Abstract
Previous studies have demonstrated that there are persistent changes in dopamine systems following withdrawal from methamphetamine (METH). This study examined changes in striatal dopamine transporter (DAT), tyrosine hydroxylase (TH) and dopamine receptor 2 (D2) 72 hours after withdrawal from METH intravenous self-administration (IVSA). Rats were given limited (1 hour) or extended (6 hour) access to METH IVSA (0.05 mg/kg/0.1 ml infusion) for 22 days. Controls did not receive METH IVSA. The rats given extended access to IVSA displayed higher METH intake during the first hour of drug access compared to rats given limited access. Extended access to METH also produced a concomitant increase in striatal DAT levels relative to drug-naïve controls. There were no changes in TH or D2 levels across groups. Previous studies have reported a decrease in striatal DAT levels during protracted periods (>7 days) of withdrawal from METH IVSA. This study extends previous work by showing an increase in striatal DAT protein expression during an earlier time point of withdrawal from this drug. These results are an important first step toward understanding the dynamic changes in dopamine systems that occur during different time points of withdrawal from METH IVSA.
Keywords: withdrawal, abstinence, striatum, methamphetamine, dopamine transporter, IVSA
1. Introduction
Clinical reports have shown that chronic exposure to methamphetamine (METH) produces a profound reduction in dopamine transporter (DAT) expression in the striatum [1, 2]. Such alterations in DAT have been associated with METH addiction [3]. Consistent with clinical reports, METH intravenous self-administration (IVSA) in rodents produces long-term down-regulation of many dopaminergic markers within the striatum, including DAT, tyrosine hydroxylase (TH) and dopamine receptor 2 (D2). Although the latter effects have been largely observed following protracted periods of withdrawal (7–30 days) from METH IVSA [4, 5, 6, 7], earlier time points of withdrawal (< 7 days) have not been thoroughly investigated.
While there are species-specific differences in the rate and pathways of METH metabolism, the clearance of this drug and its psychoactive metabolites generally occurs 72 hours after METH administration [8, 9]. Also, the strongest symptoms of withdrawal are reported 24–72 hours after abstinence from chronic METH use [10, 11]. These studies suggest that 72 hours after the initiation of METH withdrawal is a physiologically and psychologically relevant time point to examine changes in dopaminergic markers. A recent report also demonstrated that early in withdrawal from METH IVSA, rats given extended access (6 hour) displayed a larger increase in depressive-like states as compared to rats given limited (1 hour) access [12]. Thus, the present study compared DAT, TH and D2 receptor levels in the striatum 72 hours after withdrawal from limited and extended access to METH IVSA.
2. Methods
2.1. Subjects
Adult male Wistar rats (Harlan Inc.) weighing 300–350g were pair-housed in a reversed light cycle vivarium. Food and water were provided ad libitum. However, the rats did not have access to food during the IVSA sessions and for approximately one hour prior to the food training sessions. All procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals and approved by the UTEP Institutional Animal Care and Use Committee. Ten rats were removed from the study due to catheter patency and/or mechanical issues.
2.2. Operant procedures
IVSA procedures have been described previously, with a few minor modifications in the current study [13]. Briefly, rats were trained to press for food pellets prior to IV catheterization. Following surgical recovery, the rats were given 6 sessions of METH IVSA (0.05 mg/kg/0.1 ml infusion) each lasting 1 hour in order to establish baseline intake. Following each drug infusion, a 20-second cue light was illuminated over the drug lever that signaled a time-out period where responses on the lever had no scheduled consequences. The initial baseline values were similar across groups of rats that were given limited (1 hour; mean baseline value = 1.56 mg/kg) or extended (6 hour; mean baseline value=1.83 mg/kg) access to METH IVSA. Following baseline testing, the rats were given limited or extended access to METH IVSA for 22 additional days. The dose and number of sessions were based on the work of Kitamura et al. [14]. Control rats were catheterized and post-operatively cared for in the same manner as the rats that received METH IVSA. The control rats did receive operant training for food pellets and they remained drug naïve throughout the duration of the study.
2.3. Protein analyses
Seventy-two hours after the final IVSA session, the rats were sacrificed and striatal tissue was bilaterally isolated. Homogenates were subsequently adjusted to 30 μg of protein in 20 μl volume and subjected to SDS-PAGE and Western blotting for DAT (SC-1433, Santa Cruz Biotechnologies), TH and D2 receptors (MAB318 and AB5084P respectively; Millipore Corporation) or actin (Sigma-Aldrich). Bands were visualized by chemiluminescence and evaluated via densitometry using the NIH ImageJ program. Total DAT (72 kDa), TH (56 kDa) and D2 receptor (52 kDa) protein levels were normalized to total actin (42 kDa).
2.4. Statistics
METH intake (mg/kg/day) was analyzed using repeated measures ANOVA with time (days of IVSA) as a repeated measure and access condition (limited versus extended) as a between subject variable. This approach was first applied to the 6 baseline days. Subsequent analyses were made using the first hour of METH access across 22 days of IVSA in limited versus extended access conditions. Separate analyses were conducted on each protein marker using one-way ANOVA, with subsequent post-hoc comparisons of group differences where appropriate. Significance was determined throughout our analyses at the p≤0.05 level.
3. Results
3.1. METH IVSA
Figure 1 depicts METH consumption during baseline and subsequent METH IVSA days. Prior to separation into limited or extended access conditions, all rats were given 6 days of 1-hour access to METH IVSA. Our analysis of METH consumption during baseline revealed that there were no pre-existing differences between rats that received limited or extended access [F (1, 15) = 0.54, p = 0.47]. Upon separation into the different access conditions, there were notable differences in METH intake across experimental conditions. An analysis of the first hour of METH intake across all experimental sessions, revealed a significant difference between limited and extended access conditions [F (1, 15) = 6.7, p < 0.02]. Rats given extended access to METH IVSA displayed higher total METH intake as compared to rats given limited access (p < 0.05). The rats given extended access to METH IVSA displayed a general increase in total drug intake across time, but this strong trend did not reach statistical significance at individual time points as compared to Day 1 of drug access. There were no significant differences in body weights across experimental conditions upon entry into the IVSA phase of the study [F (2, 22) = 2.8, p = ns]. The mean body weights prior to IVSA were as follows (controls= 377g±0.01; limited access= 371g±0.02; extended access= 348g±0.05).
Figure 1.
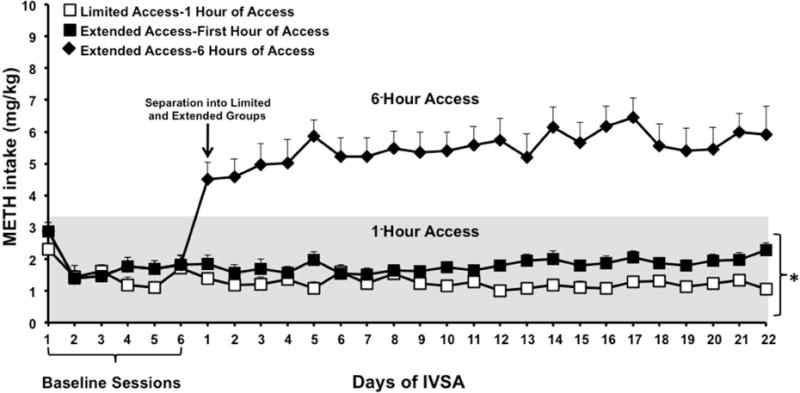
These data reflect daily consumption (mg/kg±SEM) during 6 baseline sessions and after the animals were separated into limited (n=7) and extended (n=10) access conditions. The data are presented during the first hour of drug access (shaded region) and during 6 hours of IVSA in the extended access group. Rats that were given extended access displayed higher levels of METH intake during the first hour of METH access as compared to rats given limited access to this drug (*p≤0.05).
3.2. Protein analyses
Figure 2 depicts DAT, TH and D2 levels in the striatum. When examining protein expression of striatal TH and D2 receptors there were no changes in our measures of TH [F (2, 22) = 1.70, p = ns] or D2 [F (2, 22) = 2.53, p = ns] for either access condition as compared to controls. However, there was a significant difference in DAT [F (2, 22) = 7.19, p < 0.004] across groups. Specifically, DAT levels in the striatum of extended access animals were significantly higher as compared to controls (p < 0.05).
Figure 2.
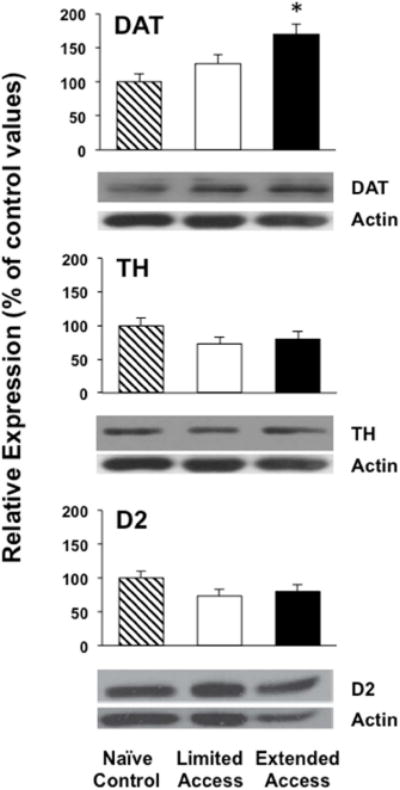
Relative expression of the dopaminergic markers DAT, TH and D2 in controls (n=8) and rats that were given limited (n=7) or extended (n=10) access to METH IVSA. The data reflects a percent change from controls (±SEM), such that a value of 100% indicates no change from the average control value. Representative blots are shown for striatal DAT, TH and D2 and the band intensity normalized to actin.
4. Discussion
The major finding of this report is that striatal DAT levels are increased 72 hours after the initiation of withdrawal from extended (but not limited) access to METH IVSA. Other laboratories have demonstrated a decrease in striatal DAT following more protracted periods of withdrawal from METH IVSA than were examined here (7 and 14 days [4]; 14 days [7]; 30 days [14]). Krasnova et al. also demonstrated a suppression of striatal TH that was accompanied by a concomitant decrease in DAT levels following 14-days of withdrawal from METH IVSA [4]. The latter effects were interpreted to reflect neurotoxicity following protracted withdrawal from high doses of METH IVSA. Without confirmation of neurotoxicity, a more conservative interpretation may be that the decreases in DAT and TH reflect a down-regulation in DA production and clearance mechanisms during late withdrawal. The experimental conditions employed in the present study revealed that there were no changes in striatal TH, but increased DAT levels following 72 hours of METH withdrawal. This suggests that DA production mechanisms remain intact but the clearance potential for DA is increased during early time points of withdrawal from METH IVSA, likely representing a compensatory response to high levels of synaptic dopamine.
To our knowledge, only one other study has examined striatal DAT levels 72 hours after the initiation of withdrawal from a bolus injection of METH. Specifically, they compared monomeric versus complexed DAT levels of expression during several time points of withdrawal [15]. At the 72-hour time point, complexed DAT levels were increased relative to the monomeric form and relative to earlier time points [15]. The present results agree with the latter finding that total striatal DAT levels are increased 72 hours after the initiation of withdrawal from METH IVSA. Taken together, these studies suggest that there is a dynamic change in DAT expression during different time points of withdrawal from METH. Namely, the present study found that DAT is increased during a pharmacologically significant period of early METH withdrawal, which is in contrast to most studies that have found a decrease in DAT following more protracted periods of withdrawal. Indeed, DAT levels are decreased 7 days after withdrawal from contingent [4] and non-contingent [16] administration of METH. Our finding that DAT levels are increased only in rats that received extended access to METH IVSA suggests that the alterations in DAT are dependent on dose and/or access conditions.
The increases in striatal DAT levels observed in the present study during an early time point of withdrawal contrasts with the unaltered expression of TH and D2 receptors. This finding highlights DAT as a dynamic and responsive element in the neurobiological consequences that occur during early time periods of METH withdrawal. Future research is needed to systematically examine dynamic changes in the expression and functional profile of DAT in response to METH exposure and withdrawal from this drug.
Highlights.
Extended access to METH IVSA produces larger intake versus limited access to this drug
Extended access to METH IVSA up-regulates dopamine transporters
Extended access to METH IVSA does not alter TH or D2 levels
Acknowledgments
This research was supported by the National Institute on Drug Abuse Diversity-promoting Institutions Drug Abuse Research Program (R24-DA029989). NIDA also provided support for personnel working on this project (R01-DA021274 and R25-DA033613). This work was also supported by the National Institute of Minority Health Disparities (G12MD007592) as part of the UTEP Border Biomedical Research Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wilson JM, Kalasinsky KS, Levey AI, Bergeron C, Schmunk GA, Shannak K, Haycock JW, Kish SJ. Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat Med. 1996;2:699–703. doi: 10.1038/nm0696-699. [DOI] [PubMed] [Google Scholar]
- 2.Volkow ND, Chang L, Wang G, Fowler JS, Franceschi D, Sedler M, Gatley SJ, Miller E, Hitzemann R, Ding Y, Logan J. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci. 2001;21:9414–9418. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang GJ, Smith L, Volkow ND, Telang F, Logan J, Tomasi D, Wong CT, Hoffman W, Jayne M, Alia-Klein N, Thanos P, Fowler JS. Decreased dopamine activity predicts relapse in methamphetamine abusers. Mol Psychiatry. 2012;9:918–925. doi: 10.1038/mp.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krasnova IN, Justinova Z, Ladenheim B, Jayanthi S, McCoy MT, Warner JE, Goldberg SR, Cadet JL. Methamphetamine self-administration is associated with persistent biochemical alterations in striatal and cortical dopaminergic terminals in the rat. PLoS One. 2010;5:e8790. doi: 10.1371/journal.pone.0008790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laćan G, Hadamitzky M, Kuczenski R, Melega WP. Alterations in the striatal dopamine system during intravenous methamphetamine exposure: Effects of contingent and noncontingent administration. Synapse. 2013;67:476–488. doi: 10.1002/syn.21654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McFadden LM, Hadlock GC, Allen SC, Vieira-Brock PL, Stout KA, Ellis JD, Hoonakker AJ, Andrenvak DM, Nielsen SM, Wilkins DG, Hanson GR, Fleckenstein AE. Methamphetamine self-administration causes persistent striatal dopaminergic alterations and mitigates the deficits caused by subsequent methamphetamine exposures. J Pharmacol Exp Ther. 2012;340:295–303. doi: 10.1124/jpet.111.188433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwendt M, Rocha A, See RE, Pacchioni AM, McGinty JF, Kalivas PW. Extended methamphetamine self-administration in rats results in a selective reduction of dopamine transporter accompanied by marked monoaminergic depletion. J Pharmacol Exp Ther. 2009;331:555–562. doi: 10.1124/jpet.109.155770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cook C, Jeffcoat A, Hill J, Pugh D, Sadler B, White W, Perez-Reyes M. Pharmacokinetics of methamphetamine self-administered to human subjects by smoking S-(+)-methamphetamine hydrochloride. Drug Metab Dispos. 1993;21(4):717–723. [PubMed] [Google Scholar]
- 9.Caldwell J, Dring LG, Williams RT. Metabolism of [14C]methamphetamine in man, the guinea pig, and the rat. Biochem J. 1972;129:11–22. doi: 10.1042/bj1290011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGregor C, Srusurapanont M, Jittiwutikarn J, Laobhripatr S, Wongtan T, White JM. The nature, time course and severity of methamphetamine withdrawal. Addiction. 2005;100:132–1329. doi: 10.1111/j.1360-0443.2005.01160.x. [DOI] [PubMed] [Google Scholar]
- 11.Zorick T, Nestor L, Miotto K, Sugar C, Hellemann G, Scanion G, Rawson R, London ED. Withdrawal symptoms in abstinent methamphetamine-dependent subjects. Addiction. 2010;105(10):1809–1818. doi: 10.1111/j.1360-0443.2010.03066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jang CG, Whitfield T, Schulteis G, Koob GF, Wee S. A dysphoric-like state during early withdrawal from extended access to methamphetamine self-administration in rats. Psychopharmacology. 2013;225:753–63. doi: 10.1007/s00213-012-2864-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Dell LE, Koob GF. ‘Nicotine deprivation effect’ in rats with intermittent 23-hour access to intravenous nicotine self-administration. Pharmacol Biochem Beh. 2007;86:346–353. doi: 10.1016/j.pbb.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kitamura O, Wee S, Specio SE, Koob GF, Pulvirenti L. Escalation of methamphetamine self-administration in rats: A dose-effect function. Psychopharmacology. 2006;186:48–53. doi: 10.1007/s00213-006-0353-z. [DOI] [PubMed] [Google Scholar]
- 15.Shepard JD, Dohuang DT, Shaman Y, Morales M. Effect of methamphetamine self-administration on tyrosine hydroxylase and dopamine transporter levels in mesolimbic and nigrostriatal dopamine pathways of the rat. Psychopharmacology. 2006;185(4):505–513. doi: 10.1007/s00213-006-0316-4. [DOI] [PubMed] [Google Scholar]
- 16.German CL, Hanson GR, Fleckenstein AE. Amphetamine and Methamphetamine reduce striatal dopamine transporter function without concurrent dopamine transporter relocalization. J Neurochem. 2012;123(2):28297. doi: 10.1111/j.1471-4159.2012.07875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


