Abstract
Retinal progenitors in the circumferential margin zone (CMZ) and Müller glia-derived progenitors have been well-described in the eyes of fish, amphibians and birds. However, there is no information regarding a CMZ and the nature of retinal glia in species phylogenetically bridging amphibians and birds. Thus, the purpose of this study was to examine the retinal glia and investigate whether a CMZ is present in the eyes of reptilian species. We used immuno-histochemical analyses to study retinal glia, neurons that could influence CMZ-progenitors, the retinal margin, and non-pigmented epithelium (NPE) of ciliary body of garter snakes, queen snakes, anole lizards, snapping turtles, and painted turtles. We compare our observations in reptile eyes to the CMZ and glia of fish, amphibians and birds. In all species, Sox9, Pax6 and the glucocorticoid receptor are expressed by Müller glia and cells at the retinal margin. However, proliferating cells were found only in the CMZ of turtles, but not in the eyes of anoles and snakes. Similar to eyes of chickens, the retinal margin in turtles contains accumulations of GLP1/glucagonergic neurites. We find that filamentous proteins, vimentin and GFAP, are expressed by Müller glia, but have different patterns of sub-cellular localization in the different species of reptiles. We provide evidence that the reptile retina may contain Non-astrocytic Inner Retinal Glial (NIRG) cells, similar to those described in the avian retina. We conclude that the retinal glia, glucagonergic neurons and CMZ of turtles appears to be the most similar to that of fish, amphibians and birds.
Keywords: retina, glia, progenitor, glucagon, AB_2155784, AB_2160651, AB_528427, AB_528504, AB_528490, AB_291611, AB_2314539, AB_1143173, AB_2110656, AB_2195807, AB_2239761
Introduction
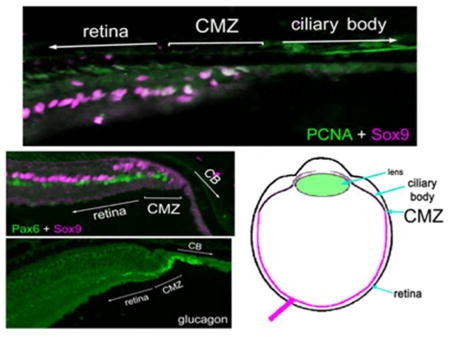
In fish and amphibians, there is a tremendous amount of ocular growth during the lifetime of the animal. Ocular growth is a function of scleral remodeling and connective tissue deposition, and this growth requires the retina to grow in parallel. The retina grows by the addition of concentric rings of newly generated cells produced by neural stem cells that are clustered within the circumferential marginal zone (CMZ), which is found at the peripheral retina (reviewed by (Hitchcock and Raymond, 1992; Perron et al., 1998; Raymond and Hitchcock, 1997; Reh and Levine, 1998). Similar to the retinas of fish and amphibians, CMZ-progenitors have been described in birds. Nearly 40 years ago, Morris and colleagues detected H3-thymidine-labeled cells at the margin of the post-hatch chick retina (Morris et al., 1976), suggesting on-going retinal growth long after the completion of embryonic histogenesis, which is complete about 9 days before hatching (Prada et al., 1991). More recently, CMZ-progenitors have been described in detail in the eyes of chickens (Fischer and Reh, 2000). The CMZ-progenitors of the chicken accumulate BrdU, and express PCNA, Pax6, Chx10, Sox9, Sox2, Egr1, N-cadherin, and transitin/nestin (Fischer and Omar, 2005; Fischer and Reh, 2000; Fischer et al., 2009). Similarly, studies in the quail have identified progenitors organized into a CMZ wherein proliferation can be stimulated by intraocular injections of insulin (Kubota et al., 2002). In the chicken, the proliferation of CMZ-progenitors is, in part, regulated by visual cues and input from distinct types of glucagonergic retinal neurons (Fischer et al., 2005; Fischer et al., 2008; Fischer et al., 2006). Although the proliferation of CMZ-progenitors is prominent during post-hatch development, evidence exists for persistent proliferation of CMZ-progenitors in the eyes of adult chickens and quails (Fischer and Reh, 2000; Kubota et al., 2002).
It has been suggested that the CMZ has been diminished through the course of evolution from fish to mammals (Kubota et al., 2002). Although fish, amphibian and bird retinas possess a CMZ, the addition of new neurons from CMZ-progenitors is diminished in chickens compared to that of fish and amphibians. For example, CMZ-progenitors in amphibians are multipotent (Wetts and Fraser, 1988), whereas CMZ-progenitors in birds are restricted to producing primarily amacrine and bipolar cells (Fischer and Reh, 2000). The peripheral retina of the marsupial contains a CMZ-like region, whereas the mammalian retina lacks a CMZ (Fischer and Reh, 2000; Kubota et al., 2002). The presence or absence of a CMZ in reptiles remains unexplored. Accordingly, one purpose of this study is to examine whether CMZ-progenitors are found in the retinas of different reptiles.
In addition to CMZ-progenitors, Müller glia are known to become retinal progenitors in the retinas of fish, birds and mammals (reviewed by (Gallina et al., 2014a). Müller glia are the predominant type of support cell in the vertebrate retina and are the only glial cell that is derived from neuroepithelial retinal stem cells. Müller glia in the fish, chicken, and mammalian retina possess the capacity to re-enter the cell cycle, dedifferentiate into retinal progenitors and regenerate retinal neurons (Gallina et al., 2014a). The regenerative capacity of Müller glia is remarkably robust in zebrafish while it is reduced in chicken and mammalian retina. There are few studies describing the characteristics of retinal glia in reptiles (Casanas et al., 2011; Dahl et al., 1985; Gaur et al., 1988; Romero-Aleman et al., 2012; Romero-Aleman et al., 2010). Therefore, another purpose of this study is to characterize the glial cells in the retinas of lizards, snakes, and turtles.
Methods and Materials
Animals
The use of animals in these experiments was in accordance with the guidelines established by the National Institutes of Health, The Ohio State University and Wittenberg University. Newly hatched leghorn chickens (Gallus gallus domesticus) were obtained from Meyer Hatchery (Polk, Ohio). Chickens were kept on a cycle of 12 hours light, 12 hours dark (lights on at 7:00 am). Chickens were housed in a stainless steel brooder at about 25°C and received water and Purinatm chick starter ad libitum. Reptiles were obtained from Dr. Kevin Gribbins and consisted of: (1) two adult male Queen Snakes (Regina sepemvittata) (2) two adult male and female Eastern Garter Snakes (Thamnophis sirtalis). (3) three adult male Brown Anoles (Anolis segrei). (4) three adult midland painted turtles (Chrysemys picta). (5) two adult snapping turtles (Chelydra serpentina). Each specimen was >1 year of age. All reptiles were collected and sent to Wittenberg University, where animals were sacrificed, according to IACUC-approved guidelines, with either injection or submersion within MS222 (Tricaine Methanesulfonate, Sigma-Aldrich).
Tissue dissection, fixation, sectioning and immunolabeling
Eyes were enucleated and fixed in 4% paraformaldehyde in 0.1M PB, pH 7.4 with 3% sucrose for 30 minutes. After washing with PBS (0.05M phosphate buffer + 0.154 mM NaCl) eyecups were cryoprotected by soaking in 30% sucrose in PBS with 0.01% NaN3 overnight. Transverse retinal sections were cut at 12 μm and thaw mounted onto Superfrost-Plustm slides (Fisher Scientific). The slides were air dried and stored at −20°C until use. For whole-mount labeling of the retina, sclera and choroid were dissected away and the retina cryoprotected in 20% sucrose (w/v) in PBS. As described previously (Fischer et al., 2006; Fischer et al., 2007; Ritchey et al., 2011; Stanke and Fischer, 2010). Sections were warmed to room temperature and ringed with rubber cement. After washing with PBS, slides were then incubated overnight in 250 μl primary antibody (antisera diluted in PBS with 0.2% Triton-X with 0.01% NaN3 with 5% blocking serum). Working dilutions and manufacture information for antibodies used in this study are provided in Table 1. Retinas for flat-mount preparations were frozen and thawed 3 times prior to incubation in primary antibody overnight followed by incubation with secondary antibodies overnight.
Table 1.
Antibodies
| Antibody | Immunogen structure | Manufacturer, Catalog Number, Species, Monoclonal or Polyclonal | Concentration | RRID |
|---|---|---|---|---|
| anti-GCR | Human synthetic peptide | Santa Cruz Biotechnology, H-300, Rabbit, Polyclonal | 1:250 | AB_2155784 |
| anti-PCNA | Rat fusion protein | Dako, MO879, Mouse, monoclonal | 1:1000 | AB_2160651 |
| anti-Pax6 | Recombinant chicken Pax6 | Developmental Studies Hybridoma Bank-DSHB, PAX6 Mouse | 1:50 | AB_528427 |
| anti-Vimentin | Adult canary brain | Developmental Studies Hybridoma Bank, h5, mouse, monclonal | 1:400 | AB_528504 |
| anti-Top AP | E13 Chicken Embyros | Gift from Dr. Paul Linser, | 1:80 | |
| anti-Tyrosine Hydroxylase | Quail fusion protein | Developmental Studies Hybridoma Bank, aTH, mouse | 1:50 | AB_528490 |
| anti-Pax2 | Recombinant Pax2 | Covance, PRB-276, Rabbit, polyclonal | 1:250 | AB_291611 |
| anti-GFAP | Cow spinal cord | Dako, N1506, Rabbit polyclonal | 1:2000 | AB_2314539 |
| anti-Substance P | Full length human Substance P | Abcam, ab7340, Rat, monoclonal | 1:50 | AB_1143173 |
| anti-GS | Purified full length sheep protein from brain | Abcam, ab125724, Mouse, monoclonal | 1:2000 | AB_2110656 |
| anti-Sox2 | Recombinant C-terminus of human SOX2 | Santa Cruz Biotechnology, Y-17, Goat, polyclonal | 1:1000 | AB_2195807 |
| anti-Sox9 | Synthetic peptide human Sox9 | Millipore, AB5535, Rabbit, polyclonal | 1:2000 | AB_2239761 |
| anti-GLP1 | Human recombinant GLP1 | Biogenesis Ltd., 4660-1604, Rabbit | 1:400 | |
| anti-n-Cadherin | Embryonic chick heart | Developmental Studies Hybridoma Bank, 6B3, mouse, monoclonal | 1:20 |
Antibody Characterization
(1) Goat anti-GCR was raised to recombinant human peptide LEESIANLNRSTSVPEN PKSSASTAVSAAPTEKEFPKTHSDVSSEQQHLKGQTGTNGGNVKLYTTDQSTFDILQD LEFSSGSPGKETNESPWRSDLLIDENCLLSPLAGEDDSFLLEGNSNEDCKPLILPDTKP KIKDNGDLVLSSPSNVTLPQVKTEKEDFIELCTPGVIKQEKLGTVYCQASFPGANIIGNK MSAISVHGVSTSGGQMYHYDMNTASLSQQQDQKPIFNVIPPIPVGSENWNRCQGSGD DNLTSLGTLNFPGRTVFSNGYSSPSMRPDVSSPPSSSSTATTGPPPKL (amino acids 121–420) and produces a ~90 kDA and 95 kDA in western blot analysis (manufactures information). Anti-GCR produced a pattern of labeling similar to that observed in the rodent, dog, chicken and human retina, using different antibodies (Gallina et al., 2014b) (2) Mouse anti-PCNA was raised to rat synthetic peptide amino acid sequence: LVFEAPNQEK. Mouse anti-PCNA recognizes a single band at ~36 kDa on western blot analysis of rat retinal extract (Gordon et al., 2002). This antibody has been shown to recognize proliferating cells in the rodent retina (Sigulinsky et al., 2008). (3) Mouse anti-Pax6 was raised to chicken recombinant Pax6 MQNSHSGVNQLGGVFVNGRPLPDS TRQKIVELAHSGARPCDISRILQTHADAKVQVLDNQNVSNGCVSKILGRYYETGSIRPR AIGGSKPRVATPEVVSKIAQYKRECPSIFAWEIRDRLLSEGVCTNDNIPSVSSINRVLRN LASEKQQMGADGMYDKLRMLNGQTGTWGTRPGWYPGTSVPGQPAQDGCPQQEGG GENTNSISSNGEDSDEAQMRLQLKRK (amino acids 1–233). Western blot analysis of retinal extracts of embryonic chickens indicated that the Pax6 monoclonal labels two major (~55 kDa) and two minor (~40 kDa) bands at the expected molecular masses of known Pax6 splice variants (Kawakami et al., 1997). The Pax6 antibody produced a cellular pattern and distribution of labeling that was consistent with previous studies in the chicken retina (Fischer and Reh, 2001). (4) Mouse anti-vimentin was raised to homogenized adult canary brain and the specificity has been confirmed with the detection of a single band at ~50 kDa, using western blot analysis (Alvarez-Buylla et al., 1987). The vimentin antibody produced a staining pattern similar to previous reports in the retina (Fischer et al., 2010b). (5) The 2M6 mouse monoclonal recognizes TopAp, an intracellular membrane-associated protein of the sarcolemmal-membrane-associated protein family (Ochrietor et al., 2010). The 2M6 monoclonal recognizes a 40–46 kDa protein in western blot analysis (Schlosshauer and Wild, 1991). Affinity purification of detergent-treated chick retina lysates and mass spectrometry have been performed to confirm the specificity of the 2M6 monoclonal (Ochrietor et al., 2010). (6) The mouse anti-tyrosine hydroxylase (TH) antibody was raised to quail TH and recognizes TH in a variety of mammalian species (Fauquet and Ziller, 1989). Western blot analysis using this antibody detected a band of ~63 kDa in quail adrenal extract and failed to detect expression in quail muscle tissue (Fauquet and Ziller, 1989). (7) Rabbit anti-Pax2 was raised to amino acids: GILGIPRSNGEKRKRDEDVSEGSVPNGDSQSGVDSLRKHLRA DTFTQQQLEALDRVFERPSYPDVFQASEHIKSEQGNEYSLPALTPGLDEVKSSLSAST NPELGSNVSGTQTYPVVTGRDMASTTLPGYPPHVPPTGQGSYPTSTLAGMVPGSEFS GPYSHPQYTAYNEAWRFSNPALLSSPYYYSAAPRSAPAA (188–385) of human Pax2 and is known to recognize both Pax2a and Pax2b isoforms (manufacturer’s information). The specificity of anti-Pax2 has been confirmed by western blot analysis wherein two distinct bands at ~44 and ~51 kDa were detected, by comparison of patterns of immunolabeling with different antibodies to Pax2 (Boije et al., 2010; Sehgal et al., 2008; Sehgal et al., 2009), and with patterns of expression seen with in situ hybridization (Stanke et al., 2010). The Pax2 antibodies produced a cellular pattern and distribution of labeling that was consistent with previous studies regarding optic nerve glia in the developing chick retina (Sehgal et al., 2008). (8) The rabbit anti-GFAP identifies a 51 kDA band in immunoblots of rat brain extract (Wishcamper et al., 2001) and produces a similar pattern of glial staining across species (Hendrickson et al., 2006; Pecchi et al., 2007). (9) Rat anti-substance P was raised to full-length of human substance P conjugated, via carbodiimide, to bovine serum albumin. This antibody recognizes the 5–8 C-terminal fragment of substance P (Cuello et al., 1979) and is known to selectively label a subset of amacrine cells and bullwhip neurons in the chicken retina (Fischer et al., 2006). Our labeling patterns in the retina are identical to that seen with different anti-Substance P antibodies tested in turtle and amphibian retina (Cuenca and Kolb, 1989; Uchiyama et al., 1988) (10) The mouse anti-GS antibody specificity was confirmed by western blot analysis which revealed a 45 kDa band as expected (manufacture information). The GS antibody was raised to the sheep glutamine synthetase MATSASSHLNKGIKQVYMALPQGEKVQAMYIWIDGTGEGLR CKTRTLDSEPKCIEELPEWNFDGSSTFQSEGSNSDMYLVPAAMFRDPFRKDPNKLVF CEVFKYNRKPAETNLRHTCKRIMDMVSNQRPWFGMEQEYTLMGTDGHPFGWPSNG FPGPQGPYYCGVGADKAYGRDIVEAHYRACLYAGIKIGGTNAEVMPAQWEFQIGPCE GIDMGDHLWVARFILHRVCEDFGVIATFDPKPIPGNWNGAGCHTNFSTKAMREENGLK YIEEAIEKLSKRHQYHIRAYDPKGGLDNARRLTGFHETSNINDFSAGVANRGASIRIPRT VGQEKKGYFEDRRPSANCDPFAVTEALIRTCLLNETGDEPFQYKN (amino acids 1–373) and produced a staining in the reptile retina similar to that observed in the mouse and chicken (Chua et al., 2013; Fischer et al., 2010b) (11) Goat anti-Sox2 was raised to the recombinant C-terminus of human Sox2 and recognizes a single 34-kDa band in Western blot analysis of lysate from mouse embryonic stem cells (manufacturer’s information). The Sox2 antibodies recognize amino acids YLPGAEVPEPAAPSRL (277–293) of human Sox2, as determined by pre-absorption controls and mass spectrometry analysis of blocking peptide (Poche et al., 2008). The Sox2 antibodies produced a pattern of labeling in the retina consistent with previous studies (Fischer et al., 2009; Poche et al., 2008). (12) Rabbit anti-Sox9 was raised against human synthetic peptide amino acids VPSIPQTHSPQWEQPVYTQLTRP. Rabbit anti-Sox9 detects a 60–65 kDa band on Western blot analysis of mouse brain tissue (manufactures information). In situ hybridization analysis of Sox9 mRNA in the embryonic retina produces an identical pattern to that seen using the Sox9 antibody (Poche et al., 2008; Wright et al., 1995). (13) Rabbit anti-GLP1 was raised to amino acids MKSIYFVAGLFVMLVQGSW (1–19 of human GLP1) and is known to cross-react with glucagon (Fischer et al., 2005). Our GLP1 antibody produced a pattern of labeling identical to that previously observed in the chick retina by a different GLP1 antibody (Vessey et al., 2005b). (14) The mouse anti-chick N-Cadherin (6B3) antibody was isolated from nonionic detergent extract of chick embryos by affinity chromatography using a monoclonal antibody to the intracellular domain of N-Cadherin (Knudsen et al., 1995). The 6B3 antibody produced a staining pattern in the chicken embryo identical to that produced by a rat monoclonal antibody against N-Cadherin (NCD-2) (George-Weinstein et al., 1997; Hatta and Takeichi, 1986). The specificity of this antibody was further confirmed with western blot analysis where the 6B3 antibody recognized a 100 kDa extracellular fragment as well as the 135 kDa full length N-cadherin (George-Weinstein et al., 1997).
None of the observed labeling was due to non-specific binding of secondary antibody or auto-fluorescence because sections labeled with secondary antibodies alone had little or no fluorescence. Secondary antibodies included donkey-anti-goat-Alexa488/568, goat-anti-rabbit-Alexa488/568/647, goat-anti-mouse-Alexa488/568/647, and goat-anti-rat-Alexa488 (Invitrogen) diluted to 1:1000 in PBS plus 0.2% Triton X-100.
Microscopy
Wide-field photomicrographs were obtained by using a Leica DM5000B microscope and Leica DC500 digital camera. Images were optimized for brightness and contrast, multiple-channel images overlaid, and figures constructed by using Adobe Photoshop™6.0. To avoid the possibility of region-specific differences within the retina, images were consistently taken from the same region of retina for each data set.
Results
Glucagon and Glucagon-like peptide-1 in amacrine cells and the retinal margin
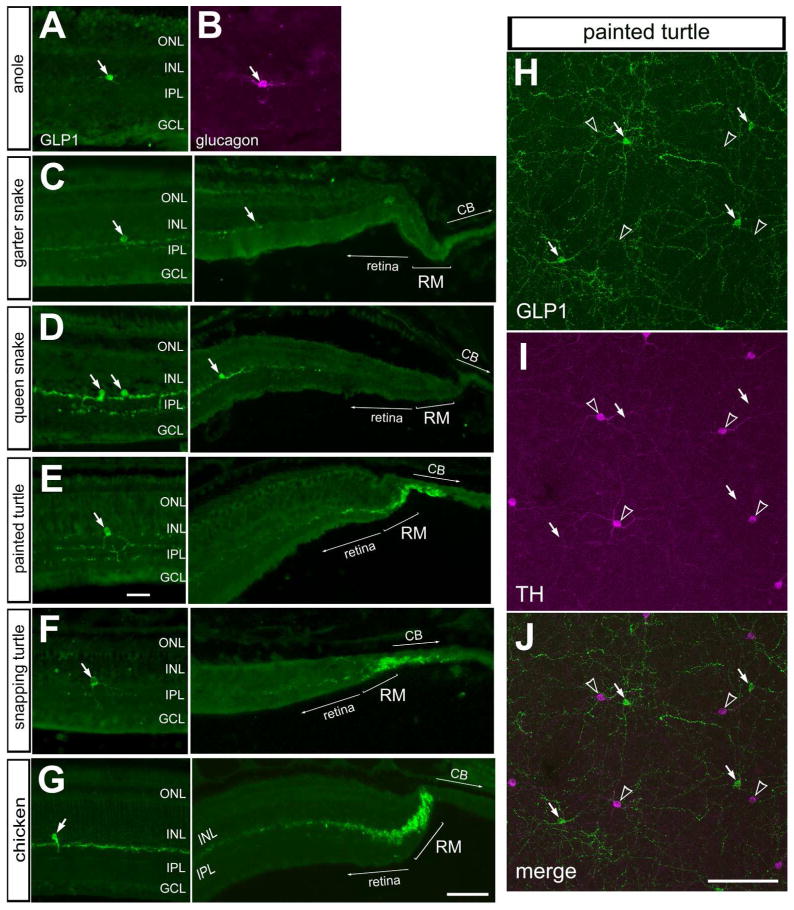
In the bird retina, unique types of amacrine cells are known to densely ramify neuronal processes in the CMZ and release peptides, glucagon and GLP1, to inhibit the proliferation of progenitors in the CMZ (Fischer et al., 2005; Fischer et al., 2006). These glucagonergic neurons coordinate equatorial scleral growth with retinal growth in response to defocus cues (Fischer et al., 2008). Thus, we sought to examine glucagonergic cells in the retina and whether there is an accumulation of neurites in the peripheral retina in reptiles. In all reptiles examined, we found GLP1-positive amacrine cells in the INL (Fig. 1). Similar to the glucagonergic amacrine cells described in different species, the glucagonergic amacrine cells in anoles, snakes and turtles had sparsely distributed cell bodies in the inner INL and neurites ramified within two or three strata in the distal IPL (Figs. 1A–G). In whole-mounts of the turtle retina, the glucagonergic cells had large cell bodies that appeared regularly spaced, and had large dendritic arbors, reminiscent of dopaminergic, tyrosine hydroxylase-positive amacrine cells (Fig. 1H–J). The patterns of expression of glucagon and GLP1 at the retinal margin were not uniform across reptile species. In anoles (not shown), garter and queen snakes there was no accumulation of glucagon/GLP1-positive neurites (Figs. 1C, D). Similar to the chicken CMZ, the retinal margin of painted and snapping turtles contained accumulations of glucagon/GLP1-positive neurites (Figs. 1E–G). These findings are consistent with a previous report describing a dense accumulation of glucagon-containing processes in the peripheral retina of the read-eared slider turtle (Wetzel and Eldred, 1997).
Figure 1.
Immunoreactivities for glucagon and Glucagon-Like Peptide 1 (GLP1) are present in retinal amacrine cells and neurites clusters in the retinal margin. Sections were obtained from the eyes of anoles (A,B), garter snake (C), queen snake (D), painted turtle (E,H), snapping turtle (F) and chicken (G). Sections of the retina were labeled with antibodies to GLP1 (A,C–G; green) or glucagon (B; magenta). H–J; Whole-mounts of the painted turtle retina were labeled with antibodies to GLP1 (green) and TH (magenta). Arrows indicate amacrine cells labeled for glucagon/GLP1, and hollow arrow-heads indicate TH-positive amacrine cells. The scale bar (50 μm) in panel G applies to A–G, and the bar in J applies to G and H. Abbreviations: ONL – outer nuclear layer, INL – inner nuclear layer, IPL – inner plexiform layer, GCL – ganglion cell layer, RM – retinal margin, CB – ciliary body, TH – tyrosine hydroxylase.
Pax6 and Sox9 expression in the retina and retinal margin
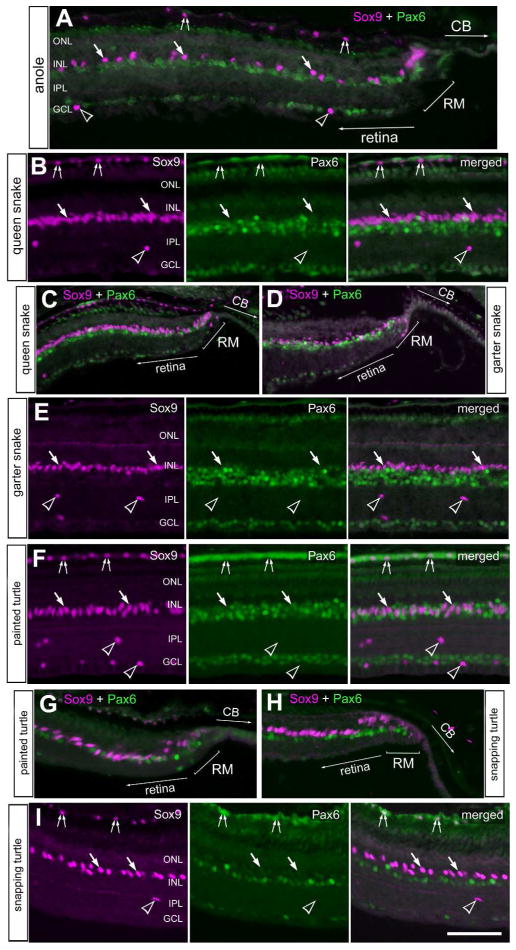
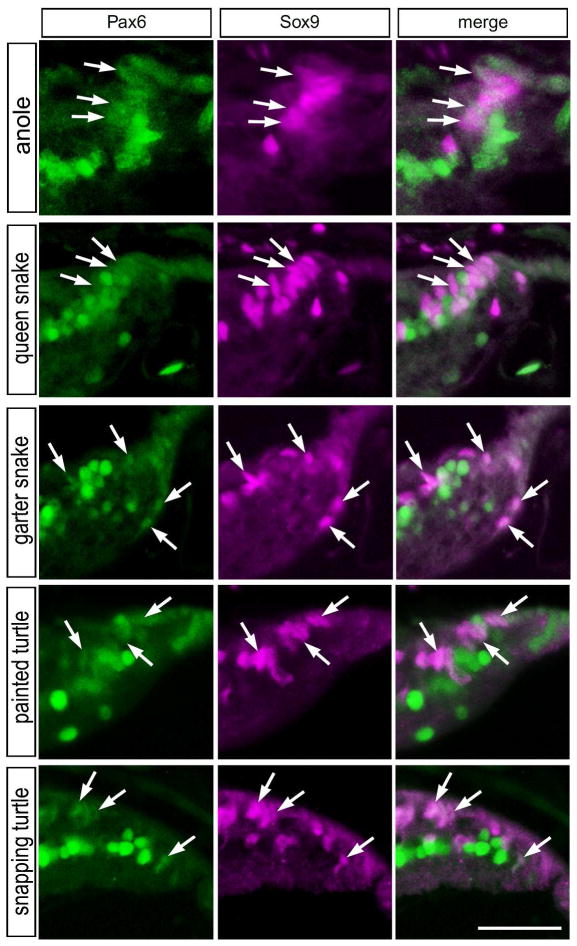
Multipotent retinal progenitor cells in the CMZ of fish, amphibians and birds are known to express complements of transcription factors that are also expressed by embryonic retinal stem cells, such as Pax6 and Sox9 (Fischer et al., 2014; Zuber et al., 2003). In addition, these transcription factors are known to be expressed by different types of mature retinal neurons or glia (Fischer et al., 2010a; Fischer et al., 2010b). Accordingly, we probed for the expression of Pax6 and Sox9 in the reptilian retina. Consistent with patterns of expression seen in different vertebrates, high levels of Pax6 was detected in amacrine cells in the vitread half of the INL in all reptiles examined (Fig. 2). Sox9-immunoreactivity was found in the nuclei of Müller glia in the middle of the INL (Fig 2). Müller glia nuclei were densely packed in the retinas of queen snake and garter snake, whereas these nuclei were spatially separated in the retinas of anoles and turtles (Fig. 2). In addition, Sox9 was detected in the nuclei of cells scattered across the inner retinal layers; these cells were presumed to be glial cells similar to the Non-astrocytic Inner Retinal Glial (NIRG) cells that have been described in the chick retina (Fischer et al., 2010a; Rompani and Cepko, 2010). Sox9 was also detected in the nuclei of pigmented retinal epithelial (RPE) cells, with the exception of the RPE in garter snake (Fig. 2). The Sox9-positive Müller glia were very weakly positive for Pax6 in the snake retinas, whereas levels of Pax6 were modestly more apparent in the Müller glia of the turtle retinas (Fig. 2). In all reptiles examined, we observed clusters of cells within the peripheral retina that expressed Sox9 and low levels of Pax6 (Fig. 3). These cells had nuclei that appeared elongated and fusiform in shape, reminiscent of the nuclei of neuroepithelial retinal stem cells.
Figure 2.
Sox9 and Pax6 are expressed by cells in central and peripheral regions of the retina. Sections of peripheral (A,C,D,G,H) and central (B,E,F,I) retina were labeled with antibodies to Sox9 (magenta) and Pax6 (green). Retinas were obtained from the eyes of anoles (A), queen snake (B,C), garter snake (D,E), painted turtle (F,G) and snapping turtle (H,I). Arrows indicate the nuclei of Müller glia, small double-arrows indicate the nuclei of RPE cells, and hollow arrow-heads indicate presumptive NIRG-like cells. The scale bar (50 μm) in panel C applies to C–E, the bar J applies to H–J and the bar in O applies to A,B,F,G,K and O. Abbreviations: ONL – outer nuclear layer, INL – inner nuclear layer, IPL – inner plexiform layer, GCL – ganglion cell layer, RM –retinal margin, CB – ciliary body.
Figure 3.
Sox9 and Pax6 are co-expressed by cells in the retinal margin. Vertical sections through the retinal margin were obtained from the eyes of anoles, queen snake, garter snake, painted turtle and snapping turtle. Arrows indicate cells in the retinal margin and/or presumptive CMZ that are co-labeled for Pax6 (green) and Sox9 (magenta). The scale bar (50 μm) in the bottom right panel applies to all panels.
Substance-P, N-Cadherin and the glucocorticoid receptor (GCR) in the retinal margin
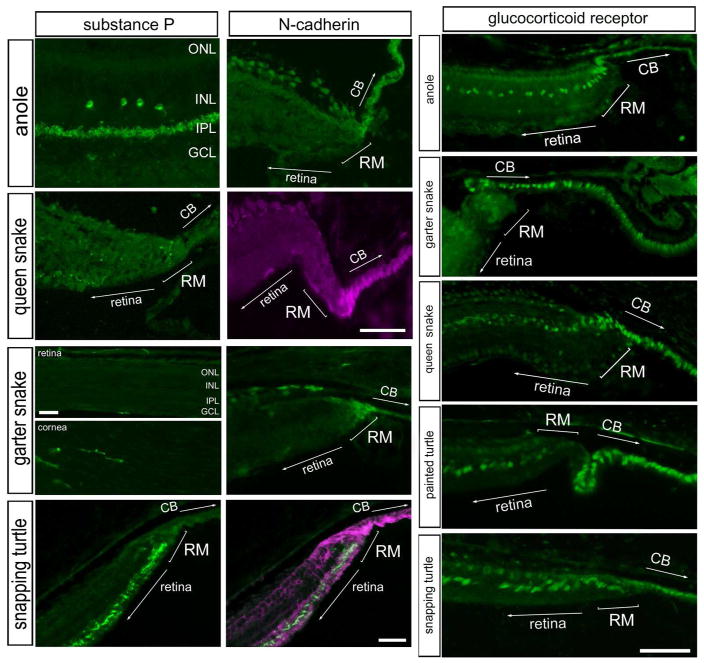
Substance-P is a neuropeptide that is expressed by distinct types of amacrine cells in the retinas of different species (reviewed by (Brecha and Karten, 1983; Karten and Brecha, 1983), and in glucagonergic neurons that ramify in the CMZ in the chicken retina (Fischer et al., 2006). Accordingly, we probed for substance-P immunofluorescence in far peripheral regions of the retina. In the anole retina, we found a population of amacrine cells that were positive for substance P, with relatively large cell bodies near the middle of the INL and neurites that were densely ramified in the distal IPL (Fig. 4). We failed to detect substance P-immunoreactivity in far peripheral regions of the anole retina (not shown). Immunoreactivity for substance-P was not detected in the retina of garter snakes (Fig. 4). As a positive control, the antibodies to substance-P labeled neurites in the cornea of the garter snake (Fig. 4), consistent with reports of substance-P-positive innervation of the cornea (Nishida, 2010). In the queen snake, a few sparsely distributed neurites were observed in the IPL, but there was no accumulation at the retinal margin (Fig. 4). In the snapping turtle, substance P-positive neurites appeared to accumulate in far peripheral regions of the retina near the margin (Fig 4). This pattern of substance P-immunoreactivity in the far peripheral retina is reminiscent of the distribution of substance P-positive neurites in far peripheral regions of the chicken retina (Fischer et al., 2006).
Figure 4.
Substance-P, N-cadherin and the glucocorticoid receptor are present at the retinal margin of the reptile retina. Sections of the retina, retinal margin and cornea were labeled with antibodies to substance-P, N-cadherin or glucocorticoid receptor (GCR). Although, substance-P immunoreactivity was observed in the cornea of garter snakes, no labeling was observed in the retina or retinal margin. The scale bar (50 μm) in panel C applies to A–C, and the bar in J applies to E–J. Abbreviations: ONL – outer nuclear layer, INL – inner nuclear layer, IPL – inner plexiform layer, GCL – ganglion cell layer.
We next examined whether N-cadherin is expressed at the margin of the reptile retina. N-cadherin is a cell surface adhesion molecule that is expressed within the CMZ of fish and birds (Ghai et al., 2008; Raymond, 1991). We found N-cadherin-immunoreactivity in the peripheral retina of anole, snakes, and turtles (Fig. 4). In snake and turtle retinas, N-cadherin-immunoreactivity was concentrated at the retinal margin and extended into the non-pigmented epithelium of the ciliary body (Fig. 4). This pattern of expression is similar to that seen in the chicken CMZ (Ghai et al., 2008).
GCR has recently been reported to be expressed by Müller glia and CMZ-progenitors in the eyes of chickens (Gallina et al., 2014b). Thus, we probed for the expression of GCR in reptiles. In the anole lizard retina, GCR-immunoreactivity was observed in the nuclei of presumptive Müller glia in the far peripheral retina, and in nuclei of non-pigmented epithelium (NPE) cells that extended into the ciliary body (Fig. 4). A similar pattern of GCR-expression was detected in the snake retina, where GCR appeared to be expressed at higher levels in cells at the retinal margin than in Müller glia within the neural retina (Figs. 4). In all species examined, GCR-immunoreactive nuclei extended through the retinal margin into the non-pigmented epithelium of the ciliary body (Figs. 4). Taken together, these data suggests that similar to the chicken retina, GCR expression is enriched in the retinal margin and the NPE cells of the ciliary body in the eyes of different reptiles.
Cellular proliferation at the retinal margin
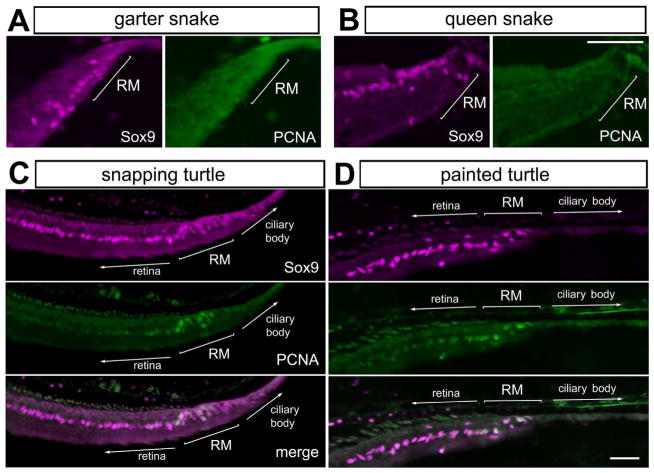
Progenitor cells in the CMZ of the fish, amphibian, and chicken proliferate and add new cells to the peripheral retina (reviewed by (Fischer et al., 2014)). We sought to detect proliferating cells in the retinal margin of reptiles by assaying for expression of PCNA. We failed to detect PCNA-positive cells in the anole lizard (not shown), garter snake, and queen snake (Figs. 5A, B). By comparison, PCNA was detected in Sox9-positive cells at the retinal margin of painted and snapping turtles (Figs. 5C, D). This finding is consistent with the hypothesis that there are proliferating progenitors in the CMZ of the turtle, and that a CMZ does not exist at the retinal margin of adult lizards and snakes.
Figure 5.
Proliferating cells are found at the retinal margin of turtles, but not snakes. Sections of the peripheral retina were obtained from the eyes of garter snakes (A), queen snakes (B), snapping turtle (C) and painted turtle (D). Tissues were labeled with antibodies to Sox9 (magenta) and PCNA (green). The scale bar (50 μm) in panel B applies to A and B, and the bar in D applies to D and E. Abbreviations: ONL – outer nuclear layer, INL – inner nuclear layer, IPL – inner plexiform layer, GCL – ganglion cell layer, RM – retinal margin, PCNA – proliferating cell nuclear antigen.
Expression patterns of glial markers
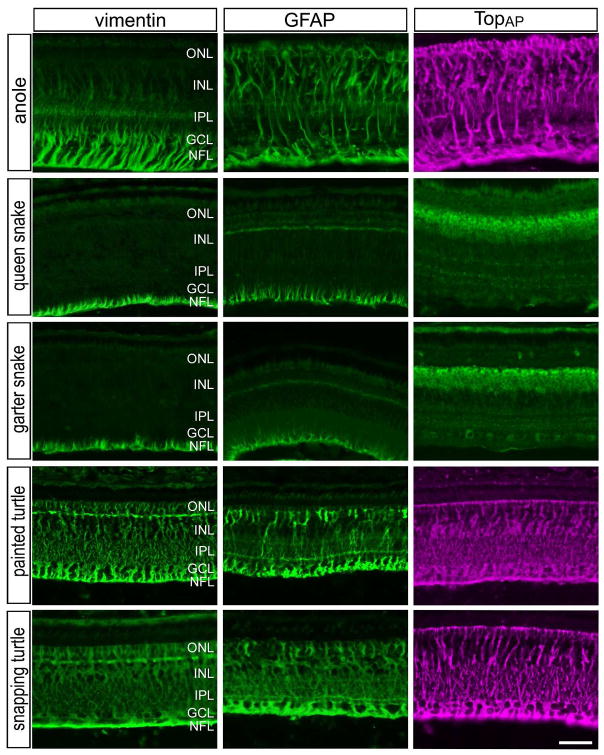
Müller glia in most species express the intermediate filaments vimentin and glial fibrillary acidic protein (GFAP). In the reptile retina, we found that vimentin and GFAP were expressed by the Müller glia, but the sub-cellular distribution of these filaments varied between species (Fig. 6). In the retinas of queen and garter snakes, both vimentin and GFAP were detected in the vitread endfeet of Müller glia (Fig. 6). By comparison, vimentin- and GFAP-immunoreactivities were present throughout the inner and outer processes of the Müller glia of anole lizards and turtles (Fig. 6). In the anole retina, vimentin expression was concentrated in vitread regions and spread into sclerad regions of the Müller glia at diminished levels (Fig. 6). Perhaps unique to the chick retina, TopAP, an intracellular membrane-associated protein of the sarcolemmal-membrane-associated family, is highly expressed by the Müller glia (Ochrietor et al., 2010). We found that TopAP was expressed by Müller glia in the retinas of anoles and turtles, whereas TopAP-immunoreactivity was observed in presumptive bipolar cells in snake retinas (Fig. 6). It remains uncertain whether the patterns of TopAP immunolabeling represent specific labeling or cross-reactivity with a TopAP–like epitope. In all species of reptiles, we found no specific immunolabeling for the nestin-related filament transitin, which is known to be expressed by normal and activated Müller glia in the chick retina (Fischer and Omar, 2005).
Figure 6.
Patterns of expression of vimentin, GFAP and TopAP in the reptile retina. Antibodies to vimentin, GFAP and TopAP were applied to vertical sections of central regions of retinas from anole, queen snake, garter snake, painted turtle and snapping turtle. The scale bar (50 μm) in panel in the bottom-right panel applies to all panels. Abbreviations: ONL – outer nuclear layer, INL – inner nuclear layer, IPL – inner plexiform layer, GCL – ganglion cell layer.
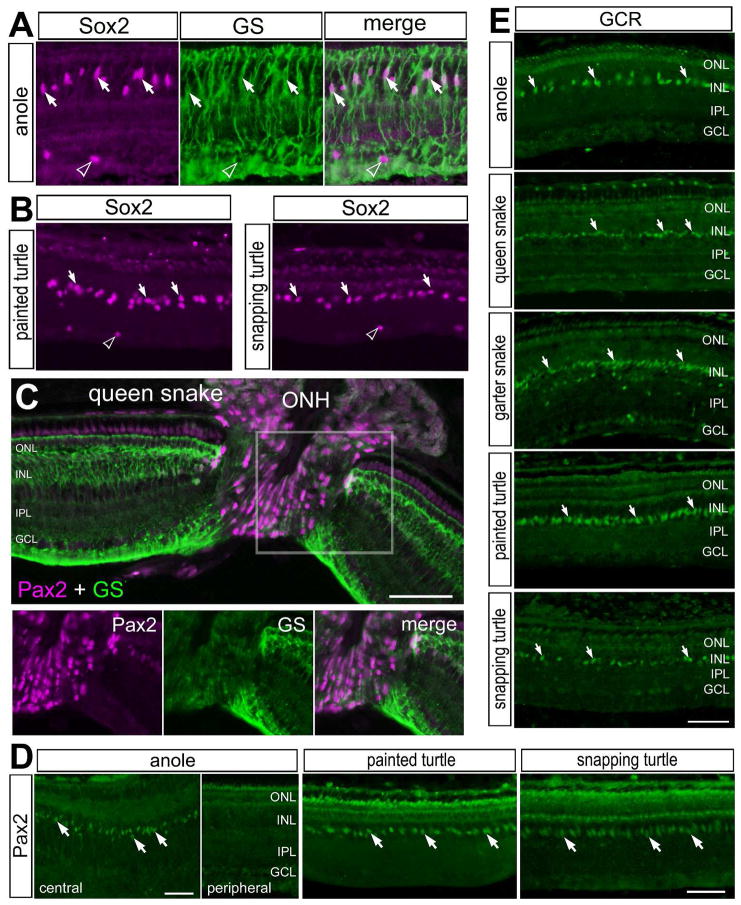
In all species examined, Sox9-immunoreactivity was observed in the nuclei of Müller glia (see Fig. 2). We then further examined the expression of transcription factors in Müller glia. In the chicken and fish retina, Müller glia are known to express the transcription factors Pax2 and Sox2 (Boije et al., 2010; Stanke et al., 2010). Accordingly, we probed for the expression of Pax2 and Sox2 in the eyes of reptiles. Antibodies to Sox2 failed to produce specific labeling in the retinas of snakes (not shown), despite high levels of conservation of Sox2 across vertebrates. By comparison, in the retinas of anoles and turtles, antibodies to Sox2 labeled the nuclei of Müller glia in the middle of the INL (Figs. 7A, B), identical to the patterns of Sox9-immunolabeling. The pattern of labeling for Sox2 was nearly identical to that seen in the retinas of chickens, mice, guinea pigs, dogs and primates (Fischer et al., 2010b). In addition to Müller glia in the chicken retina, an atypical type glial cell, derived from optic nerve progenitors, has been identified (Fischer et al., 2010a; Rompani and Cepko, 2010). These cells are scattered across the IPL, GCL and NFL, do not express GFAP, but are positive for Olig2, Sox2, Sox9 and Nkx2.2 (Fischer et al., 2010a; Rompani and Cepko, 2010). These cells have been termed Non-astrocytic Inner Retinal Glial (NIRG) cells. Similar to the chicken retina, we found Sox2/Sox9-positive cells scattered across the IPL, GCL and NFL (Figs. 2 and 7A,B), suggesting that NIRG cells may be present in the retinas of reptiles. However, we failed to find specific labeling for Olig2 or Nkx2.2 in the retinas or optic nerves of all species of reptiles (data not shown).
Figure 7.
Patterns of expression of Sox2, GS, Pax2 and GCR in the reptile retina. Vertical sections of the retina were labeled with antibodies to Sox2 (magenta) and GS (green; A), Sox2 alone (B), GS (magenta) and Pax2 (green; C), Pax2 alone (green; D), and GCR alone (D). The area boxed-out in yellow in panel C is separated into individual channels; Pax2 (magenta) and GS (green). Arrows indicate the nuclei of Müller glia and hollow arrow-heads indicate the nuclei of presumptive NIRG-like cells. The scale bar (50 μm) in panel C applies to A–C, the bars in D apply to D, and the bar in E applies to E alone. Abbreviations: ONL – outer nuclear layer, INL – inner nuclear layer, IPL – inner plexiform layer, GCL – ganglion cell layer, GS – glutamine synthetase, ONH – optic nerve head.
We found immunolabeling for Pax2 in the nuclei of cells in the optic nerve head of the queen snake (Fig. 7C). In addition, there were high-levels of Pax2-immunoreactivity in peripapillary glia and low levels of Pax2-immunoreactivity in GS-positive Müller glia flanking the optic nerve head (Fig. 7C). The pattern of labeling for Pax2 was significantly different from that described in the chicken retina (Boije et al., 2010; Stanke et al., 2010). By comparison, in the retinas of the anole lizard and turtles, we observed Pax2-immunoreactivity in fusiform nuclei in the middle of the INL, but only in central regions of the retina (Fig. 7D). The shape and patterns of distribution of labeled nuclei are consistent with Pax2 expression in Müller glia. This pattern of labeling for Pax2 was very similar to that described in the chicken and zebrafish retinas (Boije et al., 2010; Stanke et al., 2010). However, the antibody to Pax2 appeared to cross-react with an antigen at the cell surface of photoreceptors, but only in the retinas of turtles (Fig. 7D).
We have recently report that GCR is expressed by Müller glia in the retinas of chickens, mice, guinea pigs, dogs and humans (Gallina et al., 2014b). Similar to the patterns of GCR-expression inc warm-blooded vertebrates, we consistently observed GCR-immunoreactivity in fusiform nuclei found in the center of the INL in the retina of anole lizards, snakes and turtles (Fig. 7E). In the garter snake, GCR-immunoreacitivity was also observed in cells scattered across the IPL and GCL (Fig. 7E), consistent with the hypothesis that GCR may be expressed by NIRG cells. This pattern of immunolabeling is consistent with that seen in the nuclei of Müller glia and NIRG cells in the retinas of birds and mammals (Gallina et al., 2014b).
Discussion
Our findings provide the first evidence of a CMZ-like region of cells in the reptile retina. The CMZ was first characterized in amphibians more than 40 years ago (Hollyfield, 1968; Hollyfield, 1971; Straznicky and Gaze, 1971). The CMZ was next described in the goldfish eye, where it was estimated that approximately 12,000 cells are generated per day (Johns, 1977). More recently, the CMZ of the zebrafish has been thoroughly characterized (Raymond et al., 2006; Wehman et al., 2005). Recently, a CMZ has been described in cartilaginous fish. Proliferating cellular nuclear antigen (PCNA) -positive cells were found at the peripheral retinal margin of two different species of sharks (Ferreiro-Galve et al., 2010). In addition, progenitor cells organized into a CMZ have been described in the eyes of birds, wherein proliferation and addition of new cells to the edge of the retina is most prevalent during the first few weeks of post-hatch development. The avian CMZ persists into adulthood, but the neurogenic capacity of CMZ-progenitors is limited (Fischer et al., 2002; Fischer and Reh, 2000; Kubota et al., 2002). By comparison, marsupials and mammals do not appear to maintain a zone of progenitors that adds cells to the retinal margin (Kubota, et al., 2002).
Our data suggest that among different species of reptiles, only turtles possess a CMZ with proliferating progenitors that persist into adulthood. We detected PCNA-positive proliferating cells at the margin of the turtle retina. This is consistent with the hypothesis that turtles and chickens are close evolutionary relatives compared to snakes and lizards (Crawford et al., 2012). However, we cannot exclude the possibility that proliferating CMZ-progenitors are found only during early stages of post-hatch development in snakes and lizards. It is possible that CMZ-progenitors were not observed in the eyes of anoles and snakes because tissues were obtained from adults, after the need for retinal growth that accompanies overall eye growth. Alternatively, there may be species of lizards and snakes with ocular growth that continues into adulthood and requires an active CMZ. Another alternative is that CMZ-progenitors in anoles and snakes may be quiescent, but could be stimulated by growth factors, as in the eyes of adult quails (Kubota et al., 2002). It remains uncertain whether the CMZ-progenitors in turtles proliferate at increased rates in response to injury, a phenomenon known to occur in amphibians (Reh, 1987) and fish (Stenkamp et al., 2001), but not birds (Fischer, 2005). Further, it remains uncertain whether the CMZ-progenitors in turtles proliferate at increased rates in response to accelerated rates of ocular growth, as has been described in birds (Fischer et al., 2008).
Our findings suggest that the glucagonergic amacrine cells, that mediate vision-guided ocular growth in the bird retina (Fischer et al., 1999; Fischer et al., 2005; Fischer et al., 2008), have similar patterns and distributions in the turtle eye. The glucagonergic amacrine cells in the avian retina detect the sign of defocus and release glucagon peptide to slow rates of axial ocular growth in response to plus-defocus (Feldkaemper and Schaeffel, 2002; Fischer et al., 1999; Vessey et al., 2005a; Vessey et al., 2005b). In addition, unique types of glucagonergic retinal neurons and bullwhip cells, ramify neurites in the CMZ and mediate vision guided retinal growth and equatorial ocular growth (Fischer et al., 2005; Fischer et al., 2008; Fischer et al., 2006). In rodent, canine and primate retinas, neurons that express glucagon or GLP1 have not been detected (unpublished observations). In all reptile species examined herein, we find that GLP1-positive cells are found in a distinct type of amacrine cell. Furthermore, accumulations of GLP1/glucagon-positive terminal endings are observed in the CMZ/retinal margin of red-eared turtles (Wetzel and Eldred, 1997), painted turtles and snapping turtles (current study). These data are consistent with the hypothesis that glucagonergic cells may influence CMZ proliferation and vision guided ocular growth in turtles, similar to the chicken retina (Fischer et al., 1999; Fischer et al., 2005; Fischer et al., 2008). However, we did not find compelling evidence for bullwhip cells in the reptilian retina, even though we found accumulations of glucagonergic neurites within the CMZ of turtles.
To date, only one report has described the expression of GCR in the CMZ (Gallina, et al. 2014b). Our data suggest that glucocorticoid signaling may influence CMZ-progenitors in reptiles. Studies are required to determine whether glucocorticoid signaling impacts the activity of progenitors in the CMZ of birds, reptiles, amphibians and fish. Similar to patterns of expression in CMZ of birds, we found co-expression of Pax6 and Sox9 by cells at the margin of the retina of all reptiles species examined. Additionally, we identified cells at the retinal margin that expressed low levels of Pax6 compared to levels seen in mature amacrine cells. Embryonic retinal progenitors and those found in the CMZ are known to express Pax6 (Fischer and Reh, 2000; Marquardt et al., 2001) and Sox9 (Fischer et al., 2014; Poche et al., 2008). Although cells co-expressing Pax6 and Sox9 were found in all reptiles, we failed to find evidence of proliferating cells at the margin of retinas in anoles and snakes. In the chicken eye, NPE cells of the pars plana of the ciliary body are known to co-express the transcription factors Pax6, Chx10, Sox2 and Sox9 (Fischer and Reh, 2003; Fischer et al., 2009). Further, the NPE cells can be stimulated by different growth factors to proliferate and produce neurons in vivo (Fischer and Reh, 2003). The neurogenic potential of NPE cells in reptiles remains unknown.
Müller glia have the capacity to become proliferating progenitor cells when stimulated by retinal damage or growth factors (reviewed by (Gallina et al., 2014a)). This capacity has been described in fish, chicken and mammalian model systems (Gallina et al., 2014a). It is presumed that Müller glia retain the capacity to become progenitor cells because of transcription factors such as Pax6, Sox2 and Sox9 which are also expressed by embryonic retinal stem cells. We report here that Sox2, Sox9 and Pax6 are present in the Müller glia of reptiles. However, the potential of Müller glia to become progenitor cells in the reptile retina remains a topic for future investigation. Interestingly, in fish and chicken models, where the re-programming of Müller glia into progenitors has been well-described (reviewed by (Fischer and Bongini, 2010; Goldman, 2014)), these glia express Pax2 in central regions of the retina (Boije et al., 2010; Stanke et al., 2010). Similarly, we find that the Müller glia in central regions of the retina in anoles and turtles express Pax2, whereas the Müller glia in the retinas of snakes do not express detectable levels of Pax2.
Our findings suggest that NIRG cells may be present in the retinas of reptiles. The NIRG cells in the bird retina are scattered across inner retinal layers, and express transitin/nestin, vimentin, Olig2, Sox9, Sox2 and Nkx2.2, but do not express GFAP even in damaged retinas (Fischer et al., 2010a; Rompani and Cepko, 2010; Zelinka et al., 2012). In the reptile retina, we found GFAP-expression in Müller glia, but not in cells scattered across inner retinal layers. This is similar to expression patterns in the chicken retina, wherein NIRG cells do not express GFAP, unlike astrocytes in mammalian retinas (Fischer et al., 2010b). In the reptile retina, we consistently observed Sox9-positive cells, and in the turtle retina Sox2-positive cells, scattered across inner retinal layers; consistent with the notion that NIRG cells are present in the eyes of reptiles. However, antibodies to nestin/transitin, Nkx2.2 and Olig2 failed to produce specific labeling in the reptile retina (not shown). Further studies are required to determine unambiguously whether NIRG cells are present in the retinas of reptiles.
Our findings may provide some insight into the phylogenetic relationships between different reptiles and birds. For example, the position of Testudines (turtles) within Reptilia is a controversial topic. Reptiles have traditionally been placed into phylogenetic positions within amniotes based on morphological criteria such as skull type. Turtles are referred to as anapsids as they contain no temporal fossae within the skull (Chiari et al., 2012). All other reptiles are placed with birds in the diapsids (two fossae within the temporal skull), and mammals are positioned within amniotes as synapsids with one temporal fossa. Turtles have traditionally been thought to be the most basal taxon within amniotes based on morphological phylogenetic trees (Zardoya and Meyer, 2001). However, the most recent microRNA data suggest that turtles should be grouped within diapsids and share their most recent ancestor with Archosauria, birds and crocodilians (Field et al., 2014), not Lepidosauria, snakes and lizards. Field and colleagues (2014) describe the phylogenetic relationship of birds and the different reptiles used in the current study. Collectively, our findings suggests that turtles are most similar to birds based on patterns of expression of different genes in retinal glia, amacrine cells and the organization of a CMZ that contains proliferating progenitors.
Conclusions
We conclude that patterns of labeling at the retinal margin, CMZ-progenitors, retinal glia and glucagonergic amacrine cells reveal that the retinas of turtles are most similar to that of birds. We find evidence for a CMZ in the eyes of turtles, but not in the eyes of snakes or lizards. In snakes and the anole, cells at the retinal margin expressed Sox9, Pax6, GCR and N-cadherin, similar to the margin described in other orders of vertebrates. However, we found no evidence of proliferating cells in the retinas of snakes and anoles. It is possible that these Sox9/Pax6-positive cells represent a “latent” CMZ that do not proliferate or generate new retinal neurons in mature animals, but could be stimulated to do so. We conclude that the pattern of labeling for glucagonergic amacrine cells, Müller glia and inner retinal glia (astrocytes or NIRG cells) is consistent across reptiles, with the exception of filamentous proteins in snake retinas, and is similar to patterns of expression described in fish and birds. Our observations suggest that a CMZ-like region, retinal glia and glucagonergic amacrine cells in turtles, among other types of reptiles, are the most similar to those of birds; consistent with zoological and genetic analyses (Crawford et al., 2012).
Table 2.
Summary of patterns of labeling.
| Anole lizard | Garter snake | Queen snake | Snapping turtle | Painted turtle | Birds | Fish & Amphibians | |
|---|---|---|---|---|---|---|---|
| Glucagon/GLP1 Amacrine cells | +++ | +++ | +++ | +++ | +++ | +++ | ? |
| Glucagon/GLP1 Endings in CMZ | − | − | − | +++ | +++ | +++ | ? |
| Pax6 amacrine | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| Pax6 Müller glia | − | − | + | + | + | + | +? |
| Pax6 CMZ or retinal margin | + | + | + | + | + | + | + |
| Sox9 Müller glia | +++ | +++ | +++ | +++ | +++ | +++ | ? |
| Sox9 IPL glia (presumptive NIRG cells) | ++ | ++ | ++ | ++ | ++ | ++ | ? |
| Sox9 CMZ or retinal margin | + | + | + | + | + | + | ? |
| Sox9 RPE | ++ | ++ | ++ | ++ | ++ | ++ | ? |
| Substance P amacrine cells | +++ | − | − | + | +? | +++ | +++ |
| Substance P CMZ or retinal margin | − | − | − | +? | +? | +++ | ? |
| N-cadherin CMZ or retinal margin | + | ++ | ++ | ++ | ++ | ++ | ++ |
| GCR CMZ or retinal margin | +++ | +++ | +++ | ++ | ++ | +++ | ? |
| GCR Müller glia | +++ | ++ | ++ | ++ | +++ | +++ | ? |
| Vimentin Müller glia + end feet ++ through-out | + | + | + | +++ | +++ | +++ | ++ |
| GFAP Müller glia + end feet ++ low levels +++ through-out | +++ | + | + | ++ | +++ | ++ | ++ |
| TOPAP ++ Müller glia ? bipolar cells | +++ | ? | ? | +++ | +++ | +++ | − |
| Sox2 Müller glia | +++ | − | − | +++ | +++ | +++ | +++ |
| Sox2 IPL glia (presumptive NIRG cells) | ++ | − | − | ++ | ++ | ++ | − |
| Pax2 +++ Müller glia + peripapillary glia | +++ | − | + | +++ | +++ | +++ and + | +++ and +? |
| Proliferating cells in the CMZ | − | − | − | + | + | ++ | +++ |
+++ distinct immunolabeling and/or numerous cells, ++ moderate immunolabeling and/or many cells, + weak immunolabeling and/or few cells, − no detectable labeling
Acknowledgments
The authors thank Leo Volkov for technical assistance. The Pax6, N-Cadherin, and Tyrosine Hydroxylase antibody was obtained from the Developmental Studies Hybridoma Bank developed under auspices of the NICHD and maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA 52242. This work was supported by a grant from the National Institutes of Health RO1 EY022030-03.
Footnotes
Conflict of Interest Statement
The authors declare they have no competing financial interests.
Role of Authors
Study concept and design: LT, AF. Acquisition of data: LT, NS, LS, CZ, KG. Analysis and interpretation of data: LT, AF. Drafting of the manuscript: LT, CZ, AF. Funding obtained: AF.
References
- Alvarez-Buylla A, Buskirk DR, Nottebohm F. Monoclonal antibody reveals radial glia in adult avian brain. J Comp Neurol. 1987;264(2):159–170. doi: 10.1002/cne.902640203. [DOI] [PubMed] [Google Scholar]
- Boije H, Ring H, Lopez-Gallardo M, Prada C, Hallbook F. Pax2 is expressed in a subpopulation of Muller cells in the central chick retina. Dev Dyn. 2010;239(6):1858–1866. doi: 10.1002/dvdy.22309. [DOI] [PubMed] [Google Scholar]
- Brecha NC, Karten HJ. Identification and localization of neuropeptides in the vertebrate retina. In: Kreiger D, Brownstein M, Martin J, editors. Brain Peptides. New York: Wiley; 1983. [Google Scholar]
- Casanas MN, Santos E, Yanes C, Romero-Aleman MM, Vinoly R, Alfayate MC, Monzon-Mayor M. Development of astroglia heterogeneously expressing Pax2, vimentin and GFAP during the ontogeny of the optic pathway of the lizard (Gallotia galloti): an immunohistochemical and ultrastructural study. Cell Tissue Res. 2011;345(3):295–311. doi: 10.1007/s00441-011-1211-9. [DOI] [PubMed] [Google Scholar]
- Chiari Y, Cahais V, Galtier N, Delsuc F. Phylogenomic analyses support the position of turtles as the sister group of birds and crocodiles (Archosauria) BMC Biol. 2012;10:65. doi: 10.1186/1741-7007-10-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua J, Nivison-Smith L, Fletcher EL, Trenholm S, Awatramani GB, Kalloniatis M. Early remodeling of Muller cells in the rd/rd mouse model of retinal dystrophy. J Comp Neurol. 2013;521(11):2439–2453. doi: 10.1002/cne.23307. [DOI] [PubMed] [Google Scholar]
- Crawford NG, Faircloth BC, McCormack JE, Brumfield RT, Winker K, Glenn TC. More than 1000 ultraconserved elements provide evidence that turtles are the sister group of archosaurs. Biol Lett. 2012;8(5):783–786. doi: 10.1098/rsbl.2012.0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuello AC, Galfre G, Milstein C. Detection of substance P in the central nervous system by a monoclonal antibody. Proc Natl Acad Sci U S A. 1979;76(7):3532–3536. doi: 10.1073/pnas.76.7.3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuenca N, Kolb H. Morphology and distribution of neurons immunoreactive for substance P in the turtle retina. J Comp Neurol. 1989;290(3):391–411. doi: 10.1002/cne.902900308. [DOI] [PubMed] [Google Scholar]
- Dahl D, Crosby CJ, Sethi JS, Bignami A. Glial fibrillary acidic (GFA) protein in vertebrates: immunofluorescence and immunoblotting study with monoclonal and polyclonal antibodies. J Comp Neurol. 1985;239(1):75–88. doi: 10.1002/cne.902390107. [DOI] [PubMed] [Google Scholar]
- Fauquet M, Ziller C. A monoclonal antibody directed against quail tyrosine hydroxylase: description and use in immunocytochemical studies on differentiating neural crest cells. J Histochem Cytochem. 1989;37(8):1197–1205. doi: 10.1177/37.8.2569003. [DOI] [PubMed] [Google Scholar]
- Feldkaemper MP, Schaeffel F. Evidence for a potential role of glucagon during eye growth regulation in chicks. Vis Neurosci. 2002;19(6):755–766. doi: 10.1017/s0952523802196064. [DOI] [PubMed] [Google Scholar]
- Ferreiro-Galve S, Rodriguez-Moldes I, Anadon R, Candal E. Patterns of cell proliferation and rod photoreceptor differentiation in shark retinas. J Chem Neuroanat. 2010;39(1):1–14. doi: 10.1016/j.jchemneu.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Field DJ, Gauthier JA, King BL, Pisani D, Lyson TR, Peterson KJ. Toward consilience in reptile phylogeny: miRNAs support an archosaur, not lepidosaur, affinity for turtles. Evol Dev. 2014;16(4):189–196. doi: 10.1111/ede.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ. Neural regeneration in the chick retina. Prog Retin Eye Res. 2005;24(2):161–182. doi: 10.1016/j.preteyeres.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Bongini R. Turning Muller glia into neural progenitors in the retina. Mol Neurobiol. 2010;42(3):199–209. doi: 10.1007/s12035-010-8152-2. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Bosse JL, El-Hodiri HM. The ciliary marginal zone (CMZ) in development and regeneration of the vertebrate eye. Exp Eye Res. 2014;116:199–204. doi: 10.1016/j.exer.2013.08.018. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Dierks BD, Reh TA. Exogenous growth factors induce the production of ganglion cells at the retinal margin. Development. 2002;129(9):2283–2291. doi: 10.1242/dev.129.9.2283. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, McGuire JJ, Schaeffel F, Stell WK. Light- and focus-dependent expression of the transcription factor ZENK in the chick retina. Nat Neurosci. 1999;2(8):706–712. doi: 10.1038/11167. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Omar G. Transitin, a nestin-related intermediate filament, is expressed by neural progenitors and can be induced in Muller glia in the chicken retina. J Comp Neurol. 2005;484(1):1–14. doi: 10.1002/cne.20406. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Omar G, Walton NA, Verrill TA, Unson CG. Glucagon-expressing neurons within the retina regulate the proliferation of neural progenitors in the circumferential marginal zone of the avian eye. J Neurosci. 2005;25(44):10157–10166. doi: 10.1523/JNEUROSCI.3247-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ, Reh TA. Identification of a proliferating marginal zone of retinal progenitors in postnatal chickens. Dev Biol. 2000;220(2):197–210. doi: 10.1006/dbio.2000.9640. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Reh TA. Muller glia are a potential source of neural regeneration in the postnatal chicken retina. Nat Neurosci. 2001;4(3):247–252. doi: 10.1038/85090. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Reh TA. Growth factors induce neurogenesis in the ciliary body. Dev Biol. 2003;259(2):225–240. doi: 10.1016/s0012-1606(03)00178-7. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Ritchey ER, Scott MA, Wynne A. Bullwhip neurons in the retina regulate the size and shape of the eye. Dev Biol. 2008;317(1):196–212. doi: 10.1016/j.ydbio.2008.02.023. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Scott MA, Tuten W. Mitogen-activated protein kinase-signaling stimulates Muller glia to proliferate in acutely damaged chicken retina. Glia. 2009;57(2):166–181. doi: 10.1002/glia.20743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ, Scott MA, Zelinka C, Sherwood P. A novel type of glial cell in the retina is stimulated by insulin-like growth factor 1 and may exacerbate damage to neurons and Muller glia. Glia. 2010a;58(6):633–649. doi: 10.1002/glia.20950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ, Skorupa D, Schonberg DL, Walton NA. Characterization of glucagon-expressing neurons in the chicken retina. J Comp Neurol. 2006;496(4):479–494. doi: 10.1002/cne.20937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ, Stanke JJ, Ghai K, Scott M, Omar G. Development of bullwhip neurons in the embryonic chicken retina. J Comp Neurol. 2007;503(4):538–549. doi: 10.1002/cne.21404. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Zelinka C, Scott MA. Heterogeneity of glia in the retina and optic nerve of birds and mammals. PLoS One. 2010b;5(6):e10774. doi: 10.1371/journal.pone.0010774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallina D, Todd L, Fischer AJ. A comparative analysis of Muller glia-mediated regeneration in the vertebrate retina. Exp Eye Res. 2014a;123:121–130. doi: 10.1016/j.exer.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallina D, Zelinka C, Fischer AJ. Glucocorticoid receptors in the retina, Müller glia and the formation of Müller glia-derived progenitors. Development. 2014b;141:3340–3351. doi: 10.1242/dev.109835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaur VP, Eldred W, Sarthy PV. Distribution of Muller cells in the turtle retina: an immunocytochemical study. J Neurocytol. 1988;17(5):683–692. doi: 10.1007/BF01260995. [DOI] [PubMed] [Google Scholar]
- George-Weinstein M, Gerhart J, Blitz J, Simak E, Knudsen KA. N-cadherin promotes the commitment and differentiation of skeletal muscle precursor cells. Dev Biol. 1997;185(1):14–24. doi: 10.1006/dbio.1997.8542. [DOI] [PubMed] [Google Scholar]
- Ghai K, Stanke JJ, Fischer AJ. Patterning of the circumferential marginal zone of progenitors in the chicken retina. Brain Res. 2008;1192:76–89. doi: 10.1016/j.brainres.2007.01.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D. Muller glial cell reprogramming and retina regeneration. Nat Rev Neurosci. 2014 doi: 10.1038/nrn3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon WC, Casey DM, Lukiw WJ, Bazan NG. DNA damage and repair in light-induced photoreceptor degeneration. Invest Ophthalmol Vis Sci. 2002;43(11):3511–3521. [PubMed] [Google Scholar]
- Hatta K, Takeichi M. Expression of N-cadherin adhesion molecules associated with early morphogenetic events in chick development. Nature. 1986;320(6061):447–449. doi: 10.1038/320447a0. [DOI] [PubMed] [Google Scholar]
- Hendrickson A, Troilo D, Possin D, Springer A. Development of the neural retina and its vasculature in the marmoset Callithrix jacchus. J Comp Neurol. 2006;497(2):270–286. doi: 10.1002/cne.20996. [DOI] [PubMed] [Google Scholar]
- Hitchcock PF, Raymond PA. Retinal regeneration. Trends Neurosci. 1992;15(3):103–108. doi: 10.1016/0166-2236(92)90020-9. [DOI] [PubMed] [Google Scholar]
- Hollyfield JG. Differential addition of cells to the retina in Rana pipiens tadpoles. Dev Biol. 1968;18(2):163–179. doi: 10.1016/0012-1606(68)90041-9. [DOI] [PubMed] [Google Scholar]
- Hollyfield JG. Differential growth of the neural retina in Xenopus laevis larvae. Dev Biol. 1971;24(2):264–286. doi: 10.1016/0012-1606(71)90098-4. [DOI] [PubMed] [Google Scholar]
- Johns PR. Growth of the adult goldfish eye. III. Source of the new retinal cells. J Comp Neurol. 1977;176(3):343–357. doi: 10.1002/cne.901760304. [DOI] [PubMed] [Google Scholar]
- Karten HJ, Brecha N. Localization of neuroactive substances in the vertebrate retina: evidence for lamination in the inner plexiform layer. Vision Res. 1983;23(10):1197–1205. doi: 10.1016/0042-6989(83)90033-0. [DOI] [PubMed] [Google Scholar]
- Kawakami A, Kimura-Kawakami M, Nomura T, Fujisawa H. Distributions of PAX6 and PAX7 proteins suggest their involvement in both early and late phases of chick brain development. Mech Dev. 1997;66(1–2):119–130. doi: 10.1016/s0925-4773(97)00097-x. [DOI] [PubMed] [Google Scholar]
- Knudsen KA, Soler AP, Johnson KR, Wheelock MJ. Interaction of alpha-actinin with the cadherin/catenin cell-cell adhesion complex via alpha-catenin. J Cell Biol. 1995;130(1):67–77. doi: 10.1083/jcb.130.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota R, Hokoc JN, Moshiri A, McGuire C, Reh TA. A comparative study of neurogenesis in the retinal ciliary marginal zone of homeothermic vertebrates. Brain Res Dev Brain Res. 2002;134(1–2):31–41. doi: 10.1016/s0165-3806(01)00287-5. [DOI] [PubMed] [Google Scholar]
- Marquardt T, Ashery-Padan R, Andrejewski N, Scardigli R, Guillemot F, Gruss P. Pax6 is required for the multipotent state of retinal progenitor cells. Cell. 2001;105(1):43–55. doi: 10.1016/s0092-8674(01)00295-1. [DOI] [PubMed] [Google Scholar]
- Morris VB, Wylie CC, Miles VJ. The growth of the chick retina after hatching. Anat Rec. 1976;184(1):111–113. doi: 10.1002/ar.1091840109. [DOI] [PubMed] [Google Scholar]
- Nishida T. Commanding roles of keratocytes in health and disease. Cornea. 2010;29(Suppl 1):S3–6. doi: 10.1097/ICO.0b013e3181f2d578. [DOI] [PubMed] [Google Scholar]
- Ochrietor JD, Moroz TP, Linser PJ. The 2M6 antigen is a Muller cell-specific intracellular membrane-associated protein of the sarcolemmal-membrane-associated protein family and is also TopAP. Mol Vis. 2010;16:961–969. [PMC free article] [PubMed] [Google Scholar]
- Pecchi E, Dallaporta M, Charrier C, Pio J, Jean A, Moyse E, Troadec JD. Glial fibrillary acidic protein (GFAP)-positive radial-like cells are present in the vicinity of proliferative progenitors in the nucleus tractus solitarius of adult rat. J Comp Neurol. 2007;501(3):353–368. doi: 10.1002/cne.21259. [DOI] [PubMed] [Google Scholar]
- Perron M, Kanekar S, Vetter ML, Harris WA. The genetic sequence of retinal development in the ciliary margin of the Xenopus eye. Dev Biol. 1998;199(2):185–200. doi: 10.1006/dbio.1998.8939. [DOI] [PubMed] [Google Scholar]
- Poche RA, Furuta Y, Chaboissier MC, Schedl A, Behringer RR. Sox9 is expressed in mouse multipotent retinal progenitor cells and functions in Muller glial cell development. J Comp Neurol. 2008;510(3):237–250. doi: 10.1002/cne.21746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prada C, Puga J, Perez-Mendez L, Lopez R, Ramirez G. Spatial and Temporal Patterns of Neurogenesis in the Chick Retina. Eur J Neurosci. 1991;3(6):559–569. doi: 10.1111/j.1460-9568.1991.tb00843.x. [DOI] [PubMed] [Google Scholar]
- Raymond PA. Retinal regeneration in teleost fish. Ciba Found Symp. 1991;160:171–186. doi: 10.1002/9780470514122.ch9. discussion 186–191. [DOI] [PubMed] [Google Scholar]
- Raymond PA, Barthel LK, Bernardos RL, Perkowski JJ. Molecular characterization of retinal stem cells and their niches in adult zebrafish. BMC Dev Biol. 2006;6:36. doi: 10.1186/1471-213X-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond PA, Hitchcock PF. Retinal regeneration: common principles but a diversity of mechanisms. Adv Neurol. 1997;72:171–184. [PubMed] [Google Scholar]
- Reh TA. Cell-specific regulation of neuronal production in the larval frog retina. J Neurosci. 1987;7(10):3317–3324. doi: 10.1523/JNEUROSCI.07-10-03317.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reh TA, Levine EM. Multipotential stem cells and progenitors in the vertebrate retina. J Neurobiol. 1998;36(2):206–220. [PubMed] [Google Scholar]
- Ritchey ER, Code K, Zelinka CP, Scott MA, Fischer AJ. The chicken cornea as a model of wound healing and neuronal re-innervation. Mol Vis. 2011;17:2440–2454. [PMC free article] [PubMed] [Google Scholar]
- Romero-Aleman MM, Monzon-Mayor M, Santos E, Lang DM, Yanes C. Neuronal and glial differentiation during lizard (Gallotia galloti) visual system ontogeny. J Comp Neurol. 2012;520(10):2163–2184. doi: 10.1002/cne.23034. [DOI] [PubMed] [Google Scholar]
- Romero-Aleman MM, Monzon-Mayor M, Santos E, Yanes C. Expression of neuronal markers, synaptic proteins, and glutamine synthetase in the control and regenerating lizard visual system. J Comp Neurol. 2010;518(19):4067–4087. doi: 10.1002/cne.22444. [DOI] [PubMed] [Google Scholar]
- Rompani SB, Cepko CL. A common progenitor for retinal astrocytes and oligodendrocytes. J Neurosci. 2010;30(14):4970–4980. doi: 10.1523/JNEUROSCI.3456-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosshauer B, Wild M. Generation of monoclonal antibodies specific for developmentally regulated antigens of the chicken retina. Brain Res Dev Brain Res. 1991;59(2):197–208. doi: 10.1016/0165-3806(91)90100-w. [DOI] [PubMed] [Google Scholar]
- Sehgal R, Karcavich R, Carlson S, Belecky-Adams TL. Ectopic Pax2 expression in chick ventral optic cup phenocopies loss of Pax2 expression. Dev Biol. 2008;319(1):23–33. doi: 10.1016/j.ydbio.2008.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal R, Sheibani N, Rhodes SJ, Belecky Adams TL. BMP7 and SHH regulate Pax2 in mouse retinal astrocytes by relieving TLX repression. Dev Biol. 2009;332(2):429–443. doi: 10.1016/j.ydbio.2009.05.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigulinsky CL, Green ES, Clark AM, Levine EM. Vsx2/Chx10 ensures the correct timing and magnitude of Hedgehog signaling in the mouse retina. Dev Biol. 2008;317(2):560–575. doi: 10.1016/j.ydbio.2008.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanke J, Moose HE, El-Hodiri HM, Fischer AJ. Comparative study of Pax2 expression in glial cells in the retina and optic nerve of birds and mammals. J Comp Neurol. 2010;518(12):2316–2333. doi: 10.1002/cne.22335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanke JJ, Fischer AJ. Embryonic retinal cells and support to mature retinal neurons. Invest Ophthalmol Vis Sci. 2010;51(4):2208–2218. doi: 10.1167/iovs.09-4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenkamp DL, Powers MK, Carney LH, Cameron DA. Evidence for two distinct mechanisms of neurogenesis and cellular pattern formation in regenerated goldfish retinas. J Comp Neurol. 2001;431(4):363–381. doi: 10.1002/1096-9861(20010319)431:4<363::aid-cne1076>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Straznicky K, Gaze RM. The growth of the retina in Xenopus laevis: an autoradiographic study. J Embryol Exp Morphol. 1971;26(1):67–79. [PubMed] [Google Scholar]
- Uchiyama H, Reh TA, Stell WK. Immunocytochemical and morphological evidence for a retinopetal projection in anuran amphibians. J Comp Neurol. 1988;274(1):48–59. doi: 10.1002/cne.902740106. [DOI] [PubMed] [Google Scholar]
- Vessey KA, Lencses KA, Rushforth DA, Hruby VJ, Stell WK. Glucagon receptor agonists and antagonists affect the growth of the chick eye: a role for glucagonergic regulation of emmetropization? Invest Ophthalmol Vis Sci. 2005a;46(11):3922–3931. doi: 10.1167/iovs.04-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vessey KA, Rushforth DA, Stell WK. Glucagon- and secretin-related peptides differentially alter ocular growth and the development of form-deprivation myopia in chicks. Invest Ophthalmol Vis Sci. 2005b;46(11):3932–3942. doi: 10.1167/iovs.04-1027. [DOI] [PubMed] [Google Scholar]
- Wehman AM, Staub W, Meyers JR, Raymond PA, Baier H. Genetic dissection of the zebrafish retinal stem-cell compartment. Dev Biol. 2005;281(1):53–65. doi: 10.1016/j.ydbio.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Wetts R, Fraser SE. Multipotent precursors can give rise to all major cell types of the frog retina. Science. 1988;239(4844):1142–1145. doi: 10.1126/science.2449732. [DOI] [PubMed] [Google Scholar]
- Wetzel RK, Eldred WD. Specialized neuropeptide Y- and glucagon-like immunoreactive amacrine cells in the peripheral retina of the turtle. Vis Neurosci. 1997;14(5):867–877. doi: 10.1017/s0952523800011603. [DOI] [PubMed] [Google Scholar]
- Wishcamper CA, Coffin JD, Lurie DI. Lack of the protein tyrosine phosphatase SHP-1 results in decreased numbers of glia within the motheaten (me/me) mouse brain. J Comp Neurol. 2001;441(2):118–133. [PubMed] [Google Scholar]
- Wright E, Hargrave MR, Christiansen J, Cooper L, Kun J, Evans T, Gangadharan U, Greenfield A, Koopman P. The Sry-related gene Sox9 is expressed during chondrogenesis in mouse embryos. Nat Genet. 1995;9(1):15–20. doi: 10.1038/ng0195-15. [DOI] [PubMed] [Google Scholar]
- Zardoya R, Meyer A. The evolutionary position of turtles revised. Naturwissenschaften. 2001;88(5):193–200. doi: 10.1007/s001140100228. [DOI] [PubMed] [Google Scholar]
- Zelinka CP, Scott MA, Volkov L, Fischer AJ. The Reactivity, Distribution and Abundance of Non-Astrocytic Inner Retinal Glial (NIRG) Cells Are Regulated by Microglia, Acute Damage, and IGF1. PLoS One. 2012;7(9):e44477. doi: 10.1371/journal.pone.0044477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber ME, Gestri G, Viczian AS, Barsacchi G, Harris WA. Specification of the vertebrate eye by a network of eye field transcription factors. Development. 2003;130(21):5155–5167. doi: 10.1242/dev.00723. [DOI] [PubMed] [Google Scholar]