Abstract
A total synthetic strategy of 20S-hydroxyvitamin D3 [20S-(OH)D3] involving modified synthesis of key intermediates 7 and 12, Grignard reaction to stereoseletively generate 20S-OH and Wittig-Horner coupling to establish D3 framework, was completed in 16 steps with an overall yield of 0.4 %. The synthetic 20S-(OH)D3 activated vitamin D receptor (VDR) and initiated the expression of downstream genes. In addition, 20S-(OH)D3 showed similar inhibitory potency as calcitriol [1,25(OH)2D3] on proliferation of melanoma cells.
Keywords: 20S-hydroxyvitamin D3, total chemical synthesis, PCR, reporter assay, thymidine incorporation, VDR activation
1. Introduction
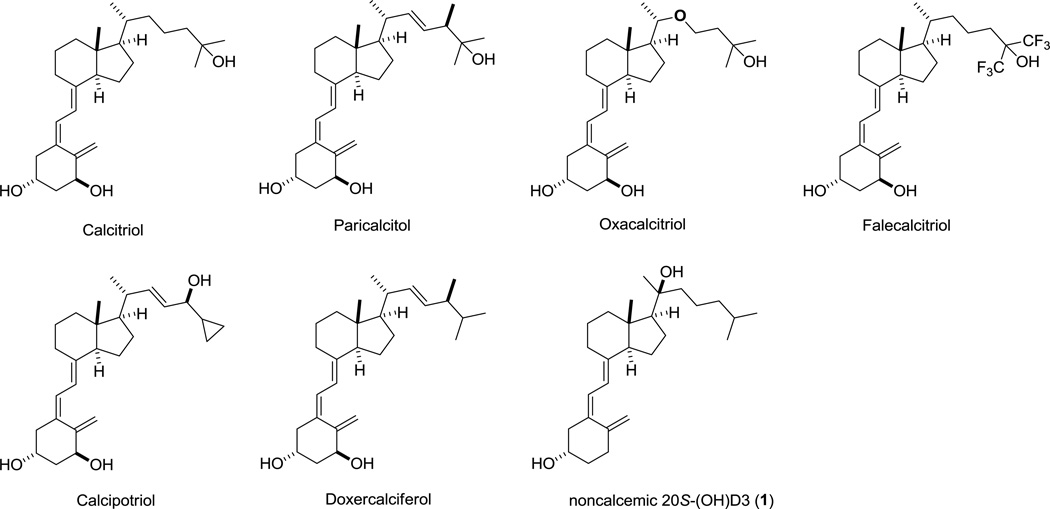
25-hydroxylase (CYP2R1 or CYP27A1) in the liver and 1α-hydroxylase (CYP27B1) in the kidney are the enzymes responsible for sequential metabolism of vitamin D3 to produce bioactive 1α,25-dihydroxyvitamin D3 [1,25(OH)2D3, calcitriol] [1, 2]. 1,25(OH)2D3 activates nuclear vitamin D receptor (VDR), which is found in almost all tissues of the body, to trigger numerous cellular effects including but not limited to stimulation of cell differentiation and/or apoptosis, inhibition of cell proliferation, regulation of secretory and immune factors of many cells, and cell protective functions, in a context dependent fashion [3–7]. Therefore, active forms of vitamin D can be used in therapy of cancer, hyperproliferative, autoimmune and metabolic disorders [8–10]. However, these applications are limited by hypercalcemic (toxic) effect of calcitriol at pharmacological concentrations [11]. This side effect has led to the development of more than 3,000 synthetic vitamin D3 analogs showing low calcemic activity [12]. Some of these analogs such as paricalcitol, oxacalcitriol, falecalcitriol, tacalcitol and doxercalciferol were used to treat secondary hyperparathyroidism and psoriasis [8, 13]. Besides psoriasis, calcipotriol is also a promising agent for the treatment of pancreatic cancer and is currently undergoing human clinical trial [14] (Figure 1).
Fig. 1.
Marketed vitamin D analogs and noncalcemic 20S-(OH)D3
Mammalian cytochrome P450 side-chain cleavage enzyme (P450scc or CYP11A1) not only cleaves the side chain of cholesterol to produce pregnenolone (precursor of all steroids) [15, 16] but also hydroxylates vitamin D3 in a sequential fashion [16–20] starting from C20 to form 20S-hydroxyvitamin D3 [20S-(OH)D3] (1, Figure 1), which is subsequently converted to di- and trihydroxy metabolites [17, 19–22]. Functional studies showed that 20S-(OH)D3 not only stimulated keratinocyte differentiation program but also inhibited NF-κB activity in human keratinocytes [23]. In addition, it has shown anti-inflammatory activities, strong anti-proliferative effects, anti-leukemia and tumorostatic effects [23–27], protective effects against ultra-violet B (UVB) induced damage [28], as well as antifibrotic activity in vivo [29]. These activities are mediated either through activation of the VDR [23, 30] or inhibition of RORα and RORγ transcriptional activities [31]. More importantly, while having comparable anti-proliferative potency with 1,25(OH)2D3 which has strong hypercalcemic toxicity at a concentration of 1µg/kg, 20S-(OH)D3 is not hypercalcemic at concentrations as high as 60 µg/kg [24, 26, 28, 32].
Unfortunately, in-depth evaluation of the biological activity for 20S-(OH)D3 or its analogs was hampered by lack of an efficient synthetic method of its production [33] without using costly enzymatic approaches [20, 21, 34]. 20S-(OH)D3 was firstly chemically synthesized from pregnenolone acetate via Grignard reaction and low yielding UVB irradiation to afford low milligram scale of 20S-(OH)D3 stereo-specifically. However, this method also generated structurally similar and physicochemically active by-products including previtamin D3, lumisterol and tachysterol which presented a significant challenge in the purification of 20S-(OH)D3 even through preparative HPLC [33]. Due to these disadvantages, scope of synthesis through UVB irradiation of 5,7-dienal precursor, 20S-(OH)-7-dehydrocholesterol was confined to very few derivatives, if not to almost exclusive production of 20S-(OH)D3. Therefore, an approach that could easily generate large quantity of 20S-(OH)D3 with high quality (> 98% purity) would facilitate further modifications on the side chain to determine the structure-activity relationships of this promising 20S-hydroxyl scaffold. Herein, we report an efficient total synthetic route for stereospecific 20S-(OH)D3.
2. Experimental
2.1. General methods
Tetrahydrofuran was distilled from sodium-benzophenone. All other solvents and chemical reagents were obtained from commercial sources and directly used without further purification. Ergocalciferol was purchased from Chem Impex International Inc. Glassware was oven-dried before use. All reactions were performed under an argon atmosphere. TLC was performed on silica gel 60 GF254 and monitored under UV light or visualized using phosphomolybdic acid reagent. Flash chromatography was performed on 230–400 mesh silica gel (Fisher Scientific). Preparative TLC was performed on Analtech TLC Uniplates (250 µm). Melting points were recorded on a MPA100 Automated Melting Point Apparatus. NMR spectra were obtained on a Bruker Ascend 400 (Billerica, MA) spectrometer or a Varian Inova-500 spectrometer (Agilent Technologies, Santa Clara, CA). Chemical shifts are given in ppm with tetramethylsilane (TMS) as an internal reference. All coupling constants (J) are given in Hertz (Hz).
2.2. Chemistry
2.2.1. (4R,7aR)-1-((2R,5R,E)-5,6-dimethylhept-3-en-2-yl)-4-((R,Z)-1-hydroxy-2-((S)-5-hydroxy-2-methylenecyclohexylidene)ethyl)-7a-methyloctahydro-1H-inden-4-ol (2)
Following a reported procedure [35], to a solution of ergocalciferol (20 g, 50.5 mmol) in ethanol (2 L) at −45 °C was added dropwise solution of potassium permanganate (9.0 g, 58.7 mmol) in water (300 mL), the mixture was stirred for half an hour and further 1 hour at −15 °C and then warmed to room temperature. After 3 hours, the precipitate was filtered off and the solution was evaporated to give yellowish crude oil. The crude oil was purified on silica gel column chromatography eluting with hexane/ethyl acetate (3:1) to give pure triol compound 2 as white solid (10.5 g, 65%) and unreacted starting material (5.1 g). Mp: 170–172 °C (MeOH). 1H NMR (400 MHz, CD3OD): δ 5.52 (dd, J = 9.9, 1.7 Hz, 1H, 6-H), 5.30 – 5.09 (m, 2H, 22/23-H), 5.02 – 4.98 (m, 2H, 7/19-H), 4.90 (d, J = 9.9 Hz, 1H, 19-H), 3.65 (m, 1H, 3-H), 2.55 (ddd, J = 12.4, 4.5, 1.9 Hz, 1H), 2.49 – 2.38 (m, 1H), 2.17 – 1.90 (m, 5H), 1.90 – 1.68 (m, 4H), 1.62 (m, 1H), 1.56 – 1.39 (m, 4H), 1.39 – 1.03 (m, 5H), 1.00 (d, J = 6.6 Hz, 3H, 21-H), 0.94 (d, J = 6.8 Hz, 3H, 28-H), 0.89 – 0.81 (t, J = 6.4 Hz, 9H, 18/26/27-H). 13C NMR (101 MHz, CD3OD): δ 147.35, 140.78, 137.06, 133.12, 126.72, 111.72, 76.25 (C7), 71.42 (C8 or C3), 71.39 (C3 or C8), 61.04, 58.79, 47.60, 45.14, 44.36, 41.70, 41.53, 39.23, 37.21, 34.50, 34.38, 29.13, 22.87, 21.76, 21.29, 20.52, 20.12, 18.27, 13.59. ESI-HRMS: calculated for C28H46O3Na [M+Na]+ 453.3345, found 453.3352.
2.2.2. (S,Z)-3-(2-hydroxyethylidene)-4-methylenecyclohexanol(3) and (4S,7aR)-1-((2R,5R,E)-5,6 - dimethylhept-3-en-2-yl)-7a-methyloctahydro-1H-inden-4-ol (4)
To a suspension of 2 (6.0 g, 13.9 mmol) and sodium carbonate (14.6 g, 138 mmol) in dichloromethane (50 mL) was added lead tetraacetate (6.7 g, 15.3 mmol) in portions at 0 °C. After 2 hours, the reaction was quenched with ethylene glycol and the mixture was vigorously stirred at 0 °C for 10 minutes. Water was then added and the mixture was extracted with dichloromethane for five times, washed with brine and dried with Na2SO4. The combined extracts were evaporated to give crude oil mixture, which was used for next step without further purification. To a solution of the above crude oily mixture in benzene (100 mL) at 0 °C under argon with stirring, Red-Al (28.7 mmol, 8.6 mL) was added dropwise and the mixture was stirred for 2 hours. On completion reaction was quenched with water. The mixture was filtered off and extracted with ethyl acetate, washed with brine and dried with anhydrous Na2SO4. Organic phase was evaporated to generate crude oil mixture which was purified by flash chromatography on silica. Elution with hexane/ethyl acetate (10:1) gave pure alcohol compound 4 as transparent oil (3.3 g, 86% for two steps), further elution with hexane/ethyl acetate (1:2) gave pure allylic alcohol 3 as colorless oil (0.91 g, 43% for two steps). compound 3: 1H NMR (400 MHz, CDCl3): δ 5.45 (t, J = 6.6 Hz, 1H, 6-H), 4.97 (dd, J = 2.4, 1.3 Hz, 1H, 19-H), 4.59 (d, J = 2.3 Hz, 1H, 19-H), 4.25 (ddd, J = 12.7, 7.0, 1.2 Hz, 1H, 7-H), 4.14 (ddd, J = 12.6, 6.2, 1.3 Hz, 1H, 7-H), 3.94 (m, 1H, 3-H), 2.56 – 2.33 (m, 2H), 2.24 (dd, J = 13.2, 7.1 Hz, 1H), 2.19 – 2.06 (m, 1H), 1.87 (m, 1H), 1.75 – 1.60 (m, 1H). 13C NMR (101 MHz, CDCl3): δ 144.51 (C10), 139.11 (C5), 125.95 (C6), 112.23 (C19), 68.64 (C3), 59.34 (C7), 44.82, 34.62, 31.45. ESI-HRMS: calculated for C9H11, [M-2H2O+H]+ 119.0861, found 119.0861. compound 4: 1H NMR (400 MHz, CDCl3): δ 5.25 – 5.02 (m, 2H, C22/23-H), 4.02 (d, J = 2.8 Hz, 1H, C8-H), 2.04 – 1.88 (m, 2H), 1.86 – 1.73 (m, 3H), 1.73 – 1.60 (m, 1H), 1.56 – 1.33 (m, 5H), 1.33 – 1.02 (m, 5H), 0.96 (d, J = 6.6 Hz, 3H, 22-H), 0.92 (s, 3H, C18-H), 0.89 (d, J = 11.2 Hz, 3H, 23-H), 0.80 (t, J = 6.6 Hz, 6H, C26/27-H). 13C NMR (101 MHz, CDCl3): δ 135.59, 131.70, 69.14 (C8), 56.48, 52.72, 42.78, 41.65, 40.32, 39.75, 33.53, 33.00, 27.57, 22.46, 20.71, 19.88, 19.55, 17.57, 17.40, 13.61. ESI-HRMS: calculated for C19H33 [M-H2O+H]+ 261.2582, found 261.2582.
2.2.3. (S,Z)-tert-butyl(2-(5-((tert-butyldimethylsilyl)oxy)-2-methylenecyclohexylidene)ethoxy)dimethylsilane (5)
Imidazole (1.4 g, 21.2 mmol) and tert-butyldimethylsilyl chloride (4.8 g, 31.6 mmol) was sequentially added to a solution of compound 3 (0.82 g, 5.3 mmol) in dichloromethane (50 mL) in a round bottom flask with stirring. The resulting suspension was stirred for 2 hours. Water was then added and the mixture was extracted with dichloromethane, washed with brine and dried with anhydrous Na2SO4. Evaporation under vacuum gave the oily residue which was subjected to flash chromatography on silica. Elution with hexane/ethyl acetate (80:1) gave pure compound 5 as colorless oil (1.8 g, 88%). 1H NMR (400 MHz, CDCl3): δ 5.38 (ddt, J = 6.8, 5.3, 1.3 Hz, 1H, 6-H), 4.95 (p, J = 1.1 Hz, 1H, 19-H), 4.57 (dd, J = 2.4, 1.3 Hz, 1H, 19-H), 4.31 (ddt, J = 13.0, 7.2, 0.9 Hz, 1H, 7-H), 4.21 (ddd, J = 12.9, 5.3, 2.1 Hz, 1H, 7-H), 3.82 (tt, J = 8.9, 3.9 Hz, 1H, 3-H), 2.47 – 2.31 (m, 2H), 2.22 – 2.13 (m, 1H), 2.06 (m, 1H), 1.93 – 1.80 (m, 1H), 1.56 (m, 1H), 0.89 (d, J = 4.7 Hz, 18H, TBS), 0.08 – 0.03 (s, 12H, TBS). 13C NMR (101 MHz, CDCl3): δ 144.89 (C10), 138.24 (C5), 126.29 (C6), 111.95 (C19), 70.11 (C3), 60.59 (C7), 45.93, 36.03, 32.15, 26.01 (TBS), 25.86 (TBS), 18.43, 18.18, −4.65 (TBS), −4.69 (TBS), −4.99 (TBS), −5.05 (TBS). ESI-HRMS: calculated for C21H42O2Si2Na [M+Na]+ 405.2621, found 405.2629.
2.2.4. (S,Z)-2-(5-((tert-butyldimethylsilyl)oxy)-2-methylenecyclohexylidene)ethanol (6)
To a solution of compound 5 (1.7 g, 4.4 mmol) in anhydrous THF (20 mL) in a round bottom flask at −5 °C under nitrogen, tetra-n-butylammonium fluoride (1.0 M in THF, 4.8 mmol) was added dropwise with stirring. The resulting mixture was stirred for 3 hours, water was then added and the brown mixture was extracted with dichloromethane, washed with brine and dried with anhydrous Na2SO4. The combined extracts were evaporated under vacuum to give the oily residue which was purified with flash chromatography on silica. Pure allylic alcohol 6 was eluted out with hexane/ethyl acetate (50:1) as colorless oil (0.93 g, 78%). 1H NMR (400 MHz, CDCl3): δ 5.44 (ddt, J = 7.3, 5.9, 1.3 Hz, 1H, 6-H), 4.96 (d, J = 1.2 Hz, 1H, 19-H), 4.62 (t, J = 2.4 Hz, 1H, 19-H), 4.43 – 4.01 (m, 2H, 7-H), 3.84 (m, 1H, 3-H), 2.48 – 2.33 (m, 2H), 2.26 – 2.14 (m, 1H), 2.13 – 2.01 (m, 1H), 1.87 (m, 1H), 1.58 (m, 1H), 0.88 (s, 9H, TBS), 0.06 (d, J = 2.9 Hz, 6H, TBS). 13C NMR (101 MHz, CDCl3): δ 144.83, 140.52, 124.88, 111.91, 70.11 (C3), 59.82 (C7), 46.02, 35.98, 32.26, 25.82 (TBS), 18.15, −4.67 (TBS), −4.71 (TBS). ESI-HRMS: calculated for C15H28O2SiNa [M+Na]+ 291.1756, found 291.1755.
2.2.5. (S,Z)-(2-(5-((tert-butyldimethylsilyl)oxy)-2-methylenecyclohexylidene)ethyl)diphenylphosphine oxide (7)
To a solution of the allylic alcohol 6 (0.81 g, 3.0 mmol) in anhydrous THF (20 mL) was added n-BuLi (2.5 M in hexanes, 30.0 mmol) under argon at 0 °C with stirring. After 5 minutes, a solution of p-tosyl chloride (598 mg, 3.15 mmol) dissolved in anhydrous THF (10 mL) was added to the allylic alcohol-BuLi solution. On the other side, to a solution of diphenylphosphine (1.02 mL, 6.0 mmol) in anhydrous THF (5 mL) under argon at 0 °C with stirring n-BuLi (2.5 M in hexanes, 2.2 mL, 5.7 mmol) was added dropwise to provide a red solution. After 10 minutes 4.1 mL of the red solution was then drawn to add to the solution of tosylate. The mixture was stirred for 30 minutes at 0 °C and quenched with H2O (1 mL). Evaporation of the solution under vacuum gave a phosphine residue. To the phosphine residue in dichloromethane (15 mL) at 0 °C with stirring was added 10% H2O2 (1.1 mL). Reaction was quenched with water, extracted with dichloromethane, washed with cold saturated sodium sulfite solution and brine and dried with Na2SO4. Organic solvent was removed under vacuum to give crude residue which was subjected to flash chromatography on silica for purification. Elution with hexane/ethyl acetate (3:1) gave pure phosphine oxide compound 7 as white solid (800 mg, 59%). Mp: 94–96 °C (EtOAc). 1H NMR (400 MHz, CDCl3): δ 7.71 (m, 4H, Ph), 7.56 – 7.36 (m, 6H, Ph), 5.35 (q, J = 7.2 Hz, 1H, 6-H), 4.92 (t, J = 1.9 Hz, 1H, 19-H), 4.68 (dd, J = 2.4, 1.3 Hz, 1H, 19-H), 3.52 (m, 1H, 3-H), 3.36 (m, 1H, 7-H), 3.20 (m, 1H, 7-H), 2.44 – 2.31 (m, 1H), 2.22 (dt, J = 13.3, 4.7 Hz, 1H), 2.15 – 2.05 (m, 1H), 1.81 – 1.71 (m, 1H), 1.66 (m, 1H), 1.47 (m, 1H), 0.83 (s, 9H, TBS), −0.00 (d, J = 2.0 Hz, 6H, TBS); 13C NMR (101 MHz, CDCl3): δ 145.03, 145.01, 142.62, 142.50, 133.49, 132.99, 132.52, 132.00, 131.75, 131.73, 131.61, 131.58, 131.20, 131.11, 130.95, 130.86, 128.59, 128.48, 128.44, 128.32, 113.19, 113.11, 111.60, 111.59, 70.46 (C3), 70.43, 46.68, 46.66, 36.21, 32.48, 32.46, 31.44, 30.74, 25.82 (TBS), 18.12, −4.69 (TBS), −4.75 (TBS). ESI-HRMS: calculated for C27H37O2PSiNa [M+Na]+ 475.2198, found 475.2203.
2.2.6. (4S,7aR)-1-((2R,5R,E)-5,6-dimethylhept-3-en-2-yl)-7a-methyloctahydro-1H-inden-4-yl acetate (8)
Pyridine (1.3 mL, 16.1 mmol), 4-dimethylaminopyridine (352 mg, 2.88 mmol) and acetyl anhydride (0.98 mL, 104 mmol) was sequentially added to a solution of compound 4 (3.2 g, 11.5 mmol) in anhydrous dichloromethane (30 mL) in a round bottom flask under argon. The resulting mixture was stirred for 3 hours. Water was then added and the mixture was extracted with dichloromethane, washed with saturated NaHCO3, 0.1 M HCl and brine and then dried with Na2SO4. Evaporation under vacuum gave the oily residue which was purified with flash chromatography on silica. Elution with hexane/ethyl acetate (30:1) gave pure compound 8 as colorless oil (3.4 g, 92%). 1H NMR (400 MHz, CDCl3): δ 5.31 – 5.00 (m, 3H, 8/22/23-H), 2.12 – 1.92 (m, 5H), 1.92 – 1.53 (m, 4H), 1.53 – 1.03 (m, 10H), 0.99 (d, J = 2.4 Hz, 3H, 22-H), 0.94 – 0.85 (m, 6H), 0.82 (t, J = 6.8 Hz, 6H, 26/27-H). 13C NMR (101 MHz, CDCl3): δ 170.73 (acetyl), 135.47, 131.90, 71.35 (C8), 56.31, 51.41, 42.79, 41.82, 39.90, 39.84, 33.05, 30.50, 27.47, 22.61, 21.31, 20.77, 19.93, 19.60, 17.90, 17.60, 13.17. ESI-HRMS: calculated for C21H36O2Na [M+Na]+ 343.2613, found 343.2614.
2.2.7. (4S,7aR)-1-((2S,3R,4R,5R)-3,4-dihydroxy-5,6-dimethylheptan-2-yl)-7a-methyloctahydro-1H-inden-4-yl acetate (9)
To a solution of the compound 8 (3.3 g, 10.3 mmol) in acetone (40 mL) was added N-methylmorpholine N-oxide (6.0 g, 51.5 mmol) and 2.5% osmium tetroxide in tert-butanol (6.5 mL, 0.52 mmol) at room temperature with stirring. After 48 hours, water and ethyl acetate was added and the organic phase was separated, washed with brine and dried with Na2SO4. Evaporation under vacuum gave the oily residue which was purified with flash chromatography on silica. Elution with hexane/ethyl acetate (10:1) gave pure compound 9 as colorless oil (2.44 g, 67%). 1H NMR (400 MHz, CDCl3): δ 5.14 (d, J = 2.6 Hz, 1H, 8-H), 3.71 (dd, J = 2.4, 4.4 Hz, 1H, 22-H), 3.59 (t, J = 2.8 Hz, 1H, 23-H), 2.39 (s, 1H), 2.04 (s, 4H), 1.94 – 1.79 (m, 2H), 1.70 (m, 5H), 1.55 – 1.09 (m, 9H), 1.00 (d, J = 6.9 Hz, 3H, 21-H), 0.96 (d, J = 6.7 Hz, 3H, 28-H), 0.92 – 0.84 (m, 9H, 18/26/27-H). 13C NMR (101 MHz, CDCl3): δ 170.80 (acetyl), 73.26 (C23), 71.19 (C8), 70.26 (C22), 53.02, 51.04, 44.11, 43.93, 42.45, 41.65, 40.00, 30.49, 29.89, 29.75, 26.91, 22.82, 21.44, 21.34, 18.94, 17.88, 13.91, 13.52, 12.83, 9.91. ESI-HRMS: calculated for C21H38O4Na [M+Na]+ 377.2668, found 377.2668.
2.2.8. (4S,7aR)-7a-methyl-1-((S)-1-oxopropan-2-yl)octahydro-1H-inden-4-yl acetate (10)
To a suspension of compound 9 (2.2 g, 6.2 mmol) in THF-H2O (4:1, 10 mL), sodium periodate (1.45 g, 6.82 mmol) was added at 0 °C in portions with stirring. After 1 hour, the reaction was quenched with ethylene glycol and the mixture was stirred at 0 °C for 10 minutes. Water was then added and the mixture was extracted with ethyl acetate for three times, washed with brine and dried with Na2SO4. The combined extracts were evaporated to give crude oil, which was purified with flash chromatography on silica. Elution with hexane/ethyl acetate (5:1) gave pure compound 10 as transparent oil (1.03 g, 66%). 1H NMR (400 MHz, CDCl3): δ 9.57 (d, J = 3.2 Hz, 1H, CHO), 5.17 (d, J = 2.0 Hz, 1H, 8-H), 2.36 (m, 1H, 20-H), 2.05 (s, 3H), 1.97 (dt, J = 12.3, 3.3 Hz, 1H), 1.89 – 1.71 (m, 3H), 1.59 – 1.24 (m, 8H), 1.11 (d, J = 6.8 Hz, 3H, 21-H), 0.93 (s, 3H, 18-H). 13C NMR (101 MHz, CDCl3): δ 204.78 (CHO), 170.70 (acetyl), 70.89 (C8), 51.37, 50.74, 49.17, 42.47, 39.75, 30.47, 26.00, 22.95, 21.32, 17.83, 13.42, 13.35. ESI-HRMS: calculated for C15H23O2 [M-H2O+H]+ 235.1698, found 235.1696.
2.2.9. (1S,4S,7aS)-1-acetyl-7a-methyloctahydro-1H-inden-4-yl acetate (12)
To a stirred solution of compound 10 (1.0 g, 3.9 mmol) in benzene (8 mL) was added morpholine (0.524 mL, 4.8 mmol) and p-toluenesulfonic acid (34mg, 0.195 mmol). The mixture was refluxed over a Dean-Stark water separator overnight. During the course of the reaction the theoretical amount of water (~70 mg) was separated. Upon completion as indicated by mass spectrum, the reaction mixture was cooled to room temperature and solvent was removed under vacuum to afford yellowish oil which was immediately used for next step.
To a stirred solution of the above crude mixture in acetone (20 mL) was added alumina (481 mg, 4.72 mmol) supported potassium permanganate (616 mg, 3.9 mmol) in portions. The reaction mixture was filtered after 2 hours and washed with ethyl acetate. Solvents were removed under vacuum to afford crude oily product which was purified with flash chromatography on silica. Elution with hexane/ethyl acetate (6:1) gave pure compound 12 as colorless oil (659 mg, 70% for two steps). 1H NMR (400 MHz, CDCl3): δ 5.16 (d, J = 2.8 Hz, 1H, 8-H), 2.48 (dd, J = 10.0, 8.0 Hz, 1H, 17-H), 2.20 – 1.99 (m, 8H), 1.92 – 1.82 (m, 1H), 1.70 – 1.39 (m, 8H), 0.81 (s, 3H, 18-H). 13C NMR (101 MHz, CDCl3): δ 208.52 (C20), 170.26 (acetyl), 70.27 (C8), 63.54, 51.15, 43.06, 38.85, 31.26, 30.08, 22.54, 21.46, 20.98, 17.64, 14.51 (C18). ESI-HRMS: calculated for C14H22O3Na [M+Na]+ 261.1467, found 261.1467.
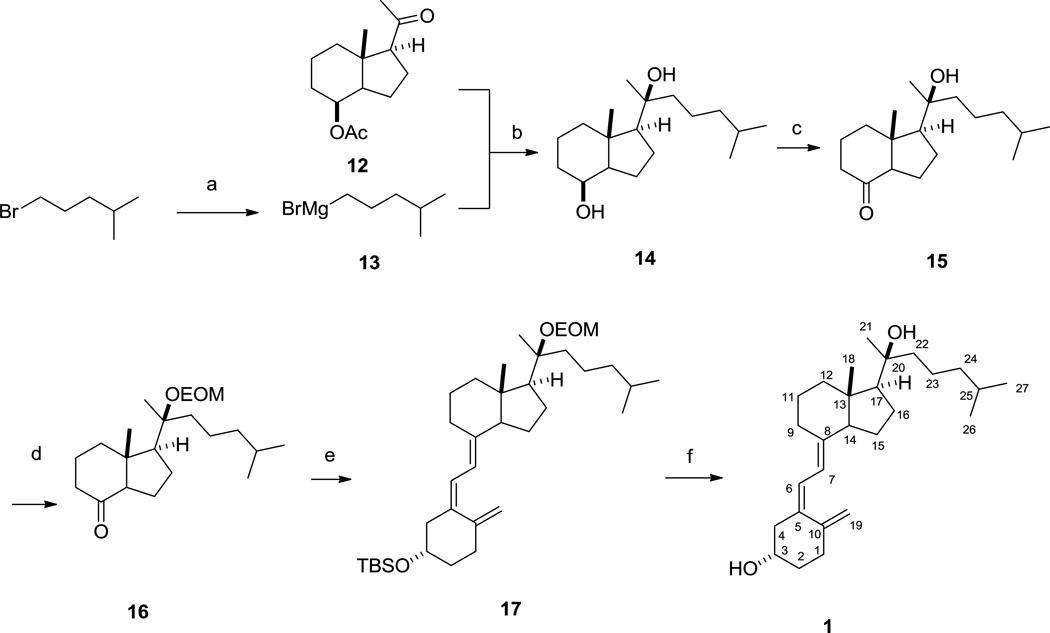
2.2.10. (1S,4S,7aS)-1-((S)-2-hydroxy-6-methylheptan-2-yl)-7a-methyloctahydro-1H-inden-4-ol (14)
To a stirred suspension of magnesium (181 mg, 7.5 mmol) in anhydrous THF (10 mL) was added 1-bromo-4-methyl-pentane (0.733 mL, 5.0 mmol) under argon at room temperature. The mixture was then refluxed for 2 hours and cooled down in ice bath to give Grignard reagent 13 which was used without further purification. Compound 12 (100 mg, 0.42 mmol) in 2 mL THF was subsequently added to the reaction mixture which was warmed to room temperature after 30 minutes and stirred overnight. Cooled saturated NH4Cl solution was added to quench the reaction and the mixture was extracted with ethyl acetate for three times, washed with brine and dried with Na2SO4. The combined extracts were evaporated to give crude oil, which was purified with flash chromatography on silica. Elution with hexane/ethyl acetate (3:1) afforded pure compound 14 as colorless oil (94 mg, 80%). 1H NMR (400 MHz, CDCl3): δ 4.09 (d, J = 2.8 Hz, 1H, 8-H), 2.14 – 2.02 (m, 1H), 1.91 – 1.74 (m, 3H), 1.74 – 1.37 (m, 9H), 1.37 – 1.08 (m, 14H), 0.87 (dd, J = 6.7, 0.8 Hz, 6H, 26/27-H). 13C NMR (101 MHz, CDCl3): δ 75.14 (C20), 69.41 (C8), 57.82, 52.55, 44.10, 42.52, 40.77, 39.56, 33.42, 27.92, 26.29, 22.71, 22.55, 22.21, 21.94, 21.30, 17.39, 15.44. ESI-HRMS: calculated for C18H31 [M-2H2O+H]+ 247.2426, found 247.2430.
2.2.11. (1S,7aR)-1-((S)-2-hydroxy-6-methylheptan-2-yl)-7a-methylhexahydro-1H-inden-4(2H)-one (15)
To a stirred solution of compound 14 (84 mg, 0.3 mmol) in dichloromethane (10 mL) was added pyridinium dichromate (225 mg, 0.6 mmol) at room temperature. The reaction mixture was stirred for 1 hour and filtered through celite. The celite was washed with dichloromethane and the combined solvent was removed under vacuum to give crude oily product which was purified with flash chromatography on silica. Elution with hexane/ethyl acetate (8:1) gave pure compound 15 as colorless oil (74 mg, 89%). 1H NMR (400 MHz, CDCl3): δ 2.43 (dd, J = 10.5, 6.9 Hz, 1H, 9-H), 2.33 – 2.13 (m, 3H, 9/12/14-H), 2.07 – 1.83 (m, 2H), 1.82 – 1.66 (m, 4H), 1.66 – 1.40 (m, 4H), 1.35 – 1.10 (m, 9H), 0.87 (d, J = 6.6 Hz, 6H, 26/27-H), 0.80 (s, 3H, 18-H)). 13C NMR (101 MHz, CDCl3): δ 211.99 (C8), 74.63 (C20), 62.10, 58.04, 49.84, 44.07, 40.80, 39.47, 39.26, 27.89, 26.53, 23.90, 22.67, 22.52, 21.95, 21.77, 18.73, 14.37. ESI-HRMS: calculated for C18H31O [M-H2O+H]+ 263.2375, found 263.2377.
2.2.12. (1S,7aR)-1-((S)-2-(ethoxymethoxy)-6-methylheptan-2-yl)-7a-methylhexahydro-1H-inden-4(2H)-one (16)
To a stirred solution of compound 15 (67 mg, 0.24 mmol), N,N-diisopropylethylamine (83 µL, 0.48 mmol) and 4-dimethylaminopyridine (12 mg, 0.096 mmol) in anhydrous dichloromethane (3 mL) was added chloromethyl ethyl ether (45 µL, 0.48 mmol) dropwise at ice temperature. The resulting mixture was stirred for overnight at room temperature. Water was then added and the reaction mixture was extracted with dichloromethane, washed with brine and dried with Na2SO4. The combined extracts were evaporated under vacuum to give the oily residue which was separated with flash chromatography on silica. Elution with hexane/ethyl acetate (30:1) gave pure compound 16 as colorless oil (73 mg, 90%). 1H NMR (400 MHz, CDCl3): δ 4.81 – 4.64 (m, 2H, EOM), 3.69 – 3.45 (m, 2H, EOM), 2.41 (dd, J = 11.5, 7.0 Hz, 1H), 2.32 – 2.06 (m, 3H), 2.03 – 1.43 (m, 11H), 1.31 (s, 3H, 21-H), 1.26 – 1.09 (m, 7H), 0.86 (dd, J = 6.6, 0.9 Hz, 6H, 26/27-H), 0.76 (s, 3H, 18-H). 13C NMR (101 MHz, CDCl3): δ 212.17 (C8), 88.76 (EOM), 79.75 (C20), 63.20 (EOM), 62.19, 57.31, 49.72, 40.83, 40.26, 39.70, 39.40, 27.87, 23.92, 23.21, 22.70, 22.57, 22.50, 21.70, 18.74, 15.14, 14.66. ESI-HRMS: calculated for C21H38O3Na [M+Na]+ 361.2719, found 361.2719.
2.2.13. tert-butyl(((1S,Z)-3-((E)-2-((1S,7aS)-1-((S)-2-(ethoxymethoxy)-6-methylheptan-2-yl)-7a-methylhexahydro-1H-inden-4(2H)-ylidene)ethylidene)-4-methylenecyclohexyl)oxy)dimethylsilane (17)
To a stirred solution of compound 7 (51 mg, 0.15 mmol) in anhydrous THF (3 mL) was added phenyllithium (1.8 M in hexanes, 0.37 mmol) under argon at −78 °C. The mixture was stirred for another 30 minutes at this temperature and was added precooled compound 16 (167 mg, 0.37 mmol) in 100µL anhydrous THF. Reaction was kept stirring at this temperature for 3 hours and further 12 hours at room temperature. Water was then added and the reaction mixture was extracted with ethyl acetate, washed with brine and dried with Na2SO4. The combined extracts were evaporated under vacuum to give the oily residue which was purified with flash chromatography on silica. Elution with hexane/ethyl acetate (30:1) gave pure compound 17 as colorless oil (45 mg, 52%). 1H NMR (400 MHz, CDCl3): δ 6.16 (d, J = 11.2 Hz, 1H, 6-H), 6.01 (d, J = 11.2 Hz, 1H, 7-H), 5.02 (s, 1H, 19-H), 4.85 – 4.64 (m, 3H, 19-H and EOM), 3.81 (m, 1H, H-3), 3.71 – 3.50 (m, 2H, EOM), 2.82 (d, J = 12.4 Hz, 1H, 9-H), 2.44 (dd, J = 13.0, 4.2 Hz, 1H), 2.36 (dt, J = 13.5, 4.8 Hz, 1H), 2.28 – 2.17 (m, 1H), 2.16 – 1.93 (m, 3H), 1.93 – 1.79 (m, 2H), 1.77 – 1.44 (m, 13H), 1.39 – 1.08 (m, 12H), 0.87 (d, J = 7.7 Hz, 15H, 26/27-H and TBS), 0.67 (s, 3H, 18-H), 0.06 (d, J = 2.2 Hz, 6H, TBS); 13C NMR (101 MHz, CDCl3): δ 145.34 (C10), 141.18 (C8), 136.36 (C5), 121.32 (C6), 118.21 (C7), 112.13 (C19), 88.80 (EOM), 80.42 (C20), 70.54 (C3), 63.14 (EOM), 57.38, 56.63, 46.84, 45.73, 40.98, 40.30, 39.79, 36.34, 32.73, 28.80, 27.94, 25.99, 25.96, 25.91, 25.88, 23.32, 22.97, 22.77, 22.55, 21.98, 21.91, 18.18, 15.19, 14.15, −4.59 (TBS), −4.63 (TBS). ESI-HRMS: calculated for C36H64O3SiNa [M+Na]+ 595.4522, found 595.4509.
2.2.14. (1S,Z)-3-((E)-2-((1S,7aS)-1-((S)-2-hydroxy-6-methylheptan-2-yl)-7a-methylhexahydro-1H-inden-4(2H)-ylidene)ethylidene)-4-methylenecyclohexanol (1)
To a stirred solution of compound 17 (10 mg, 0.017 mmol) in MeOH-DCM (5:1, 3 mL) was added camphorsulfonic acid (4.3 mg, 0.020 mmol) at room temperature. The mixture was stirred for 3 hours and saturated NaHCO3 was added. The mixture was extracted with dichloromethane, washed with brine and dried with Na2SO4. The combined extracts were evaporated under vacuo to give the oily residue which was purified with preparative TLC (hexane/ethyl acetate = 5:1) to afford pure compound 1 as colorless solid (2.6 mg, 37%). Mp: 91–93 °C (MeOH). 1H NMR (400 MHz, CD3OD): δ 6.22 (d, J = 11.2 Hz, 1H, 6-H), 6.02 (d, J = 11.2 Hz, 1H, 7-H), 5.08 – 5.00 (s, 1H, 19-H), 4.74 (d, J = 1.6 Hz, 1H, 19-H), 3.76 (m, 1H, 3-H), 2.85 (dd, J = 11.9, 4.0 Hz, 1H, 9-H), 2.58 – 2.48 (dd, 13.0, 4.2, 1H, 4-H), 2.40 (dt, J = 13.6, 4.9 Hz, 1H, 1-H), 2.25 – 1.91 (m, 5H), 1.81 – 1.62 (m, 5H), 1.61 – 1.43 (m, 6H), 1.43 – 1.09 (m, 11H), 0.89 (d, J = 6.6 Hz, 6H, 26/27-H), 0.69 (s, 3H, 18-H). 13C NMR (101 MHz, CD3OD): δ 147.00 (C10), 142.30 (C8), 137.34 (C5), 122.61 (C6), 119.37 (C7), 112.65 (C19), 75.99 (C20), 70.55 (C3), 59.89, 57.80, 47.02, 45.11, 42.25, 40.95, 36.60, 33.59, 29.84, 29.17, 26.15, 24.44, 23.16, 23.06, 23.02, 22.97, 14.14. ESI-HRMS: calculated for C27H44O2Na [M+Na]+ 423.3239, found 423.3235.
2.3. VDRE luciferase reporter assay
Dulbecco’s Modified Eagle Medium (DMEM) supplemented with glucose, L-gultamin, pyridoxine hydrochloride, together with 5% fetal bovine serum (FBS) and 1% penicillin/streptomycin/amphotericin antibiotic solution (Ab) (Sigma-Aldrich, St. Louis, MO) was used to culture immortalized human keratinocytes (HaCaT). Jurkat cells were cultured in RPMI 1640 medium supplemented with 10% FBS and 1% Ab. All cells were cultured at 37 °C in a humidified atmosphere containing 5% CO2. Jurkat and HaCaT cells were transduced with lentiviral VDRE luciferase using Cignal Lenti VDRE Reporter (luc) Kit according to manufacturer’s protocol (QIAGEN, Valencia, CA). After one week selection by puromycin (1 µg/mL), transduced HaCaT, Jurkat and Caco-2 cells were seeded in 96-well plate (1000 cells/well) using partial media (without FBS) for 24 h incubation. DMSO solutions of test compounds were added to cells, which were then incubated for another 24 h and measured for luciferase signal following the manufacturer’s procedure of ONE-Glo™ Luciferase Assay System (Promega, Madison, WI). The concentrations of DMSO were maintained consistently at 0.1% for compound treatment, and 0.1% DMSO were used as negative control. Concentration (100 nM) of 20S-(OH)D3 and 1,25(OH)2D3 was used and tested in triplicates.
2.4. PCR
HaCaT cells were cultured as described above. The RNA from HaCaT keratinocytes treated with 20S-(OH)D3, 1,25(OH)2D3 or DMSO, was isolated using the Absolutely RNA Miniprep Kit (Stratagen). Reverse transcription (100 ng RNA/reaction) was performed using the Transcriptor First Strand cDNA Synthesis Kit (Roche Inc, Mannheim, Germany). Real-time PCR was performed using cDNA diluted 10-fold in sterile water and a SYBR Green PCR Master Mix. Reactions (in triplicate) were performed at 50 °C for 2 min, 95 °C for 10 min and then 40 cycles of 95 °C for 15 sec, 60 °C for 30 sec and 72 °C for 30 sec. Data were collected on a Roche Light Cycler 480. The amount of amplified product for each gene was compared to that of β-actin as a housekeeping gene using a comparative Ct method [25].
2.5. Anti-proliferation assay
To test anti-proliferative effect of 20S-(OH)D3 in comparison to 1,25(OH)2D3 we measured [3H]-thymidine (Moravek Biochemicals Inc. Brea, CA) incorporation into the DNA of human SKEML-188 melanoma line as described previously[30]. Briefly, the cells were grown in 24-well plates using Ham’s F10 media (Mediatech, Inc. Manassas, VA, USA) containing 5% charcoal treated FBS (Atlanta Biologicals, Lawrenceville, GA, USA) at 37°C in 5% CO2 until 30% confluence. The cells were synchronized in serum free medium for 1 day and then grown in the media containing serum and 10−8 to 10−10 M of the compounds or vehicle for 72 hours. For the final 4 hours, the cells were incubated with [3H]-thymidine (final concentration of 0.5 µCi/mL). Afterwards media were replaced with 10% TCA in PBS (phosphate-buffered saline) and plates were washed twice with 1 mL PBS. The cells were lysed with 1 N NaOH/1% SDS, the extracts mixed with scintillation cocktail, and radioactivity was counted using Packard Matrix 9600 direct beta-counter (Packard, Meridan, CT, USA) as described previously [33].
3. Results
3.1. Chemistry
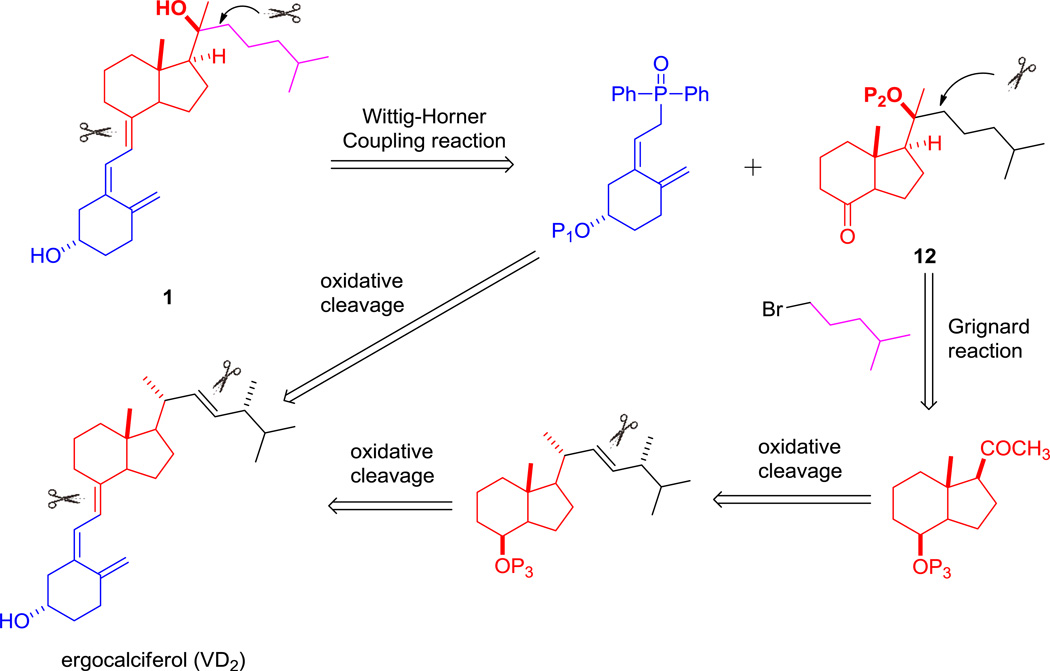
A retrosynthetic strategy was proposed as Scheme 1. The vitamin D3 scaffold could be established by Wittig-Horner coupling reaction between phosphine oxide 7 and C/D-ring fragment 16. Due to steric effect of 18-methyl, stereoselective generation of 20S-hydroxy of 16 could be achieved using Grignard reagent 13 to attack 12. In addition, introducing a relatively bulky protecting group on 8S-hydroxy was envisioned to assist the stereoselectivity of Grignard reaction. Conversion of VD2 to 7 and 12 could be carried out through oxidative cleavages.
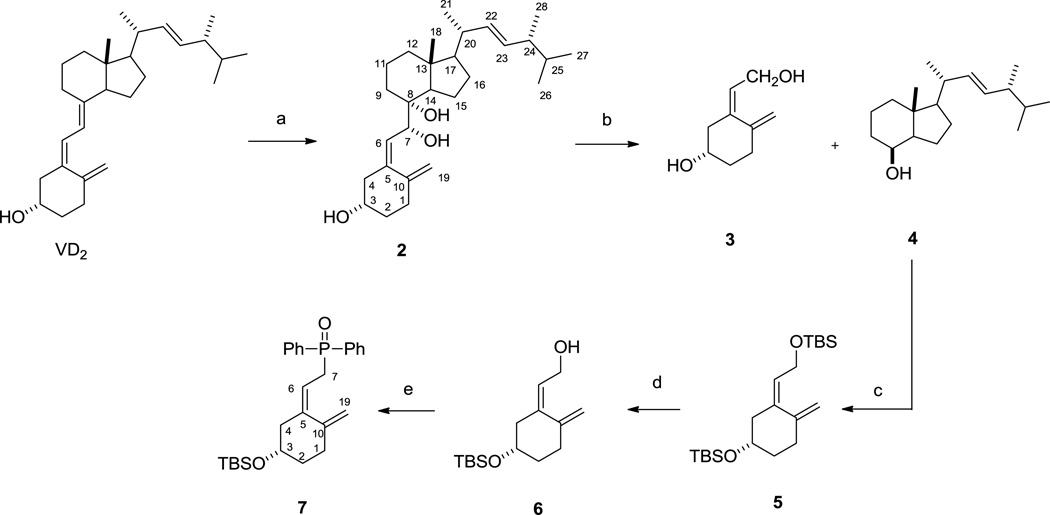
The synthesis of phosphine oxide 7 and protected C/D-ring ketone 12 were performed following literature reports [35, 36]. To prepare 7 and 12 in large scale so as to facilitate future structure-activity relationship studies, we modified the synthesis which were outlined as Scheme 2 and Scheme 3. Initially, commercially available ergocalciferol was regioselectively dihydroxylated on the triene linkage to provide triol 2 with a yield of 65% when treating with potassium permanganate in ethanol at low temperature. Due to steric effect of 18-methyl, single 7R,8R-diostereoisomer was exclusively isolated over chromatography [35]. It is worth mentioning that when 3-OH was protected with TBS, dihydroxylation of the triene did not proceed. Subsequent treatment of vicinol 2 with lead tetraacetate in dichloromethane in the presence of sodium carbonate led to formation of corresponding C/D-ring ketone and A-ring allylic aldehyde. Although complete consumption of 2 was indicated on TLC, after workup procedures and purification using flash chromatography, A-ring allylic aldehyde ended up with poor yield (<20%) while C/D-ring ketone was obtained in 94% yield. We found that A-ring allylic aldehyde was unstable when exposed to air and moisture as clearly indicated by its spot on TLC and it should be used immediately. Thus, after quenching the oxidation reaction with ethylene glycol followed by simple workup procedure the crude oily mixture was directly used without purification to the following reduction step using excess equivalent of sodium bisaluminumhydride (65% in toluene) in benzene at 0 °C. Gratifyingly, these modified procedures afforded pure A-ring allylic alcohol 3 and C/D-ring alcohol 4 with yield of 43% and 86% for two steps after chromatography, respectively. It is worth noting that after TLC indicated completion of the oxidation reaction, quenching the reaction by adding ethylene glycol was crucial to prevent further oxidation of the aldehyde to carboxylic acid. In addition, compared with lithium aluminum hydride (LAH) and diisobutylaluminium hydride (DIBAL-H in toluene or tetrahydrofuran), Red-Al (>65% in toluene) was more efficient and regioselective toreduce only aldehyde rather than conjugated diene to form 3. Compound 3 was subsequently protected with TBS to generate 5, which underwent selective removal of TBS on the allylic moiety to produce alcohol 6 in 71% yield for two steps when stirring with tetra-n-butylammonium fluoride (TBAF) in THF under low temperature (−5 °C to ice temperature). The final A-ring intermediate phosphine oxide 7 was prepared following literature procedures in three steps with 59% overall yield [36].
Fig. 2.
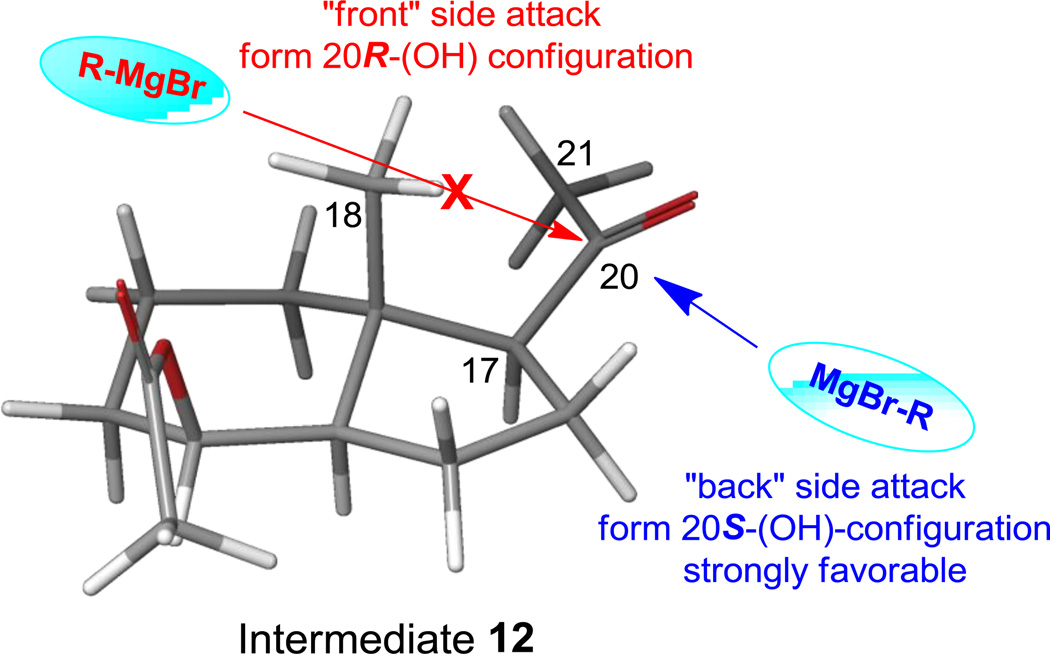
Preferred conformation of the intermediate (compound 12) calculated with density function theory with 6–31G** baseset. “Front” side attack by the bulky Grignard agent 13 to form 20R isomer of 14 is prohibited due to the steric hindrance from the 18-methyl and other moieties in compound 12, while the “back” side attack to form 20S isomer is strongly favored.
Fig. 3.
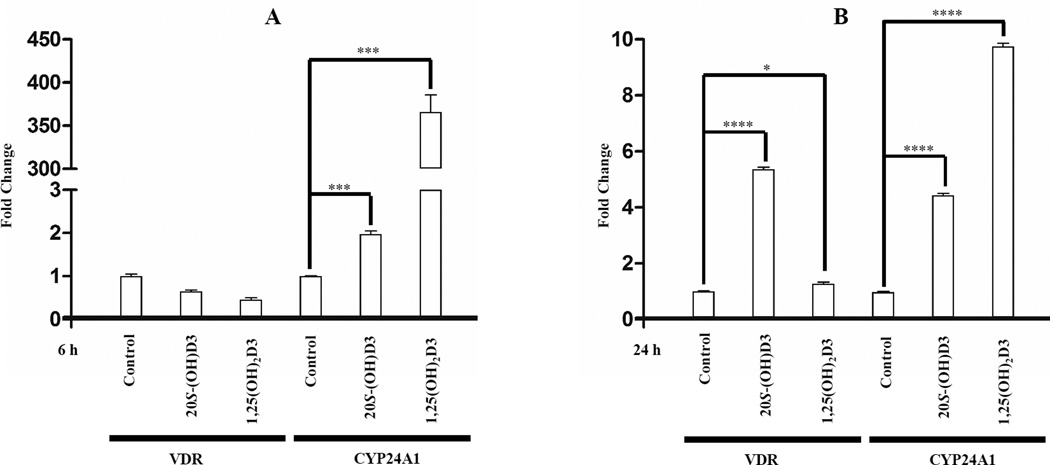
20S-(OH)D3 stimulates mRNA levels of VDR and CYP24A1 at 6 h (A) and 24 h (B). HaCaT cells were treated for 6 h with 100 nM of 20S-(OH)D3, 1,25(OH)2D3 or DMSO only (solvent) as a control. The mRNA was isolated and qPCR performed using specific primers for VDR and CYP24A1 genes. Data are presented as mean ± SE (n = 3, * p < 0.05, *** p < 0.005, **** p < 0.0001).
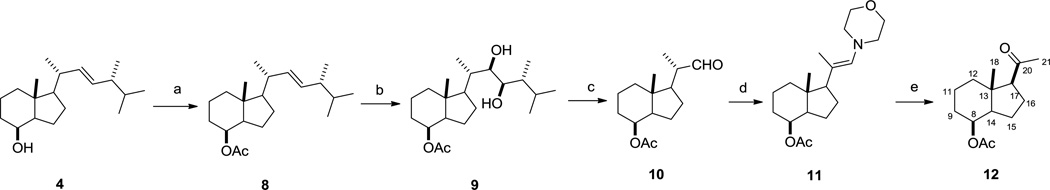
Acetate 8 was produced when secondary alcohol 4 was treated with acetic anhydride and pyridine. Compound 8 was subjected to Upjohn reaction stirring with osmium tetroxide (0.5% wt in t-butanol) and N-methylmorpholine N-oxide in acetone at ambient temperature for 48 hr to form the major diastereoisomer 9 due to strong steric effect of 21- and 24-methyl groups. While TLC indicated existence of the other diastereomer as minor product, 9 was isolated in 67% yield. Cleavages of vicinol 9 in the presence of sodium periodate and sodium carbonate in H2O-THF (1:4) provided aldehyde 10 in 66% yield. Aldehyde 10 was then refluxed in a Dean-Stark apparatus stirring with morpholine and catalytic amount of p-toluenesulfonic acid in benzene to form crude enamine 11 as, after dried on vacuum, slightly yellowish oil. Due to its instability and sensitivity to moisture, the crude enamine mixture was used for next step immediately without further purification. While the removal of C-22 on the chiron could be accomplished when treating 11 with singlet oxygen [36], this photo-oxygenation procedure was rather tedious. By referring to an alternative strategy [37], the yellowish oil was dissolved in acetone and treated with alumina supported potassium permanganate that was made prior to use, to provide the other key intermediate 12 in 70% for two steps after chromatography.
As shown in Scheme 4, Grignard reagent 13 was readily accessible by refluxing commercially available 1-bromo-4-methyl-pentane with magnesium in anhydrous THF. After 2 hours, 13 was immediately subjected to Grignard reaction to form the diol 14 with 80% yield. Similar to our previous studies [33], only the 20S-epimer was isolated over chromatography due to the steric effect of both 18-methyl and 8-acetyl. This stereo-specificity is further supported by quantum mechanical calculations employing density functional theory (DFT) using Schrodinger Molecular Modeling Suite 2015. Figure 2 shows the thermodynamically most stable and the preferred orientation of the acetyl group for the intermediate 12. This conformational preference dictated the strongly favored back-side attack by the Grignard reagent 13 to form 14 with 20S-OH configuration. The attack from the front side to form 20R-OH configuration is sterically prohibited, consistent with what we found previously [33, 38]. Oxidation of diol 14 using Cornforth reagent pyridinium dichloromate (PDC) gave 20S-hydroxy Grundmann`s ketone 15 in 89% yield. Compound 15 was subsequently introduced protecting group EOM on its tertiary alcohol to form ketone 16, the other key intermediate for the final coupling step. With both C/D-ring fragment 16 and phosphine oxide 7 in hand, construction of the 20S-(OH)D3 framework was successfully accomplished by employing Wittig-Horner reaction in the presence of phenyllithium (0.18 M, diluted from commercial source) in THF to form 17 in 52% yield. Simultaneously removal of EOM and TBS was achieved using camphorsulfonic acid (CSA) to produce the target compound 20S-(OH)D3 in 37% yield.
Fig. 4.
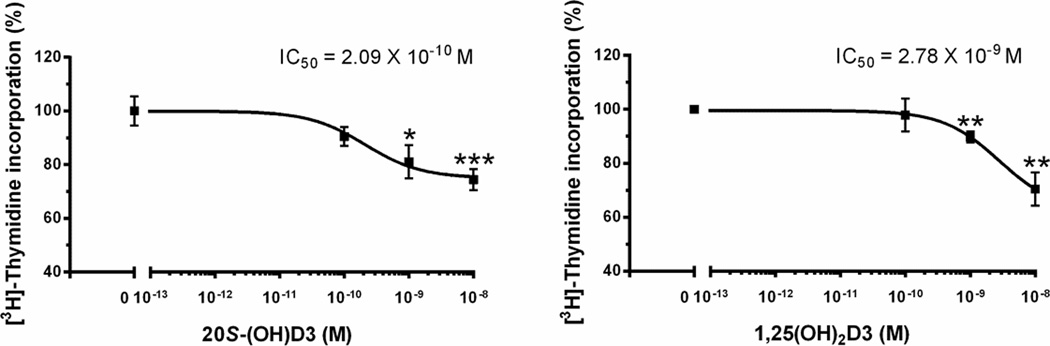
Anti-proliferative effects of 20S-(OH)D3 and 1,25(OH)2D3 on human SKEML-188 melanoma cells. Cells were treated with 20S-(OH)D3, 1,25(OH)2D3 or DMSO (solvent) as a control to assess their inhibitory effects on DNA synthesis. Data are presented as mean ± SE (n = 3, * p < 0.05, ** p < 0.01, *** p < 0.005).
3.2. Biological assays
3.2.1. 20S-(OH)D3 activates VDR
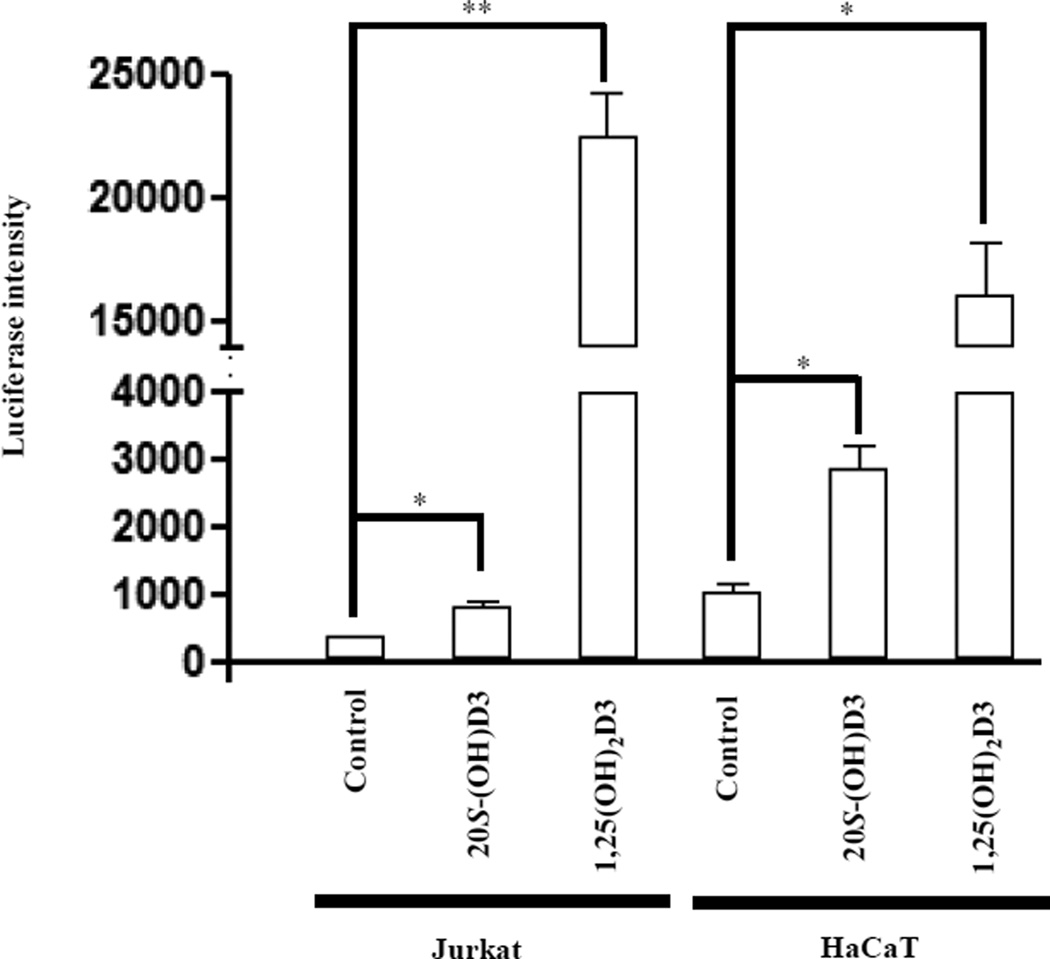
To confirm that the synthetic 20S-(OH)D3 can activate VDR, two cell lines (Jurkat and HaCaT) were transduced by VDRE luciferase vector to establish corresponding reporter assays. We compared the VDR activation activity of 20S-(OH)D3 with that of 1,25(OH)2D3 as shown in Figure 3. At a concentration of 100 nM, both 20S-(OH)D3 and 1,25(OH)2D3 can activate VDR as compared with control in both cell lines, however, 1,25(OH)2D3 showed much stronger activity than 20S-(OH)D3 suggesting deficiency in coupling of 20S-(OH)D3 with activation of genes having VDRE consensus in their promoter. These results are consistent with results obtained using enzymatically generated 20S-(OH)D3 [25] indicating that 20S-(OH)D3 acts as a biased agonist on the VDR [23].
3.2.2. 20S-(OH)D3 stimulates the mRNA levels of VDR and CYP24A1
Since the CYP24A1 gene is an important target of 1,25(OH)2D3 activation, we compared the action of 20S-(OH)D3 with that of 1,25(OH)2D3 on the expression of VDR itself and CYP24A1 genes in HaCaT cells at different time points as shown in Figure 3. After 6 hrs of treatment with 100 nM of the compound, cells exposed to 1,25(OH)2D3 showed 350 fold increased expression of CYP24A1 and only 2- to 3-fold after treatment with 20S-(OH)D3, which is consistent with previously reported data [23]. Interestingly, 20S-(OH)D3 is much stronger inducer of VDR gene expression than 1,25(OH)2D3 (Fig. 3B).
3.2.3. Anti-proliferation activity of 20S-(OH)D3
As shown in Figure 4,20S-(OH)D3 showed potent anti-proliferative and dose-dependent effect on SKMEL-188 melanoma cells comparable to classical active form of vitamin D3, 1,25(OH)2D3. 20S(OH)D3 gave an IC50 of 2.09 × 10−10 M while the IC50 of 1,25(OH)2D3 was 2.78 × 10−9 M. This result is consistent with previous studies showing that 20S-(OH)D3 shows equal or higher potency for inhibition of melanoma cell proliferation in comparison to 1,25(OH)2D3 [27, 39].
4. Discussion
20S-(OH)D3, a noncalcemic vitamin D3 analog, was enzymatically converted from vitamin D3 by P450scc and was first chemically synthesized via UVB irradiation [33]. However, this photochemical method also produces structurally similar and physicochemically active by-products that present a significant challenge for purification and further SAR studies for the promising 20S-hydroxyl scaffold. Therefore, we seek to develop an alternative synthetic method. The total synthetic strategy described in this report involves Wittig-Horner coupling and Grignard reaction and is successful in generating 20S-(OH)D3 in 0.4% overall yield in 16 steps. A distinct advantage of this method over our previously reported method is that after we generate the two key intermediates phosphine oxide 7 and C/D-ring ketone 12 on gram scale from a common starting material vitamin D2, we can efficiently make side chain derivatives for in-depth SAR studies of this 20S-hydroxyl scaffold.
As the native ligand of VDR, 1,25(OH)2D3 activated VDR (Figure 5) and initiated the rapid transcriptional activity inside the nucleus. This included highly enhanced expression of CYP24A1 (~ 350-fold) after 6 h treatment. In contrast, 20S-(OH)D3 showed weak stimulation of CYP24A1 gene expression consistent with our previous data identifying 20S-(OH)D3 as the biased agonist for the VDR [23]. Enhanced expression of VDR by treatment with 20S-(OH)D3 with higher potency than that of 1,25(OH)2D3 further emphasized certain differences with this classical agonist, and suggested amplification of VDR mediated effects, a subject warranting further studies.
Fig. 5.
20S-(OH)D3 activates luciferase activity in VDRE-LUC transduced Jurkat and HaCaT cells. Cells were treated for 24 h with 100 nM 20S-(OH)D3, 1,25(OH)2D3 or DMSO as a control. Data are presented as mean ± SE (n = 3, * p < 0.05, ** p < 0.01).
In conclusion, we have established an efficient total synthesis of biologically active 20S-(OH)D3, which involved divergently generation of key intermediates 7 and 12 from a common starting material VD2 via modified methods, stereoselective formation of the 20S-hydroxyl scaffold through Grignard reaction and simultaneously removal of EOM and TBS using CSA. Results from various biological assays demonstrated the similarity of 20S-(OH)D3 produced through this total synthesis method with the authentic compound generated enzymatically. Further modifications on the side chain to generate 20S-(OH)D3 analogs for SAR study will be carried out via nucleophilic attack on intermediate ketone 12 before it will be coupled to the other key intermediate 7.
Supplementary Material
Scheme 1.
Retrosynthetic analysis of 20S-(OH)D3
Scheme 2.
Synthesis of intermediate 7 Reagents: (a): KMnO4, EtOH-H2O; 65%; (b): i. Pb(OAc)4, Na2CO3, DCM; ii. Red-Al, benzene; 43% for 3 and 86% for 4 in two steps; (c): TBSCl, Imidazole, DCM; 88%; (d): TBAF, THF; 78%; (e): i. n-BuLi, p-TsCl, THF; ii. n-BuLi, Ph2PH, THF; iii. H2O2, H2O, DCM; 59% in three steps.
Scheme 3.
Synthesis of intermediate 12 Reagents: (a): Ac2O, Pyr, DMAP, DCM; 92%; (b): OsO4, NMO, Acetone/t-BuOH; 67%; (c): NaIO4, H2O-THF; 66% (d): Morpholine, p-TsOH, PhH, reflux; (e): KMnO4/Al2O3, Acetone; 70% in two steps.
Scheme 4.
Synthesis of 20S-(OH)D3 Reagents: (a): Mg. THF; (b): THF;80% (c): PDC, DCM; 89%; (d): EOMCl, DMAP, TEA, DCM; 90%; (e): 7, PhLi, THF; 53%; (f): CSA, DCM-MeOH; 37%.
Highlights.
Key intermediates 7 and 12 were generated in parallel via modified synthetic ways
Simultaneous removal of EOM and TBS was completed using camphorsulfonic acid
20S-hydroxyvitamin D3 was synthesized in 16 steps with an overall yield of 0.4%
Synthetic 20S-(OH)D3 activated VDR and initiated expression of downstream genes
20S-(OH)D3 was potent in inhibition of melanoma cells proliferation
Acknowledgements
This work was supported by NIH grants 1R21AR063242-01A1 (W.L. and D.D.M.), 1S10OD010678-01 (W.L.), 1S10RR026377-01 (W.L.), 2R01AR052190-(A.S.) and R21 AR066505-01A1 (A.S.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- D3
Vitamin D3
- 20S-(OH)D3
20S-hydroxyvitamin D3
- 1,25(OH)2D3
1α,25-dihydroxyvitamin D3
- EOM
Ethoxymethyl
- TBS
t-butyldimethysilyl
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:
References
- 1.Cheng JB, Motola DL, Mangelsdorf DJ, Russell DW. De-orphanization of cytochrome P450 2R1: a microsomal vitamin D 25-hydroxilase. J Biol Chem. 2003;278:38084–38093. doi: 10.1074/jbc.M307028200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeLuca HF. Vitamin D: the vitamin and the hormone. Fed Proc. 1974;33:2211–2219. [PubMed] [Google Scholar]
- 3.Pike JW. Intracellular receptors mediate the biologic action of 1,25-dihydroxyvitamin D3. Nutr Rev. 1985;43:161–168. doi: 10.1111/j.1753-4887.1985.tb02406.x. [DOI] [PubMed] [Google Scholar]
- 4.Korf H, Decallonne B, Mathieu C. Vitamin D for infections. Curr Opin Endocrinol Diabetes Obes. 2014;21:431–436. doi: 10.1097/MED.0000000000000108. [DOI] [PubMed] [Google Scholar]
- 5.Bikle DD. Vitamin D receptor, a tumor suppressor in skin. Can J Physiol Pharmacol. 2015;93:349–354. doi: 10.1139/cjpp-2014-0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiang KC, Chen TC. The anti-cancer actions of vitamin D. Anticancer Agents Med Chem. 2013;13:126–139. [PubMed] [Google Scholar]
- 7.Li YC. Vitamin D receptor signaling in renal and cardiovascular protection. Semin Nephrol. 2013;33:433–447. doi: 10.1016/j.semnephrol.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plum LA, DeLuca HF. Vitamin D, disease and therapeutic opportunities. Nature Reviews Drug Discovery. 2010;9:941–955. doi: 10.1038/nrd3318. [DOI] [PubMed] [Google Scholar]
- 9.Kulie T, Groff A, Redmer J, Hounshell J, Schrager S. Vitamin D: an evidence-based review. J Am Board Fam Med. 2009;22:698–706. doi: 10.3122/jabfm.2009.06.090037. [DOI] [PubMed] [Google Scholar]
- 10.Cheung FS, Lovicu FJ, Reichardt JK. Current progress in using vitamin D and its analogs for cancer prevention and treatment. Expert Rev Anticancer Ther. 2012;12:811–837. doi: 10.1586/era.12.53. [DOI] [PubMed] [Google Scholar]
- 11.Jones G. Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr. 2008;88:582S–586S. doi: 10.1093/ajcn/88.2.582S. [DOI] [PubMed] [Google Scholar]
- 12.Stein MS, Wark JD. An update on the therapeutic potential of vitamin D analogues. Expert Opin Investig Drugs. 2003;12:825–840. doi: 10.1517/13543784.12.5.825. [DOI] [PubMed] [Google Scholar]
- 13.Leyssens C, Verlinden L, Verstuyf A. The future of vitamin D analogs. Front Physiol. 2014;5:122. doi: 10.3389/fphys.2014.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sherman MH, Yu RT, Engle DD, Ding N, Atkins AR, Tiriac H, et al. Vitamin d receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell. 2014;159:80–93. doi: 10.1016/j.cell.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev. 2011;32:81–151. doi: 10.1210/er.2010-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tuckey RC. Progesterone synthesis by the human placenta. Placenta. 2005;26:273–281. doi: 10.1016/j.placenta.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 17.Slominski AT, Li W, Kim TK, Semak I, Wang J, Zjawiony JK, et al. Novel activities of CYP11A1 and their potential physiological significance. J Steroid Biochem Mol Biol. 2014 doi: 10.1016/j.jsbmb.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slominski A, Zjawiony J, Wortsman J, Semak I, Stewart J, Pisarchik A, et al. A novel pathway for sequential transformation of 7-dehydrocholesterol and expression of the P450scc system in mammalian skin. Eur J Biochem. 2004;271:4178–4188. doi: 10.1111/j.1432-1033.2004.04356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slominski A, Semak I, Wortsman J, Zjawiony J, Li W, Zbytek B, et al. An alternative pathway of vitamin D metabolism. Cytochrome P450scc (CYP11A1)-mediated conversion to 20-hydroxyvitamin D2 and 17,20-dihydroxyvitamin D2. FEBS J. 2006;273:2891–2901. doi: 10.1111/j.1742-4658.2006.05302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slominski A, Semak I, Zjawiony J, Wortsman J, Li W, Szczesniewski A, et al. The cytochrome P450scc system opens an alternate pathway of vitamin D3 metabolism. FEBS J. 2005;272:4080–4090. doi: 10.1111/j.1742-4658.2005.04819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tuckey RC, Li W, Zjawiony JK, Zmijewski MA, Nguyen MN, Sweatman T, et al. Pathways and products for the metabolism of vitamin D3 by cytochrome P450scc. FEBS J. 2008;275:2585–2596. doi: 10.1111/j.1742-4658.2008.06406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guryev O, Carvalho RA, Usanov S, Gilep A, Estabrook RW. A pathway for the metabolism of vitamin D3: unique hydroxylated metabolites formed during catalysis with cytochrome P450scc (CYP11A1) Proc Natl Acad Sci U S A. 2003;100:14754–14759. doi: 10.1073/pnas.2336107100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slominski AT, Kim TK, Li W, Yi AK, Postlethwaite A, Tuckey RC. The role of CYP11A1 in the production of vitamin D metabolites and their role in the regulation of epidermal functions. J Steroid Biochem Mol Biol. 2014;144(Pt A):28–39. doi: 10.1016/j.jsbmb.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janjetovic Z, Zmijewski MA, Tuckey RC, DeLeon DA, Nguyen MN, Pfeffer LM, et al. 20-Hydroxycholecalciferol, product of vitamin D3 hydroxylation by P450scc, decreases NF-kappaB activity by increasing IkappaB alpha levels in human keratinocytes. PLoS One. 2009;4:e5988. doi: 10.1371/journal.pone.0005988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zbytek B, Janjetovic Z, Tuckey RC, Zmijewski MA, Sweatman TW, Jones E, et al. 20-Hydroxyvitamin D3, a product of vitamin D3 hydroxylation by cytochrome P450scc, stimulates keratinocyte differentiation. J Invest Dermatol. 2008;128:2271–2280. doi: 10.1038/jid.2008.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Slominski AT, Janjetovic Z, Fuller BE, Zmijewski MA, Tuckey RC, Nguyen MN, et al. Products of vitamin D3 or 7-dehydrocholesterol metabolism by cytochrome P450scc show anti-leukemia effects, having low or absent calcemic activity. PLoS One. 2010;5:e9907. doi: 10.1371/journal.pone.0009907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Slominski AT, Janjetovic Z, Kim TK, Wright AC, Grese LN, Riney SJ, et al. Novel vitamin D hydroxyderivatives inhibit melanoma growth and show differential effects on normal melanocytes. Anticancer Res. 2012;32:3733–3742. [PMC free article] [PubMed] [Google Scholar]
- 28.Chen J, Wang J, Kim TK, Tieu EW, Tang EK, Lin Z, et al. Novel vitamin D analogs as potential therapeutics: metabolism, toxicity profiling, and antiproliferative activity. Anticancer Res. 2014;34:2153–2163. [PMC free article] [PubMed] [Google Scholar]
- 29.Slominski A, Janjetovic Z, Tuckey RC, Nguyen MN, Bhattacharya KG, Wang J, et al. 20S-hydroxyvitamin D3, noncalcemic product of CYP11A1 action on vitamin D3, exhibits potent antifibrogenic activity in vivo. J Clin Endocrinol Metab. 2013;98:E298–E303. doi: 10.1210/jc.2012-3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Slominski AT, Kim TK, Janjetovic Z, Tuckey RC, Bieniek R, Yue J, et al. 20-Hydroxyvitamin D2 is a noncalcemic analog of vitamin D with potent antiproliferative and prodifferentiation activities in normal and malignant cells. Am J Physiol Cell Physiol. 2011;300:C526–C541. doi: 10.1152/ajpcell.00203.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Slominski AT, Kim TK, Takeda Y, Janjetovic Z, Brozyna AA, Skobowiat C, et al. RORalpha and ROR gamma are expressed in human skin and serve as receptors for endogenously produced noncalcemic 20-hydroxy- and 20,23-dihydroxyvitamin D. FASEB J. 2014;28:2775–2789. doi: 10.1096/fj.13-242040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim TK, Wang J, Janjetovic Z, Chen JJ, Tuckey RC, Nguyen MN, et al. Correlation between secosteroid-induced vitamin D receptor activity in melanoma cells and computer-modeled receptor binding strength. Molecular and Cellular Endocrinology. 2012;361:143–152. doi: 10.1016/j.mce.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li W, Chen J, Janjetovic Z, Kim TK, Sweatman T, Lu Y, et al. Chemical synthesis of 20S-hydroxyvitamin D3, which shows antiproliferative activity. Steroids. 2010;75:926–935. doi: 10.1016/j.steroids.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tuckey RC, Janjetovic Z, Li W, Nguyen MN, Zmijewski MA, Zjawiony J, et al. Metabolism of 1 alpha-hydroxyvitamin D3 by cytochrome P450scc to biologically active 1 alpha,20-dihydroxyvitamin D3. Journal of Steroid Biochemistry and Molecular Biology. 2008;112:213–219. doi: 10.1016/j.jsbmb.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kutner A, Zhao H, Fitak H, Wilson SR. Synthesis of Retiferol Rad(1) and Rad(2), the Lead Representatives of a New Class of Des-Cd Analogs of Cholecalciferol. Bioorganic Chemistry. 1995;23:22–32. [Google Scholar]
- 36.Posner GH, Crawford K, Siu-Caldera ML, Reddy GS, Sarabia SF, Feldman D, et al. Conceptually new 20-epi-22-oxa sulfone analogues of the hormone 1alpha,25-dihydroxyvitamin D(3): synthesis and biological evaluation. J Med Chem. 2000;43:3581–3586. doi: 10.1021/jm000215j. [DOI] [PubMed] [Google Scholar]
- 37.Harris CE, Chrisman W, Bickford SA, Lee LY, Torreblanca AE, Singaram B. Enamine oxidations.2. Selective oxidative cleavage of beta, beta-disubstituted enamines using alumina supported permanganate. Synthesis of one-carbon dehomologated carbonyl compounds from enamines. Tetrahedron Letters. 1997;38:981–984. [Google Scholar]
- 38.Lu Y, Chen J, Janjetovic Z, Michaels P, Tang EK, Wang J, et al. Design, synthesis, and biological action of 20R-hydroxyvitamin D3. J Med Chem. 2012;55:3573–3577. doi: 10.1021/jm201478e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Janjetovic Z, Brozyna AA, Tuckey RC, Kim TK, Nguyen MN, Jozwicki W, et al. High basal NF-kappaB activity in nonpigmented melanoma cells is associated with an enhanced sensitivity to vitamin D3 derivatives. Br J Cancer. 2011;105:1874–1884. doi: 10.1038/bjc.2011.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.