Abstract
Background
Estrogen is an important risk factor for cholesterol cholelithiasis not only in women of childbearing age taking oral contraceptives and postmenopausal women undergoing hormone replacement therapy, but also in male patients receiving estrogen therapy for prostatic cancer. In women, hormonal changes occurring during pregnancy markedly increase the risk of developing gallstones. We investigated whether the potent cholesterol absorption inhibitor ezetimibe could prevent the formation of estrogen-induced cholesterol gallstones in mice.
Design
Following ovariectomy, female AKR mice were implanted subcutaneously with pellets releasing 17β-estradiol at 6 μg/day and fed a lithogenic diet supplemented with ezetimibe in doses of 0 or 8 mg/kg/day for 8 weeks. Cholesterol crystallization and gallstone prevalence, lipid concentrations and composition in bile, and biliary lipid output were analyzed by physical-chemical methods. Intestinal cholesterol absorption efficiency was determined by fecal dual-isotope ratio methods.
Results
Ezetimibe inhibited intestinal cholesterol absorption, while significantly reducing hepatic secretion of biliary cholesterol. Consequently, bile was desaturated through the formation of numerous unsaturated micelles and gallstones were prevented by ezetimibe in mice exposed to high doses of estrogen and fed the lithogenic diet. Ezetimibe did not influence mRNA levels of the classical estrogen receptors α (ERα) and ERβ, as well as a novel estrogen receptor the G protein-coupled receptor 30 (GPR30) in the liver.
Conclusions
Ezetimibe protects against the estrogen-mediated lithogenic actions on gallstone formation in mice. Our finding may provide an efficacious novel strategy for the prevention of cholesterol gallstones in high-risk subjects, especially those exposed to high levels of estrogen.
Keywords: bile flow, bile salts, biliary secretion, cholesterol absorption, Lith gene, lithogenic bile, micelle, nutrition
INTRODUCTION
Estrogen is a critical risk factor for enhancing cholesterol cholelithogenesis because gallstone prevalence is twice as high in women as in men at all ages in every population studied [1]. Clinical studies also found that the use of oral contraceptives and conjugated estrogens in premenopausal women doubles the incidence of cholesterol gallstones [2–4]. The administration of estrogen to postmenopausal women for hormone replacement therapy significantly increases the prevalence of gallstones [5–7]. Furthermore, hormonal changes that occur during pregnancy put women at even higher risk for gallstone formation [8–10]. Estrogen therapy to men with prostatic cancer also leads to similar lithogenic effects [11, 12]. All of these observations clearly demonstrate that the increased risk of gallstone formation in women compared to men is related to differences in how the liver metabolizes cholesterol in response to estrogen. Although high levels of estrogen have a significant effect on the pathogenesis of cholesterol cholelithiasis, it is also essential for the production of eggs and for the preparation of the uterus for pregnancy [13], as well as has a crucial therapeutic role in postmenopausal women with a drop in estrogen levels [14] and in male patients with prostatic cancer [11, 12]. Thus, an ideal approach is to identify a drug that could prevent the formation of estrogen-induced gallstones, without interfering with the biological role of estrogen in women and its therapeutic effects in patients.
Compelling evidence has established a central role for estrogen in promoting gallstone formation by activating the hepatic estrogen receptor α (ERα), but not ERβ [15]. Because of activation of ERα by estrogen, mice continue to synthesize cholesterol in spite of its excess availability from high dietary cholesterol, which reflects a loss in controlling the negative feedback regulation of cholesterol synthesis that is determined possibly by an “estrogen-ERα-SREBP-2” pathway [16]. These alterations lead to excess amounts of newly synthesized cholesterol available for biliary hypersecretion and enhance the lithogenicity of bile. Estrogen increases intestinal cholesterol absorption by up-regulating Abcg5/g8 mRNA levels on the apical membrane of enterocytes through the ERα pathway. In addition, the G protein-coupled receptor 30 (GPR30) has been identified to be a novel estrogen receptor, and molecular and genetic analyses in mice supported the candidacy of Gpr30 for a new gallstone gene Lith18 [17–19]. In the previous studies, we have found that GPR30 produces a synergistic lithogenic action with ERα to promote the formation of estrogen-induced gallstones in mice [20].
The formation of lithogenic bile is induced by persistent hepatic hypersecretion of biliary cholesterol, which has both hepatic and small intestinal components [21]. The small intestine is a unique organ providing dietary and reabsorbed biliary cholesterol to the body. The potent cholesterol absorption inhibitor ezetimibe has been discovered to be able to prevent gallstone formation by effectively reducing intestinal cholesterol absorption and biliary cholesterol secretion, and protect gallbladder motility function by desaturating bile in gallstone-susceptible mice carrying Lith1 and Lith2 genes [22]. Furthermore, ezetimibe significantly reduces biliary cholesterol saturation and retards cholesterol crystallization in gallbladder bile of patients with gallstones [22]. In the present study, we investigated whether ezetimibe could prevent the formation of estrogen-induced cholesterol gallstones in ovariectomized female AKR mice exposed to high levels of estrogen and fed a lithogenic diet for 8 weeks.
MATERIALS AND METHODS
Chemicals
Radioisotopes [5,6-3H]sitostanol and [4-14C]cholesterol were purchased from American Radiolabeled Chemicals (St. Louis, MO) and NEN Life Science Products (Boston, MA), respectively. 17β-estradiol (E2)-releasing pellets were purchased from Innovative Research of America (Sarasota, FL). Ezetimibe was produced by Merck/Schering-Plough Pharmaceuticals (North Wales, PA). Medium-chain triglyceride was purchased from Mead Johnson (Evansville, IN).
Animals and diets
AKR/J mice of both genders obtained from the Jackson Laboratory (Bar Harbor, ME) were bred to generate female mice for the studies because they are susceptible to estrogen-induced gallstone formation [15]. All mice were provided free access to water and normal rodent chow containing trace (<0.02%) cholesterol (Teklad Rodent Diet, Madison, WI). To exclude possible interindividual differences in endogenous estrogen concentrations, all female mice, at the age of 4 weeks, were ovariectomized (OVX). At the age of 8 weeks, the mice were implanted subcutaneously with pellets releasing 17β-estradiol (E2) at 6 μg/day for 8 weeks [15]. As we have found before, E2 at the dose of 6 μg/day significantly enhances cholelithogenesis in OVX female AKR mice with intact expression of the Erα, Erβ and Gpr30 genes in the liver [15]. For gallstone prevention studies, the mice were fed a lithogenic diet (1% cholesterol, 0.5% cholic acid and 15% butter fat) supplemented with ezetimibe in doses of 0 or 8 mg/kg/day for 8 weeks. All animals were maintained in a temperature-controlled room (22±1°C) with a 12-hour day cycle (0600 h – 1800 h). All procedures were in accordance with current National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committee of Saint Louis University (St. Louis, MO).
Microscopic study of gallbladder bile and gallstones
At 8 weeks on the lithogenic diet supplemented with or without ezetimibe, mice (n=10 per group) were fasted overnight with free access to water. Following cholecystectomy, gallbladder volume was measured by weighing the whole gallbladder and equating gallbladder weight with gallbladder volume. Fresh gallbladder bile was examined by polarizing light microscopy for the presence of mucin gel, liquid crystals, plate-like solid cholesterol monohydrate crystals, sandy stones, and real gallstones according to previously established criteria [23]. Pooled gallbladder bile was frozen and stored at −20°C for further lipid analysis (see below).
Determination of biliary lipid output
To perform biliary lipid secretion studies, the common bile duct was cannulated via a PE-10 polyethylene catheter. The first hour sample of hepatic bile was collected by gravity in additional groups of mice (n=5 per group) [24]. Subsequently, each hourly collection of hepatic bile was examined by polarizing light microscopy and the volumes were determined. All bile samples were frozen and stored at −20°C for further lipid analyses (see below).
Lipid analysis
Biliary cholesterol, phospholipids, and total and individual bile salts, as well as plasma total cholesterol and HDL-, LDL- and VLDL-cholesterol concentrations were determined as described previously [25, 26]. Cholesterol saturation indexes (CSI) in gallbladder and hepatic bile were calculated from the critical tables [27].
Measurement of intestinal cholesterol absorption by the fecal dual-isotope ratio method
Under chow diet feeding conditions, nonfasted and nonanesthetized mice (n=5 per group) were given by gavage an intragastric bolus of 150 μl of medium-chain triglyceride containing 1 μCi of [14C]cholesterol and 2 μCi of [3H]sitostanol. We used [3H]sitostanol as the reference compound because this saturated sterol is poorly absorbed (<3%) in mice, as demonstrated in our previous studies by the fecal recovery method [28]. The radioactive isotopes from 4-day pooled fecal samples were saponified, extracted, and counted. The ratios of the two radiolabels in the fecal extracts and in the dosing mixture were used for calculating the percent cholesterol absorption:
Quantitative real-time PCR assay
Total RNA was extracted from fresh liver and intestinal tissues of mice (n=4 per group) using RNeasy Midi (Qiagen, Valencia, CA). Reverse-transcription reaction was performed using the SuperScript II First-Strand Synthesis System (Invitrogen, Carlsbad, CA) with 5 μg of total RNA and random hexamers to generate cDNA. Primer Express Software (Applied Biosystems, Foster City, CA) was used to design the primers based on sequence data available from GenBank. Quantitative real-time PCR assays of the hepatic ER genes and the genes involved in the regulation of hepatic and intestinal lipid metabolism were performed in triplicate according to previously established methods [16]. Relative mRNA levels were calculated using the threshold cycle of an unknown sample against a standard curve with known copy numbers. To obtain a normalized target value, the target amount was divided by the endogenous reference amount of rodent Gapdh as internal control.
Statistical method
All data are expressed as means±SD. Statistically significant differences among groups of mice were assessed by Student’s t-test, Mann-Whitney U-tests, or Chi-square tests. If the F-value was significant, comparisons among groups of mice were further analyzed by a multiple comparison test. Analyses were performed with a SuperANOVA software (Abacus Concepts, Berkeley, CA). Statistical significance was defined as a two-tailed probability of less than 0.05.
RESULTS
Ezetimibe inhibits intestinal cholesterol absorption and prevents E2-induced cholesterol gallstones
Under E2 treatment, ezetimibe at 8 mg/kg/day significantly (P<0.001) reduced the percentage of intestinal cholesterol absorption in chow-fed OVX mice compared to OVX control mice receiving no ezetimibe, as measured by the fecal dual-isotope ratio method (Figure 1A). Feeding the lithogenic diet for 8 weeks led to the formation of cholesterol gallstones in 80% of OVX mice treated with E2 and receiving no ezetimibe. By contrast, administration of ezetimibe completely protected against the formation of gallstones in OVX mice exposed to high doses of E2 (Figure 1B). Examinations by polarizing light microscopy (Figure 1C) found a lot of cholesterol monohydrate crystals and gallstones in E2-treated OVX mice receiving no ezetimibe, whereas gallbladder bile was isotropic (transparent) and no cholesterol crystals or gallstones were ever detected in ezetimibe-fed OVX mice even on the lithogenic diet for 8 weeks.
Figure 1.
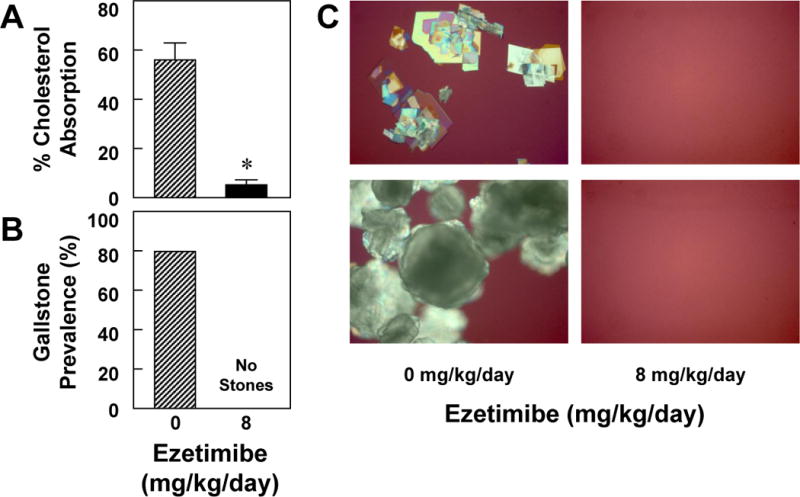
Ezetimibe prevents E2-induced cholesterol gallstones. (A) When ezetimibe is increased from 0 to 8 mg/kg/day, it significantly (P<0.001) reduces intestinal cholesterol absorption from 56±7% to 5±2% in E2-treated OVX female mice fed the chow diet, as measured by the fecal dual-isotope ratio method. (B) At 8 weeks on the lithogenic diet, the prevalence of gallstones is 80% in E2-treated mice receiving no ezetimibe. However, no gallstones are found in E2-treated mice receiving ezetimibe at 8 mg/kg/day. (C) Representative photomicrographs of plate-like solid cholesterol monohydrate crystals and gallstones as observed by polarizing light microscopy in gallbladder bile of mice receiving no ezetimibe and fed the lithogenic diet for 8 weeks. Of note, no solid cholesterol crystals or gallstones were detected in mice treated with ezetimibe. All magnifications are ×800, except for the photos from mice receiving no ezetimibe, which are ×400 (top panel) and ×200 (bottom panel).
As expected, the lithogenic diet significantly increased mole percent cholesterol in pooled gallbladder bile, leading to the formation of cholesterol-supersaturated bile in OVX mice treated with E2 (Table 1). By contrast, mole percent cholesterol in pooled gallbladder bile was markedly reduced by ezetimibe. As a result, the CSI value of pooled gallbladder bile was obviously reduced from 1.51 to 0.44 by ezetimibe. This protective effect of ezetimibe was confirmed by phase analysis in E2-treated OVX mice fed the lithogenic diet for 8 weeks (Figure 2).
Table 1.
Biliary lipid composition of pooled gallbladder bile
| Ezetimibe (mg/kg/day) |
Cholesterol (Mole%) |
Phospholipids (Mole%) |
Bile Salts (Mole%) |
Cholesterol Phospholipid Ratio |
Cholesterol Bile Salt Ratio |
Total Lipid Concentration (g/dL) |
CSI |
|---|---|---|---|---|---|---|---|
| 0 | 9.23 | 16.76 | 74.01 | 0.55 | 0.13 | 10.12 | 1.51 |
| 8 | 2.20 | 13.92 | 83.88 | 0.16 | 0.03 | 9.53 | 0.44 |
Values were determined from pooled gallbladder bile (n=10 per group). Abbreviation: CSI, cholesterol saturation index.
Figure 2.
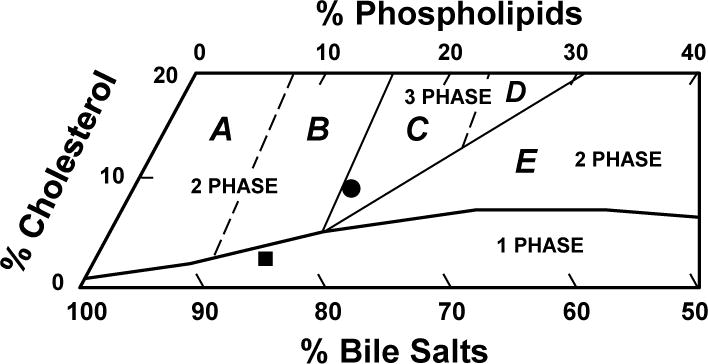
The relative biliary lipid composition (moles per 100 moles) of pooled gallbladder bile (n=10 per group) is plotted on a condensed phase diagram according to average total lipid concentration (~10.0 g/dL) of the bile (see Table 1). One-phase micellar zone is enclosed by a solid curved line. Above it, two solid and two dashed lines divide the phase diagram into Regions A–E with different crystallization sequences. The relative biliary lipid composition of pooled gallbladder bile from mice receiving no ezetimibe and fed the lithogenic diet for 8 weeks is located in the central three-phase zone, where bile is composed of solid cholesterol monohydrate crystals, liquid crystals, and saturated micelles at equilibrium. By contrast, ezetimibe administration leads to relative biliary lipid composition of pooled gallbladder bile plotted in the one-phase micellar zone, even upon the lithogenic diet feeding for 8 weeks. By phase analysis, this bile consists of only unsaturated micelles at equilibrium. Symbols ● and ■ represent relative biliary lipid composition of pooled gallbladder bile in E2-treated OVX mice at 8 weeks on the lithogenic diet supplemented with ezetimibe at 0 and 8 mg/kg/day, respectively.
In addition, ezetimibe significantly (P<0.01) reduced plasma total cholesterol from 179±12 mg/dL to 94±8 mg/dL in E2-treated OVX mice challenged to the lithogenic diet. Moreover, plasma HDL (70±11 mg/dL) and VLDL+LDL cholesterol (110±5 mg/dL) concentrations were significantly (P<0.01) decreased in ezetimibe-treated mice compared to mice receiving no ezetimibe (49±12 mg/dL and 45±11 mg/dL).
Physical-chemical analysis of gallbladder bile
The relative lipid composition of pooled gallbladder bile from E2-treated OVX mice fed the lithogenic diet and receiving no ezetimibe was located in the central three-phase area denoted Region C (Figure 2). By phase analysis, the bile consisted of solid cholesterol monohydrate crystals, liquid crystals and saturated micelles [29]. By contrast, with ezetimibe treatment, the relative lipid composition of pooled gallbladder bile was plotted within the one-phase micellar zone of the phase diagram. The bile was composed of one phase, namely unsaturated micelles, consistent with the results observed experimentally in model bile [29]. These alterations were induced by a significant reduction in cholesterol content of bile (Table 1). These results imply that ezetimibe can successfully prevent the formation of E2-induced cholesterol gallstones in OVX mice challenged to the lithogenic diet.
Ezetimibe reduces hepatic output of biliary lipids
As shown in Figure 3, at 8 weeks on the lithogenic diet, compared to control mice receiving no ezetimibe, administration of ezetimibe to E2-treated OVX mice resulted in a significant (P<0.01) reduction in biliary cholesterol output during the first hour of biliary secretion of interrupted enterohepatic circulation. However, hepatic output of biliary phospholipids and bile salts slightly varied and was not significantly different between two groups of mice. Also, cholesterol/phospholipid and cholesterol/bile salt ratios were significantly reduced, indicating that bile cholesterol saturation was significantly decreased by ezetimibe. Bile flow rates were comparable in two groups of mice.
Figure 3.
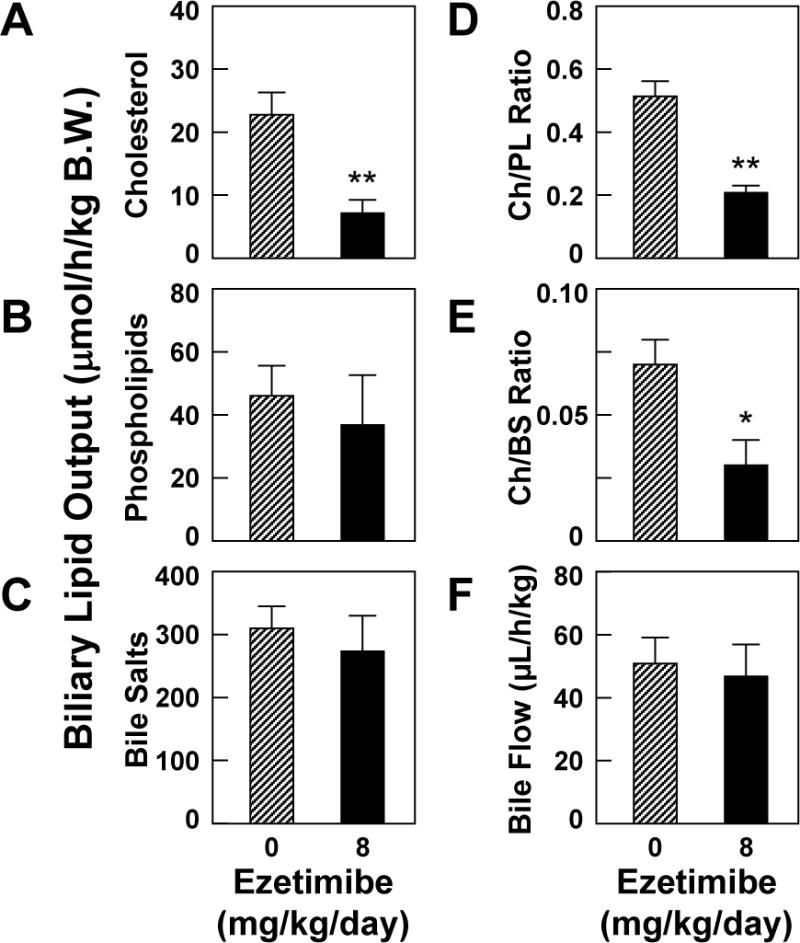
Ezetimibe significantly reduces hepatic output (μmol/h/kg B.W.) of biliary cholesterol (A) and phospholipids (B), but not bile salts (C) during the first hour of biliary secretion in E2-treated OVX mice at 8 weeks on the lithogenic diet. The ratios of Ch/PL (D) and Ch/BS (E) are reduced in ezetimibe-fed OVX mice exposed to E2. However, bile flow rates (F) are comparable in two groups of mice. *P<0.05, and **P<0.01, compared with E2-treated OVX mice receiving no ezetimibe and fed the lithogenic diet for 8 weeks. Abbreviations: Ch, cholesterol; PL, phospholipids; BS, bile salts.
Effect of ezetimibe on bile salt species of hepatic bile
Analyses of individual bile salt species by high-performance liquid chromatography (HPLC) revealed that all bile salts in hepatic bile were taurine conjugated with a similar distribution of bile salt composition between two groups of mice. At 8 weeks on the lithogenic diet, taurocholate (51.6–58.3%) became the major bile salt of biliary pool, followed by taurodeoxycholate (9.1–23.8%) and taurochenodeoxycholate (6.5–11.4%). There was a low concentration in tauro-ß-muricholate (8.7–9.0%), tauro-ω-muricholate (5.4–6.7%) and tauroursodeoxycholate (4.1–5.5%). Hydrophobicity indexes of bile salts in hepatic bile were basically the same (−0.10 to +0.04) between two groups of mice. These results indicate that ezetimibe does not influence bile salt species in bile.
Effects of ezetimibe on mRNA levels of hepatic estrogen receptor genes and the genes involved in the regulation of hepatic and intestinal lipid metabolism
Although it did not influence mRNA levels of three hepatic ER members: Erα, Erβ and Gpr30 (Figure 4A), treatment of ezetimibe at 8 mg/kg/day for 8 weeks significantly reduced expression levels of ATP-binding cassette (ABC) transporter G5 (Abcg5), Abcg8 and Abcb4, but not Abcb11 in the liver, which are responsible for hepatic secretion of biliary cholesterol, phospholipids and bile salts, respectively. Moreover, mRNA levels of the genes encoding two rate-limiting enzymes, cholesterol 7α-hydroxylase (Cyp7a1) and sterol 27-hydroxylase (Cyp27a1), in the classic and the alternative pathways of bile salt synthesis were significantly decreased by ezetimibe. By contrast, mRNA levels of the genes involved in the cholesterol biosynthesis, including 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Hmgcr), farnesyl diphosphate synthase (Fdps), lanosterol synthase (Lss), 3-hydroxy-3-methylglutaryl-coenzyme synthase 1 (Hmgcs1), and sterol regulatory element-binding protein 2 (Srebp2) were significantly increased by ezetimibe. These alterations might be secondary to reduced amounts of the intestinal source of cholesterol entering the liver in E2-treated OVX mice fed the lithogenic diet for 8 weeks. Low-density lipoprotein receptor (Ldlr) m RNA levels were markedly increased in ezetimibe-treated mice. No significant changes in mRNA levels of acyl-coenzyme A:cholesterol acyltransferase 2 (Acat2), liver X receptor α (Lxrα), and scavenger receptor class B type I (Sr-b1) were observed in two groups of mice. As expected, ezetimibe significantly reduced mRNA levels of the intestinal Niemann-Pick C1-like 1 protein (Npc1l1) in E2-treated OVX mice on the lithogenic diet (Figure 4B). Upon the lithogenic diet feeding, ezetimibe-treated mice displayed a significant reduction in mRNA levels of intestinal Abcg5, Abcg8, Acat2 and Lxrα, but not Hmgcr compared with control mice receiving no ezetimibe. This is mostly attributable to secondary reaction in response to decreased amounts of the absorbed cholesterol entering the enterocyte in ezetimibe-treated mice.
Figure 4.
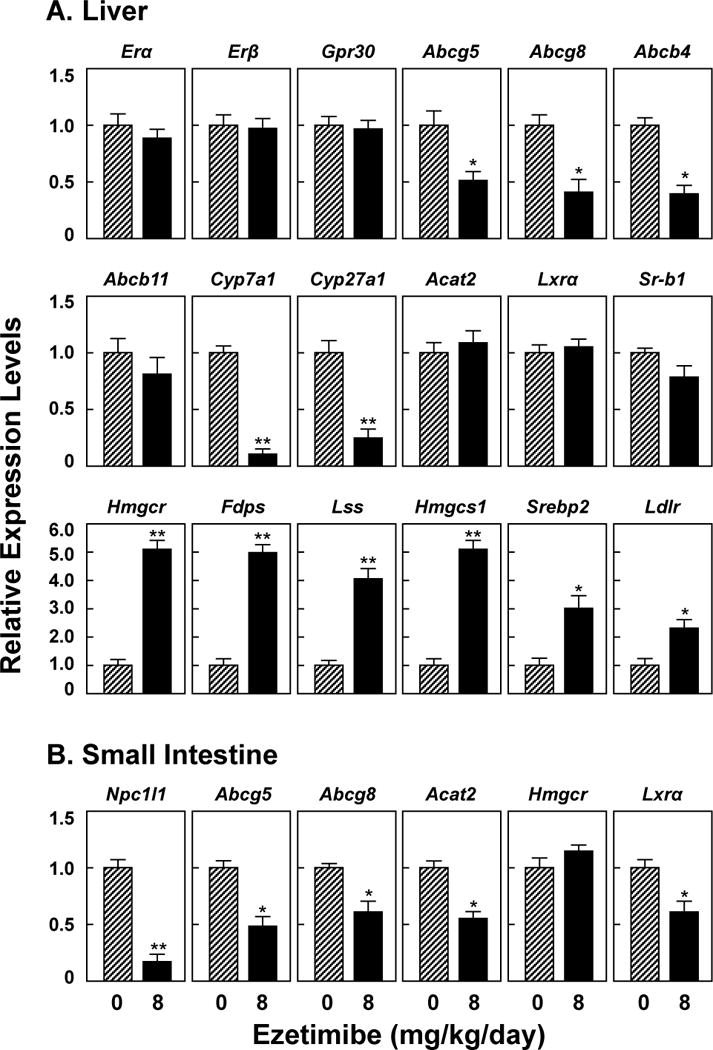
(A) Ezetimibe does not influence mRNA levels of two classical ER members, the Erα and Erβ genes, and a new ER member, the Gpr30 gene in the liver. (B) Ezetimibe treatment significantly reduces mRNA levels of Npc1l1 in the small intestine. See text for further description of other genes. *P<0.01 and **P<0.001, compared with E2-treated OVX mice receiving no ezetimibe and fed the lithogenic diet for 8 weeks.
DISCUSSION
The major findings of this study are that (i) the lithogenic actions of E2 on gallstone formation are inhibited by ezetimibe through a mechanism involving in the formation of numerous unsaturated micelles in bile so that biliary cholesterol is dissolved predominately in micellar phase; and (ii) ezetimibe does not influence mRNA levels of three ER members such as Erα, Erβ and Gpr30 in the liver. The results of this study in mice may have highly relevant translational value, as ezetimibe could prevent the formation of E2-induced cholesterol gallstones and this effect seems independent of estrogen signaling. In principle, ezetimibe may be effective in preventing E2-induced gallstones in at least three groups of subjects: (i) postmenopausal women undergoing hormone replacement therapy; (ii) male patients receiving estrogen therapy for prostatic cancer; and (iii) pregnant and postpartum women because they are at a high risk of predisposing to gallstone formation.
Ursodeoxycholic acid (UDCA), a hydrophilic bile acid, has been recommended as first-line pharmacological therapy in a subgroup of symptomatic patients with small, radiolucent cholesterol gallstones, and its long-term administration has been shown to promote the dissolution of cholesterol gallstones. A major limitation of oral litholysis by UDCA, however, is the high recurrence rate of gallstones. It is, therefore, imperative to search for a new cholelitholytic agent. Besides its striking inhibitory effect on intestinal cholesterol absorption, ezetimibe prevented gallstone formation by reducing biliary cholesterol output in gallstone-susceptible mice, even under high dietary cholesterol loads [22, 30]. It also protects gallbladder motility function by desaturating bile. Moreover, in a pilot study on gallstone patients, ezetimibe reduced biliary cholesterol saturation and retarded cholesterol crystallization in bile [22]. The current studies clearly show that ezetimibe is a potential cholelitholytic agent for preventing the formation of E2-induced gallstones in mice. Because ezetimibe and UDCA promote the dissolution of cholesterol gallstones by two distinct mechanisms via the formation of an unsaturated micelle and a liquid crystalline mesophase, respectively [22], it is highly likely that cholesterol gallstones could be dissolved faster by a combination therapy of ezetimibe and UDCA in mice exposed to high levels of E2.
Of note, NPC1L1 is detected in the liver of humans, but not mice and its expression levels are markedly lower in the liver than in the small intestine. Compared to WT mice, biliary cholesterol concentrations were reduced in mice transgenic for the human NPC1L1 gene, suggesting that the inhibition of NPC1L1 in the liver by ezetimibe may increase CSI values in bile by reducing biliary cholesterol taking back into the hepatocyte [31]. However, we found that after 30 day of ezetimibe treatment at 20 mg/day in patients with gallstones, besides a marked decrease in plasma LDL cholesterol concentrations, cholesterol concentrations and CSI values of gallbladder bile were significantly reduced [22]. Accordingly, detection time of cholesterol monohydrate crystals was significantly delayed. It has been proposed that the secretion efficiency of biliary cholesterol is determined by the net effect between efflux and influx of cholesterol molecules across the canalicular membrane of hepatocyte, which could be regulated by the ABCG5/G8-dependent and independent pathways, as well as the NPC1L1 pathway [32]. However, because biliary cholesterol secretion is a unique path for excretion of cholesterol from the body in humans and animals, hepatic ABCG5/G8 may play a stronger role in determining biliary cholesterol secretion than NPC1L1. Moreover, in the gut-liver axis, the intestinal NPC1L1 has a crucial role in providing dietary and reabsorbed biliary cholesterol to the body and the inhibition of its function by ezetimibe significantly decreases cholesterol absorption [21]. As a result, the bioavailability of intestinal source of cholesterol for biliary secretion is reduced dramatically.
Although the risks and benefits of hormone replacement therapy have been a question of great interest over the past years, conjugated estrogen alone or conjugated estrogens plus progesterone have been used to alleviate the symptoms of decline of endogenous estrogen levels in postmenopausal women [14, 33–36]. The risk of developing cholesterol gallstones is markedly increased, with a 38% increased risk for biliary tract surgery, by hormone replacement therapy [5–7]. Long-term administration of oral contraceptive steroids and conjugated estrogens also induces similar lithogenic effects in premenopausal women [2–4]. Younger women with surgical menopause or premature ovarian failure may use hormone replacement therapy for many years, until the age that natural menopause would be expected to occur. Although hormone replacement therapy is often given as a short-term relief (often 1 or 2, usually less than 5 years) from menopausal symptoms, biliary tract surgery is common in postmenopausal women. The association of hormone replacement therapy with gallbladder disease is well established, but the possibility of any pharmacological therapy for gallstone formation has not been addressed so far. The clear anti-lithogenic effect of ezetimibe seen in this study, therefore, raises further therapeutic possibilities in a subgroup of postmenopausal women undergoing hormone replacement therapy.
It is well-known that men with prostatic cancer have to undergo postoperative estrogen therapy, leading to a high risk of developing gallstones [11, 12]. These patients are recommended having a prophylactic cholecystectomy before estrogen therapy although their gallbladders function well. It is conceivable that ezetimibe treatment could prevent the formation of E2-induced gallstones without interfering with the therapeutic effect of E2 on prostatic cancer.
Elevated estrogen levels are associated with a marked increase in hepatic cholesterol secretion from the first to the third trimester of pregnancy, along with a progressive increase in the incidence of biliary sludge (a precursor to gallstones) and gallstones [37–39]. Clinical studies in the USA and Europe have found that during pregnancy and postpartum, the incidence rates of biliary sludge and gallstones are up to 30% and 12%, respectively. Although most women remain asymptomatic, 1–3% of pregnant women undergo cholecystectomy due to clinical symptoms or complications within the first year postpartum [10]. Because more than 4 million women give birth annually in the USA, it is estimated that at least 40,000 young healthy women require postpartum cholecystectomy each year. Moreover, biliary sludge and gallstones often disappear spontaneously after parturition in approximately 60% of cases mostly due to a sharp decrement in estrogen levels [10]. Therefore, for this reason, a special attention should be paid to pregnant women to reduce risk for the development of cholesterol gallstones. Of special note, no indication exists for drug prescription as preventive measure of biliary sludge and gallstones, and in principle, oral litholysis is not indicated in pregnancy. Moreover, there is currently no indication for UDCA as a preventive drug for gallstone disease in the general population, apart from high risk groups, and not enough evidence to embark on gallstone/sludge prophylaxis with UDCA in pregnancy because of transient characteristics of gallstones. Prospective clinical studies would be required in this special subgroup of women [10].
In this study, we observed that ezetimibe does not influence hepatic mRNA levels of Erα, Erβ and Gpr30. The quantitative assessments, by Western blot analysis, of changes in protein levels of ERs in response to E2 were not studied further because we have found that cellular protein levels of ERs are dynamic and are particularly sensitive to changes in circulating levels of E2 [22]. The long-term administration of E2 reduces protein concentrations of hepatic ERs. As a result, the ER proteins have a short half-life in the presence of high levels of E2 and be degraded through the proteasome-mediated proteolysis [40–42]. So, Western blot analysis is not an appropriate approach to quantitating changes in protein concentrations of ERs in the liver.
In summary, the results reported herein are consistent with the effect of ezetimibe on the prevention of gallstones in other strains of gallstone-susceptible mice [22, 30]. Our findings support the concept that ezetimibe prevents the formation of E2-induced cholesterol gallstones by inhibiting intestinal cholesterol absorption. The bioavailability of intestinal source of cholesterol for biliary secretion is markedly reduced and bile is desaturated in mice even on the lithogenic diet. Also, ezetimibe does not influence mRNA levels of the Erα, Erβ and Gpr30 genes in the liver. Therefore, we conclude that ezetimibe is a potential cholelitholytic agent for preventing or treating E2-induced gallstones. Our findings may provide an effective novel strategy for the prevention of cholesterol gallstones, particularly in women and patients exposed to high levels of estrogen. Further clinical studies are warranted in this respect.
Acknowledgments
This work was supported in part by research grants DK73917 (to D.Q.-H.W.) and DK92779 (to M.L.) from the National Institutes of Health (US Public Health Service) and research grant MRAR08P011-2012 (to P.P.) from the Italian Agency of Drug (AIFA).
Abbreviations
- ABC
ATP-binding cassette (transporter)
- CSI
cholesterol saturation index
- E2
17β-estradiol
- ER
estrogen receptor
- ERα
ER subtype α
- ERβ
ER subtype β
- GPR30
the G protein-coupled receptor 30
- NPC1L1
Niemann-Pick C1 like 1 (protein)
- OVX
ovariectomized
Footnotes
There is no conflict of interest to disclose for all authors.
All authors do not have a financial or other affiliation with Merck/Schering-Plough.
References
- 1.Wang HH, Liu M, Clegg DJ, Portincasa P, Wang DQ. New insights into the molecular mechanisms underlying effects of estrogen on cholesterol gallstone formation. Biochim Biophys Acta. 2009;1791:1037–1047. doi: 10.1016/j.bbalip.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grodstein F, Colditz GA, Hunter DJ, Manson JE, Willett WC, Stampfer MJ. A prospective study of symptomatic gallstones in women: relation with oral contraceptives and other risk factors. Obstet Gynecol. 1994;84:207–214. [PubMed] [Google Scholar]
- 3.Bennion LJ, Ginsberg RL, Gernick MB, Bennett PH. Effects of oral contraceptives on the gallbladder bile of normal women. N Engl J Med. 1976;294:189–192. doi: 10.1056/NEJM197601222940403. [DOI] [PubMed] [Google Scholar]
- 4.Stinton LM, Myers RP, Shaffer EA. Epidemiology of gallstones. Gastroenterol Clin North Am. 2010;39:157–169. doi: 10.1016/j.gtc.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Simon JA, Hunninghake DB, Agarwal SK, Lin F, Cauley JA, Ireland CC, Pickar JH. Effect of estrogen plus progestin on risk for biliary tract surgery in postmenopausal women with coronary artery disease. The Heart and Estrogen/progestin Replacement Study. Ann Intern Med. 2001;135:493–501. doi: 10.7326/0003-4819-135-7-200110020-00008. [DOI] [PubMed] [Google Scholar]
- 6.Cirillo DJ, Wallace RB, Rodabough RJ, Greenland P, LaCroix AZ, Limacher MC, Larson JC. Effect of estrogen therapy on gallbladder disease. JAMA. 2005;293:330–339. doi: 10.1001/jama.293.3.330. [DOI] [PubMed] [Google Scholar]
- 7.Grodstein F, Colditz GA, Stampfer MJ. Postmenopausal hormone use and cholecystectomy in a large prospective study. Obstet Gynecol. 1994;83:5–11. [PubMed] [Google Scholar]
- 8.Ko CW, Beresford SA, Schulte SJ, Matsumoto AM, Lee SP. Incidence, natural history, and risk factors for biliary sludge and stones during pregnancy. Hepatology. 2005;41:359–365. doi: 10.1002/hep.20534. [DOI] [PubMed] [Google Scholar]
- 9.Maringhini A, Ciambra M, Baccelliere P, Raimondo M, Orlando A, Tine F, Grasso R, et al. Biliary sludge and gallstones in pregnancy: incidence, risk factors, and natural history. Ann Intern Med. 1993;119:116–120. doi: 10.7326/0003-4819-119-2-199307150-00004. [DOI] [PubMed] [Google Scholar]
- 10.De Bari O, Wang TY, Liu M, Paik C-N, Portincasa P, Wang DQ. Cholesterol cholelithiasis in pregnant women: Pathogenesis, prevention and treatment. Ann Hepatol. 2014 (in press) [PubMed] [Google Scholar]
- 11.Angelin B, Olivecrona H, Reihner E, Rudling M, Stahlberg D, Eriksson M, Ewerth S, et al. Hepatic cholesterol metabolism in estrogen-treated men. Gastroenterology. 1992;103:1657–1663. doi: 10.1016/0016-5085(92)91192-7. [DOI] [PubMed] [Google Scholar]
- 12.Henriksson P, Einarsson K, Eriksson A, Kelter U, Angelin B. Estrogen-induced gallstone formation in males. Relation to changes in serum and biliary lipids during hormonal treatment of prostatic carcinoma. J Clin Invest. 1989;84:811–816. doi: 10.1172/JCI114240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gruber CJ, Tschugguel W, Schneeberger C, Huber JC. Production and actions of estrogens. N Engl J Med. 2002;346:340–352. doi: 10.1056/NEJMra000471. [DOI] [PubMed] [Google Scholar]
- 14.Rivera-Woll LM, Davis SR. Postmenopausal hormone therapy: the pros and cons. Intern Med J. 2004;34:109–114. doi: 10.1111/j.1444-0903.2004.00555.x. [DOI] [PubMed] [Google Scholar]
- 15.Wang HH, Afdhal NH, Wang DQ. Estrogen receptor alpha, but not beta, plays a major role in 17beta-estradiol-induced murine cholesterol gallstones. Gastroenterology. 2004;127:239–249. doi: 10.1053/j.gastro.2004.03.059. [DOI] [PubMed] [Google Scholar]
- 16.Wang HH, Afdhal NH, Wang DQ. Overexpression of estrogen receptor alpha increases hepatic cholesterogenesis, leading to biliary hypersecretion in mice. J Lipid Res. 2006;47:778–786. doi: 10.1194/jlr.M500454-JLR200. [DOI] [PubMed] [Google Scholar]
- 17.Lyons MA, Korstanje R, Li R, Sheehan SM, Walsh KA, Rollins JA, Carey MC, et al. Single and interacting QTLs for cholesterol gallstones revealed in an intercross between mouse strains NZB and SM. Mamm Genome. 2005;16:152–163. doi: 10.1007/s00335-004-2446-5. [DOI] [PubMed] [Google Scholar]
- 18.Lyons MA, Wittenburg H. Cholesterol gallstone susceptibility loci: a mouse map, candidate gene evaluation, and guide to human LITH genes. Gastroenterology. 2006;131:1943–1970. doi: 10.1053/j.gastro.2006.10.024. [DOI] [PubMed] [Google Scholar]
- 19.Krawczyk M, Wang DQ, Portincasa P, Lammert F. Dissecting the genetic heterogeneity of gallbladder stone formation. Semin Liver Dis. 2011;31:157–172. doi: 10.1055/s-0031-1276645. [DOI] [PubMed] [Google Scholar]
- 20.Wang HH, Portincasa P, Afdhal NH, Wang DQ. Lith genes and genetic analysis of cholesterol gallstone formation. Gastroenterol Clin North Am. 2010;39:185–207. doi: 10.1016/j.gtc.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 21.Wang HH, Portincasa P, de Bari O, Liu KJ, Garruti G, Neuschwander-Tetri BA, Wang DQ. Prevention of cholesterol gallstones by inhibiting hepatic biosynthesis and intestinal absorption of cholesterol. Eur J Clin Invest. 2013;43:413–426. doi: 10.1111/eci.12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang HH, Portincasa P, Mendez-Sanchez N, Uribe M, Wang DQ. Effect of ezetimibe on the prevention and dissolution of cholesterol gallstones. Gastroenterology. 2008;134:2101–2110. doi: 10.1053/j.gastro.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang DQ, Paigen B, Carey MC. Phenotypic characterization of Lith genes that determine susceptibility to cholesterol cholelithiasis in inbred mice: physical-chemistry of gallbladder bile. J Lipid Res. 1997;38:1395–1411. [PubMed] [Google Scholar]
- 24.Wang DQ, Lammert F, Paigen B, Carey MC. Phenotypic characterization of lith genes that determine susceptibility to cholesterol cholelithiasis in inbred mice. Pathophysiology Of biliary lipid secretion. J Lipid Res. 1999;40:2066–2079. [PubMed] [Google Scholar]
- 25.Wang DQ, Schmitz F, Kopin AS, Carey MC. Targeted disruption of the murine cholecystokinin-1 receptor promotes intestinal cholesterol absorption and susceptibility to cholesterol cholelithiasis. J Clin Invest. 2004;114:521–528. doi: 10.1172/JCI16801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang DQ, Carey MC. Susceptibility to murine cholesterol gallstone formation is not affected by partial disruption of the HDL receptor SR-BI. Biochim Biophys Acta. 2002;1583:141–150. doi: 10.1016/s1388-1981(02)00194-4. [DOI] [PubMed] [Google Scholar]
- 27.Carey MC. Critical tables for calculating the cholesterol saturation of native bile. J Lipid Res. 1978;19:945–955. [PubMed] [Google Scholar]
- 28.Wang DQ, Carey MC. Measurement of intestinal cholesterol absorption by plasma and fecal dual-isotope ratio, mass balance, and lymph fistula methods in the mouse: an analysis of direct versus indirect methodologies. J Lipid Res. 2003;44:1042–1059. doi: 10.1194/jlr.D200041-JLR200. [DOI] [PubMed] [Google Scholar]
- 29.Wang DQ, Carey MC. Complete mapping of crystallization pathways during cholesterol precipitation from model bile: influence of physical-chemical variables of pathophysiologic relevance and identification of a stable liquid crystalline state in cold, dilute and hydrophilic bile salt-containing systems. J Lipid Res. 1996;37:606–630. [PubMed] [Google Scholar]
- 30.Zuniga S, Molina H, Azocar L, Amigo L, Nervi F, Pimentel F, Jarufe N, et al. Ezetimibe prevents cholesterol gallstone formation in mice. Liver Int. 2008;28:935–947. doi: 10.1111/j.1478-3231.2008.01808.x. [DOI] [PubMed] [Google Scholar]
- 31.Temel RE, Tang W, Ma Y, Rudel LL, Willingham MC, Ioannou YA, Davies JP, et al. Hepatic Niemann-Pick C1-like 1 regulates biliary cholesterol concentration and is a target of ezetimibe. J Clin Invest. 2007;117:1968–1978. doi: 10.1172/JCI30060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang DQ, Cohen DE, Carey MC. Biliary lipids and cholesterol gallstone disease. J Lipid Res. 2009;50(Suppl):S406–411. doi: 10.1194/jlr.R800075-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhavnani BR, Strickler RC. Menopausal hormone therapy. J Obstet Gynaecol Can. 2005;27:137–162. doi: 10.1016/s1701-2163(16)30186-4. [DOI] [PubMed] [Google Scholar]
- 34.Wren BG. The benefits of oestrogen following menopause: why hormone replacement therapy should be offered to postmenopausal women. Med J Aust. 2009;190:321–325. doi: 10.5694/j.1326-5377.2009.tb02423.x. [DOI] [PubMed] [Google Scholar]
- 35.Nelson HD. Assessing benefits and harms of hormone replacement therapy: clinical applications. JAMA. 2002;288:882–884. doi: 10.1001/jama.288.7.882. [DOI] [PubMed] [Google Scholar]
- 36.Nelson HD, Humphrey LL, Nygren P, Teutsch SM, Allan JD. Postmenopausal hormone replacement therapy: scientific review. JAMA. 2002;288:872–881. doi: 10.1001/jama.288.7.872. [DOI] [PubMed] [Google Scholar]
- 37.Everson GT, McKinley C, Lawson M, Johnson M, Kern F., Jr Gallbladder function in the human female: effect of the ovulatory cycle, pregnancy, and contraceptive steroids. Gastroenterology. 1982;82:711–719. [PubMed] [Google Scholar]
- 38.Braverman DZ, Johnson ML, Kern F., Jr Effects of pregnancy and contraceptive steroids on gallbladder function. N Engl J Med. 1980;302:362–364. doi: 10.1056/NEJM198002143020702. [DOI] [PubMed] [Google Scholar]
- 39.Maringhini A, Marceno MP, Lanzarone F, Caltagirone M, Fusco G, Di Cuonzo G, Cittadini E, et al. Sludge and stones in gallbladder after pregnancy. Prevalence and risk factors. J Hepatol. 1987;5:218–223. doi: 10.1016/s0168-8278(87)80576-7. [DOI] [PubMed] [Google Scholar]
- 40.Preisler-Mashek MT, Solodin N, Stark BL, Tyriver MK, Alarid ET. Ligand-specific regulation of proteasome-mediated proteolysis of estrogen receptor-alpha. Am J Physiol. 2002;282:E891–898. doi: 10.1152/ajpendo.00353.2001. [DOI] [PubMed] [Google Scholar]
- 41.Nawaz Z, Lonard DM, Dennis AP, Smith CL, O’Malley BW. Proteasome-dependent degradation of the human estrogen receptor. Proc Natl Acad Sci USA. 1999;96:1858–1862. doi: 10.1073/pnas.96.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alarid ET, Bakopoulos N, Solodin N. Proteasome-mediated proteolysis of estrogen receptor: a novel component in autologous down-regulation. Mol Endocrinol. 1999;13:1522–1534. doi: 10.1210/mend.13.9.0337. [DOI] [PubMed] [Google Scholar]


