Abstract
Alcohol and nicotine (in the form of tobacco) are often taken together, with increased negative health consequences. Co-use may modify intake of one or both of the drugs, or the effects of drugs used to treat nicotine or alcohol addiction. Varenicline is commonly prescribed as an aid to enhance quitting smoking. More recently it has been shown to reduce alcohol intake in humans and laboratory animals. There is little work investigating the role of co-exposure to alcohol and nicotine in the effects of varenicline. In pilot clinical studies, it has been reported that smoking enhances varenicline’s effectiveness as a treatment for alcohol misuse, but this relationship has not been systematically investigated. To help resolve this, we examined if the effects of varenicline on alcohol and nicotine self-administration (SA) in rats are modified when the two drugs are taken together. Rats were trained on alcohol SA, and some were implanted with i.v. catheters for nicotine SA. Groups of animals then lever pressed for alcohol or nicotine alone, and another group lever pressed for alcohol and nicotine, using a two lever choice procedure. Varenicline did not affect alcohol SA. Varenicline reduced nicotine SA modestly. Access to both alcohol and nicotine reduced self-administration of either drug, but did not change the effects of varenicline. We found that in rats with a history of alcohol SA, varenicline reduced reinstatement of extinguished alcohol seeking induced by exposure to an alcohol prime combined with cues previously associated with alcohol.
Keywords: alcohol, nicotine, varenicline, self-administration, reinstatement, cues
1. Introduction
Addictions to alcohol and nicotine (most often in the form of smoked tobacco) have major impacts on health and society. Roughly 4% of American adults are alcohol-dependent and 8% can be classified as having an alcohol use disorder [1], while 18% are classified as smokers [2]. Alcohol and nicotine are often taken together [3, 4], intake of one can increase intake of the other [5, 6] and their negative health effects are greatly increased with co-abuse [7, 8]. Co-use also has important treatment implications as it may change the effects of drugs used to treat alcohol and nicotine addiction [9–11]. With the exception of our recent report [12], there is no preclinical work systematically exploring this interaction.
Varenicline is prescribed to aid quitting smoking [12]. It is a full agonist at the α7, and a partial agonist at the α4β2 nicotinic cholinergic receptor (nAChR) and can reduce nicotine self-administration (SA) in laboratory animals responding on fixed [13–15] and progressive ratio schedules [16], although there are inconsistencies in the effective dose. Varenicline can affect reinstatement of nicotine seeking in animal models of relapse, but its effects may depend on the dose of varenicline, the nature of the reinstating stimulus or other factors [14–16].
Varenicline may also be useful in treating alcohol dependence or misuse. In a human experimental study, it reduced the number of drinks consumed during an alcohol SA period and reduced craving for alcohol and its positive effects after a priming drink [17]. Data from more extensive clinical trials support these observations [18, 19]. Work using animal models of alcohol SA and relapse are consistent with this human data. Varenicline can reduce drinking and operant SA of alcohol, but as is the case for nicotine SA, there is variability in the effective doses reported [15, 20–22].
Co-use of alcohol and nicotine can modify the effects of drugs used to treat addiction to either drug [9–11]. For example, in heavy drinkers that smoke, naltrexone is more effective in reducing alcohol intake [9, 11] and is also more effective in enhancing smoking quit rates in smokers treated with nicotine replacement that also drink heavily [9]. In support of this, we found that co-exposure to alcohol and nicotine potentiated the inhibitory effect of naltrexone on alcohol SA in rats (Le et al. 2014).
There is little systematic work on the role of alcohol and nicotine co-exposure in the effects of varenicline. Varenicline was reported in one clinical study to be more effective in reducing drinking in smokers than in nonsmokers [19], but was unrelated to smoking status in another [18]. Varenicline reduced the increases in alcohol SA and drinking produced by acute injections of nicotine in rats. [23]. Varenicline reduced both alcohol and nicotine intake in rats when presented under two lever choice conditions [24], but there were no single drug alone control groups in that study to evaluate the effects of co-exposure.
To help resolve these issues, we will examine the effects of varenicline on alcohol and nicotine SA when the drugs are administered separately or when co-administered using a two lever choice procedure. We then examined the effects of varenicline on reinstatement of alcohol seeking induced by exposure to alcohol-associated cues combined with an alcohol prime.
2. Materials and methods
2.1 Animals
Male Wistar rats, 200–225 g were obtained from Charles River (Montreal, Quebec). Rats were individually housed and fed 25 g of lab chow in the home cage after the daily operant sessions. This mild food restriction was used to prevent excessive weight gain in the animals, but allows normal growth. Tap water was available ad libitum in the home cage. The vivarium temperature was 21°C and lights were on from 7 p.m. to 7 a.m. Procedures followed the National Institutes of Health “Principles of laboratory animal care” (Eighth edition, 2011) and were approved by the animal care committee of the Centre for Addiction and Mental Health.
2.2 Apparatus
Self-administration (SA) of alcohol and/or nicotine was done in 16 chambers operated by a Med Associates (Georgia, VT) interface. Each chamber (30 × 21 × 21 cm) was equipped with two retractable levers and two infusion pumps, one for delivering alcohol into a drinking receptacle, and one for delivering nicotine solution through an i.v. catheter line. For alcohol SA, lever responding activated the infusion pump (Razel Sci., Stamford, CT) for 5 sec., delivering 0.19 ml of alcohol solution (12% w/v) into the receptacle, with each delivery accompanied by a flashing white cue light above the lever (0.5 s on, 0.5 s off) for 5 s. For nicotine SA, lever responding activated the infusion pump for 0.5 s delivering the nicotine solution (30 μg/kg/delivery) via the i.v. catheter that was accompanied by a continuously illuminated white cue light above the lever for 5 s. In sessions when only alcohol or nicotine was available, an inactive lever was also present; responses on this lever had no programmed consequences, but were recorded. In sessions when both alcohol and nicotine were available, there was no inactive lever. Positions of the alcohol and nicotine-associated levers or inactive levers were counterbalanced across animals. The chambers also had a red house light illuminated during the SA session. At the end of SA sessions involving alcohol, receptacles were checked for unconsumed alcohol; if present, it was measured and used to calculate the deliveries consumed [12]. In the present experiments, unconsumed alcohol was rarely found.
2.3 Catheter or sham catheter surgery
Rats were anaesthetized using isoflurane/oxygen. Incision sites were treated with a local anesthetic (0.1 ml bupivacaine, 0.125%, s.c.). Penicillin (30 000 U, i.m.) was administered prior to surgery and buprenorphine (0.01 mg/kg, s.c.) as an analgesic afterwards. Catheters were constructed in-house and implanted into the right jugular vein as previously described [25] and exited between the scapulae and were attached to a 22-gauge cannula connected to the fluid swivel system. They were capped with a short length of heat-sealed plastic tubing. Catheters were flushed daily with 0.1 ml of a sterile heparin-saline solution (50 U/ml); their patency was tested weekly by i.v. injections of sodium methohexital (0.05 mg/kg). Data from animals that did not show rapid anesthesia following i.v. methohexital injection were excluded. Rats receiving sham catheter surgery (the alcohol alone group in Exp. 1) were treated in the same way except they received a 1 cm long incision between the scapulae that was then sutured. Animals recovered for 1 week before initiation of experiments.
2.4 Procedures
2.4.1 Experiment 1: Effects of varenicline on SA of alcohol, nicotine or alcohol + nicotine
All rats were trained to self-administer alcohol. They received daily limited access sessions (30 min) with the choice between water and alcohol in Richter tubes, with 5 d each at 3, 6 and 12 % (w/v) and were then trained on operant alcohol SA (12%) in daily 1 h sessions. Animals received 5 daily sessions each at FR-1, FR-2 and FR-3 with a 5 s timeout, with only the alcohol lever present. They then received 5 daily sessions at FR-3, but after each alcohol delivery, the lever retracted for a 30 s timeout period.
Animals were assigned to 3 groups, matched according to the mean numbers of alcohol deliveries received over the last 2 days of alcohol SA. Animals in groups that would self-administer nicotine alone (n=12) or both drugs (n=12) received catheter surgery, while those that would self-administer alcohol alone (n=12) received sham catheter surgery. After recovery, further operant training occurred as follows: Alcohol alone: rats continued to receive daily 2 h alcohol SA sessions at FR-3 with levers retracting during the 30 s timeout. Nicotine alone: rats were trained to self-administer nicotine in 2 h daily sessions at FR3 with lever retraction after each nicotine delivery for the 30 s timeout. Alcohol + Nicotine: rats were trained to self-administer nicotine for 15 days at FR-3 with lever retraction after delivery for the 30 s timeout, and then received daily 2 h sessions with opportunity to self-administer alcohol and nicotine concurrently, both at FR-3. After delivery of either drug, both levers retracted for the 30 s timeout, and the ratio was reset to FR-3 for both drugs. Testing for the effects of varenicline commenced after alcohol, nicotine or alcohol + nicotine SA became stable (about 10 days). Two rats that consumed less than 0.4 g/kg alcohol during the alcohol SA sessions, 3 rats that earned less than 10 reinforcements during the nicotine SA sessions and 4 rats with blocked catheters were excluded from analysis.
Animals were administered vehicle (saline) and varenicline i.p. at doses of 1 and 2 mg/kg 15 min prior to the 2 h test sessions. The doses used are based on previous reports [15, 21, 22]. The vehicle and doses of varenicline were administered in counterbalanced order with each test separated by at least 2 d; on these intervening days, rats received the same SA condition as during training.
2.4.2 Experiment 2: Effects of varenicline on reinstatement of alcohol seeking induced by cue+alcohol prime
Twelve rats were trained to self-administer alcohol to FR-3 in daily 1 h sessions as described above, except that the active and inactive levers did not retract, and the cue associated with alcohol delivery was a compound 2800 Hz tone and cue light illuminated for 5 s. After stable SA occurred at FR-3, responding for alcohol was extinguished under similar conditions, except lever responding led to neither alcohol delivery nor cue presentation. Tests for reinstatement were conducted when the rats reached the extinction criterion of < 12 presses on the previously active lever/1 h (about 10 d). Reinstatement tests involved presentation of the light/tone cue and one delivery of alcohol; subsequently, responding led to presentation of the compound cue only on an FR-3 schedule. Saline and two doses of varenicline (0.5 and 1 mg/kg) were administered 15 min prior to the 1 h tests, in counterbalanced order, with 2–3 d between each test. The days before the cue+prime tests were used as baselines. We used lower doses of varenicline (0.5 and 1 mg/kg) than in Exp. 1 because in a pilot dose-response experiment, varenicline at 2 mg/kg or higher suppressed responding below baseline under extinction conditions (Lê at al., unpublished observations). A further reason is that reinstatement responding is more sensitive to drug-induced reductions than is SA [26, 27]. Two rats were excluded from Exp. 2 as they self-administered less than 0.4 g/kg of alcohol during training.
2.5 Drugs
Alcohol (95%, Commercial Alcohols, Tiverton, ON) was diluted in tap water. Nicotine solutions (Sigma-Aldrich, Oakville, ON, Canada) were prepared fresh daily in sterile saline (pH 7) and the dose for i.v. nicotine SA was 30 μg/kg per delivery. Varenicline (7,8,9,10-Tetrahydro-6,10-methano-6H-pyrazino[2,3-h] [3]benzazepine tartrate) was obtained from Pfizer (Kirkland, Quebec, Canada) and dissolved in saline and administered in a volume of 1 ml/kg i.p. Drug doses are expressed as base.
2.6 Data analysis and presentation
The effects of varenicline on nicotine and/or alcohol SA in Exp. 1 were determined by mixed analysis of variance (ANOVA) on numbers of drug deliveries using the within factor of Varenicline dose (0, 1, 2 mg/kg) and the between factor of SA condition (single drug, co-access) separately for alcohol and nicotine. Determination of the effects of varenicline on cue + alcohol prime-induced reinstatement of alcohol seeking (Exp. 2), were done using the within factors of Varenicline dose (0, 0.5, 1 mg/kg) and Test condition (baseline, cue + alcohol prime) on the active and inactive lever data, with baseline referring to responding on the day before each test. In the within analyses, sphericity was assessed with Mauchly’s test, and corrected where necessary using the Greenhouse-Geisser method. Significant effects from the ANOVAs (p values < 0.05) were followed by post-hoc tests using the Newman-Keuls procedure.
3. Results
3.1 Experiment 1
3.1.1 Effects of varenicline on SA of alcohol and effects of co-access to nicotine
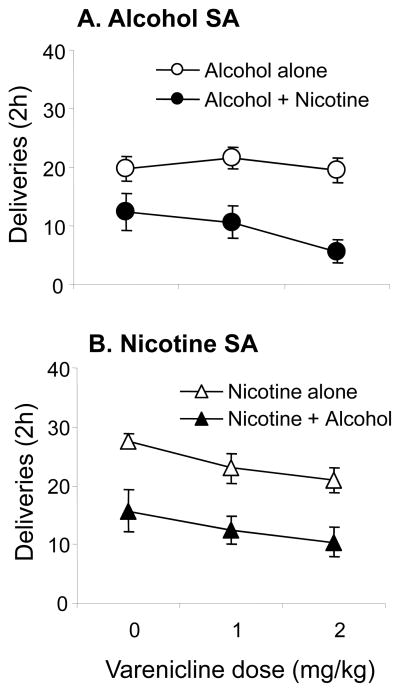
Varenicline did not significantly affect alcohol SA (Fig. 1A). Co-access to nicotine did not alter this null effect. Rats given access to both alcohol and nicotine self-administered significantly less alcohol than those with access to alcohol alone, which was reflected in a significant effect of SA group (p < 0.05) (Fig. 1A). Table 1 (left panel) shows the results of the ANOVA on the effects of Varenicline dose and SA group on number of alcohol deliveries in rats self-administering alcohol alone, or with co-access to nicotine in SA sessions.
Figure 1. Effects of varenicline on self-administration (SA) of alcohol and/or nicotine in animals receiving daily sessions with access to alcohol alone, nicotine alone, or both drugs simultaneously.
A. Mean (± sem) numbers of deliveries of alcohol in rats with access to alcohol alone (n=11) or alcohol + nicotine (n=8) administered vehicle (0) or varenicline (1 and 2 mg/kg) 15 min prior to 2 h SA sessions, in counterbalanced order with 2–3 days of SA between each dose. B. Mean (± sem) number of deliveries of nicotine in rats with access to nicotine alone (n=8) or nicotine + alcohol (n=8). Open circles: alcohol alone, closed circles: alcohol + nicotine, open triangles: nicotine alone, closed triangles: nicotine + alcohol.
Table 1.
| Alcohol | Nicotine | |||||
|---|---|---|---|---|---|---|
| df | F | p | df | F | p | |
| SA Group | 1,34 | 16.5 | .001* | 1,28 | 13.5 | .002* |
| Dose | 2,34 | 2.89 | .07 | 2,28 | 4.13 | .027* |
| SA group x Dose | 2,34 | 1.74 | .19 | 2,28 | 0.05 | .95 |
3.1.2 Effects of varenicline on SA of nicotine and effects of co-access to alcohol
Varenicline reduced nicotine SA. This was reflected in a significant main effect of Varenicline dose on numbers of nicotine deliveries (p<0.05) (Fig. 1 B and Table 1). Post-hoc tests conducted on the data collapsed across SA groups showed that nicotine SA was significantly reduced by the 2 mg/kg dose compared to vehicle (p<0.05). Co-access to alcohol significantly reduced numbers of nicotine deliveries (p’s<0.05) but did not change the effects of varenicline. Table 1 (right) shows the results of the ANOVA done on the nicotine SA data.
3.2 Experiment 2
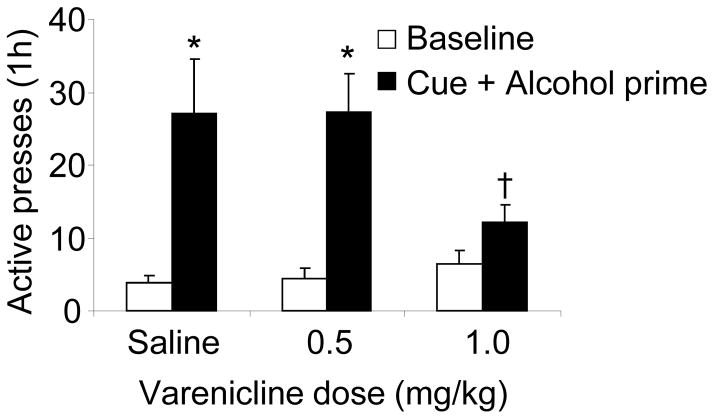
In rats treated with saline vehicle, exposure to alcohol-associated cues combined with an alcohol prime significantly reinstated responding on the active lever, which was reflected in a significant main effect of Test condition (F(1,18)=23.8, p<0.05) (Fig. 2). Post-hoc tests showed significant differences in responding between the baseline day prior to the test and the cue+prime test (p<0.05). Varenicline significantly reduced this reinstatement, as there was a significant Varenicline dose x Test condition interaction (F(2,18)=4.5, p<0.05). Animals treated with 1 mg/kg varenicline prior to cue+prime exposure pressed significantly less on the active lever than animals treated with vehicle (p<0.05). The 0.5 mg/kg dose was ineffective on cue+prime-induced reinstatement. Inactive lever presses did not differ as a function of reinstatement condition or varenicline dose (not shown).
Figure 2. Effects of varenicline on cue + alcohol prime-induced reinstatement of alcohol seeking.
Data are mean (± sem) presses on the previously active lever. Rats were trained for alcohol SA, their lever pressing extinguished and were injected with vehicle (0) or one of the doses of varenicline (0.5 and 1 mg/kg) 15 min before reinstatement test sessions (1 h). At the beginning of the sessions animals received 1 delivery of alcohol solution accompanied by the cue (light and tone); subsequently, responding resulted in cue presentation at FR3. Vehicle and varenicline injections were given in counterbalanced order with 2–3 days of regular extinction sessions between each. N=10. Open bars: baseline, closed bars: cue + alcohol prime. * Different from baseline (p<0.05). † Different from vehicle condition (p < 0.05).
4. Discussion
Nicotine SA was modestly reduced by the 2 mg/kg dose of varenicline, while the 1 mg/kg dose was without effect. These findings are contrary to previous reports [13–16, 28–30] describing more marked decreases in nicotine SA at 1 mg/kg or higher. Only one of these studies failed to find significant inhibition of nicotine SA at 1 mg/kg [31]; this study reported decreases only at a high dose (3 mg/kg). We did not find varenicline to affect alcohol SA. This contrasts with studies reporting significant reductions in alcohol SA at these doses [15, 22, 23].
There are several potential explanations for the relatively small effects of varenicline on nicotine SA, and the null effects on alcohol SA we observed. One is that the doses we used were in an ineffective range. However, this is unlikely as they were selected based on previous reports that doses of 1–2 mg/kg significantly reduce alcohol and nicotine SA [14–16, 22, 28, 29, 32], without having the non-specific motor inhibitory effects seen at higher doses (3 mg/kg) [21].
Another may be differences in operant procedures. In our study, levers were programmed to retract for a 30 s timeout period after drug delivery. In the studies reporting significant effects of lower doses of varenicline (1 mg/kg), animals could respond at any time throughout the session, by lever pressing or nose poking.
Rat strain may also be involved in the small effects of varenicline we found. In the case of nicotine, the majority of studies finding inhibitory effects of lower doses of varenicline on nicotine SA used Long Evans and Sprague Dawley rats. The one published study failing to find effects of varenicline at 1 mg/kg used Wistar rats [31], as in our study. There is less consistency on the potential role of rat strain on varenicline’s effects on alcohol SA. Most studies on the effects of varenicline on alcohol SA used Sprague Dawley and Long Evans rats and across these studies, variable effects of the 1 mg dose were reported. Only one study [15] used Wistar rats as in our study, and found a significant inhibitory effect at 1.5 mg/kg.
Differences in drug intake may also be a possible explanation for the lack of effect of varenicline on alcohol SA and the small effect on nicotine SA we observed. This consideration is especially important in the case of alcohol as inhibitory responses to drugs tend to be larger with higher basal alcohol intakes [33, 34]. However, differences in the alcohol intake of rats between our study and previous work do not appear to account for this. In the studies reporting effects of the lower dose of varenicline (1 mg/kg), there was a relatively narrow range in alcohol intakes (from about 0.7–1.0 g/kg). In our study, rats self-administering alcohol alone consumed ~1.1 g/kg in the 2 h SA sessions. Neither does duration of experience with alcohol SA appear to contribute to the effects of varenicline. The first study reporting effects of varenicline on alcohol SA used a long (5 month) SA training period and found significant effects with the 1 mg/kg dose [22]. However, more recent studies using alcohol SA training of both similar and shorter durations have reported both decreases and null effects with this dose [15, 21].
Another variable to be considered is pretreatment time. Varenicline (1.5–2.5 mg/kg), given 15 min prior to the operant session did not affect food pellet or sucrose SA, or sucrose SA when concurrently available with i.v. nicotine [15, 35, 36]. In contrast, 3 mg/kg given at the same pretreatment time disrupted responding for food [14], and reduced alcohol SA in the first 10 min of the session [21]. Only one study investigated a longer pretreatment time and reported that varenicline (0.3–3 mg/kg) given 2 h prior to the test sessions significantly reduced nicotine infusions received on a PR schedule, but did not affect responding for food pellets [16].
Another possible factor is session length. If inhibitory effects of varenicline occur relatively early after administration, test sessions of long duration may obscure its effects. In support of this, we noted that treatment of rats in the alcohol alone group with 2 mg/kg varenicline resulted in a small but significant decrease in alcohol SA in the first 30 min of the 2h test session. This agrees with a previous report that a higher dose of varenicline (3 mg/kg) reduced responding for alcohol in the first 10 min of the session[21]. This may suggest that varenicline has relatively short-lived effects on alcohol SA that may have been obscured by the 2h session duration we used, which was employed to insure adequate levels of nicotine and/or alcohol SA. Arguing against this, however, is the significant reduction in nicotine SA over the 2 h sessions produced by varenicline, that were unaccompanied by such early effects.
In the present study, co-access to alcohol and nicotine did not significantly alter the effects of varenicline on responding for either drug. Our findings therefore do not agree with the greater efficacy of varenicline on alcohol intake reported in alcohol-dependent patients who smoke [37], or that smoking can increase its inhibitory effects on drinking [19], although there have been negative reports on this relationship as well [18]. There is little preclinical data on the effects of varenicline on simultaneous responding for two reinforcers. One study demonstrated that varenicline (1.5 mg/kg) significantly reduced responding for both alcohol and IV nicotine when they could be self-administered together [24]; there were, however, no single drug controls in that study, so co-access effects per se could not be determined. Another reported that varenicline (1 mg/kg) significantly increased alcohol SA when it was self-administered at the same time as food pellets [38], while higher doses (1.8 mg/kg or greater) reduced SA of both reinforcers, but this study was also without single reinforcer controls. A later study by this same group extended these data in showing that varenicline selectively reduced responding for alcohol vs. food when both were available on a multiple schedule [39].
In a previous study, we found that simultaneous access to nicotine and alcohol affected intake depending on which drug animals were trained with first [40]. Co-access reduced responding for nicotine in rats trained with alcohol first, but if they were trained with nicotine first, responding for both drugs was reduced. Since in the present study, animals were trained with alcohol first, we would predict a reduction in nicotine intake with co-access, whereas we found reductions in responding for both drugs. Potential reasons for this discrepancy may be differences in session duration and lever access conditions between this and the present study. In our previous study [40], the session length was 1 h, with the levers continuously available, while in the present study the session length was 2 h, and the levers retracted after drug delivery for the duration of the timeout period. In contrast, our most recent work on the interaction of alcohol with nicotine using a multiple schedule of drug presentation, showed increases in alcohol SA when both drugs were made available in alternating 5 min periods during a 2 h session [12]. Taken together, these results suggest that the effects of co-access to alcohol and nicotine on responding may depend on SA schedule, session duration, lever retraction and order of training (alcohol or nicotine first). Our present data can also be interpreted as consistent with the matching law that would predict decreases in responding if two reinforcers of similar reinforcement value are available simultaneously [41, 42].
We found that rats trained to self-administer alcohol, extinguished without cues and then exposed to a cue + alcohol prime showed robust reinstatement of responding, in agreement with previous research [15, 43]. Varenicline blocked this reinstatement at a dose of 1 mg/kg, while a lower dose of 0.5 mg/kg had no effect. Overall, our findings are consistent with a previous study showing reductions in cue+prime induced reinstatement with a slightly higher dose of varenicline (1.5 mg/kg) [15]. We did not see evidence for any enhancement of cue-induced reinstatement of alcohol seeking with the low dose of varenicline (i.e. 0.5 mg/kg), that has been reported for nicotine [15, 16]. Given that exposure to alcohol and alcohol-related cues are key precipitants of relapse episodes, our findings are clinically relevant, and indicate the further study of varenicline as a treatment to help prevent relapse in abstinent alcoholics or problem drinkers.
The mechanism of action of varenicline is poorly understood. The drug is a partial nicotinic receptor agonist, and as an aid to quitting smoking, is thought to work by substituting for nicotine, thereby reducing craving. Consistent with this is the observation that varenicline substitutes for nicotine in drug discrimination studies[16], increases dopamine release in the nucleus accumbens [44], and potentiates drug-induced DA release in the NAC, at least in the case of alcohol [45]. In support of the idea that varenicline acts by substituting for drugs of abuse, it has been shown to be self-administered i.v. [24, 30], although another recent study suggests that this may be conditional on the presence of cues [46]. Although we did not specifically investigate this issue, the present results are consistent with the idea that varenicline may act to reduce nicotine SA by substituting for the drug.
Our future work will include determination of the effects of varenicline on co-administration of alcohol and nicotine using an alternating access procedure we recently developed [12], with which we showed that co-access enhanced both alcohol SA and the inhibitory effects of naltrexone on alcohol intake. Another series of studies are to determine the effects of varenicline on reinstatement of nicotine seeking induced by cue or cue + nicotine prime exposure, and if co-access would modify its effects on reinstatement of alcohol and/or nicotine seeking induced by cues, cues + drug prime, or other factors involved in relapse such as stress exposure.
5. Conclusions
Varenicline modestly reduced nicotine SA but did not affect alcohol SA. Simultaneous access to alcohol and nicotine did not change the effects of varenicline on SA of either drug. Varenicline blocked reinstatement of extinguished alcohol seeking induced by exposure to alcohol-associated cues combined with an alcohol prime. Taken together with previous work, these results suggest further investigation of varenicline as a treatment for relapse to alcohol.
Highlights.
Alcohol self-administration in rats was not affected by varenicline.
Nicotine self-administration in rats was modestly reduced by varenicline.
Nicotine did not modify the effects of varenicline on alcohol self-administration
Alcohol did not modify the effects of varenicline on nicotine self-administration
Reinstatement of alcohol seeking induced by alcohol cues+prime was blocked by varenicline.
Acknowledgments
This work was supported by grants from the NIAAA (AA13108) and Canadian Psychiatric Research Foundation to A.D. Lê.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
D. Funk, Email: douglas.funk@camh.ca.
K. Coen, Email: kathy.coen@camh.ca.
A.D. Lê, Email: anh.le@camh.ca.
References
- 1.Kinzeler NR, Travers SP. mu-Opioid modulation in the rostral solitary nucleus and reticular formation alters taste reactivity: evidence for a suppressive effect on consummatory behavior. Am J Physiol Regul Integr Comp Physiol. 2011;301(3):R690–700. doi: 10.1152/ajpregu.00142.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marchant NJ, Furlong TM, McNally GP. Medial dorsal hypothalamus mediates the inhibition of reward seeking after extinction. J Neurosci. 2010;30(42):14102–15. doi: 10.1523/JNEUROSCI.4079-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Istvan J, Matarazzo JD. Tobacco, alcohol, and caffeine use: a review of their interrelationships. Psychol Bull. 1984;95(2):301–326. [PubMed] [Google Scholar]
- 4.Kandel D, et al. Prevalence and demographic correlates of symptoms of last year dependence on alcohol, nicotine, marijuana and cocaine in the U.S. population. Drug Alcohol Depend. 1997;44(1):11–29. doi: 10.1016/s0376-8716(96)01315-4. [DOI] [PubMed] [Google Scholar]
- 5.Dani JA, Harris RA. Nicotine addiction and comorbidity with alcohol abuse and mental illness. Nat Neurosci. 2005;8(11):1465–70. doi: 10.1038/nn1580. [DOI] [PubMed] [Google Scholar]
- 6.Funk D, Marinelli PW, Le AD. Biological processes underlying co-use of alcohol and nicotine: neuronal mechanisms, cross-tolerance, and genetic factors. Alcohol Res Health. 2006;29(3):186–92. [PMC free article] [PubMed] [Google Scholar]
- 7.Tallarida RJ. Revisiting the isobole and related quantitative methods for assessing drug synergism. J Pharmacol Exp Ther. 2012;342(1):2–8. doi: 10.1124/jpet.112.193474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Purohit V, Khalsa J, Serrano J. Mechanisms of alcohol-associated cancers: introduction and summary of the symposium. Alcohol. 2005;35(3):155–60. doi: 10.1016/j.alcohol.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 9.King A, et al. Naltrexone decreases heavy drinking rates in smoking cessation treatment: an exploratory study. Alcohol Clin Exp Res. 2009;33(6):1044–50. doi: 10.1111/j.1530-0277.2009.00925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.King A, et al. Neural substrates of alcohol-induced smoking urge in heavy drinking nondaily smokers. Neuropsychopharmacology. 2010;35(3):692–701. doi: 10.1038/npp.2009.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fucito LM, et al. Cigarette smoking predicts differential benefit from naltrexone for alcohol dependence. Biol Psychiatry. 2012;72(10):832–8. doi: 10.1016/j.biopsych.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le AD, et al. Operant self-administration of alcohol and nicotine in a preclinical model of co-abuse. Psychopharmacology (Berl) 2014;231(20):4019–29. doi: 10.1007/s00213-014-3541-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Costello MR, et al. Comparison of the reinforcing properties of nicotine and cigarette smoke extract in rats. Neuropsychopharmacology. 2014;39(8):1843–51. doi: 10.1038/npp.2014.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Connor EC, et al. The alpha4beta2 nicotinic acetylcholine-receptor partial agonist varenicline inhibits both nicotine self-administration following repeated dosing and reinstatement of nicotine seeking in rats. Psychopharmacology. 2010;208(3):365–76. doi: 10.1007/s00213-009-1739-5. [DOI] [PubMed] [Google Scholar]
- 15.Wouda JA, et al. Varenicline attenuates cue-induced relapse to alcohol, but not nicotine seeking, while reducing inhibitory response control. Psychopharmacology (Berl) 2011;216(2):267–77. doi: 10.1007/s00213-011-2213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le Foll B, et al. Varenicline decreases nicotine self-administration and cue-induced reinstatement of nicotine-seeking behaviour in rats when a long pretreatment time is used. Int J Neuropsychopharmacol. 2012;15(9):1265–74. doi: 10.1017/S1461145711001398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKee SA, et al. Varenicline reduces alcohol self-administration in heavy-drinking smokers. Biol Psychiatry. 2009;66(2):185–90. doi: 10.1016/j.biopsych.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Litten RZ, et al. A double-blind, placebo-controlled trial assessing the efficacy of varenicline tartrate for alcohol dependence. J Addict Med. 2013;7(4):277–86. doi: 10.1097/ADM.0b013e31829623f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plebani JG, et al. Results from a pilot clinical trial of varenicline for the treatment of alcohol dependence. Drug Alcohol Depend. 2013;133(2):754–8. doi: 10.1016/j.drugalcdep.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hendrickson LM, et al. Activation of alpha4* nAChRs is necessary and sufficient for varenicline-induced reduction of alcohol consumption. J Neurosci. 2010;30(30):10169–76. doi: 10.1523/JNEUROSCI.2601-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Randall PA, et al. The role of varenicline on alcohol-primed self-administration and seeking behavior in rats. Psychopharmacology (Berl) 2015 doi: 10.1007/s00213-015-3878-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steensland P, et al. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. Proc Natl Acad Sci U S A. 2007;104(30):12518–23. doi: 10.1073/pnas.0705368104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bito-Onon JJ, et al. Varenicline, a partial agonist at neuronal nicotinic acetylcholine receptors, reduces nicotine-induced increases in 20% ethanol operant self-administration in Sprague-Dawley rats. Addict Biol. 2011;16(3):440–9. doi: 10.1111/j.1369-1600.2010.00309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cippitelli A, et al. AT-1001: a high-affinity alpha3beta4 nAChR ligand with novel nicotine-suppressive pharmacology. Br J Pharmacol. 2015;172(7):1834–45. doi: 10.1111/bph.13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corrigall WA, Coen KM. Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology (Berl) 1989;99(4):473–8. doi: 10.1007/BF00589894. [DOI] [PubMed] [Google Scholar]
- 26.Henderson-Redmond A, Czachowski C. Effects of systemic opioid receptor ligands on ethanol- and sucrose seeking and drinking in alcohol-preferring (P) and Long Evans rats. Psychopharmacology (Berl) 2014;231(22):4309–21. doi: 10.1007/s00213-014-3571-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le AD, et al. Effects of naltrexone and fluoxetine on alcohol self-administration and reinstatement of alcohol seeking induced by priming injections of alcohol and exposure to stress. Neuropsychopharmacology. 1999;21(3):435–44. doi: 10.1016/S0893-133X(99)00024-X. [DOI] [PubMed] [Google Scholar]
- 28.Hall BJ, et al. Bupropion-varenicline interactions and nicotine self-administration behavior in rats. Pharmacol Biochem Behav. 2015;130:84–9. doi: 10.1016/j.pbb.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goeders NE, et al. Effects of the combination of metyrapone and oxazepam on intravenous nicotine self-administration in rats. Psychopharmacology (Berl) 2012;223(1):17–25. doi: 10.1007/s00213-012-2682-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rollema H, et al. Pharmacological profile of the alpha4beta2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007;52(3):985–94. doi: 10.1016/j.neuropharm.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 31.George O, et al. Varenicline blocks nicotine intake in rats with extended access to nicotine self-administration. Psychopharmacology (Berl) 2011;213(4):715–22. doi: 10.1007/s00213-010-2024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rollema H, et al. Preclinical pharmacology of the alpha4beta2 nAChR partial agonist varenicline related to effects on reward, mood and cognition. Biochem Pharmacol. 2009;78(7):813–24. doi: 10.1016/j.bcp.2009.05.033. [DOI] [PubMed] [Google Scholar]
- 33.Walker BM, Zorrilla EP, Koob GF. Systemic kappa-opioid receptor antagonism by nor-binaltorphimine reduces dependence-induced excessive alcohol self-administration in rats. Addict Biol. 2011;16(1):116–9. doi: 10.1111/j.1369-1600.2010.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walker BM, et al. alpha1-noradrenergic receptor antagonism blocks dependence-induced increases in responding for ethanol. Alcohol. 2008;42(2):91–7. doi: 10.1016/j.alcohol.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang Q, et al. Noribogaine reduces nicotine self-administration in rats. J Psychopharmacol. 2015;29(6):704–11. doi: 10.1177/0269881115584461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Panlilio LV, Hogarth L, Shoaib M. Concurrent access to nicotine and sucrose in rats. Psychopharmacology (Berl) 2014;232(8):1451–60. doi: 10.1007/s00213-014-3787-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mitchell JM, et al. Varenicline decreases alcohol consumption in heavy-drinking smokers. Psychopharmacology (Berl) 2014;223(3):299–306. doi: 10.1007/s00213-012-2717-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ginsburg BC, Lamb RJ. Effects of varenicline on ethanol- and food-maintained responding in a concurrent access procedure. Alcohol Clin Exp Res. 2013;37(7):1228–33. doi: 10.1111/acer.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ginsburg BC, Lamb RJ. Relative potency of varenicline or fluvoxamine to reduce responding for ethanol versus food depends on the presence or absence of concurrently earned food. Alcohol Clin Exp Res. 2014;38(3):860–70. doi: 10.1111/acer.12285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Le AD, et al. Coadministration of intravenous nicotine and oral alcohol in rats. Psychopharmacology (Berl) 2010;208(3):475–86. doi: 10.1007/s00213-009-1746-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jimenez-Gomez C, Shahan TA. Matching law analysis of rats’ alcohol self-administration in a free-operant choice procedure. Behav Pharmacol. 2008;19(4):353–6. doi: 10.1097/FBP.0b013e328308f1c5. [DOI] [PubMed] [Google Scholar]
- 42.McSweeney FK, Swindell S, Weatherly JN. Within-session changes in responding during concurrent schedules with different reinforcers in the components. J Exp Anal Behav. 1996;66(3):369–90. doi: 10.1901/jeab.1996.66-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fredriksson I, et al. Evaluation of guanfacine as a potential medication for alcohol use disorder in long-term drinking rats: behavioral and electrophysiological findings. Neuropsychopharmacology. 2015;40:1130–40. doi: 10.1038/npp.2014.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feduccia AA, et al. Varenicline decreases ethanol intake and increases dopamine release via neuronal nicotinic acetylcholine receptors in the nucleus accumbens. Br J Pharmacol. 2014;171(14):3420–31. doi: 10.1111/bph.12690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ericson M, et al. The smoking cessation medication varenicline attenuates alcohol and nicotine interactions in the rat mesolimbic dopamine system. J Pharmacol Exp Ther. 2009;329(1):225–30. doi: 10.1124/jpet.108.147058. [DOI] [PubMed] [Google Scholar]
- 46.Schassburger RL, et al. Differentiating the primary reinforcing and reinforcement-enhancing effects of varenicline. Psychopharmacology (Berl) 2014;232(5):975–83. doi: 10.1007/s00213-014-3732-x. [DOI] [PMC free article] [PubMed] [Google Scholar]