Abstract
Skeletal muscle has a remarkable ability to respond to different physical stresses. Loading muscle through exercise, either anaerobic or aerobic, can lead to increases in muscle size and function while, conversely, the absence of muscle loading stimulates rapid decreases in size and function. A principal mediator of this load-induced change is focal adhesion kinase (FAK), a downstream non-receptor tyrosine kinase that translates the cytoskeletal stress and strain signals transmitted across the cytoplasmic membrane by integrins to activate multiple anti-apoptotic and cell growth pathways. Changes in FAK expression and phosphorylation have been found to correlate to specific developmental states in myoblast differentiation, muscle fiber formation and muscle size in response to loading and unloading. With the capability to regulate costamere formation, hypertrophy and glucose metabolism, FAK is a molecule with diverse functions that are important in regulating muscle cell health.
Keywords: Focal adhesion kinase, Hypertrophy, Muscle development, Exercise
Introduction
Skeletal muscle is a remarkably plastic tissue that is capable of responding to changes in muscle workload. The ability of the muscle to detect changes in cytoskeletal tension and initiate the appropriate signal is the responsibility of proteins that detect cytoskeletal tension, termed mechanosensors. Mechanosensors relay the information throughout the muscle cell to change gene transcription and protein expression. Skeletal muscle contains multiple types of mechanosensors with diverse responses to changes in tension. Changes in the activity of ion channels and signaling through sarcomeric proteins like titin are the result of changes in muscle tension [Reviewed in (Burkholder 2007; Gautel 2011; Tidball 2005)]. This review will focus on the role of focal adhesion kinase (FAK), a prominent and well-studied protein that responds to changes in cellular tension. FAK is a 125 kDa non-receptor tyrosine kinase that lies within and coordinates signals through a multiprotein complex called the focal adhesion complex (Schaller et al. 1995). Focal adhesion complexes anchor the cytoplasmic tails of heterodimeric membrane-spanning proteins called integrins, forming a continuous link between the cytosol and extracellular matrix (ECM) (Hanks et al. 2003). FAK is similar to proline-rich tyrosine kinase [Pyk2; also commonly referred to as FAK2, protein tyrosine kinase 2-beta (PTK2β), cell-associated kinase-beta (CAK-β) or calcium-dependent tyrosine kinase (CADTK)] in amino acid sequence, structure and substrates. However, their cellular functions are distinct with Pyk2 being highly cell-specific and its activation being independent of ECM and integrin interactions, which are most likely the main activator of FAK in skeletal muscle (Orr and Murphy-Ullrich 2004). FAK also has similar qualities to integrin-linked kinase (ILK), an important component of the focal adhesion complex. The role of ILK in skeletal muscle is largely unknown but it has been demonstrated to respond positively in skeletal muscle following overload (Chaillou et al. 2013) and transgenic integrin overexpression (Boppart et al. 2011). Furthermore, muscle-specific HSACre-driven ILK knockout resulted in decreased hypertrophic intracellular signaling, myotendinous junction organization and insulin receptor stability (Wang et al. 2008).
In skeletal muscle, the focal adhesion complex is densely localized within the costamere (Anastasi et al. 2008; Bloch and Gonzalez-Serratos 2003) and myotendinous junction (Burkin and Kaufman 1999; Mayer et al. 1997), which are the main force transducers of skeletal muscle. The costamere, composed of the dystroglycan and focal adhesion complex (Bloch and Gonzalez-Serratos 2003), transmits sarcomeric forces laterally to the extracellular matrix, while the myotendinous junction transmits forces longitudinally through the tendon to bone. Since integrins have no known kinase activity, they must rely on the focal adhesion complex, primarily through FAK, to signal changes in cytoskeletal loading.
FAK has multiple roles within skeletal muscle. It is activated by receptor tyrosine kinases for growth factors, such as insulin (Bisht et al. 2007) and insulin-like growth factor-1 (IGF-1) (Crossland et al. 2013) and can regulate myoblast development and muscle fiber formation (Quach et al. 2009). The latter function is intriguing because the convergence of myogenesis, nutritional control of muscle homeostasis and muscle loading provide a meeting point between prominent hypertrophy and anti-apoptotic signals that reinforce the importance of FAK signaling during muscle development and homeostasis.
Regulation of FAK activation
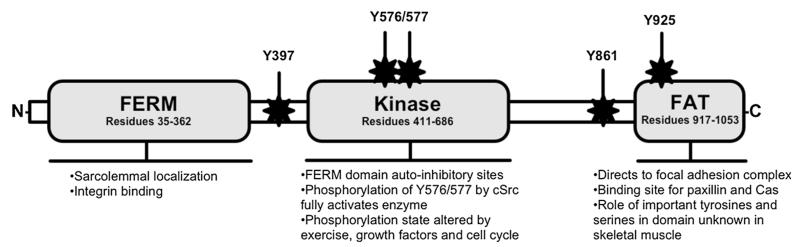
The following is a brief summary of the structure and autoregulation of FAK that is meant to provide basic structural information for the reader. For more detailed reviews, please refer to (Frame et al. 2010; Hall et al. 2011; Parsons 2003). The structure of FAK (Fig. 1) shares characteristics of other focal adhesion complex and membrane-bound proteins, such as talin. FAK is composed of three major domains: an N-terminal 4.1 ezrin, radixin, moesin (FERM) domain, a kinase domain and the C-terminal focal adhesion targeting (FAT) domain (Parsons 2003). The FERM domain targets and organizes proteins to the cell membrane (Baines et al. 2014) and is a major regulator of FAK activity. Deletion of the initial 375 residues of the FAK FERM domain leads to a constitutively active enzyme (Cooper et al. 2003). Additionally, the FERM domain of FAK can bind to the cytoplasmic portion of the β1 integrin tail (Schaller et al. 1995), creating a logical docking point to stabilize or signal through the focal adhesion complex. A linker domain, which contains the major phosphorylation site at tyrosine 397, connects the FERM domain and kinase domain. The kinase domain contains two tyrosine residues that are important for optimal FAK function, tyrosines 576 and 577 (Schaller et al. 1994). The FAT domain’s main responsibility is directing FAK to the focal adhesion complex (Hildebrand et al. 1993). FAK-related non-kinase (FRNK) is a protein transcribed from the FAT portion of the FAK gene (PTK2) and acts to inhibit FAK in many cell types, including skeletal muscle (Klossner et al. 2013).
Fig. 1.
Simplified structure, domain roles and important phosphorylation sites of FAK. Autoinhibition is a result of interactions between the FERM and kinase domains, which prevents autophosphorylation of Y397, the major phosphorylation site of FAK. Phosphorylation of Y397 creates strong binding affinities for proteins containing SH2-domains, mainly cSrc and PI3K. Binding of cSrc induces phosphorylation of Y576/577, resulting in FAK’s full activation. In skeletal muscle, the role of major tyrosine and serines in the FAT and preceding linker domain remain unknown. Figure based on (Franchini 2012; Hanks et al. 2003; Lietha et al. 2007)
Lietha and colleagues (Lietha et al. 2007) were able to crystallize an autoinhibited form of FAK and describe the mechanisms that block kinase activity in the inactive form of FAK. The structure of the autoinhibited enzyme showed binding of the FERM domain to the kinase domain, essentially burying the linker that contains tyrosine 397. The location of the FERM domain during this interaction also protects the important tyrosines of the kinase domain, tyrosines 576 and 577, from phosphorylation (Lietha et al. 2007). Movement of the FERM domain allows tyrosine 397 to become exposed to the cytosol, after which it is quickly autophosphorylated (Schaller et al. 1994). What exactly causes the initial shift in the FERM domain is unknown but there is evidence that acidic phospholipids such as phosphatidylinositol 4,5-bisphosphate can induce a change in the position of FERM and thereby activate FAK (Cai et al. 2008). Phosphorylation of tyrosine 397 creates high affinity binding sites for proteins that contain Src Homology (SH) 2 and 3 domains, including the tyrosine kinase, cSrc. cSrc phosphorylates tyrosines 576 and 577 of FAK to fully activate its kinase activity (Schaller et al. 1994).
FAK expression and function during muscle development
Muscle development is a coordinated process in which transcription factors like myogenin and MyoD induce terminal differentiation of myoblasts. Upon differentiation, these myoblasts then fuse together to form multi-nucleated myotubes. In this early form of muscle, the ECM and sarcolemmal proteins are important in regulating and coordinating proper myogenesis. Since integrins are major effectors of laminin binding and are used to stabilize muscle during myogenesis (Burkin and Kaufman 1999), it would be logical that FAK would be an important regulator of myoblasts during these states. Indeed, FAK activity is necessary for expression of MyoD and other important cell cycle regulators such as Cdo and Cdc42 in C2C12 cells (Han et al. 2011).
Myoblast proliferation and differentiation
Differentiation of myoblasts for 3 days activated FAK (Goel and Dey 2002a). However, the state of FAK activation can alter cell cycle progression and commitment to differentiation of muscle progenitor cells. Ectopically increasing expression of a constitutively active membrane-bound wild-type FAK promoted cell proliferation in quail myoblasts while a tyrosine to phenylalanine inactivating mutation of the main activation site of FAK promoted differentiation and multinucleated myotube formation (Sastry et al. 1999). Ectopic over-expression of certain α integrin subunits altered FAK phosphorylation, with the RGD-sequence binding α5 integrin having no affect on FAK phosphorylation and the laminin-binding α6 integrin decreasing FAK phosphorylation (Sastry et al. 1999), providing evidence that the evolution of the ECM and integrin to which it is bound can alter the state of FAK activation in early muscle development. The addition of insulin to myoblasts stimulated FAK phosphorylation in proliferating cells but rapidly decreased phosphorylation in differentiated muscle cells within 5 min, followed by a gradual increase in phosphorylation levels back to near-baseline after 30 min (Goel and Dey 2002a). Expression of a dominant-negative FAK in C2C12 myoblasts results in a loss of RhoA-induced α-actin promoter activity (Wei et al. 2000), suggesting that the presence of functional FAK is necessary for important fundamental gene expression in muscle cells.
The level of FAK activation also responds to cues for differentiation. FAK phosphorylation levels decreased when myoblasts were switched from proliferating medium to differentiating medium but then gradually increased when cells were maintained in differentiation medium for 1–6 days (Clemente et al. 2005; Goel and Dey 2002a). Other studies of C2C12 cells found that total FAK decreases gradually during differentiation while phosphorylated FAK gradually increases (Nguyen et al. 2014). Similarly, primary myoblasts incubated with differentiation media show decreases in total and phosphorylated FAK over the course of 96 h (Quach et al. 2009). Clemente et al (2005) localized FAK to lamellipodia and filopodia in proliferating myoblasts. However, following 24 h in differentiating medium, there were no apparent lamellipodia or filopodia and FAK was localized to large patches on the cytoplasmic surface of the cytoplasmic membrane. Transfection of differentiating myoblasts with wild-type FAK prevented differentiation-associated decreases in the cell cycle regulator cyclin D1 and prevented expression of myogenin mRNA and protein. Further, FAK overexpression reduced upregulation of creatine kinase activity (Clemente et al. 2005). The decreases in total FAK expression associated with differentiation may be due to targeting to the ubiquitin–proteasome pathway for proteolytic destruction after ubiquination by the E3 ligase mitsugumin 53 as protein levels decrease during differentiation without any change in mRNA levels (Nguyen et al. 2014). In regulating the muscle cell cycle, FAK translocation to the nucleus following binding to the heterochromatin interacting methyl CpG-binding protein 2 (MBD2) induces myogenin expression by decreasing MBD2 protein interactions within the myogenin promoter (Luo et al. 2009). In summary, FAK expression and activity are reduced early after the cell commits to differentiation and this decrease is critical to correct progression of differentiation. Once differentiation programs are initiated, FAK activity is restored, presumably to participate in other integrin functions including fusion of myoblasts with each other or with existing muscle fibers.
Myoblast fusion
There is evidence that FAK is necessary for proper myoblast fusion. FAK has been shown to be expressed at higher levels in myotubes compared to myoblasts (Fluck et al. 1999). Satellite cell-specific FAK knock out mice had a fourfold decrease in regenerating fibers 3 days after BaCl2-induced injury and inhibition of FAK by its FAT domain or siRNA prevented myoblast fusion (Quach et al. 2009). The mechanism behind this appears to be decreases in FAK-regulated expression of important myofusion proteins such as the β1D integrin and caveolin-3 (59). Furthermore, knockdown of heparan sulfate endosulfatases disrupts phosphorylated FAK sarcolemmal localization and can inhibit myoblast fusion (Tran et al. 2012).
Costamere formation
FAK has a functional role in costamere synthesis, most likely stabilizing and signaling through the protein scaffold around the cytoplasmic portion of integrins. There is no evidence that FAK routinely localizes to the dystroglycan complex but integrins have been implicated as being critical to proper costamere formation (Balaban et al. 2001; Fujita et al. 2007) and FAK’s downstream signaling ability makes it a prominent component for costamere genesis. During muscle development, costamere formation is delayed compared to myofibrillar formation (Quach and Rando 2006), meaning that there has to be a more developed myofiber in order for the maturation of the costamere. This is most likely related to muscle specific gene expression and the need for some type of contractility in the muscle cell to correctly form the costamere and sarcomere (De Deyne 2000; Fujita et al. 2007; Quach and Rando 2006). Inhibition of FAK by a dominant-negative FAT or FAK siRNA in myotubes resulted in lower levels of costamere organization as measured by immunofluorescence of vinculin, the first protein associated with costameres (Pardo et al. 1983) and a protein that connects the many proteins of the focal adhesion complex (Bloch and Gonzalez-Serratos 2003), and myofiber formation (Quach and Rando 2006).
Other proteins that can regulate FAK during development
Protein kinase C (PKC) negatively-regulates FAK phosphorylation and focal adhesion complex localization during the differentiation of myoblasts but stimulates FAK phosphorylation while in the proliferating stage (Goel and Dey 2002b). PKC increases FAK phosphorylation and cell spreading in α5-null myoblasts, suggesting that PKC may work through FAK in an inside-out signaling pathway, independent of integrins, which creates more ECM binding capabilities for the cell (Disatnik and Rando 1999). PKC-null myoblasts have lower levels of phosphorylated FAK and exogenous transfection of PKC increases FAK phosphorylation in C2C12 cells (Madaro et al. 2011).
FAK activation can also occur following ligand binding of the neogenin receptor. Neogenin is a membrane receptor protein that binds to a family of proteins called netrins that are structurally similar to laminin. Netrins are secretory proteins that aid in localizing axons and have been shown to be important in other cell types (Lai Wing Sun et al. 2011). In its cytosolic tail, neogenin can bind and activate FAK (Li et al. 2004). In mouse embryos that had neogenin knocked-out by gene trapping, myotubes were smaller and there was an almost complete inhibition of FAK activation (Bae et al. 2009). In non-gene trapped myoblasts, recombinant netrin increased FAK phosphorylation after 30 min but neogenin knockout myoblasts had no FAK phosphorylation response (Bae et al. 2009).
FAK and actin dynamics
As mentioned previously, FAK may have a role in actin dynamics (Wei et al. 2000), and in muscle, most likely functions through activation of RhoA and downstream signaling through FAK and serum response factor (SRF) (Wei et al. 1998). RhoA is a small GTPase that works in coordination with the striated muscle activator of Rho signaling (STARS) to help regulate the formation of filamentous actin and SRF is a transcription factor that can control actin dynamics and many integral proteins of the muscle cell cycle (Lamon et al. 2014). To our knowledge, there is no explicit interaction between STARS and FAK in skeletal muscle but there is a potential for RhoA-induced FAK regulation. In cardiac muscle tissue, FAK activation following cell stretch is dependent on RhoA (Torsoni et al. 2005) and inhibition of RhoA with an exoenzyme prevented FAK autophosphorylation (Del Re et al. 2008). In skeletal muscle, RhoA protein expression decreases following 3 days of hindlimb unloading and increases following reloading (McClung et al. 2004). We have seen RhoA decrease similarly to FAK following downhill running (Graham et al. 2015). SRF can colocalize with both RhoA and FAK (Sakuma et al. 2003) and unloading decreased SRF protein expression along with FAK protein expression in rat hindlimb muscles (Gordon et al. 2001). Furthermore, decreased SRF expression is associated with decreased levels of FAK in dystrophic muscle (Sakuma et al. 2004). The limited information regarding the RhoA/FAK/SRF pathway in muscle suggest that they may have a role in muscle and actin regulation but more research is needed to clearly elucidate the function of this pathway.
Cytokine regulation through FAK
The literature is sparse in regards to the ability of cytokine signaling to modulate FAK expression or activation in skeletal muscle. Exposing C2C12 cells to exogenous TNFα increases FAK expression. Silencing of FAK with siRNA in TNFα-exposed C2C12 cells, or an inactivating tyrosine to phenylalanine mutation on the main activation site of FAK, decreases IL-6 production, suggesting that FAK participates in the regulation of the expression of inflammatory cytokines (Tseng et al. 2010). This concept of IL-6 regulation by FAK is intriguing due to the large IL-6 response typically seen after intense exercise (Pedersen and Febbraio 2008).
Mechanisms for muscle hypertrophy
FAK and cSrc
Activation of cSrc by FAK has been implicated in the hypertrophy of cardiac muscle (Franchini 2012; Torsoni et al. 2003) but evidence is lacking regarding cSrc’s role in skeletal muscle. In overloaded cardiac muscle, pressure-related hypertrophy is associated with activation of FAK and extracellular signal-related kinase (ERK) 1/2 (Franchini et al. 2000). Conversely, inhibition of FAK or cSrc decreases ERK1/2 activation following cell stretch (Torsoni et al. 2003). In skeletal muscle, ERK1/2 responds to various types of muscle loading (Boppart et al. 2001; Hornberger et al. 2005; Williamson et al. 2003) and is important for muscle hypertrophy and overall homeostasis (Shi et al. 2009). Thus the prospect of a FAK/cSrc/ERK1/2 relationship warrants investigation. Cyclic strain decreases phosphorylation of cSrc at tyrosine 527, the main inhibitory site on this kinase, thus activating cSrc; this effect is maintained for 60 min in cultured C2C12 (Kumar et al. 2004). We have observed that in rat soleus, total and activated cSrc protein expression are not affected by 90 min of eccentric downhill running at 2 and 48 h post-exercise despite decreases in FAK levels (Graham et al. 2015) We have also found that cSrc activation is decreased 56 days following spinal cord injury (SCI) in rat gastrocnemius with no changes in total or activated ERK1/2 (Graham, manuscript in review). This suggests that in skeletal muscle, direct FAK and cSrc signaling through ERK1/2 may occur (1) less than 2 h following exercise; (2) during the immediate time-frame following unloading or paralysis or; (3) independently of ERK1/2 in load-induced cell signaling.
The FAK/PI3K/Akt pathway
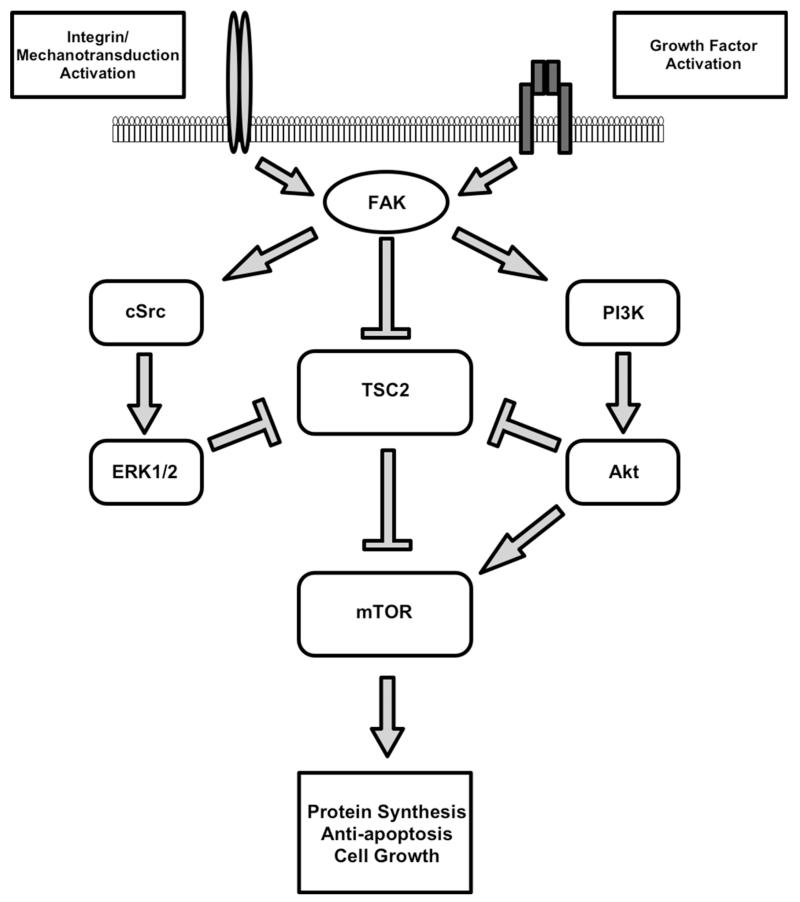
In skeletal muscle it is likely that there is cross-talk between FAK and the phosphatidylinosital-3 kinase (PI3K) pathway (Fig. 2). The PI3K pathway includes Akt, mechanistic target of rapamycin (mTOR) and p70S6 kinase (p70S6k). mTOR is a protein kinase and serves as a master controller of cell size and protein synthesis (Reiling and Sabatini 2006). Its activity responds to many signals that include nutritional changes, growth factors and changes in muscle loading (Baar and Esser 1999; Baar et al. 2006; Bodine 2006; Goodman et al. 2011). Phosphorylation of tyrosine 397 of FAK results in binding of FAK to the SH2 domain of the 85 kDa subunit of PI3K, which can lead to increases in PI3K activity (Chen et al. 1996). FAK may modulate mTOR through inhibition of tuberous sclerosis complex 2 (TSC2) by phosphorylation, which results ultimately in destabilization and proteolytic destruction of TSC2. TSC2 is a negative regulator of mTOR. The first evidence for a FAK/TSC2 interaction came from studies of 293T and NIH3T3 cells. These studies demonstrated that FAK binds and phosphorylates TSC2 on a residue between 609 and 1080; over-expression of a mutant FAK lacking kinase activity decreased phosphorylation of p70S6k and eukaryotic 4E-binding protein-1 (4E-BP1) (Gan et al. 2006). This observation has been recently reproduced in C2C12 myoblasts; incubation of these cells with IGF-1 increased FAK autophosphorylation and increased TSC2 phosphorylation as measured by Western blotting in cells incubated with scrambled siRNA. Moreover, knockdown of FAK expression using FAK-specific siRNA resulted in decreased TSC2 phosphorylation and decreased p70S6k and 4E-BP1 phosphorylation and puromycin-labeled nascent polypeptide chains compared to scrambled siRNA (Crossland et al. 2013). Conversely, overexpression of FAK increased p70S6k phosphorylation independent of Akt activation in rat tibialis anterior (Klossner et al. 2009). Consistent with these findings, FAK appears to mediate responses in rat skeletal muscle of p70S6K and 4EBP1 to administration of acetaminophen. Eight weeks of daily acetaminophen administration leads to a threefold greater expression of total and phosphorylated FAK and similar changes are seen in the downstream proteins p70S6k and 4E-BP1. These changes affected only the soleus and not the gastrocnemius, suggesting that acetaminophen may increase hypertrophic signaling through FAK in a muscle/fiber-type dependent manner (Graham, in review).
Fig. 2.
Prominent signaling through the FAK nexus. Either mechanical activation from integrins or upstream regulation from growth factor signaling can activate multiple pathways that result in protein synthesis and anti-apoptosis. Other factors not shown that may similarly regulate FAK are RhoA, neoginin and protein kinase C
FAK appears to have a role in the control of energy metabolism and glucose uptake within skeletal muscle through insulin and PI3K signaling. Adenovirus delivered anti-sense FAK blunts glucose uptake, insulin-stimulated GLUT-4 translocation and glycogen synthesis in L6 cells (Huang et al. 2006). Insulin-resistant C2C12 cells have a 40 % decrease in basal FAK phosphorylation and insulin-resistant rats had a roughly 60 % decrease in basal FAK phosphorylation with no change in total FAK expression (Bisht et al. 2007). Overexpression of FAK increases labeled glucose uptake in insulin-sensitive cells by approximately 40 % and insulin-resistant cells by 29 % while overexpression of dominant-negative mutant FAK renders wild-type cells insulin-resistant (Bisht et al. 2007). Insulin can initiate actin remodeling and colocalization of FAK with remodeled actin which increases the ability of GLUT-4 to translocate to the sarcolemma, increasing insulin sensitivity (Bisht and Dey 2008). Intriguingly, GLUT-4 translocation is blunted by wartmanin, a PI3 K inhibitor, in cells over-expressing FAK by preventing actin remodeling, suggesting crosstalk between FAK and PI3K in insulin action (Bisht and Dey 2008). Insulin sensitivity can be manipulated by changing the phosphorylation state of FAK by known PI3K pathway regulators, SHP2 and PTEN. Inhibition of SHP2 and PTEN by siRNA increases FAK phosphorylation (Gupta and Dey 2009) and, in C2C12 cells, SHP2 has been found to directly bind to FAK, preventing its activity (de Oliveira et al. 2009).
FAK and the response to altered loading
Animal and culture models
FAK is inactivated by cytoskeletal forces (Rahnert and Burkholder 2013). The cellular localization of FAK, principally along the cytoplasmic surface of the sarcolemma (Wilson et al. 2014, 2012), allows it to be readily available for activation by integrins. While a direct link between mechanotransduction-induced FAK activation and muscle hypertrophy is still speculative, it has been well established that FAK responds quickly to both overload and unloading. Roosters that had their left wings overloaded with 10 % of their body weight for 1.5 and 7 days had increased FAK expression and increased FAK autophosphorylation at tyrosine 397 (Fluck et al. 1999). Further, FAK was observed in the belly of the muscle, providing the first evidence that FAK could be localized outside of the myotendinous junction (Fluck et al. 1999). Another component of this study used gastrocnemius ablation in rats to overload the soleus. The overloaded soleus had increases in FAK expression at 1 and 8 days post-surgery (Fluck et al. 1999). Gordon et al. (Gordon et al. 2001) used hindlimb unloading of female rats to examine the FAK response to changes in muscle loading in different muscle groups. The fast twitch plantaris and predominantly fast-twitch gastrocnemius had lower baseline levels of FAK expression compared to the soleus and had lower FAK expression following 7 days of unloading while the soleus surprisingly increased its expression of total FAK. The soleus, but not the plantaris or gastrocnemius, had decreases in levels of FAK phosphorylation compared to control rats after unloading (Gordon et al. 2001). In parallel studies, the effects of muscle overloading achieved by gastrocnemius ablation were examined; the soleus had increases in autophosphorylated FAK at 1 and 8 days post-surgery. The plantaris had increases in total FAK expression both 1 and 8 days post-overload but only had increases in expression of phosphorylated FAK at 8 days post-surgery (Gordon et al. 2001). Recently, Klossner et al (Klossner et al. 2013) used multiple experiments to analyze the time course of alterations in FAK activation response in muscle loading. Increased FAK phosphorylation occurred quickly, within 20 s after a 2 s passive stretch of the soleus. Soleus overloading by gastrocnemius ablation increased FAK activation at 1 and 24 h post-surgery and total FAK expression 6 and 24 h post-surgery. FAK overexpression increased expression of the costamere-related proteins β1 integrin and vinculin following reloading and this response was ablated in the presence of a FAK inhibitor, FAK-related non-kinase (FRNK), providing support that FAK expression is necessary for load-induced costamere organization (Klossner et al. 2013). We have found that total and phosphorylated FAK are lower following 90 min of muscle damage-inducing downhill treadmill exercise in the rat soleus compared to non-exercised controls. This decrease was seen at 2 h post-exercise but not 48 h post-exercise (Graham et al. 2015). In C2C12 cells, 30 min of static stretch decreases phosphorylated FAK by roughly 50 % while FAK phosphorylation was not altered in isolated primary muscle fibers from control or γ-sarcoglycan knockout mice over the course of 4 h of static stretching (Moorwood et al. 2014). In proliferating C2C12 cells, cyclic strain activates FAK 15 min post-strain, followed by a return to baseline at 30 min post-strain (Kumar et al. 2004).
Human models
Muscular unloading can lead to skeletal muscle protein catabolism as soon as 3 days after unloading in the vastus lateralis (Tesch et al. 2008). This duration of unloading does not alter FAK mRNA levels or total FAK in the vastus lateralis or soleus (Fluck et al. 2014a) but does decrease phosphorylation of tyrosine 397 in the vastus lateralis (Fluck et al. 2014a). During a 21 day unloading period of the vastus lateralis, FAK phosphorylation was reduced at day 10, with no further change seen during the final 11 days of the study. This pattern matched well with the overall reduced myofibrillar synthesis rates of the subjects (de Boer et al. 2007). In a similar study, 14 days of knee brace unloading resulted in decreases in FAK phosphorylation. Furthermore, amino acid supplementation had no effect on FAK phosphorylation, although phosphorylation of proteins in the PI3K cascade were increased (Glover et al. 2008b). Unloading from extended bedrest led to decreased total FAK expression after 8 and 34 days of bedrest with no changes in FAK phosphorylation (Li et al. 2013). Muscle type may influence the response of FAK during unloading. In a 58 days bedrest study using resistance training or resistance training plus vibration training, there was no effect on FAK expression in the soleus of the control group or either exercise group. However, FAK expression did increase in the vastus lateralis of the control and resistance-training plus vibration group (Salanova et al. 2014).
Externally applied pressure to muscle is another mechanism for FAK activation. Manual massage treatment conducted at the conclusion of a bout of aerobic exercise to exhaustion increased FAK phosphorylation in the vastus lateralis immediately post-exercise with a return to baseline 3 h later (Crane et al. 2012). We saw no changes in total or phosphorylated FAK levels 24, 48, or 72 h after instrument-assisted massage of the gastrocnemius (Graham, in review), suggesting that FAK activation in response to massage is most likely transient and occurs within the 3 h window.
Traditional exercise, both aerobic and resistance training, are widely used interventions to increase muscle health and performance but generally provide for less force production than experimental overloading conditions. However, both can transmit forces across the sarcolemma and have the potential to activate FAK. Four sets of 10 repetitions of leg press and leg extensions maneuvers did not result in changes in FAK phosphorylation in actively resistance-trained men (Glover et al. 2008a). Another study in sedentary males saw that FAK phosphorylation was increased for both acute aerobic and anaerobic exercise immediately post-exercise with anaerobic exercise remaining elevated at 4 h post-exercise. Additionally, 10 weeks of training increased basal levels of FAK phosphorylation (Wilkinson et al. 2008). Nine weeks of resistance training (three times a week) on a flywheel ergometer that loads the muscle during the concentric and eccentric phases increased phosphorylated FAK at both the mid and end of the protocol (Li et al. 2013). Downhill skiing training has been shown to increase FAK expression by approximately twofold in elderly men while having no effect on elderly women (Flueck et al. 2011).
FAK and clinical conditions
Pathologies such as muscular dystrophies result in satellite activation and increased myoblast differentiation in an attempt at muscle regeneration. Dystrophic mice that have mutations of the α2 chain of laminins have similar levels of total FAK compared to wild-type mice 2 weeks after birth. At 12 weeks post-birth, dystrophic mice have a non-significant 25 % decrease in total FAK in the gastrocnemius and a significant 60 % decrease in the rectus femoris (Sakuma et al. 2004). The rectus femoris is not a major weight bearing muscle as compared to the gastrocnemius. Furthermore, these dystrophic mice drag their hindlimbs by a few weeks after birth and this abnormality progresses to complete hindlimb extension after 1–2 months (Sakuma et al. 2004). Thus, the decreases in FAK expression in these muscle dystrophy models may be a result of decreased physical activity and muscle recruitment.
SCI leads to immobilization and disruption of motor, sensory and autonomic nervous system function below the anatomical level of the lesion, creating a state of muscle atrophy that is distinct from that of hindlimb suspension in rodents or braces, bedrest and casts in humans. The alterations in signaling by FAK after SCI has only recently been investigated. These data suggest that individuals who are on average 22 years post-SCI have an approximately threefold elevation in phosphorylated FAK compared to able bodied subjects but no further increases in FAK activation following external electrical stimulation (Yarar-Fisher et al. 2014). Conversely, in rat gastrocnemius muscle at 56 days after spinal cord transection we have observed that total and phosphorylated FAK are reduced (Graham, manuscript in review).
Ischemic protection through FAK
In rats exposed to 4 h of complete femoral artery occlusion, total FAK was increased in the gastrocnemius, but not the soleus, 24 h post-reperfusion (Fluck et al. 2014b). FAK overexpression prevented ischemic rhabdomyolysis and reduced expression of the mitochondrial pore opening protein Bax and inflammatory marker CD68 in the predominantly fast-twitch tibialis anterior. In these studies, overexpression of FAK was less effective in the soleus but, even so, TUNEL-staining for apoptosis was decreased (Fluck et al. 2014b).
Muscle fiber type and FAK expression
There are differences in FAK expression in muscles with different fiber type characteristics, with the slow-twitch and fast-twitch oxidative muscle having higher basal levels (Gordon et al. 2001). Type I and IIa muscle fibers show the highest sarcolemmal expression of FAK as measured by immunohistochemistry (Flück et al. 2002). Additionally, soleus muscle that was cross-reinnervated with the extensor digitorum longus (EDL) nerve gradually lost FAK expression while EDL muscle cross-reinnervated with the soleus nerve and placed in the soleus cavity demonstrated increased FAK expression (Flück et al. 2002). Thus, the changes in localization and expression of FAK seem to be indicative of the properties of the innervating nerve, with early and minimal recruitment firing patterns of slow-twitch motor neurons driving FAK expression.
This neural drive of FAK is interesting in that FAK may be involved in regulating the oxidative capacity of the muscle fiber. Electrotransfer of the FAK gene into the soleus increased the expression of multiple proteins of the electron transfer chain as well as myosin heavy chain 1. Additionally, FAK overexpression was associated with a decrease in myosin heavy chain 2A mRNA and protein expression (Durieux et al. 2009). FAK overexpression decreased hybrid fiber percentage whereas FRNK, a competitive inhibitor of FAK, increased fast twitch fiber percentage (Klossner et al. 2013). However, these FAK-induced changes are not seen when the muscle is unloaded (24), indicating that FAK regulates the oxidative phenotype only when under load.
Conclusion
The role of FAK in skeletal muscle provides important insight into how myogenesis and muscle homeostasis is regulated by integrated responses of multiple signaling pathways. The role of FAK is plieotropic, with roles in regulating myogenesis, muscle phenotype, costamere formation, muscle hypertrophy and glucose uptake. Developing therapies or interventions that take advantage of these many functions of FAK in muscle homeostasis may prove to be beneficial for increasing muscle health. The role of FAK in promoting cell survival has readily been established in multiple cell types and conditions and information gained may provide insights regarding how to design exercise or pharmaceutical interventions that help protect against muscle atrophy following SCI or in cachectic and sarcopenic conditions.
Acknowledgments
This work was supported by a Veterans Affairs Rehabilitation Research and Development Service grant (B9212C) and the James J. Peters VA Medical Center, where Dr. Cardozo is a member of the Medical-Surgical Service and Dr. Graham is a member of the Research Service.
Abbreviations
- FAK
Focal adhesion kinase
- PI3K
Phosphatidylinositol-3 kinase
- ERK1/2
Extracellular signal-related kinase 1/2
- mTOR
Mechanistic target of rapamycin
- SCI
Spinal cord injury
- PKC
Protein kinase C
- ECM
Extracellular matrix
- IGF-1
Insulin-like growth factor 1
- FERM
4.1 ezrin, radixin, moesin domain
- FAT
Focal adhesion targeting
- MDB2
Methyl CpG-binding protein 2
- FRNK
Focal adhesion kinase-related non-kinase (FRNK)
References
- Anastasi G, et al. Costameric proteins in human skeletal muscle during muscular inactivity. J Anat. 2008;213:284–295. doi: 10.1111/j.1469-7580.2008.00921.x. doi:10.1111/j.1469-7580.2008.00921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baar K, Esser K. Phosphorylation of p70S6K corelates with increased muscle mass following resistance exercise. Am J Cell Physiol. 1999;276:120–127. doi: 10.1152/ajpcell.1999.276.1.C120. [DOI] [PubMed] [Google Scholar]
- Baar K, Nader G, Bodine S. Resistance exercise, muscle loading/unloading and the control of muscle mass. Essays Biochem. 2006;42:61–74. doi: 10.1042/bse0420061. doi:10.1042/bse0420061. [DOI] [PubMed] [Google Scholar]
- Bae GU, et al. Neogenin regulates skeletal myofiber size and focal adhesion kinase and extracellular signal-regulated kinase activities in vivo and in vitro. Mol Biol Cell. 2009;20:4920–4931. doi: 10.1091/mbc.E09-06-0491. doi:10.1091/mbc.E09-06-0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines AJ, Lu HC, Bennett PM. The Protein 4.1 family: hub proteins in animals for organizing membrane proteins. Biochim Biophys Acta. 2014;1838:605–619. doi: 10.1016/j.bbamem.2013.05.030. doi:10.1016/j.bbamem.2013.05.030. [DOI] [PubMed] [Google Scholar]
- Balaban NQ, et al. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat Cell Biol. 2001;3:466–472. doi: 10.1038/35074532. doi:10.1038/35074532. [DOI] [PubMed] [Google Scholar]
- Bisht B, Dey CS. Focal Adhesion Kinase contributes to insulin-induced actin reorganization into a mesh harboring Glucose transporter-4 in insulin resistant skeletal muscle cells. BMC Cell Biol. 2008;9:48. doi: 10.1186/1471-2121-9-48. doi:10.1186/1471-2121-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisht B, Goel HL, Dey CS. Focal adhesion kinase regulates insulin resistance in skeletal muscle. Diabetologia. 2007;50:1058–1069. doi: 10.1007/s00125-007-0591-6. doi:10.1007/s00125-007-0591-6. [DOI] [PubMed] [Google Scholar]
- Bloch RJ, Gonzalez-Serratos H. Lateral force transmission across costameres in skeletal muscle. Exerc Sport Sci Rev. 2003;31:73–78. doi: 10.1097/00003677-200304000-00004. [DOI] [PubMed] [Google Scholar]
- Bodine SC. mTOR signaling and the molecular adaptation to resistance exercise. Med Sci Sports Exerc. 2006;38:1950–1957. doi: 10.1249/01.mss.0000233797.24035.35. doi:10.1249/01.mss.0000233797.24035.35. [DOI] [PubMed] [Google Scholar]
- Boppart MD, Hirshman MF, Sakamoto K, Fielding RA, Goodyear LJ. Static stretch increases c-Jun NH2-terminal kinase activity and p38 phosphorylation in rat skeletal muscle. Am J Physiol Cell Physiol. 2001;280:C352–C358. doi: 10.1152/ajpcell.2001.280.2.C352. [DOI] [PubMed] [Google Scholar]
- Boppart MD, Burkin D, Kaufman SJ. Activation of AKT signaling promotes cell growth and survival in α7β1 integrin-mediated alleviation of muscular dystrophy. Biochim Biophys Acta. 2011;1812:439–446. doi: 10.1016/j.bbadis.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkholder TJ. Mechanotransduction in skeletal muscle. Front Biosci. 2007;12:174–191. doi: 10.2741/2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkin DJ, Kaufman SJ. The alpha7beta1 integrin in muscle development and disease. Cell Tissue Res. 1999;296:183–190. doi: 10.1007/s004410051279. [DOI] [PubMed] [Google Scholar]
- Cai X, et al. Spatial and temporal regulation of focal adhesion kinase activity in living cells. Mol Cell Biol. 2008;28:201–214. doi: 10.1128/MCB.01324-07. doi:10.1128/mcb.01324-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaillou T, Lee JD, England JH, Esser KA, McCarthy JJ. Time course of gene expression during mouse skeletal muscle hypertrophy. J Appl Physiol (1985) 2013;115:1065–1074. doi: 10.1152/japplphysiol.00611.2013. doi:10.1152/japplphysiol.00611.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HC, Appeddu PA, Isoda H, Guan JL. Phosphorylation of tyrosine 397 in focal adhesion kinase is required for binding phosphatidylinositol 3’-kinase. J Biol Chem. 1996;271:26329–26334. doi: 10.1074/jbc.271.42.26329. [DOI] [PubMed] [Google Scholar]
- Clemente CF, Corat MA, Saad ST, Franchini KG. Differentiation of C2C12 myoblasts is critically regulated by FAK signaling. Am J Physiol Regul Integr Comp Physiol. 2005;289:R862–R870. doi: 10.1152/ajpregu.00348.2004. doi:10.1152/ajpregu.00348.2004. [DOI] [PubMed] [Google Scholar]
- Cooper LA, Shen TL, Guan JL. Regulation of focal adhesion kinase by its amino-terminal domain through an autoinhibitory interaction. Mol Cell Biol. 2003;23:8030–8041. doi: 10.1128/MCB.23.22.8030-8041.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane JD, Ogborn DI, Cupido C, Melov S, Hubbard A, Bourgeois JM, Tarnopolsky MA. Massage therapy attenuates inflammatory signaling after exercise-induced muscle damage. Sci Transl Med. 2012;4:119ra113. doi: 10.1126/scitranslmed.3002882. doi:10.1126/scitranslmed.3002882. [DOI] [PubMed] [Google Scholar]
- Crossland H, et al. Focal adhesion kinase is required for IGF-I-mediated growth of skeletal muscle cells via a TSC2/mTOR/S6K1-associated pathway. Am J Physiol Endocrinol Metab. 2013;305:E183–E193. doi: 10.1152/ajpendo.00541.2012. doi:10.1152/ajpendo.00541.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer MD, et al. The temporal responses of protein synthesis, gene expression and cell signalling in human quadriceps muscle and patellar tendon to disuse. J Physiol. 2007;585:241–251. doi: 10.1113/jphysiol.2007.142828. doi:10.1113/jphysiol.2007.142828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Deyne PG. Formation of sarcomeres in developing myotubes: role of mechanical stretch and contractile activation. Am J Physiol Cell Physiol. 2000;279:C1801–C1811. doi: 10.1152/ajpcell.2000.279.6.C1801. [DOI] [PubMed] [Google Scholar]
- de Oliveira MV, Marin TM, Clemente CF, Costa AP, Judice CC, Franchini KG. SHP-2 regulates myogenesis by coupling to FAK signaling pathway. FEBS Lett. 2009;583:2975–2981. doi: 10.1016/j.febslet.2009.08.022. doi:10.1016/j.febslet.2009.08.022. [DOI] [PubMed] [Google Scholar]
- Del Re DP, Miyamoto S, Brown JH. Focal adhesion kinase as a RhoA-activable signaling scaffold mediating Akt activation and cardiomyocyte protection. J Biol Chem. 2008;283:35622–35629. doi: 10.1074/jbc.M804036200. doi:10.1074/jbc.M804036200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disatnik MH, Rando TA. Integrin-mediated muscle cell spreading. The role of protein kinase c in outside-in and inside-out signaling and evidence of integrin cross-talk. J Biol Chem. 1999;274:32486–32492. doi: 10.1074/jbc.274.45.32486. [DOI] [PubMed] [Google Scholar]
- Durieux AC, D’Antona G, Desplanches D, Freyssenet D, Klossner S, Bottinelli R, Fluck M. Focal adhesion kinase is a load-dependent governor of the slow contractile and oxidative muscle phenotype. J Physiol. 2009;587:3703–3717. doi: 10.1113/jphysiol.2009.171355. doi:10.1113/jphysiol.2009.171355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluck M, Carson JA, Gordon SE, Ziemiecki A, Booth FW. Focal adhesion proteins FAK and paxillin increase in hypertrophied skeletal muscle. Am J Physiol. 1999;277:C152–C162. doi: 10.1152/ajpcell.1999.277.1.C152. [DOI] [PubMed] [Google Scholar]
- Fluck M, Li R, Valdivieso P, Linnehan RM, Castells J, Tesch P, Gustafsson T. Early changes in costameric and mitochondrial protein expression with unloading are muscle specific. Biomed Res Int. 2014a;2014:519310. doi: 10.1155/2014/519310. doi:10.1155/2014/519310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluck M, von Allmen RS, Ferrie C, Tevaearai H, Dick F. Protective effect of focal adhesion kinase against skeletal muscle reperfusion injury after acute limb ischemia. Eur J Vasc Endovasc Surg. 2014b doi: 10.1016/j.ejvs.2014.11.011. doi:10.1016/j.ejvs.2014.11.011. [DOI] [PubMed] [Google Scholar]
- Flück M, Ziemiecki A, Billeter R, Müntener M. Fibre-type specific concentration of focal adhesion kinase at the sarcolemma: influence of fibre innervation and regeneration. J Exp Biol. 2002;205:2337–2348. doi: 10.1242/jeb.205.16.2337. [DOI] [PubMed] [Google Scholar]
- Flueck M, et al. Load-sensitive adhesion factor expression in the elderly with skiing: relation to fiber type and muscle strength. Scand J Med Sci Sports. 2011;21(Suppl 1):29–38. doi: 10.1111/j.1600-0838.2011.01339.x. doi:10.1111/j.1600-0838.2011.01339.x. [DOI] [PubMed] [Google Scholar]
- Frame MC, Patel H, Serrels B, Lietha D, Eck MJ. The FERM domain: organizing the structure and function of FAK. Nat Rev Mol Cell Biol. 2010;11:802–814. doi: 10.1038/nrm2996. doi:10.1038/nrm2996. [DOI] [PubMed] [Google Scholar]
- Franchini KG. Focal adhesion kinase—the basis of local hypertrophic signaling domains. J Mol Cell Cardiol. 2012;52:485–492. doi: 10.1016/j.yjmcc.2011.06.021. doi:10.1016/j.yjmcc.2011.06.021. [DOI] [PubMed] [Google Scholar]
- Franchini KG, Torsoni AS, Soares PHA, Saad MJA. Early activated of the multicomponent signaling complex associated with focal adhesion kinase induced by pressure overlaod in the rat heard. Circ Res. 2000;87:558–565. doi: 10.1161/01.res.87.7.558. [DOI] [PubMed] [Google Scholar]
- Fujita H, Nedachi T, Kanzaki M. Accelerated de novo sarcomere assembly by electric pulse stimulation in C2C12 myotubes. Exp Cell Res. 2007;313:1853–1865. doi: 10.1016/j.yexcr.2007.03.002. doi:10.1016/j.yexcr.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Gan B, Yoo Y, Guan JL. Association of focal adhesion kinase with tuberous sclerosis complex 2 in the regulation of s6 kinase activation and cell growth. J Biol Chem. 2006;281:37321–37329. doi: 10.1074/jbc.M605241200. doi:10.1074/jbc.M605241200. [DOI] [PubMed] [Google Scholar]
- Gautel M. Cytoskeletal protein kinases: titin and its relations in mechanosensing. Pflugers Arch. 2011;462:119–134. doi: 10.1007/s00424-011-0946-1. doi:10.1007/s00424-011-0946-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover EI, Oates BR, Tang TE, Moore DR, Tarnopolsky MA, Phillips SM. Resistance training decreases eIF2Bε phosphorylation and potentiates the feeding-induced stimulation of p70s6k and rpS6 in young men. Am J Physiol Regul Integr Comp Physiol. 2008a;295:604–610. doi: 10.1152/ajpregu.00097.2008. [DOI] [PubMed] [Google Scholar]
- Glover EI, et al. Immobilization induces anabolic resistance in human myofibrillar protein synthesis with low and high dose amino acid infusion. J Physiol. 2008b;586:6049–6061. doi: 10.1113/jphysiol.2008.160333. doi:10.1113/jphysiol.2008.160333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel HL, Dey CS. Focal adhesion kinase tyrosine phosphorylation is associated with myogenesis and modulated by insulin. Cell Prolif. 2002a;35:131–142. doi: 10.1046/j.1365-2184.2002.00232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel HL, Dey CS. PKC-regulated myogenesis is associated with increased tyrosine phosphorylation of FAK, Cas, and paxillin, formation of Cas-CRK complex, and JNK activation. Differentiation. 2002b;70:257–271. doi: 10.1046/j.1432-0436.2002.700604.x. doi:10.1046/j.1432-0436.2002.700604.x. [DOI] [PubMed] [Google Scholar]
- Goodman CA, Mayhew DL, Hornberger TA. Recent progress toward understanding the molecular mechanisms that regulate skeletal muscle mass. Cell Signal. 2011;23:1896–1906. doi: 10.1016/j.cellsig.2011.07.013. doi:10.1016/j.cellsig.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon SE, Fluck M, Booth FW. Selected contribution: skeletal muscle focal adhesion kinase, paxillin, and serum response factor are loading dependent. J Appl Physiol. 2001;90:1174–1183. doi: 10.1152/jappl.2001.90.3.1174. discussion 1165. [DOI] [PubMed] [Google Scholar]
- Graham ZA, Touchberry CD, Gupte AA, Bomhoff GL, Geiger PC, Gallagher PM. Changes in alpha7beta1 integrin signaling after eccentric exercise in heat-shocked rat soleus. Muscle Nerve. 2015;51:562–568. doi: 10.1002/mus.24324. doi:10.1002/mus.24324. [DOI] [PubMed] [Google Scholar]
- Gupta A, Dey CS. PTEN and SHIP2 regulates PI3K/Akt pathway through focal adhesion kinase. Mol Cell Endocrinol. 2009;309:55–62. doi: 10.1016/j.mce.2009.05.018. doi:10.1016/j.mce.2009.05.018. [DOI] [PubMed] [Google Scholar]
- Hall JE, Fu W, Schaller MD. Focal adhesion kinase: exploring Fak structure to gain insight into function. Int Rev Cell Mol Biol. 2011;288:185–225. doi: 10.1016/B978-0-12-386041-5.00005-4. doi:10.1016/B978-0-12-386041-5.00005-4. [DOI] [PubMed] [Google Scholar]
- Han JW, Lee HJ, Bae GU, Kang JS. Promyogenic function of Integrin/FAK signaling is mediated by Cdo, Cdc42 and MyoD. Cell Signal. 2011;23:1162–1169. doi: 10.1016/j.cellsig.2011.03.001. doi:10.1016/j.cellsig.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Hanks SK, Ryzhova L, Shin NY, Brabek J. Focal adhesion kinase signaling activities and their implications in the control of cell survival and motility. Front Biosci. 2003;8:D982–D996. doi: 10.2741/1114. doi:10.2741/1114. [DOI] [PubMed] [Google Scholar]
- Hildebrand JD, Schaller MD, Parsons JT. Identification of sequences required for the efficient localization of the focal adhesion kinase, pp125FAK, to cellular focal adhesions. J Cell Biol. 1993;123:993–1005. doi: 10.1083/jcb.123.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornberger TA, Armstrong DD, Koh TJ, Burkholder TJ, Esser KA. Intracellular signaling specificity in response to uniaxial vs. multiaxial stretch: implications for mechanotransduction. Am J Physiol Cell Physiol. 2005;288:C185–C194. doi: 10.1152/ajpcell.00207.2004. doi:10.1152/ajpcell.00207.2004. [DOI] [PubMed] [Google Scholar]
- Huang D, Khoe M, Ilic D, Bryer-Ash M. Reduced expression of focal adhesion kinase disrupts insulin action in skeletal muscle cells. Endocrinology. 2006;147:3333–3343. doi: 10.1210/en.2005-0382. doi:10.1210/en.2005-0382. [DOI] [PubMed] [Google Scholar]
- Klossner S, Durieux AC, Freyssenet D, Flueck M. Mechanotransduction to muscle protein synthesis is modulated by FAK. Eur J Appl Physiol. 2009;106:389–398. doi: 10.1007/s00421-009-1032-7. doi:10.1007/s00421-009-1032-7. [DOI] [PubMed] [Google Scholar]
- Klossner S, Li R, Ruoss S, Durieux AC, Fluck M. Quantitative changes in focal adhesion kinase and its inhibitor, FRNK, drive load-dependent expression of costamere components. Am J Physiol Regul Integr Comp Physiol. 2013 doi: 10.1152/ajpregu.00007.2013. doi:10.1152/ajpregu.00007.2013. [DOI] [PubMed] [Google Scholar]
- Kumar A, Murphy R, Robinson P, Wei L, Boriek AM. Cyclic mechanical strain inhibits skeletal myogenesis through activation of focal adhesion kinase, Rac-1 GTPase, and NF-kappaB transcription factor. FASEB J. 2004;18:1524–1535. doi: 10.1096/fj.04-2414com. doi:10.1096/fj.04-2414com. [DOI] [PubMed] [Google Scholar]
- Lai Wing Sun K, Correia JP, Kennedy TE. Netrins: versatile extracellular cues with diverse functions. Development. 2011;138:2153–2169. doi: 10.1242/dev.044529. doi:10.1242/dev.044529. [DOI] [PubMed] [Google Scholar]
- Lamon S, Wallace MA, Russell AP. The STARS signaling pathway: a key regulator of skeletal muscle function. Pflugers Arch. 2014;466:1659–1671. doi: 10.1007/s00424-014-1475-5. doi:10.1007/s00424-014-1475-5. [DOI] [PubMed] [Google Scholar]
- Li W, et al. Activation of FAK and Src are receptor-proximal events required for netrin signaling. Nat Neurosci. 2004;7:1213–1221. doi: 10.1038/nn1329. doi:10.1038/nn1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, et al. Costamere remodeling with muscle loading and unloading in healthy young men. J Anat. 2013 doi: 10.1111/joa.12101. doi:10.1111/joa.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lietha D, Cai X, Ceccarelli DF, Li Y, Schaller MD, Eck MJ. Structural basis for the autoinhibition of focal adhesion kinase. Cell. 2007;129:1177–1187. doi: 10.1016/j.cell.2007.05.041. doi:10.1016/j.cell.2007.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo SW, et al. Regulation of heterochromatin remodelling and myogenin expression during muscle differentiation by FAK interaction with MBD2. EMBO J. 2009;28:2568–2582. doi: 10.1038/emboj.2009.178. doi:10.1038/emboj.2009.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madaro L, et al. PKCtheta signaling is required for myoblast fusion by regulating the expression of caveolin-3 and beta1D integrin upstream focal adhesion kinase. Mol Biol Cell. 2011;22:1409–1419. doi: 10.1091/mbc.E10-10-0821. doi:10.1091/mbc.E10-10-0821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer U, et al. Absence of integrin alpha 7 causes a novel form of muscular dystrophy. Nat Genet. 1997;17:318–323. doi: 10.1038/ng1197-318. doi:10.1038/ng1197-318. [DOI] [PubMed] [Google Scholar]
- McClung JM, Thompson RW, Lowe LL, Carson JA. RhoA expression during recovery from skeletal muscle disuse. J Appl Physiol (1985) 2004;96:1341–1348. doi: 10.1152/japplphysiol.01015.2003. doi:10.1152/japplphysiol.01015.2003. [DOI] [PubMed] [Google Scholar]
- Moorwood C, Philippou A, Spinazzola J, Keyser B, Macarak EJ, Barton ER. Absence of gamma-sarcoglycan alters the response of p70S6 kinase to mechanical perturbation in murine skeletal muscle. Skelet Muscle. 2014;4:13. doi: 10.1186/2044-5040-4-13. doi:10.1186/2044-5040-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen N, Yi JS, Park H, Lee JS, Ko YG. Mitsugumin 53 (MG53) ligase ubiquitinates focal adhesion kinase during skeletal myogenesis. J Biol Chem. 2014;289:3209–3216. doi: 10.1074/jbc.M113.525154. doi:10.1074/jbc.M113.525154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr AW, Murphy-Ullrich JE. Regulation of endothelial cell function BY FAK and PYK2. Front Biosci. 2004;9:1254–1266. doi: 10.2741/1239. [DOI] [PubMed] [Google Scholar]
- Pardo JV, Siliciano JD, Craig SW. A vinculin-containing cortical lattice in skeletal muscle: transverse lattice elements (“costameres”) mark sites of attachment between myofibrils and sarcolemma. Proc Natl Acad Sci USA. 1983;80:1008–1012. doi: 10.1073/pnas.80.4.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons JT. Focal adhesion kinase: the first ten years. J Cell Sci. 2003;116:1409–1416. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev. 2008;88:1379–1406. doi: 10.1152/physrev.90100.2007. doi:10.1152/physrev.90100.2007. [DOI] [PubMed] [Google Scholar]
- Quach NL, Rando TA. Focal adhesion kinase is essential for constamerogenesis in cultured skeletal muscle cells. Dev Biol. 2006;293:38–52. doi: 10.1016/j.ydbio.2005.12.040. [DOI] [PubMed] [Google Scholar]
- Quach NL, Biressi S, Reichardt LF, Keller C, Rando TA. Focal adhesion kinase signaling regulates the expression of caveolin 3 and beta1 integrin, genes essential for normal myoblast fusion. Mol Biol Cell. 2009;20:3422–3435. doi: 10.1091/mbc.E09-02-0175. doi:10.1091/mbc.E09-02-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahnert JA, Burkholder TJ. High-frequency electrical stimulation reveals a p38-mTOR signaling module correlated with force-time integral. J Exp Biol. 2013;216:2619–2631. doi: 10.1242/jeb.080705. doi:10.1242/jeb.080705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiling JH, Sabatini DM. Stress and mTORture signaling. Oncogene. 2006;25:6373–6383. doi: 10.1038/sj.onc.1209889. doi:10.1038/sj.onc.1209889. [DOI] [PubMed] [Google Scholar]
- Sakuma K, Nishikawa J, Nakao R, Nakano H, Sano M, Yasuhara M. Serum response factor plays an important role in the mechanically overloaded plantaris muscle of rats. Histochem Cell Biol. 2003;119:149–160. doi: 10.1007/s00418-003-0499-2. doi:10.1007/s00418-003-0499-2. [DOI] [PubMed] [Google Scholar]
- Sakuma K, Nakao R, Inashima S, Hirata M, Kubo T, Yasuhara M. Marked reduction of focal adhesion kinase, serum response factor and myocyte enhancer factor 2C, but increase in RhoA and myostatin in the hindlimb dy mouse muscles. Acta Neuropathol. 2004;108:241–249. doi: 10.1007/s00401-004-0884-5. doi:10.1007/s00401-004-0884-5. [DOI] [PubMed] [Google Scholar]
- Salanova M, et al. Disuse deterioration of human skeletal muscle challenged by resistive exercise superimposed with vibration: evidence from structural and proteomic analysis. FASEB J. 2014 doi: 10.1096/fj.14-252825. doi:10.1096/fj.14-252825. [DOI] [PubMed] [Google Scholar]
- Sastry SK, Lakonishok M, Wu S, Truong TQ, Huttenlocher A, Turner CE, Horwitz AF. Quantitative changes in integrin and focal adhesion signaling regulate myoblast cell cycle withdrawal. J Cell Biol. 1999;144:1295–1309. doi: 10.1083/jcb.144.6.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller MD, Hildebrand JD, Shannon JD, Fox JW, Vines RR, Parsons JT. Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src. Mol Cell Biol. 1994;14:1680–1688. doi: 10.1128/mcb.14.3.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller M, Otey CA, Hildebrand JD, Parsons JT. Focal adhesion kinase and paxillin bind to peptides mimicking β integrin cytoplasmic domains. J Cell Biol. 1995;130:1181–1187. doi: 10.1083/jcb.130.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Scheffler JM, Zeng C, Pleitner JM, Hannon KM, Grant AL, Gerrard DE. Mitogen-activated protein kinase signaling is necessary for the maintenance of skeletal muscle mass. Am J Physiol Cell Physiol. 2009;296:C1040–C1048. doi: 10.1152/ajpcell.00475.2008. doi:10.1152/ajpcell.00475.2008. [DOI] [PubMed] [Google Scholar]
- Tesch PA, von Walden F, Gustafsson T, Linnehan RM, Trappe TA. Skeletal muscle proteolysis in response to short-term unloading in humans. J Appl Physiol (1985) 2008;105:902–906. doi: 10.1152/japplphysiol.90558.2008. doi:10.1152/japplphysiol.90558.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidball JG. Mechanical signal transduction in skeletal muscle growth and adaptation. J Appl Physiol (1985) 2005;98:1900–1908. doi: 10.1152/japplphysiol.01178.2004. doi:10.1152/japplphysiol.01178.2004. [DOI] [PubMed] [Google Scholar]
- Torsoni AS, Constancio SS, Nadruz W, Hanks SK, Franchini KG. Focal adhesion kinase is activated and mediates the early hypertrophic response to stretch in cardiac myocytes. Circ Res. 2003;93:140–147. doi: 10.1161/01.RES.0000081595.25297.1B. doi:10.1161/01.Res.0000081595.25297.1b. [DOI] [PubMed] [Google Scholar]
- Torsoni AS, Marin TM, Velloso LA, Franchini KG. RhoA/ROCK signaling is criticial to FAK activation by cyclic stretch in cardiac myocytes. Am J Physiol. 2005;289:1488–1496. doi: 10.1152/ajpheart.00692.2004. [DOI] [PubMed] [Google Scholar]
- Tran TH, Shi X, Zaia J, Ai X. Heparan sulfate 6-O-endosulfatases (Sulfs) coordinate the Wnt signaling pathways to regulate myoblast fusion during skeletal muscle regeneration. J Biol Chem. 2012;287:32651–32664. doi: 10.1074/jbc.M112.353243. doi:10.1074/jbc.M112.353243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng WP, Su CM, Tang CH. FAK activation is required for TNF-alpha-induced IL-6 production in myoblasts. J Cell Physiol. 2010;223:389–396. doi: 10.1002/jcp.22047. doi:10.1002/jcp.22047. [DOI] [PubMed] [Google Scholar]
- Wang HV, et al. Integrin-linked kinase stabilizes myotendinous junctions and protects muscle from stress-induced damage. J Cell Biol. 2008;180:1037–1049. doi: 10.1083/jcb.200707175. doi:10.1083/jcb.200707175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L, Zhou W, Croissant JD, Johansen FE, Prywes R, Balasubramanyam A, Schwartz RJ. RhoA signaling via serum response factor plays an obligatory role in myogenic differentiation. J Biol Chem. 1998;273:30287–30294. doi: 10.1074/jbc.273.46.30287. [DOI] [PubMed] [Google Scholar]
- Wei L, Zhou W, Wang L, Schwartz RJ. beta(1)-integrin and PI 3-kinase regulate RhoA-dependent activation of skeletal alpha-actin promoter in myoblasts. Am J Physiol Heart Circ Physiol. 2000;278:H1736–H1743. doi: 10.1152/ajpheart.2000.278.6.H1736. [DOI] [PubMed] [Google Scholar]
- Wilkinson SB, Phillips SM, Atherton PJ, Patel R, Yarasheski KE, Tarnopolsky MA, Rennie MJ. Differential effects of resistance and endurance exercise in the fed state on signalling molecule phosphorylation and protein synthesis in human muscle. J Physiol. 2008;586:3701–3717. doi: 10.1113/jphysiol.2008.153916. doi:10.1113/jphysiol.2008.153916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D, Gallagher P, Harber M, Hollon C, Trappe S. Mitogen-activated protein kinase (MAPK) pathway activation: effects of age and acute exercise on human skeletal muscle. J Physiol. 2003;547:977–987. doi: 10.1113/jphysiol.2002.036673. doi:10.1113/jphysiol.2002.036673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson OJ, Shaw CS, Sherlock M, Stewart PM, Wagenmakers AJ. Immunofluorescent visualisation of focal adhesion kinase in human skeletal muscle and its associated microvasculature. Histochem Cell Biol. 2012;138:617–626. doi: 10.1007/s00418-012-0980-x. doi:10.1007/s00418-012-0980-x. [DOI] [PubMed] [Google Scholar]
- Wilson OJ, Bradley H, Shaw CS, Wagenmakers AJ. Paxillin and focal adhesion kinase colocalise in human skeletal muscle and its associated microvasculature. Histochem Cell Biol. 2014;142:245–256. doi: 10.1007/s00418-014-1212-3. doi:10.1007/s00418-014-1212-3. [DOI] [PubMed] [Google Scholar]
- Yarar-Fisher C, Bickel CS, Kelly NA, Windham ST, McLain AB, Bamman MM. Mechanosensitivity may be enhanced in skeletal muscles of spinal cord-injured versus able-bodied men. Muscle Nerve. 2014;50:599–601. doi: 10.1002/mus.24248. doi:10.1002/mus.24248. [DOI] [PMC free article] [PubMed] [Google Scholar]