Abstract
OBJECTIVES
Current guidelines suggest that arteriovenous fistula (AVF) is associated with survival advantage over arteriovenous grafts (AVG). However, AVF often require months to become functional, increasing tunneled dialysis catheter (TDC) use, which can erode the benefit of AVF. We sought to compare survival in ESRD patients after creation of AVF or AVG in patients starting hemodialysis (HD) with TDC and identify patient populations that may benefit from preferential use of AVG over AVF.
METHODS
Using U.S. Renal Data System (USRDS) databases, we identified incident HD patients in 2005 through 2008 and followed them through 2008. Initial access type and clinical variables including albumin levels were assessed using USRDS data collection forms. Attempts at AVF and AVG creation in patients who started HD through TDC were identified by CPT codes. We accounted for the effect of changes in access type by truncating follow-up when additional AVF or AVG were performed. Survival curves were then constructed, and log-rank tests used for pairwise survival comparisons, stratified by age. Multivariate analysis was performed with Cox proportional hazards regressions; variables were chosen using stepwise elimination. An interaction of access type and albumin level was detected, and Cox models using differing thresholds for albumin level were constructed. The primary outcome was survival.
RESULTS
Among the 138,245 patients who started with TDC and had complete records amenable for analysis, 22.8% underwent AVF creation (Mean age±SD: 68.9±12.5 years, 27.8% mortality at 1 year) and 7.6% underwent AVG placement (70.2±12.0 years, 28.2% mortality) within 3 months of HD initiation; 69.6% remained with TDC (63.2±15.4 years, 33.8% mortality). In adjusted Cox proportional hazards regression, AVF creation is equivalent to AVG placement in terms of survival (HR 0.98, 95% CI 0.93–1.02; P=.349). AVG placement is superior to continued TDC use (HR 1.54, 95% CI 1.48–1.61; P<.001). In patients over age 80 with albumin levels greater than 4.0 g/dL, AVF creation is associated with higher mortality hazard compared to AVG creation (HR1.22, 95% CI 1.04–1.43, P=.013).
CONCLUSIONS
For patients that start HD through TDC, placement of AVF and AVG are associated with similar mortality hazard. Further study is necessary in order to determine the ideal access for patients in whom the survival advantage of AVF over AVG is uncertain.
INTRODUCTION
End-stage renal disease (ESRD) requiring chronic dialysis affects approximately 400,000 patients in the United States with 100,000 new patients starting dialysis annually. Historical analyses suggest that survival with AVF is improved compared to AVG and TDC (1,2,3,4). These provide part of the foundation for current guidelines from the National Kidney Foundation Kidney Disease Outcomes Quality Initiatives (NKF-KDOQI) and the Society for Vascular Surgery (SVS), which prioritize AVF as the ideal long-term vascular access; AVG is the next preferred vascular access choice, followed by TDC (5,6).
However, AVF frequently fail to mature adequately and often require additional interventions to become functional. This commonly leads to the temporary use of a TDC during this “bridge” period, exposing the patient to the risk of catheter related complications, including mortality (7). In patients that are receiving HD through a catheter and have failed a forearm AVF, subsequent upper arm AVF are associated with a higher primary failure rate, more interventions to achieve maturation, longer catheter dependence, and more frequent catheter-related bacteremia compared to AVG (8). Currently, 82% of US patients initiate HD patients via a catheter, despite the “Fistula-First” campaign that actively encourages AVF over AVG or TDC (9).
Prior literature that describes the benefit of AVF over AVG and TDC is based on the access first used as opposed to access first placed. Since patients with initial AVF failure are not recognized as AVF patients and are frequently represented in the TDC group, the possibility for selection bias is present and has been acknowledged in a systematic review (10). Further, patients whose AVF fail to mature likely have comorbidities that predispose to both AVF non-maturation and mortality, possibly overstating the benefit of the decision to create an AVF (8,11).
In this study of ESRD patients who receive HD, we focused on patients that start HD with TDC, since that represents the vast majority of incident HD patients, and it begins to address the selection bias described above. We sought to test the hypothesis that creation of AVF after starting HD through TDC is associated with improved survival compared to creation of AVG or remaining with a TDC.
METHODS
The United States Renal Data System collects data from multiple sources, including Medicare and Medicaid, for over 90% of all patients with ESRD in the United States (12). This is an integrated and consistent resource for investigating health outcomes for patients with End-Stage Renal Disease (ESRD). Physician services are encoded using CPT codes, which are accompanied by ICD-9-CM codes. As applied to this analysis, the USRDS defines date of first service as the earlier of the first service date entered on CMS-2728 or the first Medicare claim for dialysis (13).
A local IRB waiver from the University of Pittsburgh was obtained in addition to executing a Data Use Agreement with the US Renal Data System Coordinating Center before commencing data analysis. Explicit patient consent was not obtained, pursuant to CMS rules allowing for release of limited data sets with a Data Use Agreement executed with the USRDS Coordinating Center (14).
We identified incident HD patients in 2005, 2006, 2007, and 2008. We then followed them through the end of 2008 and determined physician services usage and survival. Initial access type was assessed using the End Stage Renal Disease Medical Evidence Report (CMS-2728). This form asks whether an AVF, AVG or catheter was used on first outpatient dialysis. If a catheter is used, it asks if a maturing AVF or AVG is present. This form also provided demographic and clinical information that were used as independent variables in regression modeling.
Data analysis
We removed patients from the analysis whose billing records were not complete in addition to patients who later underwent transplantation, as this patient population may have deferred creation of an AV access. Finally, we removed patients who recovered renal function or who had an uncertain dialysis modality.
In the remaining population, incident HD patients were initially divided into groups based on the values from item 18d from CMS-2728: patients who initiated outpatient HD through AVF, those who initiated HD through AVG, and those that initiated HD through a TDC. We retained patients who started HD with TDC. Patients who had a maturing AVF or AVG, but still started HD through TDC, were excluded, as transition to AVF or AVG could not be captured and risked access misclassification. Patients who did not have valid values for this data item were dropped from the analysis. We retained patients who survived at least 90 days after initiation of HD, consistent with other USRDS survival analyses (13,15). Demographic data were analyzed using chi-square statistics for dichotomous variables and the Kruskal-Wallis equality-of-populations rank test for continuous variables.
We then compared survival after the decision to place an AVF or AVG. In order to do this, we constructed a population that could minimize the selection bias inherent in comparing patients who have successfully matured an AVF or AVG, since these patients have better access to specialists, and are less likely suffer from heart failure or peripheral arterial disease (16, 17). This patient population is clinically relevant, since the majority of patients start HD with a catheter in place.
Patients were divided into groups based on whether an attempt at AVF or AVG was undertaken within 3 months after initiation of HD. Patients without CPT records of AVF or AVG creation were assumed to have remained with a TDC. The risk of immortal time bias was reduced since we included only patients with at least 90 days of survival after initiation of HD (18, 19). Patients who underwent both AVF and AVG creation were dropped from the analysis. CPT codes used to identify the creation of AVF or AVG are noted in Table I. The primary outcome was survival.
Table I.
CPT codes to determine AVF and AVG creation
| AVF | |
|---|---|
| CPT code | Description |
| 36818 | Arteriovenous anastomosis, open; by upper arm cephalic vein transposition |
| 36819 | Arteriovenous anastomosis, open; by upper arm basilic vein transposition |
| 36820 | Arteriovenous anastomosis, open; by forearm vein transposition |
| 36821 | Arteriovenous anastomosis, open; direct, any site (eg, Cimino type) (separate procedure) |
| 36825 | Creation of arteriovenous fistula by other than direct arteriovenous anastomosis (separate procedure); autogenous graft |
| AVG | |
|---|---|
| CPT code | Description |
| 36830 | Creation of arteriovenous fistula by other than direct arteriovenous anastomosis (separate procedure); nonautogenous graft (eg, biological collagen, thermoplastic graft) |
We followed the patients until they died, the end of 2008, or because a new AVF or AVG was placed. TDC patients were censored when a new AVF or AVG was placed. We then performed preliminary univariate analyses of the survival curves using a log-rank test to compare in pairwise fashion HD patients who underwent AVF creation, AVG placement, and remained with TDC during the first 3 months after HD initiation, stratified by age. Multivariate Cox regressions were then performed, utilizing the demographic variables available in the CMS-2728 form. Because the effect of access was different across the age groups in the preliminary univariate analysis, multivariate Cox proportional-hazards models were created for different age categories. Tests for interaction with age suggested the presence of a significant interaction of age and pre-dialysis serum albumin levels with access surgery. To explore this, Cox proportional hazards models were constructed for different threshold albumin values for patients younger and older than age 80 years. Final models for different age groups were specified using backwards stepwise removal of variables.
Significance for statistical analyses was P<.05. Statistical analyses were performed with Stata/MP 13.1 (StataCorp LP, College Station, TX).
RESULTS
Patient flow
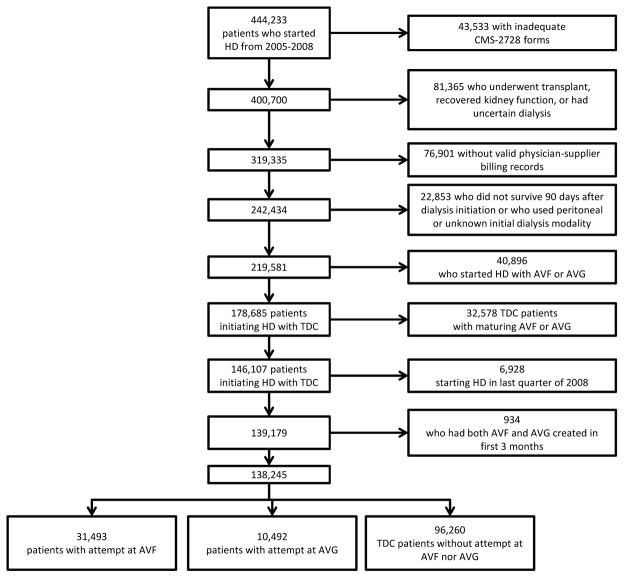
A total of 107,824 patients started HD in 2005, 111,629 in 2006, 111,622 in 2007, and 113,158 in 2008, for a total of 444,233 patients. After excluding patients without complete records, those who recovered renal function or received transplant, 219,581 patients were available (Figure 1). TDC was the initial access for 178,685 (81.4%) of the patients, 30,927 (14.1%) patients started with AVF and 9,969 (4.5%) started with AVG.
Figure 1.
Patient flow
Analysis based on attempted access type after initiation of HD through TDC
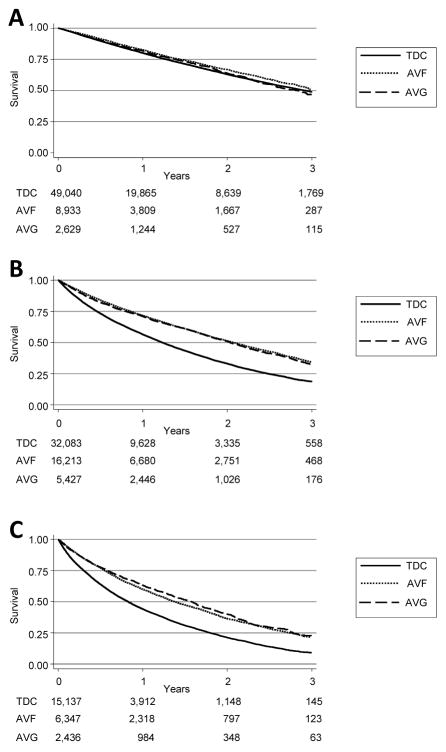
Our analysis focused on patients who started HD with TDC. A total of 138,245 patients were available for analysis (Figure 1). In this group, 31,493 (22.8%) underwent AVF creation and 10,492 (7.6%) underwent AVG creation within 3 months of starting HD. Another 96,260 (69.6%) did not have any CPT codes associated with AVF or AVG creation and were assumed to have remained with a TDC. The demographics for the groups are presented in Table II. Nearly all the studied variables demonstrated statistically significantly differences due to the large sample sizes. TDC patients were on average younger than AVF and AVG patients (Mean age ± standard deviation: AVF 68.9±12.5 years, AVG 70.2±12.0 years, TDC 63.2±15.4 years, P<.001). AVG creation was associated with patients who were more likely to be older, female, and non-Caucasian. TDC patients were less likely to be eligible for Medicare. Pairwise comparisons using log-rank tests suggest that both AVF creation and AVG placement are superior to TDC (P<.001), while AVF creation is superior to AVG placement (P=.006). Due to the difference in average ages between the three groups (AVF, AVG, TDC), we repeated pairwise comparisons after stratifying by age. In those under age 65, AVF is superior to AVG (P=.031), but this is not evident in the elderly (65–79 years, P=.089; 80 years and older, P=.119). AVG and TDC appear equivalent in patients under 65 (P=.744), but AVG was associated with improved survival in the elderly (65–79 years, and 80 years and older, both P<.001). After the 90 day mortality exclusion period, overall survival was short (Figure 2). For patients under 65 years, median survival was: AVF 3.02 years, AVG 2.84 years, TDC 2.93 years. For patients between 65 and 80 years, median survival was: AVF 2.08 years, AVG 2.03 years, and TDC 1.23 years. For patients older than 80 years, median survival was: AVF 1.38 years, AVG 1.58 years, TDC 0.83 years.
Table II.
Demographics of patients that initiated HD with TDC, based on attempted access creation within 3 months of starting HD.
| Characteristic | AVF | AVG | TDC | Total | P-value |
|---|---|---|---|---|---|
| N | 31,493 | 10,492 | 96,260 | 138,245 | |
| Average Age at dialysis initiation (years, SD) | 68.9 (12.5) | 70.2 (12.0) | 63.2 (15.4) | 65.1 (14.8) | <.001 |
| Age distribution | |||||
| Under 65 years | 8,933 (28.4%) | 2,629 (25.1%) | 49,040 (51.0%) | 60,602 (43.8%) | <.001 |
| 65–79 years | 16,213 (51.5%) | 5,427 (51.7%) | 32,083 (33.3%) | 53,723 (38.9%) | |
| 80+ years | 6,347 (20.1%) | 2,436 (23.2%) | 15,137 (15.7%) | 23,920 (17.3%) | |
| Caucasian race | 69.9% | 58.7% | 63.0% | 64.2% | <.001 |
| Male gender | 58.3% | 42.6% | 53.3% | 53.7% | <.001 |
| Hispanic ethnicity | 11.8% | 11.8% | 13.8% | 13.2% | <.001 |
| Body mass index (mean kg/m2) | 28.58 | 28.91 | 28.61 | 28.62 | .001 |
| Cause of ESRD | |||||
| Diabetes | 45.9% | 47.5% | 41.8% | 43.2% | <.001 |
| Hypertension | 28.2% | 29.9% | 27.4% | 27.8% | <.001 |
| Medical Comorbididites | |||||
| Amputation | 3.8% | 3.9% | 3.7% | 3.7% | .578 |
| Cancer | 8.3% | 8.3% | 7.5% | 7.7% | <.001 |
| Cardiovascular disease | |||||
| Atherosclerotic heart disease | 27.9% | 27.5% | 21.7% | 23.6% | <.001 |
| Congestive heart failure | 41.2% | 42.1% | 37.1% | 38.4% | <.001 |
| Peripheral vascular disease | 18.6% | 18.8% | 15.1% | 16.1% | <.001 |
| Other cardiac diseases | 19.3% | 18.3% | 17.1% | 17.7% | <.001 |
| Chronic obstructive pulmonary disease | 11.9% | 11.3% | 10.0% | 10.5% | <.001 |
| Diabetes, Insulin dependent | 36.8% | 39.1% | 36.4% | 36.7% | <.001 |
| Hypertension | 85.9% | 86.4% | 83.7% | 84.4% | <.001 |
| TIA/Stroke | 11.7% | 13.1% | 10.6% | 11.0% | <.001 |
| Tobacco smoker, current | 6.1% | 5.1% | 6.6% | 6.4% | <.001 |
| Other demographics | |||||
| Entitled to Federal Medicare benefits | 89.4% | 91.1% | 54.6% | 65.3% | <.001 |
| Inability to ambulate | 6.4% | 9.0% | 8.9% | 8.3% | <.001 |
| Inability to transfer | 2.6% | 3.8% | 4.6% | 4.1% | <.001 |
| Living in an assisted care home or other institution | 7.3% | 9.8% | 10.2% | 9.5% | <.001 |
| Medically unfit for transplant surgery | 10.2% | 12.1% | 10.9% | 10.9% | <.001 |
| Nephrologist care prior to HD initiation | 62.4% | 62.9% | 51.5% | 54.9% | <.001 |
| Dietitian care prior to HD initiation | 8.2% | 8.4% | 7.5% | 7.7% | <.001 |
| Serum Albumin level (mean g/dL) | 3.08 | 3.06 | 2.97 | 3.00 | <.001 |
| Hemoglobin A1c (mean) | 6.63 | 6.56 | 6.64 | 6.63 | .229 |
| Use of erythropoietin pre-dialysis | 33.2% | 35.7% | 25.7% | 28.2% | <.001 |
Figure 2.
Kaplan-Meier survival curves, stratified by age groups, comparing attempts at access creation among patients that started with TDC. N=138,245. (A) Under 65 years. (B) 65–79 years. (C) 80+ years. Time-to-event does not include initial 90 day mortality exclusion period. Standard error is less than 10% at all time points. Number at risk denoted beneath each Kaplan-Meier survival curve.
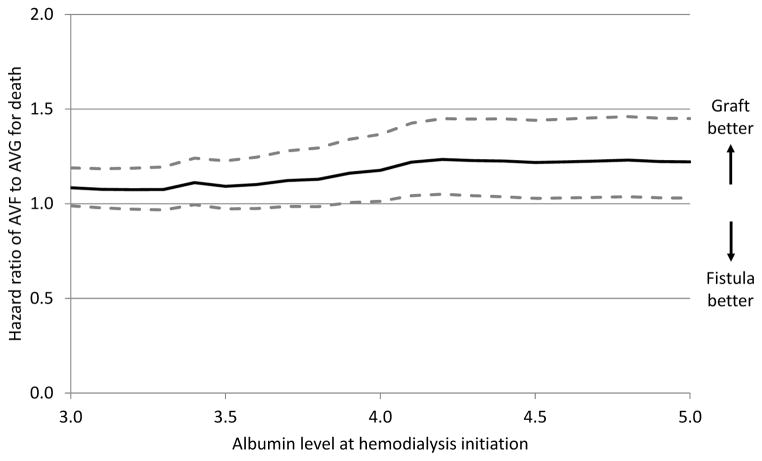
Multivariate Cox regressions adjusting for available covariates demonstrated that AVG placement is superior to remaining with TDC for patients of all ages. AVF has similar hazard as AVG for all age groups (Table III). Other significant modifiable predictors of mortality that were present in the models for all age groups included decreased albumin, lower body mass index (BMI), and the absence of a nephrologist caring for the patient pre-dialysis (all P<.001) (Table IV). Results of age-stratified models are presented in the Supplemental Tables I through III. Attempts at AV access creation were found to interact with albumin and age. Cox models for differing albumin threshold levels were created, stratified on age. In older patients who were nutritionally replete, AVG placement appeared to have lower hazard compared to AVF creation (Figure 3). In patients over age 80 with albumin levels greater than 4.0 g/dL, AVF creation is associated with higher mortality hazard compared to AVG creation (HR1.22, 95% CI 1.04–1.43, P=.013).
Table III.
Results of Cox Proportional-Hazards regression for mortality based on attempted access creation within 3 months of starting HD. Separate regressions were modeled for each age group and adjusted for other independent variables. Reference is AVG creation. HR: Hazard ratio, CI: Confidence Interval.
| AVF
|
TDC
|
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Unstratified | 0.978 | 0.933 –1.025 | .349 | 1.541 | 1.475 – 1.609 | <.001 |
|
| ||||||
| Stratified by Age | ||||||
|
| ||||||
| Less than 65 years | 0.940 | 0.844 –1.048 | .264 | 1.492 | 1.351 – 1.649 | <.001 |
| 65–79 years | 0.948 | 0.889 –1.010 | .101 | 1.529 | 1.441 – 1.624 | <.001 |
| 80 years and older | 1.021 | 0.941 –1.109 | .616 | 1.545 | 1.434 – 1.665 | <.001 |
Table IV.
Results of Cox Proportional-Hazards regression for mortality among patients starting HD with TDC.
| Variable | Hazard ratio | 95% Confidence interval | P value |
|---|---|---|---|
| Vascular access (placement of AVG is reference) | |||
| Creation of AVF | 0.978 | 0.933 – 1.025 | .349 |
| Continue with TDC | 1.541 | 1.475 – 1.609 | <0.001 |
| Increase in age (per year) | 1.024 | 1.022 – 1.025 | <0.001 |
| Caucasian race | 1.258 | 1.224 – 1.294 | <0.001 |
| Male gender | 1.017 | 0.993 – 1.041 | .170 |
| Hispanic ethnicity | 0.759 | 0.729 – 0.791 | <0.001 |
| Increase in Body Mass Index (per unit kg/m2) | 0.987 | 0.986 – 0.989 | <0.001 |
| Cause of ESRD | |||
| Diabetes | 0.924 | 0.897 – 0.953 | <0.001 |
| Hypertension | 0.943 | 0.914 – 0.972 | <0.001 |
| Medical Comorbidities | |||
| Amputation | 1.107 | 1.044 – 1.174 | .001 |
| Cancer | 1.281 | 1.235 – 1.328 | <0.001 |
| Cardiovascular disease | |||
| Congestive heart failure | 1.225 | 1.196 – 1.255 | <0.001 |
| Peripheral vascular disease | 1.086 | 1.054 – 1.119 | <0.001 |
| Other cardiac disease | 1.105 | 1.074 – 1.137 | <0.001 |
| Chronic obstructive pulmonary disease | 1.170 | 1.131 – 1.210 | <0.001 |
| Diabetes, Insulin dependent | 1.095 | 1.065 – 1.126 | <0.001 |
| Hypertension | 0.830 | 0.805 – 0.856 | <0.001 |
| TIA or stroke | 1.050 | 1.015 – 1.086 | .005 |
| Tobacco smoker, current | 1.093 | 1.042 – 1.147 | <0.001 |
| Other demographics | |||
| Entitled to Federal Medicare benefits | 1.612 | 1.559 – 1.667 | <0.001 |
| Inability to ambulate | 1.187 | 1.133 – 1.243 | <0.001 |
| Inability to transfer | 1.186 | 1.116 – 1.260 | <0.001 |
| Living in an assisted care home or other institution | 1.238 | 1.191 – 1.287 | <0.001 |
| Medically unfit for transplant | 1.214 | 1.177 – 1.253 | <0.001 |
| Serum albumin level (per g/dL) | 0.842 | 0.827 – 0.857 | <0.001 |
| Dietitian care prior to HD initiation | 0.968 | 0.927 – 1.011 | <0.001 |
| Nephrologist care prior to HD initiation | 0.905 | 0.884 – 0.928 | .139 |
Figure 3.
Hazard ratio of mortality after placement of AVF as compared to AVG in patients starting HD with TDC, as a function of pre-dialysis albumin level in patients over age 80. Hazard ratios estimated from proportional-hazards models with adjustment for available covariates. Dashed line indicate 95% confidence intervals.
DISCUSSION
Our study has several findings of interest. First, contrary to guidelines, creation of an AVF and placement of an AVG within 3 months of starting HD are associated with similar life expectancy after adjusting for available covariates and also recognizing the importance of adjustment for possible changes in access type. Creation of an internal access (AVF or AVG) is clearly associated with better survival than staying with a TDC regardless of patient age.
Our findings parallel a different study that sought to determine the impact of the decision to perform AVF or AVG surgery, which found that among elderly patients, AVF placement pre-dialysis initiation is not superior to AVG placement in terms of mortality (20). Other non-modifiable predictors of mortality that have been described in previous studies of ESRD patients include non-white race, being institutionalized, and history of stroke, CHF, peripheral arterial disease, and amputation; modifiable predictors of mortality include absence of a nephrologist prior to HD initiation and lower albumin levels (20, 21).
A novel contribution of our effort is the recognition that there may be a subset of the population where AVG placement is superior to AVF creation. Our analysis suggests that patients over age 80 who start HD through a TDC and are nutritionally replete appear to have lower mortality after AVG placement versus AVF creation; 4.7% of the population in our data set meet these criteria. While a biologically plausible mechanism for this finding is not clear, KDOQI guidelines suggest that increased serum albumin concentrations are associated with improved long-term survival, leading to a recommended goal albumin greater than 4.0 g/dL (22). Others have also commented recently on the possible benefit of AVG in select populations especially if vein quality or other anatomic challenges to successful maturation exist (23, 24).
Our study has strengths and weaknesses that can affect its interpretation. First, we were able to use the USRDS database, which is nearly comprehensive in its review of ESRD patients in the United States. Second, through the CMS-2728, we were able to use data collected at the time of outpatient HD initiation, including type of access and selected clinical variables. Third, we were able to devise a methodology to account for possible changes in dialysis access type.
At the same time our study must be interpreted with caution for multiple reasons. This analysis is a retrospective review of administrative data, and is limited by the variables available to us. Clinically important variables like anatomy and the quality and diameter of the vein conduit could not be included in our models. Also, our analysis of patients who underwent AVF or AVG creation is limited by our inability to determine if the surgery ultimately led to the creation of a usable access. While survival is a critical endpoint, other important endpoints including functional patency, infection, and reinterventions were not captured. Finally, many patients did not have complete records and were not included in the analysis, and despite our efforts to control for selection bias in our analysis, bias may still remain, potentially confounding our results. Even with these limitations, this analysis provides intriguing direction for future research.
CONCLUSION
AVG placement is equivalent to AVF creation and is superior to leaving a TDC in place in terms of survival in patients that start HD through TDC in this retrospective review of administrative data. These results suggest the need for a randomized controlled trial comparing fistulas and grafts in the subpopulation of dialysis patients that start HD with TDC, with the goal of identifying the ideal access for patients in whom the survival advantage of AVF over AVG is uncertain.
Supplementary Material
Acknowledgments
The authors wish to thank Larry Fish, PhD, for invaluable statistical guidance and review, and Steve Weisbord, MD MSc, who reviewed the manuscript. This project was supported by the National Institutes of Health through grant number KL2TR000146. Disclaimer: The data reported here have been supplied by the United States Renal Data System (USRDS). The opinions expressed are those of the authors and do not necessarily represent the views or policy positions of the National Institute of Diabetes and Digestive and Kidney Diseases nor the Centers for Medicare and Medicaid Services.
Footnotes
A preliminary version of this manuscript was presented at the Eastern Vascular Surgery Society 2013, Greenbriar, WV
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dhingra RK, Young EW, Hulbert-Shearon TE, Leavey SF, Port FK. Type of vascular access and mortality in U.S. hemodialysis patients. Kidney Int. 2001 Oct;60(4):1443–51. doi: 10.1046/j.1523-1755.2001.00947.x. [DOI] [PubMed] [Google Scholar]
- 2.Woods JD, Port FK. The impact of vascular access for haemodialysis on patient morbidity and mortality. Nephrol Dial Transplant. 1997;12:657–659. doi: 10.1093/ndt/12.4.657. [DOI] [PubMed] [Google Scholar]
- 3.Polkinghorne KR, McDonald SP, Atkins RC, Kerr PG. Vascular access and all-cause mortality: a propensity score analysis. J Am Soc Nephrol. 2004 Feb;15(2):477–86. doi: 10.1097/01.asn.0000109668.05157.05. [DOI] [PubMed] [Google Scholar]
- 4.Xue JL, Dahl D, Ebben JP, Collins AJ. The association of initial hemodialysis access type with mortality outcomes in elderly Medicare ESRD patients. Am J Kidney Dis. 2003;42:1013–1019. doi: 10.1016/j.ajkd.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 5.National Kidney Foundation. KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for 2006 Updates: Hemodialysis Adequacy, Peritoneal Dialysis Adequacy and Vascular Access. Am J Kidney Dis. 2006;48(suppl 1):S1–S322. [Google Scholar]
- 6.Sidawy AN, Spergel LM, Besarab A, Allon M, Jennings WC, Padberg FT, Jr, Murad MH, Montori VM, O’Hare AM, Calligaro KD, Macsata RA, Lumsden AB, Ascher E Society for Vascular Surgery. The Society for Vascular Surgery: clinical practice guidelines for the surgical placement and maintenance of arteriovenous hemodialysis access. J Vasc Surg. 2008 Nov;48(5 Suppl):2S–25S. doi: 10.1016/j.jvs.2008.08.042. [DOI] [PubMed] [Google Scholar]
- 7.Tamura MK, Tan JC, O’Hare AM. Optimizing renal replacement therapy in older adults: a framework for making individualized decisions. Kidney Int. 2011 Nov 16; doi: 10.1038/ki.2011.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee T, Barker J, Allon M. Comparison of survival of upper arm arteriovenous fistulas and grafts after failed forearm fistulas. J Am Soc Nephrol. 2007;18:1936–1941. doi: 10.1681/ASN.2006101119. [DOI] [PubMed] [Google Scholar]
- 9.Foley RN, Chen SC, Collins AJ. Hemodialysis access at initiation in the United States, 2005 to 2007: Still “catheter first”. Hemodial Int. 2009;13:533–542. doi: 10.1111/j.1542-4758.2009.00396.x. [DOI] [PubMed] [Google Scholar]
- 10.Murad MH, Elamin MB, Sidawy AN, Malaga G, Rizvi AZ, Flynn DN, Casey ET, McCausland FR, McGrath MM, Vo DH, El-Zoghby Z, Duncan AA, Tracz MJ, Erwin PJ, Montori VM. Autogenous versus prosthetic vascular access for hemodialysis: a systematic review and meta-analysis. J Vasc Surg. 2008 Nov;48(5 Suppl):34S–47S. doi: 10.1016/j.jvs.2008.08.044. [DOI] [PubMed] [Google Scholar]
- 11.Allon M, Lok CE. Dialysis fistula or graft: the role for randomized clinical trials. Clin J Am Soc Nephrol. 2010 Dec;5(12):2348–54. doi: 10.2215/CJN.06050710. [DOI] [PubMed] [Google Scholar]
- 12.Eggers PW. Medicare’s End Stage Renal Disease Program. Health Care Financing Review. 2000;22:55–60. [PMC free article] [PubMed] [Google Scholar]
- 13.U.S. Renal Data System Coordinating Center. USRDS 2012 Researcher’s Guide to the USRDS Database. 2012 [Google Scholar]
- 14.U.S. Department of Health & Human Services. OCR HIPAA Privacy, Research. 2003 Apr 3; Retrieved from http://www.hhs.gov/ocr/privacy/hipaa/understanding/coveredentities/research.html.
- 15.Hicks CW, Canner JK, Arhuidese I, Zarkowsky DS, Qazi U, Reifsnyder T, Black JH, 3rd, Malas MB. Mortality benefits of different hemodialysis access types are age dependent. J Vasc Surg. 2015 Feb;61(2):449–456. doi: 10.1016/j.jvs.2014.07.091. [DOI] [PubMed] [Google Scholar]
- 16.Ravani P, Palmer SC, Oliver MJ, Quinn RR, MacRae JM, Tai DJ, Pannu NI, Thomas C, Hemmelgarn BR, Craig JC, Manns B, Tonelli M, Strippoli GF, James MT. Associations between hemodialysis access type and clinical outcomes: a systematic review. J Am Soc Nephrol. 2013 Feb;24(3):465–73. doi: 10.1681/ASN.2012070643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allon M, Robbin ML. Resolved: Fistulas are preferred to grafts as initial vascular access for dialysis. Con J Am Soc Nephrol. 2008 Sep;19(9):1632–3. [PubMed] [Google Scholar]
- 18.Shariff SZ, Cuerden MS, Jain AK, Garg AX. The secret of immortal time bias in epidemiologic studies. J Am Soc Nephrol. 2008 May;19(5):841–3. doi: 10.1681/ASN.2007121354. [DOI] [PubMed] [Google Scholar]
- 19.Lévesque LE, Hanley JA, Kezouh A, Suissa S. Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ. 2010 Mar 12;340:b5087. doi: 10.1136/bmj.b5087. [DOI] [PubMed] [Google Scholar]
- 20.Desilva RN, Patibandla BK, Vin Y, Narra A, Chawla V, Brown RS, Goldfarb-Rumyantzev AS. Fistula first is not always the best strategy for the elderly. J Am Soc Nephrol. 2013 Jul;24(8):1297–304. doi: 10.1681/ASN.2012060632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bradbury BD, Fissell RB, Albert JM, Anthony MS, Critchlow CW, Pisoni RL, Port FK, Gillespie BW. Predictors of early mortality among incident US hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS) Clin J Am Soc Nephrol. 2007 Jan;2(1):89–99. doi: 10.2215/CJN.01170905. [DOI] [PubMed] [Google Scholar]
- 22.Clinical practice guidelines for nutrition in chronic renal failure. K/DOQI, NAtional Kidney Foundation. Am J Kidney Dis. 2000 Jun;35(6 Suppl 2):S1–140. doi: 10.1053/ajkd.2000.v35.aajkd03517. [DOI] [PubMed] [Google Scholar]
- 23.McGrogan DG, Field MA, Inston NG. Survival following arteriovenous fistula formation: are grafts indicated in the elderly? J Vasc Access. 2014 Nov-Dec;15(6):548. doi: 10.5301/jva.5000288. [DOI] [PubMed] [Google Scholar]
- 24.Al-Jaishi AA, Oliver MJ, Thomas SM, Lok CE, Zhang JC, Garg AX, Kosa SD, Quinn RR, Moist LM. Patency rates of the arteriovenous fistula for hemodialysis: a systematic review and meta-analysis. Am J Kidney Dis. 2014 Mar;63(3):464–78. doi: 10.1053/j.ajkd.2013.08.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.