Abstract
Large commercial laboratories in the United States were surveyed regarding the number of specimens tested for eight tickborne diseases in 2008. Seven large commercial laboratories reported testing a total of 2,927,881 specimens nationally (including Lyme disease). Of these, 495,585 specimens (17 percent) were tested for tickborne diseases other than Lyme disease. In addition to large commercial laboratories, another 1,051 smaller commercial, hospital, and government laboratories in four states (CT, MD, MN, and NY) were surveyed regarding tickborne disease testing frequency, practices, and results. Ninety-two of these reported testing a total of 10,091 specimens for four tickborne diseases other than Lyme disease. We estimate the cost of laboratory diagnostic testing for non-Lyme disease tickborne diseases in 2008 to be $9.6 million. These data provide a baseline to evaluate trends in tickborne disease test utilization and insight into the burden of these diseases.
Keywords: tickborne disease, laboratory, diagnostic testing, babesiosis, anaplasmosis
INTRODUCTION
North American ticks transmit the agents of Lyme disease and several other tickborne diseases (TBDs) of humans. Although the geographic distributions of specific diseases vary, TBDs occur throughout the United States and collectively constitute a public health problem. Overall reports of TBDs have increased over the last decade, possibly reflecting greater exposure, increased awareness, improved diagnostics, changes in surveillance practices, changes in human activities, and variation in tick distributions and infection prevalence.
Laboratory testing can be central to establishing the correct diagnosis and guiding care for patients with TBDs, including those with atypical presentations. Alternatively, testing specimens from patients with a low disease probability can lead to misinterpretation of positive results and inflate medical costs (Ramsey et al., 2004). The number of Lyme disease tests performed in 1995 was estimated at 2.8 million using marketing data (MK Associates, 1993; Tugwell et al., 1997); however, the current volume of testing for Lyme and other TBDs is expected to be much higher (Hinckley et al, 2014).
In an effort to better understand TBD diagnostic testing practice and volume, a nationwide survey of large commercial laboratories was conducted, as well as hospital-based and other smaller laboratories in four states where Lyme and other tick-borne diseases are endemic. In addition, we used the reported volume to estimate the cost of non-Lyme disease TBD testing in the United States.
MATERIAL AND METHODS
TickNET is a network created in 2007 to foster collaboration on surveillance, research, education, and prevention for tickborne diseases. Collaborators include various divisions within CDC and key state and local health departments. CDC provides extramural funding to participating health departments and partners through the Epidemiology and Laboratory Capacity for Infectious Diseases cooperative agreement, to sustain and enhance surveillance for Lyme disease, and through the Emerging Infections Program (EIP), to promote applied research. There are EIPs are located within Connecticut, Maryland, Minnesota, and New York,, and Lyme disease cases in these four states accounted for nearly 40 percent of all reported cases in the United States in 2008. In addition, 56 percent of HGA cases, 14 percent of HME cases, and 5 percent of RMSF were reported from these four states in 2008 (CDC, 2010). At the time of this study, Babesia infection was not nationally notifiable so the endemnicity of babesiosis compared to other US states could not be compared. However, in 2011, more than 50 percent of the 1,124 confirmed and probable cases of babesiosis were reported from Connecticut, Minnesota, and New York (CDC, 2011). The etiologic agents of Lyme disease, HGA, and babesiosis are all transmitted by the blacklegged tick (Ixodes scapularis).
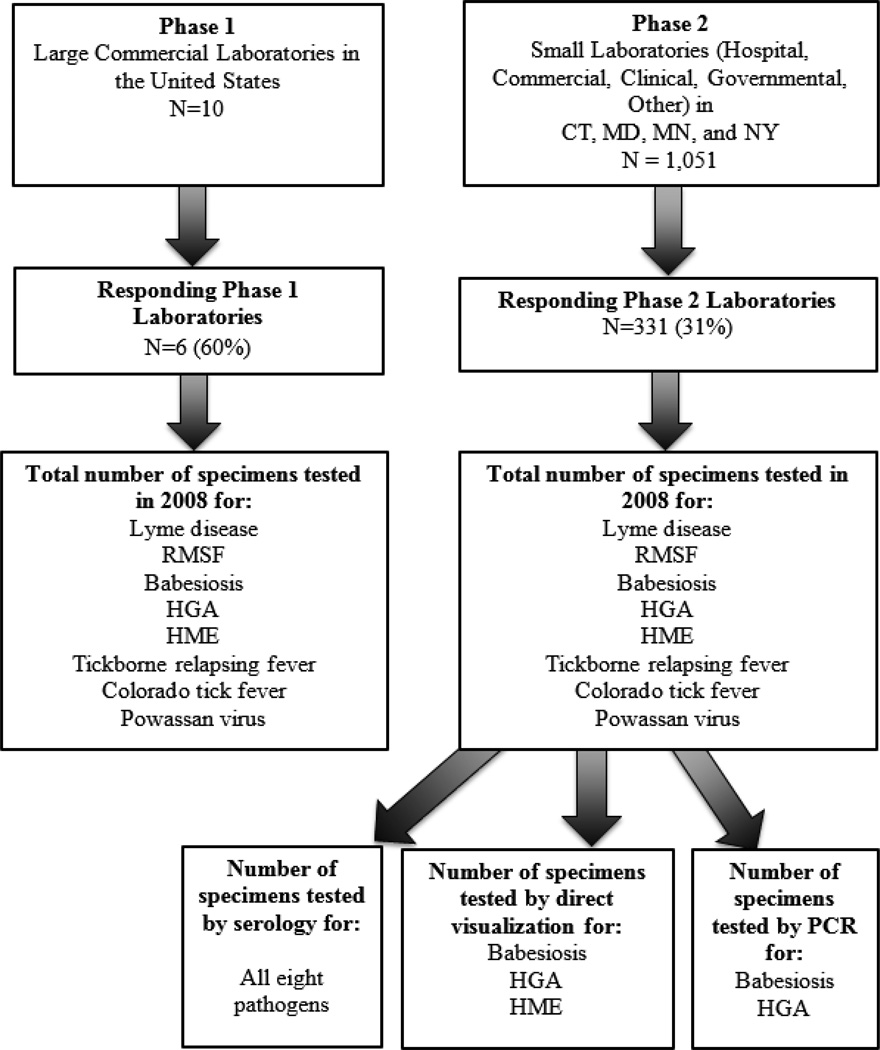
In an effort to better understand TBD diagnostic testing practice and volume, a survey of large commercial laboratories as well as hospital-based and other smaller laboratories was conducted in four TickNET states. A two-phased approach was used (Figure 1). The catchment area for this study included laboratories that were likely to test patients in Connecticut, Maryland, Minnesota, and New York, as determined by state and national disease surveillance records. The survey included commercial laboratories (Phase 1) and smaller clinical and hospital-based laboratories (Phase 2) that were likely to test patients for TBDs, as determined by a review of state disease surveillance records.
Figure 1.
A two-phased laboratory survey approach for non-Lyme tickborne disease testing.
Phase 1
The first phase of the survey was aimed at large commercial laboratories known to conduct TBD disease testing nationally. The following laboratories were contacted by email and telephone to ask for participation: ARUP, Clinical Laboratory Partners, Focus Diagnostics, Laboratory Corporation of America (LabCorp), Mayo Medical Laboratories, Quest Diagnostics, and Specialty Laboratories. These laboratories accounted for the majority of Lyme disease cases reported to health departments in the four endemic states (Connecticut, Maryland, Minnesota and New York) in 2008. Three additional laboratories known to provide alternative non-FDA approved methods of Lyme disease testing (IGeneX, MD Laboratories, Neuroimmunology Laboratory) were also contacted and asked to participate. The survey was sent to laboratories by email and returned by fax or email. Non-responding laboratories were contacted by phone and email by EIP or CDC research staff on a minimum of three occasions to request participation. Commercial laboratories were asked to report their national specimen testing volume for eight TBDs (Lyme disease, RMSF, babesiosis, HGA, HME, tickborne relapsing fever, Colorado tick fever, and Powassan virus); because single specimens may have been tested for multiple pathogens and single patients could have multiple specimens submitted, the number of specimens tested does not necessarily reflect the number of patients tested. In addition, it is possible that one specimen may have been tested for one pathogen by more than one assay.
Phase 2
The second phase of the survey targeted clinical, hospital, government, and small commercial laboratories in the four TickNET states. State health department surveillance records in Connecticut, Maryland, and Minnesota were reviewed to identify laboratories that were known to conduct TBD testing. In New York, all licensed laboratories were surveyed regardless of whether or not they were known to conduct TBD testing. Identified laboratories were asked to complete an emailed survey regarding the number of tests and types of assays performed by their laboratory in 2008 for eight TBDs. As with the Phase 1 survey, laboratories reported the total specimens tested. Therefore, because single specimens may have been tested for multiple pathogens and single patients could have multiple specimens submitted, the number of specimens tested does not necessarily reflect the number of patients tested.
Respondents to Phase 2 were asked to report the percent positive by diagnostic assay for residents of the four endemic study states (CT, MD, MN, and NY). The number of tests and percent positive by test type were aggregated across all laboratories. Assay types included serologic testing (for all pathogens), direct visualization (babesiosis, HGA, HME), and PCR (babesiosis and HGA).
Non-responding laboratories were sent two follow-up emails. If still no response was received, research staff from the EIP in each state contacted laboratory managers in their respective states by telephone to request participation and collected survey responses over the telephone. Both phases of this research effort were deemed exempt from IRB review (non-human subject research) by the Yale Human Investigations Committee and CDC.
Estimating the costs of tickborne disease testing
To estimate the direct cost of non-Lyme disease TBD testing nationally, the proportion of assay types used to diagnose specific TBDs reported by the Phase 2 laboratories (e.g., IFA, PCR, microscopy) was applied to the national specimen testing volume for each TBD reported by the Phase 1 laboratories. The national limit for reimbursement in the 2008 clinical diagnostic laboratory fee schedule (Centers for Medicare and Medicaid Services, 2008) was then used to estimate the cost of national testing for the following test types: Babesia, Anaplasma or Ehrlichia direct detection (microscopy) ($16.76), Babesia indirect fluorescent antibody ($17.32), Babesia or Anaplasma PCR ($49.04), Anaplasma or Ehrlichia indirect fluorescent antibody ($14.22), HGA/HME concurrent panel ($28.44), RMSF indirect fluorescent antibody ($27.05). Although tickborne relapsing fever and Colorado tick fever are not endemic to CT, MD, MN, and NY, the 2008 national limit for their diagnostic immunoassays were also applied to the total number of specimens tested nationally ($37.38 and $36, respectively).
RESULTS
Phase 1
Six laboratories completed the entire survey: ARUP, Clinical Laboratory Partners, Focus Diagnostics, LabCorp, Quest Diagnostics, and Specialty Laboratories. One additional laboratory, Mayo Medical Laboratories, completed only the PCR and serology sections of the survey. All data were aggregated to protect the identity of each individual laboratory.
National TBD testing volume is reported in Table 1. Of the nearly three million specimens tested for tickborne pathogens in 2008, 83 percent of the specimens were tested for Lyme disease, followed by HME (6 percent), RMSF (5 percent), babesiosis (3 percent), HGA (2 percent), tick-borne relapsing fever (<1 percent), and Colorado tick fever (<1 percent). No commercial laboratories reported testing for Powassan virus. The details of the Lyme disease testing portion of the laboratory survey are reported by Hinckley et al. (2014).
Table 1.
Testing volume for tick-borne diseases in the US, 20081
| Tickborne Disease | Specimens Tested | % |
|---|---|---|
| Lyme disease | 2,432,396 | 83 |
| Ehrlichiosis (HME) | 193,121 | 6 |
| Rocky Mountain spotted fever | 152,713 | 5 |
| Babesiosis | 85,323 | 3 |
| Anaplasmosis (HGA) | 59,943 | 2 |
| HGA/HME2 | 3,750 | <1 |
| Tick-borne relapsing fever | 405 | <1 |
| Colorado tick fever | 230 | <1 |
| Powassan encephalitis | 0 | 0 |
| Total | 2,927,881 |
Data aggregated from seven U.S. commercial laboratories.
One laboratory could not provide data specific to Anaplasma vs. Ehrlichia PCR testing.
Phase 2
A total of 1,051 laboratories were sent surveys in CT (n=30), MD (n=42), MN (n=158), and NY (n=821). In total, 331 laboratories (31 percent) responded to the survey; responding laboratories did not always provide responses to all modules in the survey. Responding laboratories were self-described as hospital (72 percent), commercial (19 percent), clinical (10 percent), governmental (5 percent), or other (6 percent). Response data from the four states were aggregated. Detailed results from the Lyme disease module were reported separately (Hinckley et al., 2014).
Babesiosis
Fifty-seven (18 percent) of the 312 laboratories that responded to the Babesia module of the survey reported conducting in-house testing for Babesia microti. The survey module queried laboratories about assays ordered specifically for Babesia. Therefore, incidental diagnoses following review of a blood smear initially ordered for other reasons were not part of this module. A total of 4,967 specimens were tested by responding laboratories in the four study states. Diagnostic tests conducted by responding laboratories included microscopy (72 percent), indirect fluorescent antibody (IFA) (28 percent) and polymerase chain reaction (PCR) (< 1 percent). Babesia testing volume and positivity by assay are detailed in Table 2.
Table 2.
Tickborne disease testing volume and positivity in four states, by diagnostic assay, 2008.
| Tickborne disease | No. Tests | No. Positive (%) |
|
|---|---|---|---|
| Babesiosis | Microscopy (DFM, Giemsa-Wright or Wright) | 3,561 | 71 (2) |
| IFA | 1,388 | 28 (2) | |
| PCR (blood, serum, CSF) | 18 | 1 (6) | |
| Total | 4,967 | ||
| Anaplasmosis (HGA) | IFA | 575 | 40 (7) |
| Microscopy (Giemsa-Wright) | 493 | 44 (9) | |
| PCR (blood) | 150 | 1 (8) | |
| Total HGA | 1,218 | ||
| Ehrlichiosis (HME) | IFA | 2,326 | 19 (8) |
| Microscopy (Giemsa-Wright) | 204 | 0 (0) | |
| Total HME | 2,530 | ||
| HGA/HME | Concurrent Panel | 120 | 5 (4) |
| Rocky Mountain spotted fever (RMSF) | IFA | 1,236 | 74 (6) |
Data from small clinical and hospital laboratories testing specimens in CT, MD, MN, and NY in 2008. Does not reflect specimens tested by large, commercial laboratories.
Anaplasmosis (HGA) and ehrlichiosis (HME)
Thirty-one (10 percent) of the 304 laboratories that responded to the HGA-HME module of the survey reported conducting in-house laboratory testing for HGA or HME (or both). A total of 1,218 specimens were tested by responding laboratories for Anaplasma phagocytophilum by IFA (47 percent), microscopy (41 percent) and PCR (12 percent). A total of 2,530 specimens were tested for Ehrlichia chaffeensis by laboratories in the four study states. Most of the specimens were tested using IFA (92 percent), and a few were tested via microscopy (8 percent). A small number of specimens were tested using a panel for concurrent HGA and HME testing. HGA and HME testing volume and positivity by assay are detailed in Table 2.
Rocky Mountain spotted fever (RMSF)
Of the 319 laboratories that responded to the RMSF module, four laboratories (1 percent) reported conducting testing for Rickettsia rickettsii on site. A total of 1,236 specimens were tested in two states (NY and MD), all by IFA, with a 6 percent positivity (Table 2).
Estimated costs of tickborne disease testing
The estimated direct cost for TBDs other than Lyme disease nationally was $9.6 million, including $4.1 million for RMSF, $2.8 million for ehrlichiosis, $1.5 million for babesiosis, $1.2 million for anaplasmosis, and $0.11 million for HME/HGA panels. Costs for diagnosis of tickborne relapsing fever and Colorado tick fever are estimated at $15,000 and $4,000, respectively (Table 3).
Table 3.
Estimated cost of tickborne disease testing, by diagnostic assay, 2008.
| Tickborne Disease | Diagnostic Method |
Specimens tested in four states* (% of total) |
National testing estimate based on four-state distribution |
National cost estimate ($) |
|---|---|---|---|---|
| Babesiosis | Microscopy | 3,561 (71.7) | 61,177 | 1,025,320 |
| IFA | 1,388 (27.9) | 23,805 | 412,305 | |
| PCR | 18 (0.4) | 341 | 16,737 | |
| Total | 4967 | 85,323 | 1,454,362 | |
| Anaplasmosis (HGA) | Microscopy | 493 (40.5) | 24,277 | 406,881 |
| IFA | 575 (47.2) | 28,293 | 402,328 | |
| PCR | 150 (12.3) | 7,373 | 361,571 | |
| Total | 1218 | 59,943 | 1,170,780 | |
| Ehrlichiosis (HME) | Microscopy | 204 (8.1) | 15,643 | 262,173 |
| IFA | 2,326 (91.9) | 177,478 | 2,523,739 | |
| Total | 2530 | 193,121 | 2,785,912 | |
| HGA/HME | Concurrent Panel | 120 (100) | 3,750 | 106,650 |
| Total | 120 | 3,750 | 106,650 | |
| Rocky Mountain Spotted Fever | IFA | 1,236 (100) | 152,713 | 4,130,887 |
| Total | 1236 | 152,713 | 4,130,887 | |
| Tick-borne Relapsing Fever | IFA | 405 | 15,139 | |
| Total | 405 | 15,139 | ||
| Colorado Tick Fever | IFA | 230 | 8,280 | |
| Total | 230 | 8,280 | ||
| TOTAL | 9,672,010 | |||
Specimens tested from CT, MD, MN, and NY in 2008. Because specimens in these states were not tested for tick-borne relapsing fever or Colorado tick fever, estimates were based upon the national limit for CMS costs in 2008.
DISCUSSION
Nearly three million specimens were tested for TBDs by responding large and small laboratories in the US in 2008. Eighty-three percent of specimens in our survey were tested for Lyme disease. Similarly, Lyme disease accounted for 86 percent of all TBDs reported to CDC in 2008 (CDC, 2010). The proportions of laboratory testing volume for RMSF, anaplasmosis and ehrlichiosis were similar to the proportions of cases reported to CDC. We estimate that the direct costs for the non-Lyme disease TBD tests to total nearly $10 million. Including the estimated $492 million estimated cost of Lyme disease testing (Hinckley et al., 2014), the estimated direct cost of TBD testing in the United States amounts to $501.6 million. Testing for TBDs other than Lyme disease accounted for approximately 17 percent of all tickborne disease testing in 2008. The nearly $10 million cost for non-Lyme disease testing is an estimate of what commercial laboratories charged in 2008 according the Center for Medicare and Medicaid Services Clinical Diagnostic Laboratory fee schedule. This figure does not include other indirect expenses that may accompany these diagnostic tests (Hinckley et al., 2014) and likely underestimates the true cost of TBD testing nationally.
More specimens were tested for ehrlichiosis than for RMSF nationally, despite the fact that more than twice the number of RMSF cases were reported to CDC in 2008 than ehrlichiosis cases (including E. chaffeensis and E. ewingii) (CDC, 2012). Because the causative agent of anaplasmosis was considered to be an Ehrlichia species until 2000, some healthcare providers may mistakenly still refer to cases of anaplasmosis as ehrlichiosis and therefore order tests for Ehrlichia instead of Anaplasma (Wormser et al., 2006). Alternatively, diagnostic assays for Ehrlichia and Anaplasma are often included on the same testing panel. Therefore, specimens counted for Ehrlichia testing may be greater in number because they include those originally submitted for Anaplasma testing or ordered as part of a panel.
The majority of specimens tested for HGA or HME, as reported in Phase 2 of the study, were done using IFA, which is common for the diagnosis of Anaplasma and Ehrlichia pathogens (Wormser et al., 2006). Although thought to be uncommon, it is possible that both acute and convalescent specimens are tested for some individuals, which may contribute to an overestimate in the positivity rate for those tests overall. In addition, a large number of specimens were examined by microscopy as a method of pathogen detection. This method may be insensitive and may require examination by a microscopist with specialized expertise in this field, and may therefore contribute to an underestimate of the true positivity rate (Ismail et al., 2010).
RMSF testing was conducted exclusively by IFA in the four study states. IFA was the most acceptable method at the time for detection of the causative agent R. rickettsia; however, timing of the sample collection is important to the sensitivity of the test (CDC, 2006). It is unclear how many of the nearly 2,000 specimens tested included acute and convalescent specimens from the same patient, as those data were not collected. Therefore, the positivity rate reported in the results may be greatly affected by this issue.
The majority of tests for babesiosis conducted in the four study states were via blood smear microscopy. Although microscopy and PCR are the recommended methods for detection of Babesia microti (CDC, 2012), the survey of Phase 2 laboratories found that nearly one-third of specimens were tested for Babesia parasites using IFA. Less than one percent of specimens were tested using PCR. Since the majority of laboratories responding to the Phase 2 survey modules were hospital-based laboratories, it is not surprising that microscopy would be used more commonly than PCR. The Babesia survey module asked about tests conducted specifically for detecting Babesia; diagnoses made from incidental detection via blood smears were not included as part of the survey.
Our findings illustrate the large volume of TBD testing in the United States. Because not every large commercial testing facility that was contacted responded to the Phase 1 survey, it is likely that these findings underrepresent the true testing burden for TBDs. However, the laboratories that did respond accounted for the majority of Lyme disease testing nationally (Hinckley et al., 2014). It is also possible that some laboratories were not identified and therefore not invited to participate in this survey, especially in Connecticut, Maryland, and Minnesota, where we targeted the survey based on state disease surveillance records. In addition, we did not collect positivity rates for diseases other than Lyme disease during Phase 1. Therefore, we cannot compare the positivity rates for TBDs at the Phase 2 laboratories to national rates.
Similarly, the assay types used to diagnose TBDs during Phase 1 were not collected. Instead, the proportion of assay types used by Phase 2 laboratories was applied to Phase 1 data to estimate the national distribution of assay type for each TBD. If testing patterns at the large commercial laboratories of Phase 1 differ greatly from the smaller commercial and hospital-based laboratories of Phase 2, our cost estimates may be inaccurate. For example, if large commercial laboratories are more likely than smaller clinical laboratories to conduct molecular tests for TBDs (e.g., PCR), it is possible that we underestimated the direct costs of testing. Additional studies could help elucidate the testing practice differences between the types of laboratories surveyed in this study. In addition, using CMS reimbursement rates provided us the minimum costs associated with TBD testing. Therefore, we conclude that the nearly $10 million cost estimate for non-Lyme TBD testing is a conservative one. We are currently unaware of any studies estimating the testing volume and costs for non-Lyme TBD testing. This study provides a baseline of testing data for these emerging arthropod-borne infectious diseases.
If done routinely, laboratory surveillance may present a more efficient and sustainable approach than current public health methods (i.e., passive surveillance whereby providers and laboratories report cases to public health agencies) for monitoring trends of TBD in the U.S. However, because positive antibody tests may only indicate exposure to a pathogen and can be falsely positive in some circumstances, it is unclear how many active infections, as indicated by incident TBD cases captured through current public health surveillance methods, could be ascertained through laboratory surveillance alone. Nevertheless, the results of this survey provide valuable insight into the true magnitude and costs of these diseases and give us a baseline to evaluate trends in TBD test utilization and positivity in the United States.
ACKNOWLEDGEMENTS
We thank Barbara Herwaldt and Patty Wilkins for guidance on Babesia questions and interpretation; Jennifer McQuiston and William Nicholson for guidance on rickettsial agent questions and interpretation. We also thank Paula Snippes Vagnone for helping coordinate survey distribution in Minnesota. This work would not have been possible without Erin Jones at the Maryland Department of Health, as well as several Yale MPH students, and graduate student workers in the Minnesota Department of Health Vector-Borne Disease program who also assisted with data collection. This work was supported by CDC Cooperative Agreements to the Connecticut, Maryland, Minnesota, and New York Emerging Infections Programs. (Cooperative Agreement # 5U01CI000307 [CT], 5U01CI00031005 [MD], 5U01C1000313 [MN], 5U01CI00031004 [NY]).
Footnotes
DISCLAIMER
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
REFERENCES
- Associates MK. Projected annual market for rapid direct tests. Owings Mills, MD: Rapid testing for infectious diseases: competitive advantage in a turbulent market; 1993. p. 277. [Google Scholar]
- Centers for Disease Control and Prevention. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever, ehrlichioses, and anaplasmosis -- United States. Morb Mortal Wkly Rep. 2006;55 [Google Scholar]
- Centers for Disease Control and Prevention. Summary of notifiable diseases - United States, 2008. Morb Mortal Wkly Rep. 2010;54:1–94. [Google Scholar]
- Centers for Disease Control and Prevention. Babesiosis surveillance-- 18 states, 2011. Morb Mortal Wkly Rep. 2012;61:505–509. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Summary of notifiable diseases - United States, 2010. Morb Mortal Wkly Rep. 2012;9:2–111. [PubMed] [Google Scholar]
- Centers for Medicare and Medicaid Services. [Accessed May 29, 2015];Current Procedural Terminology (CPT) (Fourth Edition). 2008 http://www.cms.gov/apps/ama/license.asp?file=/ClinicalLabFeeSched/downloads/08clab-b.zip.
- Hinckley AF, Connally NP, Meek JI, Johnson BJ, Kemperman MM, Feldman KA, White JL, Mead PS. Lyme disease testing by large commercial laboratories in the United States. Clin Infect Dis. 2014;59:676–681. doi: 10.1093/cid/ciu397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail N, Bloch KC, McBride JW. Human ehrlichiosis and anaplasmosis. Clin Lab Med. 2010;30:262–291. doi: 10.1016/j.cll.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey A, Belongia E, Chyou PH, Davis J. The appropriateness of Lyme disease serologic testing. Ann Family Med. 2004;2:341–344. doi: 10.1370/afm.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tugwell P, Dennis D, Weinstein A, Wells G, Shea B, Nichol G, et al. Laboratory evaluation in the diagnosis of Lyme disease. Ann Intern Med. 1997;127:1109–1123. doi: 10.7326/0003-4819-127-12-199712150-00011. [DOI] [PubMed] [Google Scholar]
- Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, Klempner MS, Krause PJ, Bakken JS, Strle F, Standek G, Bockenstedt L, Fish D, Dumler JS, Nadelman RB. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43:1089–1134. doi: 10.1086/508667. [DOI] [PubMed] [Google Scholar]