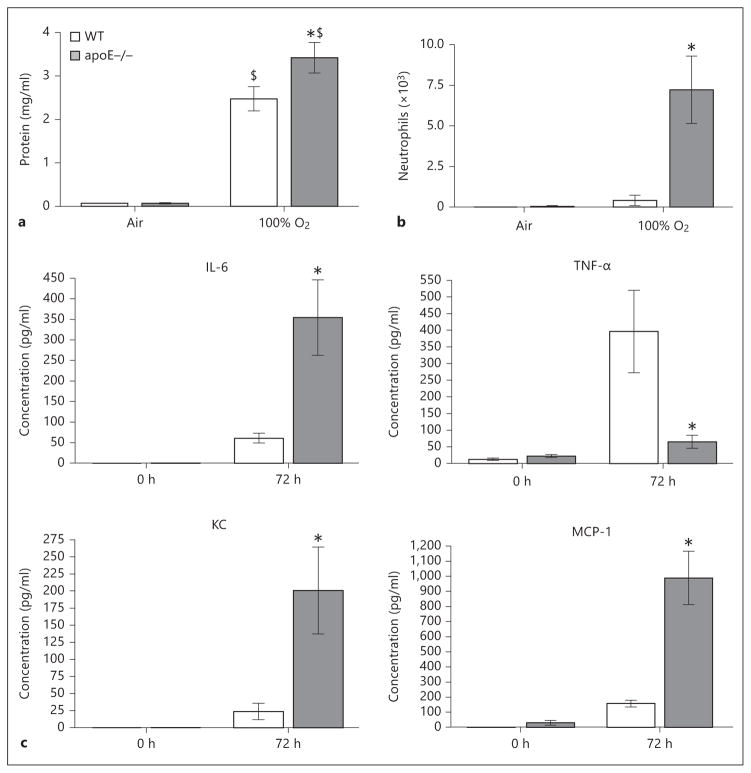
In order to discern whether the susceptibility of the apoE−/− mouse to lung injury is model specific or generalizable to other forms of ALI, WT and apoE−/− mice were studied using a hyperoxia-induced model of ALI. In control air-exposed animals, no difference in lavage protein, neutrophils, or inflammatory cytokines was observed between WT and apoE−/− mice (fig. 3a–c). After 72 h of exposure to >95% O2, there was a significant increase in lavage protein in WT and apoE−/− animals. Notably, hyperoxia-exposed apoE−/− mice had significantly higher lavage fluid concentrations of protein, neutrophil counts, IL-6, KC, and MCP-1 levels compared to their WT counterparts. In contrast, TNF-α was significantly lower in apoE−/− mice compared to WT mice after 72 h of hyperoxia exposure.
Fig. 3.
Lung lavage analysis of WT and apoE−/− mice exposed to 72 h of 100% O2. a Lavage protein (mg/ml) (WT Air = 0.07 ± 0.005, apoE−/− Air = 0.07 ± 0.014, WT O2 = 2.47 ± 0.28, apoE−/− O2 = 3.42 ± 0.35). b Lavage neutrophils (WT Air = 0.0 ± 0.0, apoE−/− Air = 0.42 ± 0.032, WT O2 = 0.42 ± 0.28, apoE−/− O2 = 7.23 ± 2.07). c Lavage cytokines (IL-6, TNF-α, KC, and MCP-1). n = 6–7 animals/group. * p < 0.05 vs. WT; $ p < 0.05 vs. Air.
Response of apoE−/− Lungs to Acid Aspiration and ex vivo Mechanical Ventilation
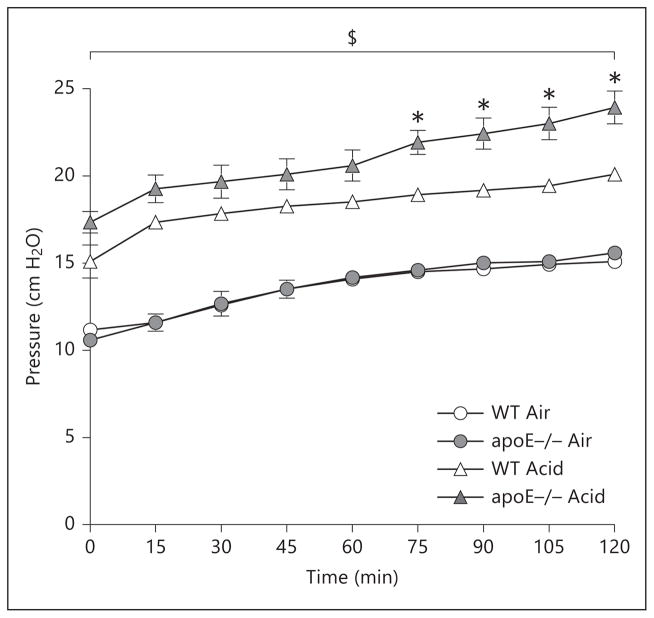
A model utilizing in vivo acid aspiration (or air control) followed by ex vivo mechanical ventilation of explanted lungs, i.e. the IPML model, was employed to determine whether apoE−/− lungs are inherently susceptible to the development of ALI. By replacing intravascular blood with media prior to ventilation, this model allows a more targeted investigation of the isolated injury response of lung parenchymal cells after their dissociation from the circulation. In animals receiving an air bolus followed by ex vivo mechanical ventilation, no differences were observed between genotypes with respect to PIP over the course of 120 min of ex vivo ventilation. On the other hand, animals receiving an intratracheal bolus of acid showed a significant rise in PIP at all time points compared to their respective genotype counterparts receiving an air bolus and mechanical ventilation. Furthermore, apoE−/− animals demonstrated a significantly greater increase in PIP compared to similarly treated WT animals from 75 to 120 min of the ventilation protocol (fig. 4).
Fig. 4.
Measurement of PIP in ex vivo mechanical ventilation using the IPML setup over 120 min. $ p < 0.05 for Acid vs. Air (genotype matched). n = 6–7 animals/group. * p < 0.05 for apoE−/− Acid vs. WT Acid.
With respect to lung lavage analysis, comparison of nonventilated animals receiving a control air bolus did not demonstrate significant differences between genotypes with respect to lavage protein, neutrophil counts, or cytokine concentrations (fig. 5a–c). In nonventilated animals receiving an acid bolus, there was a significant rise in lavage protein compared to values in air-instilled animals; however, no differences between neutrophil counts or cytokine levels were observed between groups. In animals receiving an air bolus followed by mechanical ventilation, no significant increase in lavage protein, neutrophils, or cytokines was observed compared to nonventilated controls, and no differences were observed between genotypes. In animals receiving acid instillation followed by ex vivo mechanical ventilation, there was a significant increase in lavage cytokine levels compared to air-instilled animals; however, no differences between genotypes were observed for any of the lavage outcomes measured. Similarly, no differences were observed in cytokine concentrations in lung perfusate collected at the completion of mechanical ventilation between genotypes in either air- or acid-instilled animals (data not shown). Taken together, these findings suggest the requirement for a circulating mediator in the enhanced injury response of apoE−/− lungs.
Fig. 5.
Lavage analysis of WT and apoE−/− animals exposed to acid aspiration and ex vivo mechanical ventilation. a Lavage protein (WT Non-Vent Air = 0.13 ± 0.01, apoE−/− Non-Vent = 0.15 ± 0.01, WT Non-Vent Acid = 0.72 ± 0.01, apoE−/− Non-Vent Acid = 0.55 ± 0.1, WT Vent Air = 0.60 ± 0.02, apoE−/− Vent Air = 0.42 ± 0.01, WT Vent Acid = 1.30 ± 0.21, apoE−/− Vent Acid = 1.67 ± 0.32). b Lavage neutrophils (WT Non-Vent Air = 7.0 ± 2.1, apoE−/− Non-Vent = 5.7 ± 2.8, WT Non-Vent Acid = 13.6 ± 5.2, apoE−/− Non-Vent Acid = 6.6 ± 2.1, WT Vent Air = 23.1 ± 8.6, apoE−/− Vent Air = 70.6 ± 44.9, WT Vent Acid = 5.6 ± 1.0, apoE−/− Vent Acid = 20.3 ± 11.1). c Lavage cytokines. n = 6–7 animals/group. $ p < 0.05 for Acid vs. Air, Non-Vent.
Effects of oxLDL on Lung Endothelial Cell Permeability and Inflammation
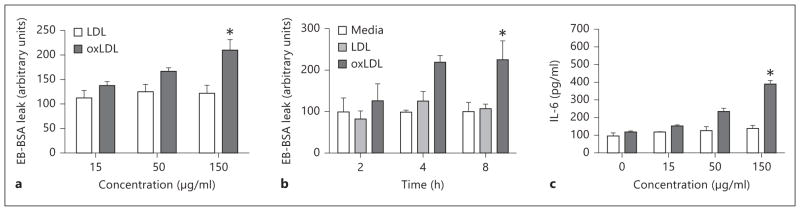
Given that serum cytokines were equivalent in WT and apoE−/− mice after acid aspiration and ventilation (table 1), we reasoned that a nonprotein circulating proinflammatory mediator might underlie the enhanced lung injury in apoE−/− mice. One candidate proinflammatory lipid mediator that has been described to be elevated in apoE−/− murine serum is oxLDL. Baseline evaluation of serum samples from WT and apoE−/− demonstrated a significant increase in serum cholesterol levels in apoE−/− compared to WT animals (11.59 ± 0.43 mmol/l vs. 1.51 ± 0.10 mmol/l, p < 0.05). Furthermore, consistent with past reports, we found an elevated concentration of MDA generated by lipid hyperoxides (TBARS assay) in apoE−/− serum, a widely used surrogate measure of oxLDL. apoE−/− mice displayed significantly greater concentrations of oxidized lipids (46.08 ± 5.20 μM MDA) compared to WT animals (26.91 ± 1.97 μM MDA, p < 0.05). Given this, the effects of oxLDL on lung endothelial cell permeability and inflammation were next evaluated in PMVEC in vitro. Compared to native (i.e. nonoxidized) LDL, oxLDL induced a significant increase in lung endothelial cell monolayer permeability in a time- and dose-dependent fashion, as shown in figure 6a–c. Furthermore, after exposure of endothelial cells to oxLDL, there was a significant rise in IL-6 levels in cell culture supernatant compared to cell culture supernatant in cells exposed to media alone or LDL. These findings thus support the notion that elevated serum oxLDL may contribute, at least in part, to the enhanced in vivo lung injury phenotype of apoE−/− mice.
Fig. 6.
Exposure of primary mouse lung endothelial cells to media, LDL, or oxLDL. Concentration-dependent changes in permeability (a), time-dependent changes in permeability (b), and changes in IL-6 concentrations (c) in cell culture supernatant. n = 3 independent experiments. * p < 0.05 vs. LDL.
Discussion
In the current study, we report that apoE−/− mice are more susceptible to the development of ALI compared to WT animals as determined by increases in lung permeability, neutrophil recruitment, and inflammatory cytokines. This susceptibility was observed across 2 independent models of lung injury: acid aspiration with mechanical ventilation and 72-hour hyperoxia exposure. Despite fundamental differences in the mechanism of injury incited by these experimental models, apoE deficiency was found to induce a similar pattern of injury in both models.
Over the past several years, the apoE−/− mouse model has been utilized extensively within the cardiovascular literature, based on the observed phenotype of hypercholesterolemia and premature atherosclerotic disease [6, 8]. In the context of lung injury, previous studies have demonstrated that exposure of apoE−/− mice to cigarette smoke resulted in premature emphysema and a more rapid decline in lung function compared to cigarette smoke-exposed, WT mice [22]. In other studies, house dust mite-challenged apoE−/− mice have displayed enhanced airway hyperreactivity and goblet cell hyperplasia compared to controls, and it has further been demonstrated that administration of an exogenous apoE-mimetic peptide could mitigate this inflammatory response [16]. Collectively, these studies, together with the current findings, suggest that absence of the apoE protein and downstream consequences of this deficiency result in an altered pulmonary inflammatory response. The present study extends the regulatory purview of apoE to the acutely injured lung by defining the response of apoE−/− mice to multiple forms of experimental, clinically relevant ALI. Moreover, we propose a molecular pathway (i.e. oxLDL) by which this enhanced susceptibility to ALI may occur.
It is worth noting, in relation to the ALI models employed in the current study, that the response of apoE−/− mice to injurious stimuli did not result in a global, non-specific enhancement of lung injury. For example, in the acid aspiration model of ALI, although lung lavage fluid demonstrated striking differences in neutrophil counts, alveolar permeability, and concentrations of selected cytokines in the apoE−/− mice, other relevant markers of lung dysfunction, such as changes in lung physiology (e.g. compliance, oxygenation) or pulmonary surfactant composition (data not shown), or serum cytokine levels did not differ significantly between the two genotypes. Additionally, analysis of the cytokine response in apoE−/− mice secondary to hyperoxia-induced ALI demonstrated a reduction in lavage TNF-α levels compared to WT controls, which contrasted with the findings for acid aspiration with the mechanical ventilation model, suggesting a complex role of apoE in modulating injury-specific inflammatory responses. One explanation for these findings may be predicated on the basis of different mechanisms of injury between the two experimental models. On the one hand, acid aspiration (±mechanical ventilation) has been shown to elicit a direct chemical injury to the airways and alveoli that culminates in alveolar hemorrhage, edema, and the recruitment of neutrophils [23, 24]. On the other hand, sustained hyperoxia has been shown to elicit lung responses through the overproduction of free oxygen radicals at the local tissue level, resulting in an NFκB-dependent mechanism of injury [25, 26]. While apoE deficiency enhanced the injury phenotype in both models, important differences in the apoE−/− phenotype between the two models (e.g. elevated BALF TNF-α after acid/ventilation vs. reduced BALF TNF-α after hyperoxia) suggest a complex, context-dependent role for apoE in modulation of the lung’s response to injury.
Although the primary focus of the current study was to elucidate whether a deficiency of apoE impacts the development of ALI, several lines of evidence suggest that decompartmentalization of the pulmonary inflammatory response to a systemic inflammatory response represents a critical stage in the natural history of this disease process [27, 28]. Despite observing a marked reduction in the pulmonary inflammatory response associated with acid aspiration and mechanical ventilation in apoE−/− mice, analysis of serum proinflammatory analytes did not reveal differences compared to WT animals. Although several possible explanations exist, including: (i) the timing of the sample collection, (ii) the possibility that pulmonary and systemic responses in the setting of apoE deficiency may be different, or (iii) that differences in other circulating factors in apoE deficiency may alter systemic responses, future studies will be required to elucidate the nature of this observation.
The differences observed in the outcomes between apoE−/− and WT mice may be particularly relevant from a clinical standpoint. For example, elevated lavage levels of IL-6 as observed in apoE−/− mice in both models utilized in the current study have been shown to be predictive of adverse outcomes in patients with ARDS [29]. Recently, Lin et al. [30] reported that, among mediators found in bronchoalveolar lavage fluid, IL-8 levels were the greatest predictor of mortality in a cohort of patients with ARDS, a finding also observed in apoE−/− animals (mouse analogue, KC) within both ALI models used in the current study [30].
Through use of the IPML model, lungs of WT and apoE−/− mice were isolated from the systemic circulation ex vivo to assess the inherent properties of the apoE−/− lungs in this setting. In contrast to observations in the initial in vivo model of ALI and those made previously [31], apoE−/− animals exposed to acid aspiration and mechanical ventilation did not exhibit differences in any outcomes compared to WT mice, including changes in lung permeability, neutrophil recruitment, and cytokine production within the alveolar airspaces. Further analysis of pooled lung perfusate circulated through the pulmonary circulation of WT and apoE−/− mice collected at the completion of mechanical ventilation did not demonstrate differences in circulating proinflammatory mediators, further supporting a lack of differences between the lungs of WT and apoE−/− animals. These findings suggest that circulating systemic factor(s) may be responsible for the observed susceptibility.
Thus, preliminary experiments were conducted in order to elucidate whether specific circulating factors may be responsible for the observed phenotype. Prior studies have shown that apoE−/− animals fed a standard chow diet (as in the current study) exhibit a significant increase in circulating levels of oxLDL [12]. Elevated levels of oxidized lipids were subsequently confirmed in the current study using a standard TBARS assay, which has been previously shown to correlate with levels of oxLDL [32]. Interestingly, the exposure of primary lung vascular endothelial cells to oxLDL resulted in an increase in both monolayer permeability and IL-6 release in both a time- and a dose-dependent fashion. Of note, LOX-1, a major endothelial receptor for oxLDL, has been shown to play a role in the development of ALI, as mice lacking endothelial expression of LOX-1 are resistant to the development of sepsis-induced ALI [33]. Taken together with our findings, this supports a potential involvement of oxLDL in the responses observed in our in vivo model; however, further studies are required to examine this in more detail, including the specific involvement of LOX-1 in the lung. Furthermore, our findings of the effects of oxLDL on pulmonary vascular cells do not preclude other potential mechanisms by which oxLDL may contribute to either the initiation or the propagation of ALI.
Despite the consistent observations made in the current study, specific limitations of the findings are noteworthy. Firstly, while the IPML experiments suggest that lung-resident cells do not account for the increased ALI susceptibility in apoE−/− mice and that a circulating factor is required, the IPML model is limited by the inherent effect of ischemia. In the current study we observed differences in PIP between acid-instilled WT and apoE−/− mice after 75 min of mechanical ventilation in this IPML model, which were not reflected by differences in lavage measures of ALI. This discrepancy in lung physiology and the markers of ALI may be due to the ‘open-chest’ setup used in ex vivo mechanical ventilation as opposed to an intact in vivo specimen. Secondly, it is notable that there were differences with respect to the timing of the mechanical ventilation after the delivery of intratracheal acid in the in vivo and IPML models. Animals received immediate mechanical ventilation in the in vivo model in order to maintain the viability of the model for the duration of ventilation as opposed to sacrificing the animal for purposes of the IPML setup, and it is therefore possible that this may have affected the outcome parameters observed within each of the models.
From a clinical perspective, the findings of the current study may have potential implications for patients with or at risk for the development of ALI. For example, the notion that oxLDL levels could influence the propagation of lung injury would have significant implications for a large population of patients with underlying hypercholesterolemia in which elevated levels of oxLDL have been demonstrated to circulate in direct proportion to serum LDL levels [34]. Unfortunately, at the current time, we are unaware of any clinical or epidemiologic data demonstrating a relationship between serum cholesterol levels and outcomes associated with ALI. This may be in part due to the high prevalence of other comorbid conditions including hypertension, obesity, and diabetes mellitus, which collectively coexist in a high proportion of these patients. Interestingly, recent data has suggested that obesity alone may be protective against the development of ALI/ARDS [35, 36], hence increasing the complexity of these so-called ‘metabolic’ risk factors. Based on evidence that apoE deficiency results in a susceptibility to ALI, a potential therapeutic role for exogenous apoE administration could be considered in future experimental models. Such an approach was previously demonstrated, whereby the administration of an apoE-mimetic peptide mitigated dust-mite-induced airway inflammation in an animal model of asthma [16].
In conclusion, the current study demonstrates the susceptibility of the apoE−/− mouse to the development of lung injury across two independent experimental models of ALI. We speculate that this increased susceptibility is driven, at least in part, by increased levels of circulating oxLDL. These findings provide a strong rationale to further investigate the role of both apoE and oxLDL in the context of ALI and to determine whether such factors may be amenable to pharmacologic intervention for patients with or at risk for ALI.
Acknowledgments
The authors would like to thank Ms. Shannon Seney of the Screening Lab for Immune Disorders, Lawson Health Research Institute, for her assistance with the multiplex analysis. This research was supported by the Ontario Thoracic Society/Ontario Lung Association (C.Y., J.F.L., and R.A.W.V.), the Western University Department of Medicine, Program of Experimental Medicine (C.Y.), and the Intramural Research Program of the National Institutes of Health NIEHS (Z01 ES102005) (M.B.F.).
References
- 1.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 2.Lewis JF, Veldhuizen R. The role of exogenous surfactant in the treatment of acute lung injury. Annu Rev Physiol. 2003;65:613–642. doi: 10.1146/annurev.physiol.65.092101.142434. [DOI] [PubMed] [Google Scholar]
- 3.Zemans RL, Matthay MA. Bench-to-bedside review: the role of the alveolar epithelium in the resolution of pulmonary edema in acute lung injury. Crit Care. 2004;8:469–477. doi: 10.1186/cc2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Villar J, Blanco J, Anon JM, Santos-Bouza A, Blanch L, et al. The ALIEN study: incidence and outcome of acute respiratory distress syndrome in the era of lung protective ventilation. Intensive Care Med. 2011;37:1932–1941. doi: 10.1007/s00134-011-2380-4. [DOI] [PubMed] [Google Scholar]
- 5.Yamashita CM, Lewis JF. Emerging therapies for treatment of acute lung injury and acute respiratory distress syndrome. Expert Opin Emerg Drugs. 2012;17:1–4. doi: 10.1517/14728214.2012.667800. [DOI] [PubMed] [Google Scholar]
- 6.Wouters K, Shiri-Sverdlov R, van Gorp PJ, van Bilsen M, Hofker MH. Understanding hyperlipidemia and atherosclerosis: lessons from genetically modified apoe and ldlr mice. Clin Chem Lab Med. 2005;43:470–479. doi: 10.1515/CCLM.2005.085. [DOI] [PubMed] [Google Scholar]
- 7.Bourdillon MC, Poston RN, Covacho C, Chignier E, Bricca G, et al. ICAM-1 deficiency reduces atherosclerotic lesions in double-knockout mice (ApoE(−/−)/ICAM-1(−/−)) fed a fat or a chow diet. Arterioscler Thromb Vasc Biol. 2000;20:2630–2635. doi: 10.1161/01.atv.20.12.2630. [DOI] [PubMed] [Google Scholar]
- 8.Moghadasian MH, McManus BM, Nguyen LB, Shefer S, Nadji M, et al. Pathophysiology of apolipoprotein E deficiency in mice: relevance to apo E-related disorders in humans. FASEB J. 2001;15:2623–2630. doi: 10.1096/fj.01-0463com. [DOI] [PubMed] [Google Scholar]
- 9.Ali K, Middleton M, Pure E, Rader DJ. Apolipoprotein E suppresses the type I inflammatory response in vivo. Circ Res. 2005;97:922–927. doi: 10.1161/01.RES.0000187467.67684.43. [DOI] [PubMed] [Google Scholar]
- 10.Ponnuswamy P, Schrottle A, Ostermeier E, Gruner S, Huang PL, et al. eNOS protects from atherosclerosis despite relevant super-oxide production by the enzyme in apoE mice. PLoS One. 2012;7:e30193. doi: 10.1371/journal.pone.0030193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weber C, Soehnlein O. ApoE controls the interface linking lipids and inflammation in atherosclerosis. J Clin Invest. 2011;121:3825–3827. doi: 10.1172/JCI60457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kato R, Mori C, Kitazato K, Arata S, Obama T, et al. Transient increase in plasma oxidized LDL during the progression of atherosclerosis in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2009;29:33–39. doi: 10.1161/ATVBAHA.108.164723. [DOI] [PubMed] [Google Scholar]
- 13.Khan BV, Parthasarathy SS, Alexander RW, Medford RM. Modified low density lipoprotein and its constituents augment cytokine-activated vascular cell adhesion molecule-1 gene expression in human vascular endothelial cells. J Clin Invest. 1995;95:1262–1270. doi: 10.1172/JCI117776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kitagawa K, Matsumoto M, Sasaki T, Hashimoto H, Kuwabara K, et al. Involvement of ICAM-1 in the progression of atherosclerosis in APOE-knockout mice. Atherosclerosis. 2002;160:305–310. doi: 10.1016/s0021-9150(01)00587-1. [DOI] [PubMed] [Google Scholar]
- 15.Goldklang M, Golovatch P, Zelonina T, Trischler J, Rabinowitz D, et al. Activation of the TLR4 signaling pathway and abnormal cholesterol efflux lead to emphysema in ApoE-deficient mice. Am J Physiol Lung Cell Mol Physiol. 2012;302:L1200–L1208. doi: 10.1152/ajplung.00454.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yao X, Fredriksson K, Yu ZX, Xu X, Raghavachari N, et al. Apolipoprotein E negatively regulates house dust mite-induced asthma via a low-density lipoprotein receptor-mediated pathway. Am J Respir Crit Care Med. 2010;182:1228–1238. doi: 10.1164/rccm.201002-0308OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walker MG, Tessolini JM, McCaig L, Yao LJ, Lewis JF, et al. Elevated endogenous surfactant reduces inflammation in an acute lung injury model. Exp Lung Res. 2009;35:591–604. doi: 10.1080/01902140902780460. [DOI] [PubMed] [Google Scholar]
- 18.Walker MG, Yao LJ, Patterson EK, Joseph MG, Cepinskas G, et al. The effect of tidal volume on systemic inflammation in acid-induced lung injury. Respiration. 2011;81:333–342. doi: 10.1159/000323609. [DOI] [PubMed] [Google Scholar]
- 19.Martin EL, Sheikh TA, Leco KJ, Lewis JF, Veldhuizen RA. Contribution of alveolar macrophages to the response of the TIMP-3 null lung during a septic insult. Am J Physiol Lung Cell Mol Physiol. 2007;293:L779–L789. doi: 10.1152/ajplung.00442.2006. [DOI] [PubMed] [Google Scholar]
- 20.Razavi HM, Wang le F, Weicker S, Rohan M, Law C, et al. Pulmonary neutrophil infiltration in murine sepsis: role of inducible nitric oxide synthase. Am J Respir Crit Care Med. 2004;170:227–233. doi: 10.1164/rccm.200306-846OC. [DOI] [PubMed] [Google Scholar]
- 21.Farley KS, Wang L, Mehta S. Septic pulmonary microvascular endothelial cell injury: role of alveolar macrophage NADPH oxidase. Am J Physiol Lung Cell Mol Physiol. 2009;296:L480–L488. doi: 10.1152/ajplung.90201.2008. [DOI] [PubMed] [Google Scholar]
- 22.Arunachalam G, Sundar IK, Hwang JW, Yao H, Rahman I. Emphysema is associated with increased inflammation in lungs of atherosclerosis-prone mice by cigarette smoke: implications in comorbidities of COPD. J Inflamm (Lond) 2010;7:34. doi: 10.1186/1476-9255-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Folkesson HG, Matthay MA, Hebert CA, Broaddus VC. Acid aspiration-induced lung injury in rabbits is mediated by interleukin-8-dependent mechanisms. J Clin Invest. 1995;96:107–116. doi: 10.1172/JCI118009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knight PR, Druskovich G, Tait AR, Johnson KJ. The role of neutrophils, oxidants, and proteases in the pathogenesis of acid pulmonary injury. Anesthesiology. 1992;77:772–778. doi: 10.1097/00000542-199210000-00023. [DOI] [PubMed] [Google Scholar]
- 25.O’Brien-Ladner AR, Nelson ME, Cowley BD, Jr, Bailey K, Wesselius LJ. Hyperoxia amplifies TNF-alpha production in LPS-stimulated human alveolar macrophages. Am J Respir Cell Mol Biol. 1995;12:275–279. doi: 10.1165/ajrcmb.12.3.7873193. [DOI] [PubMed] [Google Scholar]
- 26.Shea LM, Beehler C, Schwartz M, Shenkar R, Tuder R, et al. Hyperoxia activates NF-kappaB and increases TNF-alpha and IFN-gamma gene expression in mouse pulmonary lymphocytes. J Immunol. 1996;157:3902–3908. [PubMed] [Google Scholar]
- 27.Patterson EK, Yao LJ, Ramic N, Lewis JF, Cepinskas G, et al. Lung-derived mediators induce cytokine production in downstream organs via an NF-kappaB-dependent mechanism. Mediators Inflamm. 2013;2013:586895. doi: 10.1155/2013/586895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 29.Ranieri VM, Suter PM, Tortorella C, De Tullio R, Dayer JM, et al. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA. 1999;282:54–61. doi: 10.1001/jama.282.1.54. [DOI] [PubMed] [Google Scholar]
- 30.Lin WC, Lin CF, Chen CL, Chen CW, Lin YS. Prediction of outcome in patients with acute respiratory distress syndrome by bronchoalveolar lavage inflammatory mediators. Exp Biol Med (Maywood) 2010;235:57–65. doi: 10.1258/ebm.2009.009256. [DOI] [PubMed] [Google Scholar]
- 31.Massaro D, Massaro GD. Apoetm1Unc mice have impaired alveologenesis, low lung function, and rapid loss of lung function. Am J Physiol Lung Cell Mol Physiol. 2008;294:L991–L997. doi: 10.1152/ajplung.00013.2008. [DOI] [PubMed] [Google Scholar]
- 32.Chow SE, Hshu YC, Wang JS, Chen JK. Resveratrol attenuates oxLDL-stimulated NADPH oxidase activity and protects endothelial cells from oxidative functional damages. J Appl Physiol. 2007;102:1520–1527. doi: 10.1152/japplphysiol.00881.2006. [DOI] [PubMed] [Google Scholar]
- 33.Wu Z, Sawamura T, Kurdowska AK, Ji HL, Idell S, et al. LOX-1 deletion improves neutrophil responses, enhances bacterial clearance, and reduces lung injury in a murine polymicrobial sepsis model. Infect Immun. 2011;79:2865–2870. doi: 10.1128/IAI.01317-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van der Zwan LP, Teerlink T, Dekker JM, Henry RM, Stehouwer CD, et al. Circulating oxidized LDL: determinants and association with brachial flow-mediated dilation. J Lipid Res. 2009;50:342–349. doi: 10.1194/jlr.P800030-JLR200. [DOI] [PubMed] [Google Scholar]
- 35.Memtsoudis SG, Bombardieri AM, Ma Y, Walz JM, Chiu YL, et al. Mortality of patients with respiratory insufficiency and adult respiratory distress syndrome after surgery: the obesity paradox. J Intensive Care Med. 2012;27:306–311. doi: 10.1177/0885066611411410. [DOI] [PubMed] [Google Scholar]
- 36.Dossett LA, Heffernan D, Lightfoot M, Collier B, Diaz JJ, et al. Obesity and pulmonary complications in critically injured adults. Chest. 2008;134:974–980. doi: 10.1378/chest.08-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]