Abstract
Stressful life events and stress-related psychiatric disorders impair sustained attention, the ability to monitor rare and unpredictable stimulus events over prolonged periods of time. Despite the link between stress and attentional disruptions, the neurobiological basis for stress regulation of attention systems remains under explored. Here we examined whether corticotropin releasing factor (CRF), which orchestrates stress responses and is hypersecreted in patients with stress-related psychiatric disorders, impairs sustained attention. To this end, male and female rats received central infusions of CRF prior to testing on an operant sustained attention task (SAT), where rats were trained to discriminate signaled from non-signaled events. CRF caused a dose-dependent decrease in SAT performance in both male and female rats. Females were more impaired than males following a moderate dose of CRF, particularly during the middle part of the session. This sex difference was moderated by ovarian hormones. Females in the estrous cycle stage characterized by lower ovarian hormones had a greater CRF-induced attentional impairment than males and females in other cycle stages. Collectively, these studies highlight CRF as a critical stress-related factor that can regulate attentional performance. As sustained attention subserves other cognitive processes, these studies suggest that mitigating high levels of CRF in patients with stress-related psychiatric disorders may ameliorate their cognitive deficits.
Keywords: stress, corticotropin releasing hormone, cognition, sex difference, estrous cycle
Stressful life events can alter attention and disrupt vigilance [1]. Moreover, patients with stress-related psychiatric disorders, such as major depression and post-traumatic stress disorder (PTSD), have trouble sustaining attention [2, 3], or continuously monitoring situations for rare and unpredictably occurring events over prolonged periods of time. Sustained attention represents a fundamental component of attention that determines the effectiveness of other attentional processes, such as divided attention and selective attention, and cognitive performance in general [4]. Thus, stress-induced disruptions in sustained attention could impact many cognitive functions simultaneously, thereby contributing to a variety of cognitive changes observed during stress and in patients with stress-related psychiatric disorders. Despite the potentially devastating impacts of stress on sustained attention, how stress mediates this cognitive process is unknown.
One neuropeptide that orchestrates stress responses is corticotropin releasing factor (CRF). During a stressful event, CRF acts as a hormone to initiate the hypothalamic-pituitary-adrenal axis response to stress, as well as a neurotransmitter to centrally modulate brain circuits required for behavioral and cognitive responses to stress [5, 6]. Evidence for central CRF hypersecretion has been found in patients with depression and PTSD [7, 8], and this central elevation in CRF is thought to contribute to both the mood and cognitive disruptions that characterize these disorders [9, 10]. In rodent models, where the CRF system is easily manipulated, many mnemonic processes have been demonstrated to be regulated by CRF (for review see, [10]). However, how CRF specifically effects sustained attention has not been the focus of this previous work. The present study addressed this gap by determining whether central CRF disrupted sustained attention using a well-established sustained attention task (SAT) in which rats were trained to detect signaled from non-signaled trials [11, 12]. Both male and female rats were tested here because of previously reported increased neuronal sensitivity to CRF in females relative to males [13, 14].
Male and female adult (~60 days old) Sprague-Dawley rats (Charles River, Wilmington, MA) were housed individually with a 12h light/dark cycle (lights on at 9:00 am) and ad libitum access to food. After acclimation to the facility, rats were progressively water-restricted to 10-min/day for 7 days before beginning behavioral training (5–6 days/week). During nonperforming days, rats received 15 minutes access to water. The estrous cycle was tracked in female rats with vaginal cytology. All experimental procedures were approved by the Institutional Animal Care and Use Committee of Temple University and were conducted in accordance with the National Institute of Health guidelines.
Rats were trained on the operant SAT as described previously [11, 15, 16]. Briefly, after learning to lever press, rats were trained to discriminate between two levers: one paired to signaled events (light presentations) and one paired to non-signal events (no light). Following training sessions where rats had correction trials and the signal duration was 1s, rats were exposed to the basic SAT procedure where the duration of signal was reduced and varied (25ms, 50ms, and 500ms), the houselight was illuminated, the intertrial interval was 9 ± 3s, and no correction trials were allowed. Each session, which typical lasted 40 min, consisted of 162 trials was divided into three blocks of 54 trials that contained pseudorandomized, equal presentations of signaled (27) and nonsignaled trials (27). Following each event (2s), both levers were presented for 4 seconds, and if a rat failed to respond during this time, an omission was recorded. Other responses on signaled trials included, a correct response or “hit”, which was rewarded (0.02 mL of water) oran incorrect response or “miss”, which was not rewarded. On non-signaled trials, a correct response or “correct rejection” was rewarded (0.02 mL of water), while an incorrect response or “false alarm” was not rewarded. An additional important measure was the vigilance index, which takes into account hits and false alarms and is thought to reflect overall attentional performance, and is calculated using the following formula: vigilance index = (hits − false alarms)/[2(hits + false alarms) − (hits + false alarms)2]. [11]. Rats were defined as reaching baseline criteria after three consecutive days of 70% correct responding to the 500ms signal presentation events, 70% correct rejections, and <30% omissions.
After attaining criterion performance, aseptic stereotaxic surgery was performed to implant each rat with a cannula (22 gauge guide cannula, Plastics One, Roanoke, VA) aimed at the right lateral ventricle (A/P = −1.1mm and M/L = +1.5mm from Bregma, D/V = −4.4mm from the surface of the skull) as previously described [17]. Following recovery and reacquisition of baseline SAT criteria, rats received intracerebroventricular (i.c.v.) infusions of vehicle (artificial cerebral spinal fluid, aCSF) or one of three doses (0.1 μg, 0.5 μg, and 1.0 μg in vehicle) of ovine CRF (America Peptides, Vista, CA, USA) as previously described [17]. All infusions were made at a rate of (1 μL/min) and a total volume of 3 μL solution was infused. Ten minutes following the infusion, rats were tested for performance using the SAT procedure. As this study utilized a within subjects design, subsequent doses of CRF or vehicle were administered in a counterbalanced fashion with at least a one week wash out period, during which no infusions were given but SAT training continued. Criteria performance was met before each infusion.
Following administration of all doses and vehicle, rats were transcardially perfused, tissue was sectioned (30 μm coronal) and stained with cresyl violet, and cannula placements were verified as detailed previously [17]. One female rat was dropped from analysis due to a poor cannula placement.
First we confirmed that the dosing schedule did not affect behavioral outcomes by comparing dose, sex, and schedule for all performance variables with mixed factor ANOVAs. No significant interactions [F(9,24) <.45, p >.75] or main effect of schedules [F(3,8) <0.40, p>.76] were identified for the vigilance index or omissions (data not depicted). Thus, as expected, the counterbalancing was effective.
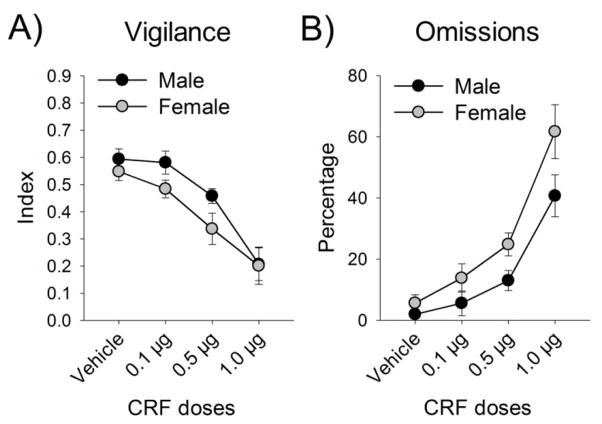
To determine whether CRF altered sustained attention, CRF dose response curves for the vigilance index and omissions were generated for male and female rats and analyzed with mixed factors ANOVAs. CRF dose-dependently decreased the vigilance index [F(3,39)=19.49, p<.001] (Fig. 1A), while it increased omissions [F(3,42)=24.42, p<.001] (Fig. 1B). Additionally, there was a main effect of sex with omissions, such that females omitted more trials than males [F(1,14)=8.14, p=.013] (Fig. 1D). To determine whether omissions increased in females more than males across the session, which could indicate that females were becoming satiated faster and thus less motivated, we conducted a sex × block analysis that revealed no sex differences in omissions over the session [F(2,28)=.914, p>.05] (data not depicted).
Figure 1.
CRF impaired SAT performance. In male and female rats, CRF administration caused a dose-dependent decrease in the vigilance index (A), while it increased omissions (B).
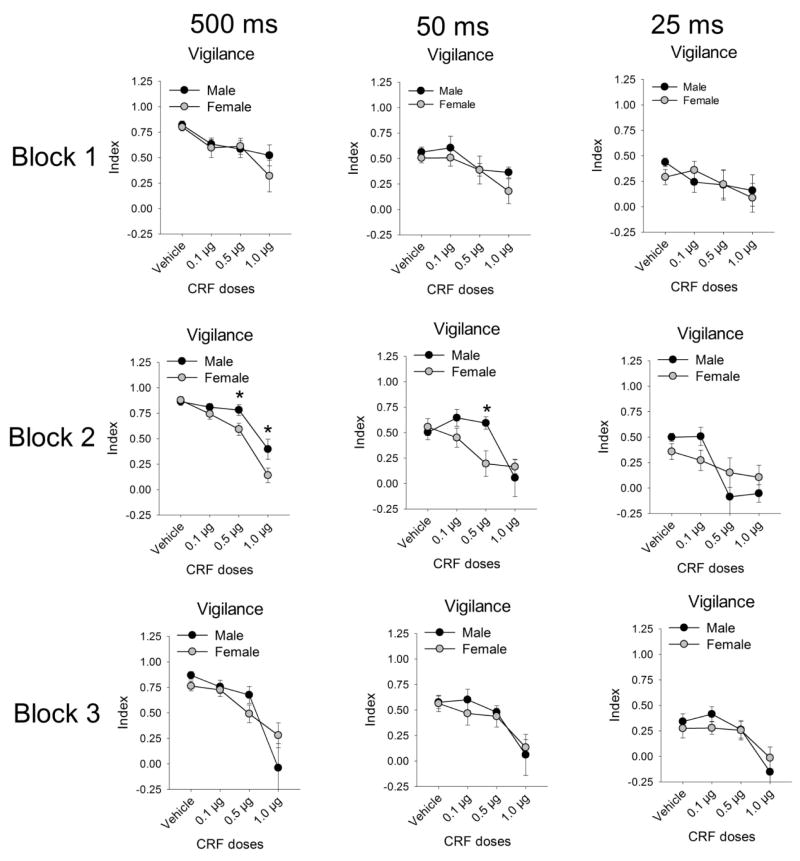
To further explore how CRF affected performance throughout the session and at different stimulus durations, we conducted a sex × block × stimulus duration × dose analysis on vigilance index, which revealed a significant interaction [F(12,168)=2.56, p=.004] (Fig. 2). Follow-up ANOVAs conducted at each block indicated that during block 2 there was a sex × dose × stimulus duration interaction [F(6,84)=2.56, p=.001]. Female rats had a lower vigilance index than males for the 0.5 μg dose to the 500ms (p=.039) and 50ms (p=.021) stimulus durations, and for the 1.0 μg dose at the 500ms stimulus duration (p=.049). There were no sex × dose × duration interactions for blocks 1 [F(6,84)=.546, p>.05] and 3 [F(6,84)=1.05, p>.05]. Next, we distinguished whether the vigilance index was impaired by CRF during block 2 in females due to sex differences in signaled (hits) or non-signaled trials (correct rejections). There were neither sex differences in hits during block 2 at the 0.5 μg dose to the 500ms [t(14)=0.22, p>.05] and 50ms [t(14)=0.94, p>.05] stimulus durations, nor for the 1.0 μg dose at the 500ms stimulus duration [t(14)=1.00, p>.05] (data not depicted). However, females made fewer correct rejections than males during the second block following administration of the 0.5 μg CRF dose [t(14)=5.88, p=.025] (data not depicted). It is noted that the dose-related decline in vigilance index observed following central administration of CRF paralleled a reduction in performance on signal trials (main effect of dose for hits: F(3,42)=4.78, p=.006). However, the sex × dose interaction for hit rate was not significant (p>0.05). Together these findings indicated that performance decrement on non-signaled trials contributed to greater attentional impairments in female rats.
Figure 2.
Analysis of the vigilance index by block (rows) and stimulus durations (columns). Sex differences emerged in the second block to the longer stimulus durations. Asterisks indicate a significant sex difference (p<.05).
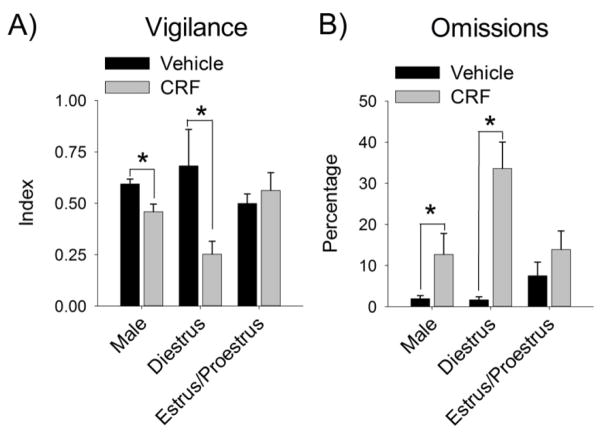
Although this study was not designed specifically to assess the effect of circulating ovarian hormones, a preliminary analysis gauged the contribution of these hormones by comparing females in the proestrus and estrus phases of the cycle (higher ovarian hormones) to females in the diestrus phase (lower ovarian hormones), and males, at both the vehicle and 0.5 μg dose, where most sex differences were observed. Because females were not necessarily in the same estrous cycle phase during both infusions, 3 (hormone) × 2 (dose) ANOVAs were conducted for performance measures. Significant interactions were found for both vigilance [F(2,26)=10.60, p<.001] (Fig. 3A) and omissions [F(2,26)=4.01, p=.030] (Fig. 3B). Post-hoc tests revealed that CRF decreased the vigilance index and increased omissions in males (p=.041, p=.051, respectively) and diestrus females (p<.001, p<.001, respectively), but not proestrus/estrus females (p=.220, p=.325, respectively).
Figure 3.
Lower levels of ovarian hormones increase female vulnerability to CRF-induced impairments in SAT performance. Males, females with low ovarian hormones (diestrus), and females with higher ovarian hormones (estrus/proestrus) were compared following central infusion of vehicle or 0.5 μg of CRF for the vigilance index (A) and omissions (B). Asterisks indicate a significant difference (p<.05).
The present study demonstrated that CRF disrupted sustained attention in both male and female rats, as indicated by the dose-dependent decrease in the vigilance index. CRF also increased the number of omissions in both sexes, and many trials were omitted following administration of the highest CRF dose. This suggests that, in addition to impairing aspects of attention, high doses of CRF may have decreased motivation to perform the task. Further analysis revealed sex differences in the vigilance index, such that females had a greater vigilance decrement than males to a moderate dose of CRF, especially during the middle part of the session. This sex difference in CRF regulation of the vigilance index was driven by a sex difference in responses to non-signaled trials. Moreover, it was moderated by ovarian hormones as females in the diestrus phase of the cycle had a larger CRF-induced impairment in sustained attention than females in other cycle stages and males. This suggests that low levels of ovarian hormones in females increase susceptibility to attentional disruptions induced by CRF.
These findings are consistent with previous studies demonstrating that central CRF administration can impair cognition in male rodents. For example, high levels of CRF release due to overexpression or central CRF infusion disrupts selective attention in males [18, 19]. Similarly, central CRF impairs cognitive flexibility in male rats [15]. Interestingly, because adequately sustaining attention is required for accurate performance on the aforementioned tasks, CRF-induced impairments in sustained attention could have contributed to these previously reported cognitive deficits. An important aspect of our design was the inclusion of females and the results demonstrate that the effects of CRF on attention are observable in females as well as males.
CRF could disrupt sustained attention via a number of different mechanisms. For example, CRF could directly regulate the basal forebrain corticopetal circuitry that is required for accurate SAT performance [4, 12, 20]. Direct regulation is possible because CRF1 receptors are found in the basal forebrain and are present on cholinergic and non-cholinergic neurons [21], which mediate responding on signaled trials and non-signaled trials (if GABAergic), respectively [22]. Additionally, there is a high density of CRF1 receptors in the prefrontal cortex [23], which is critically involved when attentional demands are high [20]. Alternatively, instead of direct effects, CRF could indirectly regulate sustained attention through afferent structures with CRF receptors [13, 23], such as the nucleus accumbens and locus coeruleus-norepinephrine system, which are thought to regulate motivation and arousal to impact performance, respectively [4]. Further studies using site specific CRF administration will be required to elucidate this circuitry.
The SAT has previously been used to test attention in both males and females, but these prior studies were conducted in males and females separately [12, 24]. This is the first time, to our knowledge, male and female rats were tested in the same study, allowing for direct comparisons between the sexes. We found a main effect of sex on omissions, such that females omitted more trials than males, regardless of dose. One explanation is that females were less motivated to engage in the task than males. However, in the vehicle and low CRF conditions, trials were rarely omitted. Moreover, omissions did not increase over time more in females than males, indicating that sex differences in satiety were not observed. Thus, the sex difference in omissions likely reflects sex differences in attentional rather than motivation factors.
Following CRF administration, a sex difference in the vigilance index, an overall measure of attention, was observed. Females, relative to male rats, had a greater attentional impairment in response to the moderate dose of CRF. This effect was most pronounced during the second trial block following the presentation of longer signal durations, perhaps because performance on the shortest signal duration was so poor that any further impairment induced by CRF in females was undetectable. Performance differences between male and female rats observed following a moderate CRF dose were attributable to impaired accuracy on non-signaled trials in females. This suggests that during moderately stressful conditions, females were more likely to claim a signal when a signal was not present and may be biased towards the detection of a visual stimulus. The significance of the sex difference emerging during the second block is not entirely clear. It could, however, be related to both the time course of CRF1 receptor signaling and the increased CRF1 receptor, Gs-mediated signaling observed in females compared to males [13]. The combination of these factors could have translated into increased CRF-induced signaling in females that had peak efficacy during the second trial block, resulting in a greater impairment in their vigilance index.
The sex differences observed in CRF regulation of sustained attention appear, at least in part, to be attributable to circulating ovarian hormones. Although a full analysis of hormones was beyond the scope of this initial study, a preliminary comparison of the effects of CRF in females in estrous cycle stages characterized by lower versus higher ovarian hormones levels revealed an interesting cycle effect. Specifically, a moderate dose of CRF impaired SAT performance in females with lower levels of ovarian hormones, but surprisingly had no effect when females had higher levels of ovarian hormones. Thus, higher hormone levels appear to protect females from the negative effect of CRF on attention. If supported by future studies, this could indicate that the effects of stress via CRF on cognition would change across the female lifespan as hormone levels change, and suggest that post-menopausal women may be particularly vulnerable to stress-induced cognitive deficits.
Clinically, patients with stress-related psychiatric disorders have difficulty sustaining attention and demonstrate other impairments in cognitive processes that rely adequately on attention such as working memory, long-term memory, and executive functions [2, 3]. Although preclinical, our data suggest that CRF is a critical factor that can contribute to these cognitive impairments. These findings may have implications for treatment because they suggest that CRF receptor antagonists, which are being developed to treat the affective symptoms, could also mitigate cognitive symptoms of stress-related psychiatric disorders [3]. If supported by future studies, this would be a great advance as commonly prescribed pharmacotherapies currently do not alleviate cognitive disruptions, even when affective symptoms are treated [25].
Hihghlights.
Corticotropin releasing factor (CRF) impairs sustained attention in rats.
Females with lower levels of ovarian hormones were more vulnerable to this effect.
CRF may contribute to cognitive deficits in stress-related psychiatric disorders.
Acknowledgments
We would like to thank Gerald Van Buskirk and Alexa Fritz for their assistance with the behavior. This work was supported by MH092438 to D.A.B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hancock PA, Warm JS. A dynamic model of stress and sustained attention. Human factors. 1989;31:519–37. doi: 10.1177/001872088903100503. [DOI] [PubMed] [Google Scholar]
- 2.Paelecke-Habermann Y, Pohl J, Leplow B. Attention and executive functions in remitted major depression patients. J Affect Disorders. 89:125–35. doi: 10.1016/j.jad.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Vasterling JJ, Brailey K, Constans JI, Sutker PB. Attention and memory dysfunction in posttraumatic stress disorder. Neuropsychology. 1998;12:125–33. doi: 10.1037//0894-4105.12.1.125. [DOI] [PubMed] [Google Scholar]
- 4.Sarter M, Givens B, Bruno JP. The cognitive neuroscience of sustained attention: where top-down meets bottom-up. Brain Res Brain Res Rev. 2001;35:146–60. doi: 10.1016/s0165-0173(01)00044-3. [DOI] [PubMed] [Google Scholar]
- 5.Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–57. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- 6.Van Bockstaele EJ, Peoples J, Valentino RJ. A.E. Bennett Research Award. Anatomic basis for differential regulation of the rostrolateral peri-locus coeruleus region by limbic afferents. Biol Psychiatry. 1999;46:1352–63. doi: 10.1016/s0006-3223(99)00213-9. [DOI] [PubMed] [Google Scholar]
- 7.Banki CM, Karmacsi L, Bissette G, Nemeroff CB. CSF corticotropin-releasing hormone and somatostatin in major depression: response to antidepressant treatment and relapse. European neuropsychopharmacology: the journal of the European College of Neuropsychopharmacology. 1992;2:107–13. doi: 10.1016/0924-977x(92)90019-5. [DOI] [PubMed] [Google Scholar]
- 8.Bremner JD, Licinio J, Darnell A, Krystal JH, Owens MJ, Southwick SM, et al. Elevated CSF corticotropin-releasing factor concentrations in posttraumatic stress disorder. Am J Psychiatry. 1997;154:624–9. doi: 10.1176/ajp.154.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gold PW, Chrousos GP. Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol Psychiatry. 2002;7:254–75. doi: 10.1038/sj.mp.4001032. [DOI] [PubMed] [Google Scholar]
- 10.Bangasser DA, Kawasumi Y. Cognitive disruptions in stress-related psychiatric disorders: A role for corticotropin releasing factor (CRF) Horm Behav. 2015 doi: 10.1016/j.yhbeh.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demeter E, Sarter M, Lustig C. Rats and humans paying attention: cross-species task development for translational research. Neuropsychology. 2008;22:787–99. doi: 10.1037/a0013712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGaughy J, Kaiser T, Sarter M. Behavioral vigilance following infusions of 192 IgG-saporin into the basal forebrain: selectivity of the behavioral impairment and relation to cortical AChE-positive fiber density. Behav Neurosci. 1996;110:247–65. doi: 10.1037//0735-7044.110.2.247. [DOI] [PubMed] [Google Scholar]
- 13.Bangasser DA, Curtis A, Reyes BA, Bethea TT, Parastatidis I, Ischiropoulos H, et al. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol Psychiatry. 2010;15:877, 96–904. doi: 10.1038/mp.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bangasser DA, Reyes BA, Piel D, Garachh V, Zhang XY, Plona ZM, et al. Increased vulnerability of the brain norepinephrine system of females to corticotropin-releasing factor overexpression. Mol Psychiatry. 2013;18:166–73. doi: 10.1038/mp.2012.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Snyder K, Wang WW, Han R, McFadden K, Valentino RJ. Corticotropin-releasing factor in the norepinephrine nucleus, locus coeruleus, facilitates behavioral flexibility. Neuropsychopharmacology. 2012;37:520–30. doi: 10.1038/npp.2011.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parikh V, Howe WM, Welchko RM, Naughton SX, D’Amore DE, Han DH, et al. Diminished trkA receptor signaling reveals cholinergic-attentional vulnerability of aging. Eur J Neurosci. 2013;37:278–93. doi: 10.1111/ejn.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bangasser DA, Lee CS, Cook PA, Gee JC, Bhatnagar S, Valentino RJ. Manganese-enhanced magnetic resonance imaging (MEMRI) reveals brain circuitry involved in responding to an acute novel stress in rats with a history of repeated social stress. Physiol Behav. 2013 doi: 10.1016/j.physbeh.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van’t Veer A, Yano JM, Carroll FI, Cohen BM, Carlezon WA., Jr Corticotropin-releasing factor (CRF)-induced disruption of attention in rats is blocked by the kappa-opioid receptor antagonist JDTic. Neuropsychopharmacology. 2012;37:2809–16. doi: 10.1038/npp.2012.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Gaalen MM, Stenzel-Poore M, Holsboer F, Steckler T. Reduced attention in mice over producing corticotropin-releasing hormone. Behavioural Brain Research. 2003;142:69–79. doi: 10.1016/s0166-4328(02)00381-9. [DOI] [PubMed] [Google Scholar]
- 20.Gill TM, Sarter M, Givens B. Sustained visual attention performance-associated prefrontal neuronal activity: evidence for cholinergic modulation. J Neurosci. 2000;20:4745–57. doi: 10.1523/JNEUROSCI.20-12-04745.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sauvage M, Steckler T. Detection of corticotropin-releasing hormone receptor 1 immunoreactivity in cholinergic, dopaminergic and noradrenergic neurons of the murine basal forebrain and brainstem nuclei--potential implication for arousal and attention. Neuroscience. 2001;104:643–52. doi: 10.1016/s0306-4522(01)00137-3. [DOI] [PubMed] [Google Scholar]
- 22.Burk JA, Sarter M. Dissociation between the attentional functions mediated via basal forebrain cholinergic and GABAergic neurons. Neuroscience. 2001;105:899–909. doi: 10.1016/s0306-4522(01)00233-0. [DOI] [PubMed] [Google Scholar]
- 23.Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, et al. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 24.McGaughy J, Sarter M. Effects of ovariectomy, 192 IgG-saporin-induced cortical cholinergic deafferentation, and administration of estradiol on sustained attention performance in rats. Behav Neurosci. 1999;113:1216–32. doi: 10.1037//0735-7044.113.6.1216. [DOI] [PubMed] [Google Scholar]
- 25.Popovic D, Vieta E, Fornaro M, Perugi G. Cognitive tolerability following successful long term treatment of major depression and anxiety disorders with SSRi antidepressants. J Affect Disord. 2015;173:211–5. doi: 10.1016/j.jad.2014.11.008. [DOI] [PubMed] [Google Scholar]