Introduction
Over the past 15 years, we and others have been investigating the use of shorter electrode-arrays to preserve residual hearing in individuals with functional low-frequency hearing and severe to profound high-frequency sensorineural hearing losses1–15. In 2014, the Nucleus® Hybrid™ L24 cochlear implant was approved by the Food and Drug Administration (FDA) for implantation in those with significant residual hearing16 and the results from that clinical trial have been published15.
While there continues to be some risk of loss of residual hearing with surgical implantation and also after activation of the device, it appears that shorter arrays preserve more hearing than longer ones3,17,18. The present case study is an example of the dilemma that can be encountered when residual hearing is preserved, but the implant fails. It does illustrate that the inner ear is more robust than once thought, and what it might be able to endure.
Methods
Subject
A 60-year old female subject with history of gradual progressive bilateral steeply sloping sensorineural hearing loss of unknown etiology presented to our clinic for a cochlear implant evaluation. Her audiogram demonstrated a severe, rapidly sloping, predominantly high-frequency sensorineural hearing loss in both ears, with the left more severe than the right. She reported that she first noticed her hearing loss in both ears at the age of 38 years and began using bilateral hearing aids (HA) at age 52.
The CNC Word recognition test19 in quiet at 60 dBA was administered in right HA, left HA, and Bilateral HA conditions. The subject scored 16% in the ear to be implanted (left ear), 57% in the contralateral ear, and 54% bilaterally. Based on the audiogram, speech perception results and demographic factors, the subject fit the criteria for an FDA trial of a Hybrid S12 (S12) Cochlear Implant.
Hybrid S12 Device Description
The subject was implanted with the S12 cochlear implant as part of a Cochlear Americas sponsored FDA IDE (IDE No. G070016) in October of 2008. The Nucleus® Hybrid™ S12 cochlear implant consists of an electrode array that is 10 millimeters in length with 10 electrode contacts spaced over 5.6 mm of the apical tip of the electrode and an internal receiver/stimulator containing Nucleus Freedom cochlear implant electronics.
Surgical procedure
Surgery was performed using soft surgery techniques, as described in Gantz, Turner, Gfeller, Lowder11. Dexamethasone (10 mg) was systemically administered intraoperatively.
The status of residual acoustic hearing was monitored during the surgical procedure using the auditory brainstem response (ABR). The stimuli were clicks delivered through an insert earphone in the left ear.
Results
Device Activation
The subject returned one month following surgery for her device activation. Figure 1, Panel A, shows the subject’s pure-tone air conduction thresholds at activation and over time. The subject’s preoperative pure-tone average (PTA; using thresholds from .125–1k Hz) was 44 dB HL and 52 dB HL at initial activation, demonstrating an 8 dB HL shift in hearing.
Figure 1.
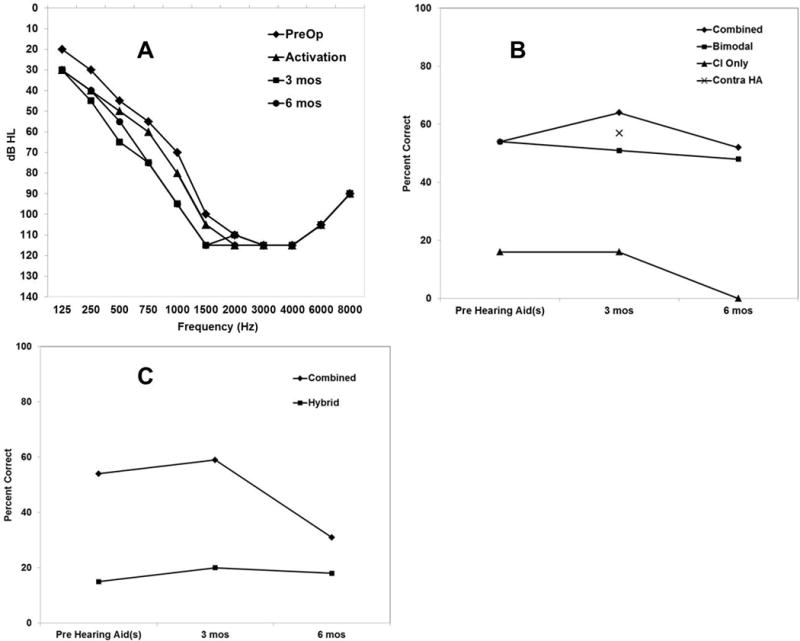
Panel A. Individual unaided thresholds over the range 125 to 8000 Hz in the implanted ear preoperatively and post-operatively at initial activation and post-operatively over time through 6 months with the S12. Panel B. Individual CNC word perception performance pre-operatively and post-operatively over time at 3 and 6 months in the Combined, Bimodal, CI Only, and Contralateral hearing aid conditions. Panel C. Individual AzBio Sentences in +5 Signal-to-noise ratio multi-talker babble at 0°-azimuth performance pre-operatively and post-operatively over time at 3 and 6 months in the Combined and Hybrid conditions.
The device was programmed with the electric lower frequency boundary of 813 Hz and an upper frequency boundary of 7938 Hz. The subject’s acoustic parameters were programmed using NAL-RP prescriptive methods20,21 to assess the degree to which real-ear targets were met for the subject.
Hearing and Speech Perception Over Time
The subject’s PTAs (Figure 1, Panel A) remained stable with a PTA at 3 months of 62 dB HL and 6 months of 59 dB HL. In Panel B, we show CNC words over time. AzBio Sentences tested in +5 signal-to-noise (S/N) ratio using multi-talker babble with noise presented at 0°-azimuth are shown in Panel C. Bilateral hearing aids (pre-operatively) are compared to the Combined and Bimodal test conditions and the unilateral hearing aid in the ear to be implanted is compared to the Hybrid and CI-only test conditions. Table 1 defines the various post-operative listening conditions. In both panels, the results show the subject’s speech perception outcomes not improving over time. In Panel B, when comparing CNC words in the Combined and Bimodal listening conditions to the contralateral hearing aid only condition at 3 months post-operatively, there was no significant difference in scores using the Binomial model. Furthermore, in the CI only condition the patient scored 16% at 3 months and declined to 0% at 6 months. The same trend is evident when evaluating the AzBio sentence scores over time in the Combined and Hybrid conditions in Panel C.
Table 1.
Definitions used to classify device use and testing conditions.
| Name | Definition |
|---|---|
| Acoustic Stimulation | Use of the word “acoustic” refers to sound delivered with or without amplification only. |
| CI Only Stimulation | Use of the word “electric” refers to sound delivered via the Hybrid cochlear implant only. |
| Hybrid Stimulation | Use of unilateral acoustic hearing, with or without amplification, in addition to electric hearing via the Hybrid cochlear implant in the same ear. |
| Bimodal Stimulation | Use of unilateral acoustic hearing, with or without amplification, in addition to electric hearing only via the Hybrid cochlear implant in the opposite ear. |
| Combined Stimulation | Use of bilateral acoustic hearing, with or without amplification, in addition to electric hearing via the Hybrid cochlear implant. That is, a combination of the Hybrid and Bimodal conditions. |
| Contra Acoustic HA | Use of contralateral acoustic hearing. That is, only acoustic hearing in the contralateral ear. Nothing in the ipsilateral implanted ear. |
Device Failure
An integrity test of the device was performed by the clinical specialist from Cochlear Americas and our electrophysiology team22. The results of the integrity test were inconclusive. A CT scan was performed and indicated that the cochlear implant was in normal position in the basal turn of the cochlea.
Because of the lack of improved speech perception with the electrical speech processing, a decision was made to explant the Hybrid S12 and reimplant with a Hybrid L24. The FDA provided appropriate permission to explant the Hybrid S12 device and reimplant the Hybrid L24 device. Device analysis of the returned Hybrid S12, provided by Cochlear Limited, indicated that the device malfunctioned with the primary fault being an electrode lead failure due to insulation damage.
Second surgery
The internal receiver stimulator of the S12 was carefully freed from its capsule with the electrode still in the cochlea. The electrode was then carefully removed. The new L24 device was then inserted through the cochleostomy. Throughout this time, ABR monitoring was performed and amplitude of wave V was preserved.
L24 Hearing Over time
Figure 2, Panel A, shows pure-tone air conduction thresholds prior to S12 explantation and with the L24 at initial activation, 3 months, 6 months and 12 months postoperatively. The PTA prior to explantation with the S12 was 59 dB HL and at activation of the L24 at one month post explantation/reimplantation was 66 dB HL. Over time, the hearing remained very stable, with PTAs at 62 dB HL, 59 dB HL, and 66 dB HL at 3, 6, and12 months, respectively. Overall, after two cochlear implant surgeries, the subject had a 22 dB HL total shift in PTA (Figure 2, Panel B).
Figure 2.
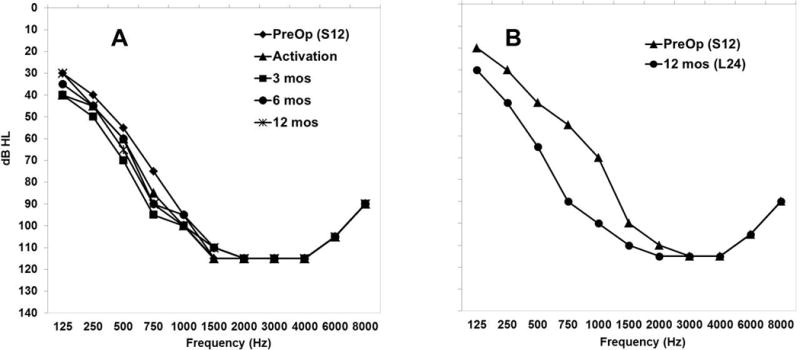
Panel A. Individual unaided thresholds over the range 125 to 8000 Hz in the implanted ear preoperatively with the S12 and post-operatively at initial activation through 12 months with the L24. Panel B. Individual unaided thresholds over the range 125 to 8000 Hz in the implanted ear preoperatively to surgery with the S12 and post-operatively at 12 months of CI experience with the L24.
L24 Speech Perception Over Time
In Figure 3, we show the speech perception results over time for CNC words (Panel A) and AzBio Sentences in noise (Panel B). The left side of the graphs in Panels A and B show the speech perception results in multiple listening conditions with the S12 device and on the right side with the L24 device over time. There were significant improvements (using the binomial confidence interval) in the CNC scores in all conditions with L24 after 3 months of device use compared to scores with S12 after 6 months of device use (Panel A). At 12 months, the Combined condition CNC score was 89%, a 37% improvement. In the Hybrid and CI only conditions, there was a 50% and 61% improvement, respectively. Similarly, there were significant changes in the AzBio sentence scores in the Combined and Hybrid conditions with L24 after 3 months of device use compared to scores with S12 after 6 months of device use (Panel B). At 12 months, the Combined condition score was 73%, a 42% improvement over the S12 at 6 months of device use. In the Hybrid condition, there was a 57% improvement in performance over the S12 after 6 months of device use.
Figure 3.
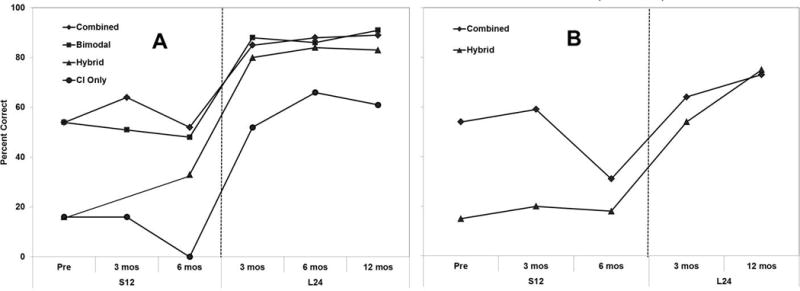
Panel A. Individual CNC word perception performance over time in the Combined, Bimodal, Hybrid, and CI-Only conditions. The left side of the graph shows the CNC word scores over time with the S12 device. On the right side of the graph are the CNC scores over time with the L24 device. Panel B. Individual AzBio Sentences in +5 Signal-to-noise ratio multi-talker babble at 0°-azimuth performance over time in the Combined and Hybrid conditions. The left side of the graph shows the sentence in noise scores over time with the S12 device. On the right side of the graph are the sentences in noise scores over time with the L24 device.
Discussion
One of the more challenging aspects of this case was that a non-functioning device was present in an ear with substantial hearing. This case study demonstrates that shorter electrodes have the ability to preserve the regions of the cochlear partition that are apical to the electrode. This finding is also relevant to the pediatric population who are implanted at very young ages. While we do not know the full life-expectancy of a cochlear implant, it is likely that a child implanted at a young age will reach the life-expectancy of the implant and have to undergo explanation/reimplantation of their CI. Furthermore, doing minimal damage to the inner ear might retain the possibility of allowing them to take advantage of future advances in the field of auditory neurobiology and hearing devices.
Finally, the results of this case study demonstrate learning outcomes and implications that are important for professionals in the field of cochlear implants. As the audiologist is providing post-operative follow-up care, they need to be cognizant and able to recognize unusual performance patterns associated with the cochlear implant. The audiologist should review impedance measurements over time, speech perception improvement, and loudness growth associated with the programming. Furthermore, at the 3, 6, and 12 month visits, it is crucial to perform a battery of speech perception tests. The most current version of the Minimum Speech Test Battery (MSTB;23) provides audiologists guidance to assessing CI candidacy and post-operative follow-up care.
Conclusion
The inner ear might be more robust than once thought. Furthermore, it is important to remain cognizant of outcomes over time in order to recognize uncommon performance growth patterns which might indicate a failed device.
Acknowledgments
This research was supported in part by research grant 2P50DC000242 from the National Institutes on Deafness and Other Communication Disorders, National Institutes of Health; the Lions Clubs International Foundation; the Iowa Lions Foundation; Cochlear Corporation for developing a cochlear implant to our specifications and providing initial devices at no cost; Multicenter Clinical Trial Group (see next page for Center name and Principal Investigator). Dr. Gantz is a consultant for Cochlear Corporation and Advanced Bionics Corporation. Dr. Dunn is a on the audiology council for Cochlear Corporation and Med-EL Corporation.
Footnotes
There are no other financial or conflicts of interest.
References
- 1.Mowry SE, Woodson E, Gantz BJ. New frontiers in cochlear implantation: acoustic plus electric hearing, hearing preservation, and more. Otolaryngol Clin North Am. 2012 Feb;45(1):187–203. doi: 10.1016/j.otc.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Kiefer J, Pok M, Adunka O, et al. Combined electric and acoustic stimulation of the auditory system: results of a clinical study. Audiology & neuro-otology. 2005 May-Jun;10(3):134–144. doi: 10.1159/000084023. [DOI] [PubMed] [Google Scholar]
- 3.Gantz B, Dunn C, Oleson J, Hansen M, Parkinson A, Turner C. Multicenter Clinical Trial of the Nucleus® Hybrid™ S8 Cochlear Implant: Final Outcomes. Laryngoscope. doi: 10.1002/lary.25572. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woodson EA, Reiss LA, Turner CW, Gfeller K, Gantz BJ. The Hybrid cochlear implant: a review. Adv Otorhinolaryngol. 2010;67:125–134. doi: 10.1159/000262604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luetje CM, Thedinger BS, Buckler LR, Dawson KL, Lisbona KL. Hybrid cochlear implantation: clinical results and critical review in 13 cases. Otol Neurotol. 2007 Jun;28(4):473–478. doi: 10.1097/RMR.0b013e3180423aed. [DOI] [PubMed] [Google Scholar]
- 6.James CJ, Fraysse B, Deguine O, et al. Combined electroacoustic stimulation in conventional candidates for cochlear implantation. Audiology & neuro-otology. 2006;11(Suppl 1):57–62. doi: 10.1159/000095615. [DOI] [PubMed] [Google Scholar]
- 7.James C, Albegger K, Battmer R, et al. Preservation of residual hearing with cochlear implantation: how and why. Acta Otolaryngol. 2005 May;125(5):481–491. doi: 10.1080/00016480510026197. [DOI] [PubMed] [Google Scholar]
- 8.Gifford RH, Dorman MF, McKarns SA, Spahr AJ. Combined electric and contralateral acoustic hearing: word and sentence recognition with bimodal hearing. Journal of speech, language, and hearing research : JSLHR. 2007 Aug;50(4):835–843. doi: 10.1044/1092-4388(2007/058). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gfeller KE, Olszewski C, Turner C, Gantz B, Oleson J. Music perception with cochlear implants and residual hearing. Audiol Neurootol. 2006;11(Suppl 1):12–15. doi: 10.1159/000095608. [DOI] [PubMed] [Google Scholar]
- 10.Gantz BJ, Turner CW. Combining acoustic and electrical hearing. The Laryngoscope. 2003 Oct;113(10):1726–1730. doi: 10.1097/00005537-200310000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Gantz BJ, Turner C, Gfeller KE, Lowder MW. Preservation of hearing in cochlear implant surgery: advantages of combined electrical and acoustical speech processing. The Laryngoscope. 2005 May;115(5):796–802. doi: 10.1097/01.MLG.0000157695.07536.D2. [DOI] [PubMed] [Google Scholar]
- 12.Gantz BJ, Turner C, Gfeller KE. Acoustic plus electric speech processing: preliminary results of a multicenter clinical trial of the Iowa/Nucleus Hybrid implant. Audiology & neuro-otology. 2006;11(Suppl 1):63–68. doi: 10.1159/000095616. [DOI] [PubMed] [Google Scholar]
- 13.Gantz BJ, Turner C. Combining acoustic and electrical speech processing: Iowa/Nucleus hybrid implant. Acta Oto-Laryngol. 2004 May;124(4):344–347. doi: 10.1080/00016480410016423. [DOI] [PubMed] [Google Scholar]
- 14.Gantz BJ, Hansen MR, Turner CW, Oleson JJ, Reiss LA, Parkinson AJ. Hybrid 10 clinical trial: preliminary results. Audiology & neuro-otology. 2009;14(Suppl 1):32–38. doi: 10.1159/000206493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roland JT, Jr, Gantz BJ, Waltzman SB, Parkinson AJ, Multicenter Clinical, Trial G. United States multicenter clinical trial of the cochlear nucleus hybrid implant system. Laryngoscope. 2015 Jul 7; doi: 10.1002/lary.25451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nucleus® Hybrid™ L24 Cochlear Implant System - P130016. http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm392836.htm. Accessed 02/16/2015.
- 17.Lenarz T, James C, Cuda D, et al. European multi-centre study of the Nucleus Hybrid L24 cochlear implant. International Journal of Audiology. 2013 Dec;52(12):838–848. doi: 10.3109/14992027.2013.802032. [DOI] [PubMed] [Google Scholar]
- 18.Van Abel KM, Dunn CC, Sladen DP, et al. Hearing preservation among patients undergoing cochlear implantation. Otol Neurotol. 2015 Mar;36(3):416–421. doi: 10.1097/MAO.0000000000000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tillman TW, Carhart R. An expanded test for speech discrimination utilizing CNC monosyllabic words. Northwestern University Auditory Test No. 6. SAM-TR-66-55. [Technical report] SAM-TR USAF School of Aerospace Medicine. 1966 Jun;:1–12. doi: 10.21236/ad0639638. [DOI] [PubMed]
- 20.Byrne D, Dillon H, Ching T, Katsch R, Keidser G. NAL-NL1 procedure for fitting nonlinear hearing aids: characteristics and comparisons with other procedures. Journal of the American Academy of Audiology. 2001 Jan;12(1):37–51. [PubMed] [Google Scholar]
- 21.Byrne D, Parkinson A, Newall P. Hearing aid gain and frequency response requirements for the severely/profoundly hearing impaired. Ear Hear. 1990 Feb;11(1):40–49. doi: 10.1097/00003446-199002000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Yamazaki H, O’Leary S, Moran M, Briggs R. Comprehensive analysis of cochlear implant failure: usefulness of clinical symptom-based algorithm combined with in situ integrity testing. Otol Neurotol. 2014 Apr;35(4):605–612. doi: 10.1097/MAO.0000000000000267. [DOI] [PubMed] [Google Scholar]
- 23.Minimum Speech Test Battery (MSTB) for Adult Cochlear Implant Users. Auditory Potential, LLC; 2011. [Google Scholar]


