Abstract
Akt kinase, a member of AGC kinases, is important in many cellular functions including proliferation, migration, cell growth and metabolism. There are three known Akt isoforms which play critical and diverse roles in the cardiovascular system. Akt activity is regulated by its upstream regulatory pathways at transcriptional and post-translational levels. beta-catenin/Tcf-4, GLI1 and Stat-3 are some of few known transcriptional regulators of AKT gene. Threonine 308 and serine 473 are the two critical phosphorylation sites of Akt1. Translocation of Akt to the cell membrane facilitates PDK1 phosphorylation of the threonine site. The serine site is phosphorylated by mTORC2. Ack1, Src, PTK6, TBK1, IKBKE and IKKε are some of the non-canonical pathways which affect the Akt activity. Protein-protein interactions of Akt to actin and Hsp90 increase the Akt activity while Akt binding to other proteins such as CTMP and TRB3 reduces the Akt activity. The action of Akt on its downstream targets determines its function in cardiovascular processes such as cell survival, growth, proliferation, angiogenesis, vasorelaxation, and cell metabolism. Akt promotes cell survival via caspase-9, YAP, Bcl-2, and Bcl-x activities. Inhibition of FoxO proteins by Akt also increases cell survival by transcriptional mechanisms. Akt stimulates cell growth and proliferation through mTORC1. Akt also increases VEGF secretion and mediates eNOS phosphorylation, vasorelaxation and angiogenesis. Akt can increase cellular metabolism through its downstream targets GSK3 and GLUT4. The alterations of Akt signaling play an important role in many cardiovascular pathological processes such as atherosclerosis, cardiac hypertrophy, and vascular remodeling. Several Akt inhibitors have been developed and tested as anti-tumor agents. They could be potential novel therapeutics for the cardiovascular diseases.
Keywords: Akt, vascular remodeling, Signal transduction
Graphical Abstract
Introduction
Akt family kinases (also known as protein kinase B /PKB) are serine/threonine kinases that belongs to the general class of AGC kinases (AMP/GMP kinase and PKC subfamily of proteins) which has 518 members in humans [1]. While there are Akt homologs from fly to humans, structure and function of mammalian Akt is highly conserved [2–6]. General pathway of Akt is called phosphoinositide-3-kinase–protein kinase B/Akt (PI3K-PKB/Akt) pathway or PI3k/AKT/mTOR pathway, named after upstream and downstream proteins involved. It was discovered by three different groups at the same time. Two of those discoveries were based on PKA or PKC homology based approaches and the other is through retroviral cloning [7–9]. The name Akt stems from the retroviral strain Akt-8 which was used for the cloning experiments. There are three Akt isoforms, Akt1, Akt2 and Akt3 (also known as PKBα, PKBβ, and PKBγ, respectively). Akt1 and Akt3 are ubiquitously expressed while Akt2 is expressed in the insulin-responsive tissues such as brown fat, skeletal muscle and liver [10]. The upstream and downstream targets of these Akt isoforms are quite similar. But there seems to be functional differences between these isoforms in different cell context. They seem to be specific in their interactions with other proteins. For example, onco-protein TCL1b forms oligomers with Akt1, not Akt2 or Akt3 [11]. These differences can be observed in the cell cycle regulation. Akt2 accumulates in the cytoplasm during mitosis [12] and in the nucleus during muscle differentiation [13]. Differences in Akt isoforms are also evident in disease development. Akt2 is indicated in many different tumors [14,15]. Ectopic expression of Akt2 has been shown to induce metastasis and invasion in human breast cancer cells and induce malignancy in mouse fibroblasts [12,16]. In human thoracic aortic dissection (TAD) and aortic aneurysm and dissection (AAD) Akt2 phosphorylation level is higher while Akt1 phosphorylation levels remain the same between disease and healthy conditions [17]. These differences are also evident in Akt isoform knockout mice. Akt2 deficient mice shows a diabetic phenotype which is not observed in Akt-1 knockout mice [18]. Importance of Akt3 in brain development has also been shown in Akt3 knockout mice. This isoform specific cell development causes smaller brain sizes in Akt3 knockout mice, but not in Akt1 knockout mice [19]. In addition, Akt1 promotes endothelial neoplasms while Akt3 acts in an opposite manner [20]. The reduction of phosphorylation of endothelial nitric oxide synthase (eNOS) by Akt1 knockout can be compensated by Akt2, though phosphorylation of angiogenic substrate by Akt1 seems to be essential for angiogenesis [21]. Since they are involved in signal pathways related to cell proliferation, cell growth, survival and aspects of intermediary metabolism, Akt has been the center of focus for many studies attempting to therapeutically control these aspects.
Akt protein structure
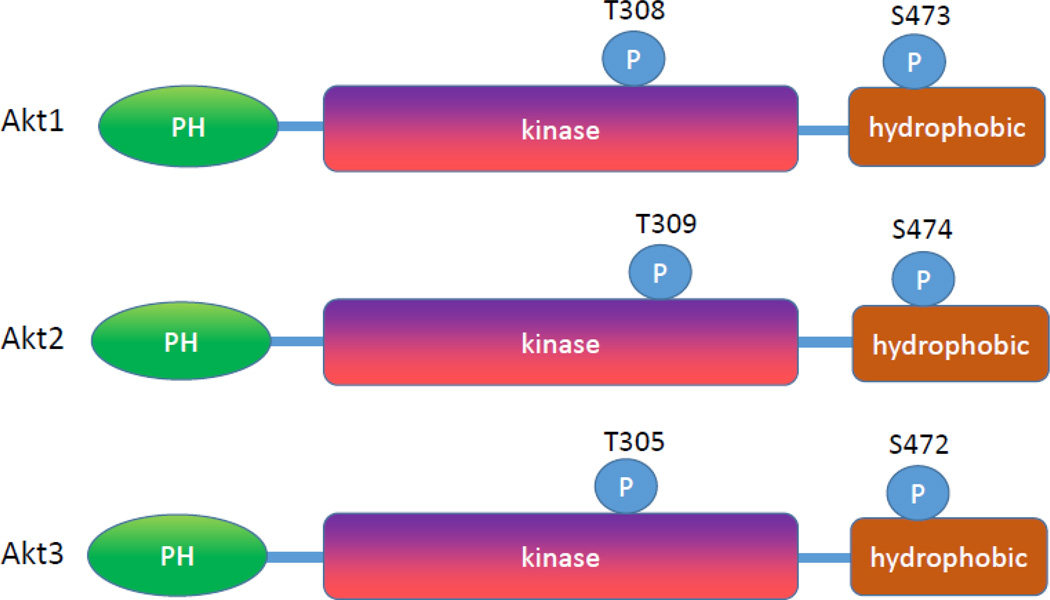
Akt has a characteristic pleckstrin homology (PH) domain at the amino terminal (~ 110 amino acids), a middle kinase domain (~260 amino acids) and a carboxy-terminal regulatory domain (~70 amino acids) (Figure 1). Of these, pleckstrin homology domain controls the membrane translocation of Akt. This structure is conserved in diverse species from flies to man. Akt has a higher sequence identity to other AGC family member proteins which made therapeutic strategies and developing specific inhibitors more challenging [22]. The region homology varies among the isoforms. Pleckstrin domain is 80% identical between Akt isoforms, while this being 30% identical to pleckstrin domains of other proteins. The region between PH domain and the catalytic domain is called the linker (LINK). This link is poorly conserved amongst Akt isoforms and lacks similarities to other human proteins. The catalytic domain shares a higher similarity within Akt isoforms (90%) and a significant similarity to other AGC family proteins. Because of the difficulty of obtaining crystal structure of the linker region, the structures of Akt protein is speculative [23].
Figure 1.
Human Akt protein structure. Akt has a characteristic pleckstrin homology (PH) domain at the amino terminal (~ 110 amino acids), a middle kinase domain (~260 amino acids) and a carboxy-terminal regulatory domain (~70 amino acids).
Upstream regulatory pathways of Akt
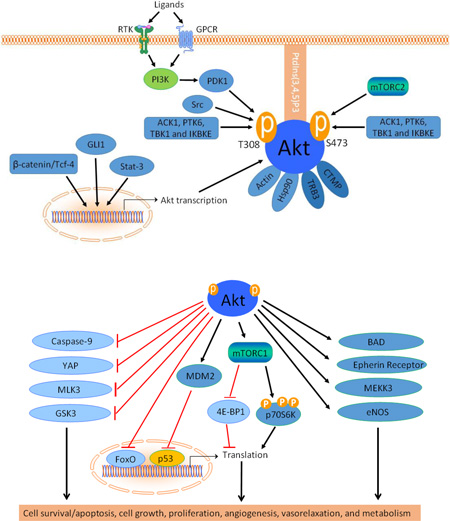
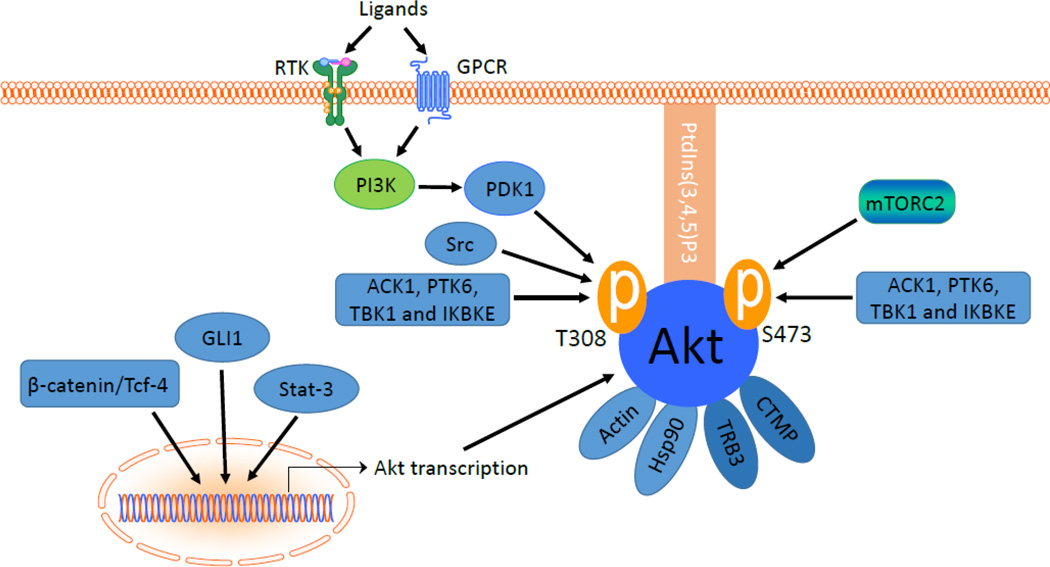
In cardiovascular system, Akt is activated by several stimuli such as insulin, platelet derived growth factor (PDGF), vascular endothelial growth factor (VEGF), epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF) [24,25]. In addition, some phosphatase inhibitors also activate Akt [26]. Reactive oxygen species (ROS) have been shown to activate Akt through angiotensin II [27,28]. These growth factors regulate Akt activity through transcriptional or posttranslational mechanisms (Figure 2).
Figure 2.
The upstream signals of Akt. Akt activity is regulated by its upstream regulatory pathways at transcriptional and post-translational levels. beta-catenin/Tcf-4, GLI1 and Stat-3 are known transcriptional regulators of AKT gene. T308 and S473 are the two critical phosphorylation sites of Akt1. Affinity of Akt to PtdIns (3,4,5) P3 is required for membrane translocation. PI3 kinase-dependent phosphorylation of T308 is through PDK1. S473 is phosphorylated by mTORC2. Ack1, Src, PTK6, TBK1, IKBKE and IKKε are some of the non-canonical pathways which affect the Akt activity. Protein-protein interactions of Akt to actin and Hsp90 increase the Akt activity while Akt binding to CTMP and TRB3 reduces the Akt activity.
Transcriptional regulation of Akt
Transcriptional regulation of Akt genes remains largely unknown. It has been shown that upregulation of total Akt protein results in an increase in Akt activity, though the expression of a kinase may not be necessarily a reflection of its activity level [29]. The Akt gene promoters contain binding sites for several signaling molecules such as Stat3, β-catenin/Tcf-4, and GLI1, indicating that Akt gene transcription may be regulated by these molecules.
The 4.2-kb region upstream of the transcription start site of the AKT1 promoter contains five putative Stat3-binding motifs. However, the promoter is not induced by Stat3 and/or Src. Actually the major Stat3 response elements are located within exon 1 and intron 1 regions of the AKT1 gene, which is upstream of the AKT1 translation initiation site. Stat-3 interacts with this region and increases the Akt1 gene transcription [30].
The transcription of Akt1 gene is induced by β-catenin/Tcf-4 [29]. Nine putative b-catenin/Tcf/Lef-binding sites (TBE) in the AKT1 gene have been found that show a high degree of homology to the core consensus sequence AGATCAAAGGG [29]. Four of these TBEs are located upstream of the transcriptional start, whereas five TBEs are situated in Exon 1. Moreover, the promoter region of the AKT2 gene contains six potential TBEs [29], and seven complete or partial TBEs have been identified within 1340 nucleotides of the promoter region of the AKT3 gene [29]. The inhibitory effect of non-steroidal anti-inflammatory drugs (NSAIDs) such as aspirin and indomethacin downregulate Akt1 gene transcription through stabilization of β-catenin phosphorylation [31].
GLI1, a zinc finger transcriptional factor of the Hedgehog signaling, increases the transcription of of AKT1, AKT2, and AKT3 genes [32]. AKT1 promoter possesses two GLI1 binding sites (BS1 and BS2) located upstream of the transcriptional start site of AKT1 gene. The homology of each GLI1 binding site to the consensus sequence was 67% for BS1 and 78% for BS2. AKT2 and AKT3 promoters also contain three and two GLI1 binding sites, respectively [32].
In addition, one possible AP-1 binding site (ACTCAGT or TGAGTCA) and two potential nuclear factor kappa B (NFκB) binding elements have been detected in the AKT1 promoter region [29]. However, the functions of these binding sites are not clear yet.
Posttranslational regulation of Akt by phosphorylation
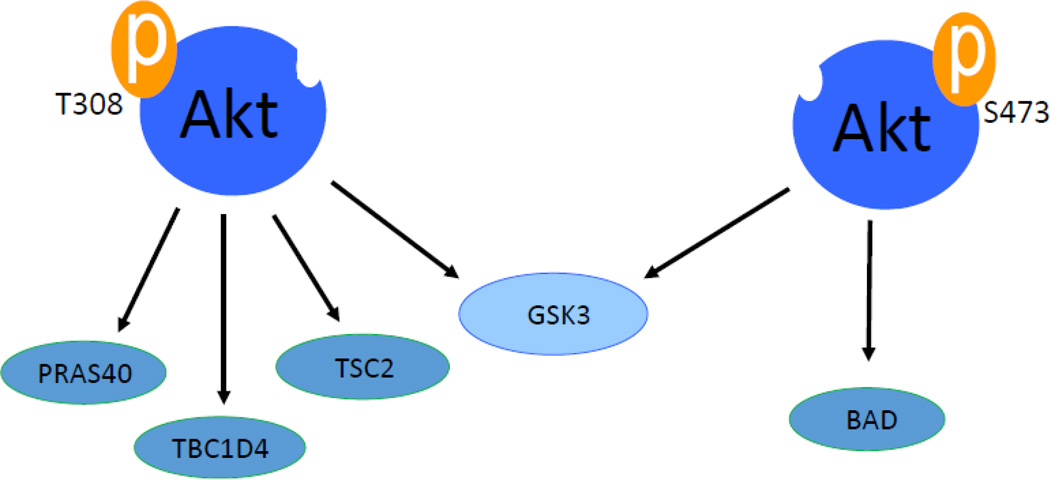
Phosphorylation is the most important posttranslational determinant in Akt activity. There are two major phosphorylation sites in Akt protein. Positions of these sites vary slightly among Akt isoforms. These phosphorylation sites are threonine residue in the kinase domain (Akt1 at 308, Akt2 at 309 and Akt3 at 305) and serine residue in the hydrophobic domain (Akt1 at 473, Akt2 at 474 and Akt3 at 472) (Figure 1). Phosphorylations of both sites positively increase the activity of Akt in varying degrees. For example, phosphorylation of threonine 308 (T308) in Akt1 increases the enzymatic activity of Akt by 100 fold and phosphorylation of serine 473 (S473) will increase Akt activity by 10 fold [33]. There is growing evidence of the diverse roles these phosphorylation sites play in Akt activation. Akt activation induced by T308 phosphorylation seems to be important in phosphorylation of substrates PRAS40, TSC2 and TBC1D4 while S473 phosphorylation doesn’t seem to have significant role in phosphorylating those substrates [34]. In contrast only S473 phosphorylation shows a positive correlations with substrates like BAD while substrates like GSK3β can be phosphorylated by Akt phosphorylation at either critical sites [35] (Figure3). These differences are manifested in disease development where one phosphorylation site shows more correlation with certain cancer [36,37]. The activation of Akt by phosphorylation follows the classical AGC family kinases behavior. The catalytic region of Akt which is located at the central region consists of a conserved threonine residue whose phosphorylation partially activates Akt [38,39]. The carboxyl region has a sequence F-X-X-F/Y-S/T-Y/F hydrophobic motif (where X is any amino acid) which is a characteristic of all AGC family kinases. It is known that phosphorylation of the serine and threonine residue in Akt2 causes a conformational change in the kinase region resulting in enzyme activation channel [40]. The activity of Akt is enhanced by the hydrophobic motif interaction of the N-terminal lobe channel [40].
Figure 3.
Two critical phosphorylation sites of Akt has different downstream targets in certain disease conditions. For example Phosphorylation of Akt at T308 significantly increases phosphorylation of PRAS40, TBC1D4 and TSC2 where Phosphorylation of S473 has less effect on these substrates. In similar manner, phosphorylation of S473 significantly increases phosphorylation of BAD. However GSK3 can be phosphorylated by any of the critical phosphorylations on Akt1.
One of the key requirements in activation of Akt is its translocation to the plasma membrane. This translocation requires the PH domain and is affected by wortmannin treatment. PH domain is not required for the activation of serine or threonine residues of Akt. Affinity of PH domains to PtdIns(3,4,5)P3 explains this membrane translocation [41–43]. Once Akt is translocated a protein kinase is capable of phosphorylating the threonine residue (T308). This protein kinase is named 3-phosphoinositide-dependent protein kinase-1 (PDK1) due to its association with PtdIns (3,4,5)P3 [44–46]. Following the activation Akt detaches from the plasma membrane and translocates into the cytosol and nucleus [39,43]. This is called the canonical pathway in which Akt activation is PI3 kinase dependent. Either tyrosine kinase receptors or G-protein coupled receptors can activate PI3K. Based on these, upstream receptor types PI3K is divided in to class IA (for receptor tyrosine kinase) and class 1B (for G-protein coupled receptors) [47]. Either of these two types of receptors recruits PIP2 and then PI3 kinase acts on its remaining OH group to form PIP3. This is negatively regulated by phosphatases like PTEN which reverses the PI3 kinase action [48]. Unlike other AGC kinases co-localization of Akt with PDK1 is necessary for phosphorylation of the threonine residue. Once Akt is translocated to the cell membrane, conditions at the cell membrane help phosphorylate both T308 and S473 [39]. The exact mechanism of how S473 is phosphorylated is poorly known. Initially it was assumed that PDK1 plays a role in the phosphorylation process. However, later research revealed that PDK1 is not essential although it can increase the S473 phosphorylation [49–51]. More recent studies revealed the importance of mammalian target of rapamycin 2 (mTOR2) in the phosphorylation of the critical S473 residue in Akt [52].
In addition to the canonical pathways, Akt can be activated by a non-canonical pathway independent of PI3K. Ser/Thr/Tyr kinases can directly activate Akt in this pathway. For example, receptor tyrosine kinases can lead to activation of Akt (at both S473 and T308) through nonreceptor tyrosine kinase Ack1 (ACK/TNK2). This activity can occur even in the presence of PI3 kinase inhibitors [53]. Moreover, certain kinases have the ability to affect the activation status of Akt through phosphorylating other critical tyrosine residues. For example, Src can phosphorylate T315 and T326, leading to increased T308 phosphorylation [54]. The effect on S473 phosphorylation by Src is not known. Protein tyrosine kinase 6 (PTK6) can phosphorylate T215 and T326 residues in low EGF concentrations in the same manner. This can activate both the critical S473 and T308 residues. However this activation is not prominent in higher EGF concentrations, suggesting that PI3K pathway to be the dominant pathway in high concentrations of growth factors [55]. Furthermore, IKBKE or IKKε (I-kappa-B kinase epsilon), TANK-binding kinase 1 (TBK1) and DNA-PKcs have been shown to directly activate Akt [56–58]. The serine/threonine kinase IKBKE is a non-canonical IKK signal transduction family member. In response to inflammatory factors like lipopolysaccharides (LPS) and phorbol myristate acetate (PMA), active IKBKE directly phosphorylate T308 and S473 as well as p65/RelA, interferon response factors 3 and 7 (IRF3 and IRF7) and STAT1 [59–61]. IKBKE-induced Akt activation is not affected by inhibition of PI3K, knockdown of PDK1 or mTORC2 complex. It is also not dependent on the PH domain of Akt as shown by Akt inhibitor studies [56]. In addition, TANK binding kinase (TBK1) has been shown to phosphorylate Akt at S473 and subsequently induce maximal activation of interferon regulatory factor 3 (IRF3) and expression of IFN-b in the antiviral cellular responses [57].
Posttranslational regulation by protein-protein interaction
There are several proteins that bind and modulate Akt activity. One of the emerging areas in the regulation of Akt activity is the subcellular movement facilitated by non-substrate ligands. Treatment of cells with PDGF increases Akt association with actin skeleton [62]. This is through direct interaction of actin with the PH domain of the Akt. Phosphorylation of both S473 and T308 is important in this process as mutation of those sites to alanine completely abrogates the Akt co-localization to actin. This co-localization of Akt with actin induced by PDGF is enhanced by small GTP-ases Rac1 and Cdc42 [62]. It has been shown that Akt is associated with heatshock protein 90 (Hsp90), which helps keep Akt active through preventing the dephosphorylation of Akt by protein phosphatase 2A (PP2A) [63]. Amino acid residues 229–309 of Akt were found to bind to the region of 327–340 of Hsp90. In similar manner Hsp27 interact with amino acids 117–128 of Akt and help phosphorylate S473 residue [64]. On the other hand, Akt association with other proteins can negatively regulate Akt activity. Carboxyl-terminal modulator protein (CTMP) binds to the carboxyl terminal regulatory domain of Akt1 at the plasma membrane. This interaction reduces phosphorylation of Akt at both T308 and S473 sites [65]. TRB3, a mammalian homolog of Drosophila tribbles, which is expressed in the liver under fasting condition, also bind to the central region of Akt and negatively regulate its activity [66].
Downstream signaling of Akt
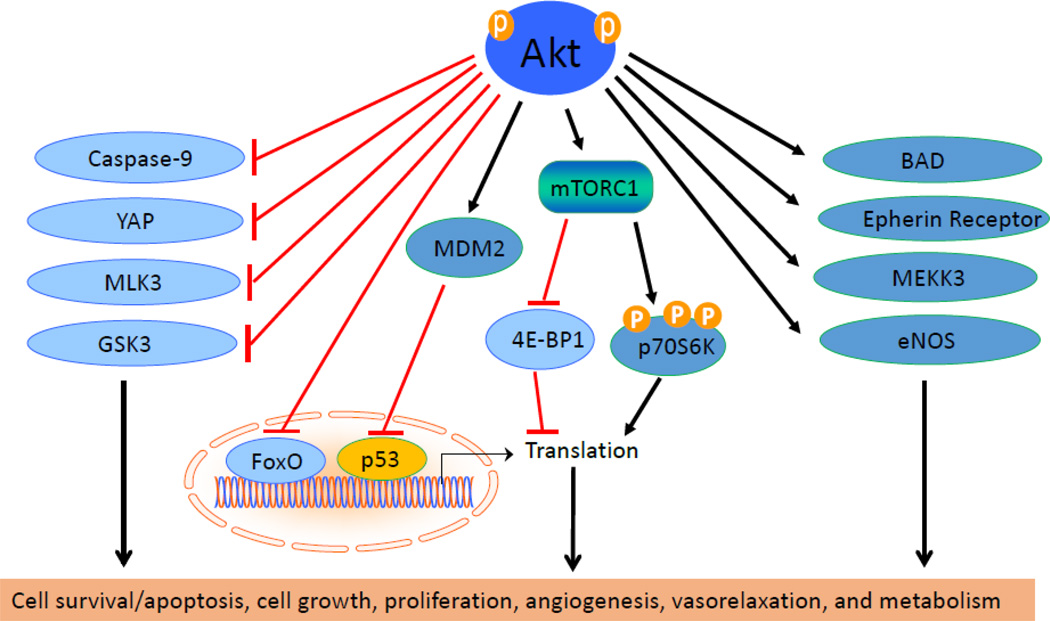
Synthetic peptide substrates related to the phosphorylation site of GSK3 paved the way to identifying minimal conditions required for Akt downstream targets. This is given as R-X-R-X-X-S/T-B where X can be any amino acid and B a bulky hydrophobic amino acid. The most effective substrate for Akt is R-P-R-T-S-S-F [67]. The action of Akt on its downstream targets determines its function in cardiovascular physiology [68]. Akt plays important roles in cell survival, growth, proliferation, angiogenesis, vasorelaxation, and cell metabolism. The downstream signaling of Akt is described in these physiological processes (Figure 3).
Cell survival and apoptosis
It is widely accepted that Akt mediates cell survival induced by growth factors. Cell survival is enhanced by blocking apoptosis. The initial event leads to apoptosis is the loss of mitochondrial integrity. Hallmark of loss of mitochondrial integrity is the release of cytochrome C to the cytoplasm. This cytochrome C can bind and activate apoptotic protease-activating factor (Apaf-1). Apaf-1 and caspase-9 together create a pro-apoptotic signaling cascade. Akt directly phosphorylates caspase-9 and inhibits activation of caspase-9. This negatively regulates cytochrome-c/Apaf-1/caspase-9 pathway [69]
Akt can phosphorylate several other target proteins which regulates apoptotic or pro-apoptotic molecules. BAD, a member of Bcl-2 family, forms a protein complex with Bcl-2 and Bcl-X and inhibits their anti-apoptotic effects. Akt can phosphorylate BAD at Ser136 which enables BAD to bind with 14-3-3 protein. This in turn releases Bcl-2 and Bcl-X to promote more anti-apoptotic behavior [70]. Moreover, Akt phosphorylates murine double minute 2 (MDM2) at S166 and S186, resulting in increased nuclear translocation of MDM2 and ubiquitination and degradation of p53 and subsequently promoting cell cycle transition and survival [71–74]. Furthermore, Akt phosphorylates Yes-associated protein (YAP) at S127 in the cytosol and increases the binding of 14-3-3 protein to phosphorylated YAP. This helps YAP exporting from the nucleus to the cytoplasm, reducing the apoptotic activity of p73 [75].
In addition to directly affecting apoptotic or pro-apoptotic proteins, Akt can increase the cell survival by indirectly regulating the expression of these proteins. Forkhead family of transcriptional factors (FoxO) induces pro-apoptotic Bcl-2 family of proteins or stimulates expression of death receptor ligands such as Fas ligand and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). It can also enhance the levels of cyclin dependent kinase inhibitors (CDKIs). Phosphorylation of FoxO by Akt inhibits the transcriptional functions of FoxOs and leads to decreased Bcl-2 and increased cell survival [76].
Akt-mediated cell survival can also be through MAP kinase pathway and JNK pathway. These two pathways are commonly known as stress activated protein kinase pathway (SAPK). Mixed lineage kinase 3 (MLK3) is a mitogen-activated protein kinase kinase kinase (MAPKKK) that activates c-jun N-terminal kinase (JNK) which activates apoptosis. MLK3 can interact with Akt, and insulin regulates this interaction. Akt phosphorylates MLK3 at S674, resulting in inhibition of MLK3-related cell death [77]. Moreover, Akt phosphorylates the apoptosis signalregulating kinase 1 (ASK1) at S83, leading to cell survival and inhibition of apoptosis [78]. Gain and loss of function of Akt has been shown to increase and decrease the survival of cardiomyocytes, respectively [79].
Cell growth and proliferation
The regulation of cell growth and proliferation by Akt requires the interplay between the mammalian target of rapamycin complex 1 (mTORC1) and the tuberous sclerosis complex 1/2 (TSC1 and TSC2) or the proline-rich Akt substrate of 40 kDa (PRAS40). TSC2 is an inhibitor of Ras-related small G protein Rheb, an activator of mTORC1. Phosphorylation of TSC1 by Akt inhibits TSC2 activity, leading to activation of mTORC1 [80]. Akt phosphorylates PRAS40 at T246 which facilitates PRAS40 phosphorylation of S183 by mTORC1. PRAS40 is known to negatively regulate mTORC1 [81,82]. Phosphorylation of PRAS40 by Akt and by mTORC1 per se results in the dissociation of PRAS40 from mTORC1 and the relief of an inhibitory constraint on mTORC1 activity [83]. This leads to phosphorylation of various mTORC1 substrates which are involved in cell proliferation including p70 s6 kinase (p70S6K1) and eukaryotic initiation factor 4E (eIF4E) binding protein 1 (4E-BP1) [84–86].
In addition to cell growth and survival, cell proliferation can be stimulated by Akt through cell-cycle regulation. Growth factors cause Akt to increase the transcription of c-Myc and reduce the degradation of c-Myc by Mad1 [87]. C-Myc prevents cell cycle arrest [88]. Akt directly inactivates GSK3β, increasing Cyclin D1 which then inhibits Forkhead family transcription factors and the tumor suppressor tuberin (TSC2), reducing p27Kip levels [89]. This positively regulates G1/S cell cycle progression.
Angiogenesis
Akt mediates angiogenesis through stimulating the secretion of vascular endothelial growth factor (VEGF) [90,91]. Akt acts on TSC1–TSC2/Rheb/mTORC1 pathway in endothelial cells and increases the protein level of hypoxia inducible factor 1α (HIF-1α) which stimulates VEGF release. Akt has been shown to increase HIF-1α protein translation by activating the translational regulatory proteins p70 S6 kinase and eIF-4E [92–94]. However, sustained Akt activation leads to the formation of functionally abnormal blood vessels [95].
Endothelial cell migration is another essential function in angiogenesis. VEGF-induced endothelial migration is through Akt-PI3K pathway [96–98]. PDK1 activity is essential for this migration [99]. Ephrins and their receptor tyrosine kinases play an important role in the migration of vascular cells. Ephrin receptors can be phosphorylated by Akt even in the absence of ligands, causing cell migration [100,101].
Proper attachment to extracellular matrix proteins such as integrins helps endothelial cell attachment. Binding to integrin αvβ3 is required for VEGF to activate Akt that blocks anoikis [102,103].
Akt-dependent endothelial survival pathway also promotes angiogenesis. Angiopoietin-1 (Ang-1) acting via Tie 2 receptor has been shown to induce Akt phosphorylation which up-regulates survivin to reduce apoptosis in endothelial cells [104]. Akt can enable endothelial cells to be resistant to Fas-mediated apoptosis by expressing the FLICE-inhibitory protein (FLIP) [105]. Constitutively active Akt has been shown to phosphorylate MEKK3 that reduces MKK3/6-and p38 MAPK-activated apoptosis [106].
The HMG-CoA reductase inhibitor statins seems to act with Akt pathway to bring pleiotropic effects. Statins have been shown to promote the proliferation and differentiation of endothelial progenitor cells (EPCs) through PI3K/Akt pathway [107].
Akt can directly phosphorylate eNOS [108]. eNOS plays a predominant role in angiogenesis and vascular permeability induced by growth factors and angiotensin II [109] [110]. Akt directly phosphorylates eNOS at S1197, enhancing its enzymatic activity and altering the sensitivity of the enzyme to Ca2+ [108,111]. Akt interacts with Hsp90, a protein that associates with and activates eNOS. Hsp90 serves a scaffolding function to facilitate Akt-mediated phosphorylation of eNOS in the calveolae [112–114].
Vasorelaxation
Akt has been shown to regulate vasomotor tone in vivo [115,116]. Since NO is an important regulator of vasomotor tone, effects of Akt on eNOS phosphorylation and activation can significantly enhance vasorelaxation. Over-expression of Akt causes a significant increase in resting vessel diameter and blood flow while inhibition of Akt attenuates endothelium-dependent vasodilatation in response to acetylcholine [115]. Therefore, dysregulation of Akt activity can lead to development of endothelial dysfunction in hypertension [117].
Metabolism
Akt can increase metabolism in cells by increasing either the uptake of nutrients or the cellular glucose and lipid metabolism. Hexose uptake and GLUT4 translocation can be increased by Akt2 activity [118]. Akt is also associated with sterol-regulatory element-binding proteins (SREBPs), a transcriptional regulator in lipid metabolism, mTORC1 and GSK3 pathway [119]. Akt might be playing a crucial role in cholesterol import and synthesis, fatty acid synthesis or SREBP production [119]. Akt is one of the major signal molecules in insulin-related signal transduction. After tyrosine phosphorylation of insulin receptors, insulin receptor substrate (IRS) family members of proteins are phosphorylated. This activates PI3K pathway and phosphorylates Akt, which leads to inactivation of GSK3 and phosphorylation of the Forkhead transcription factor. This signal leads to increases in the glucose metabolism, glycogen, lipid and protein synthesis, and other specific gene expressions [120,121].
Akt in cardiovascular pathologies
Atherosclerosis
Oxidized LDL has been shown to reduce Akt phosphorylation causing the inhibition of endothelial cell migration [122]. In atherosclerosis, instability and rupture of the plaque can lead to myocardial infarction, which can be caused through apoptosis of vascular smooth muscle cells. Akt seems to have a role in smooth muscle apoptosis via insulin-like growth factor 1 (IGF1) receptor signaling. Reduction of IGF1R signaling and dysregulation of phosphorylation of Akt, FoxO3a and GSK3 leads to apoptosis of vascular smooth muscle cells [123]. Inhibition of Akt in vascular smooth muscle cells can lead to significant increase in p-JNK and p-c-jun, pro-apoptotic proteins. This works in opposite manner in immune cells [124]. For example, lack of Akt2 in macrophages shows a decrease in pro-inflammatory genes and a reduction of cell migration. Loss of Akt2 suppresses macrophages undergoing M1 polarization. All these events on macrophages help reduce atherosclerosis [125]. Peroxisome proliferator-activated receptors (PPARs) have been shown to protect vasculature from pathological alterations like atherosclerosis. This is achieved through inhibition of VEGF-induced Akt phosphorylation pathway [126].
Cardiac hypertrophy
Cardiac hypertrophy can be defined at cellular level as increased cardiomyocyte cell volume [127,128]. Normal growth, growth induced by physical conditioning and growth induced by pathologic stimuli are the three major types of cardiac hypertrophy [129]. Cardiac hypertrophy caused by normal growth or physical conditioning such as exercise is called adaptive cardiac hypertrophy [130]. Maladaptive hypertrophy can be either caused as a response to excessive hemodynamic workload or by genetic mutations. This can eventually lead to heart failure [131–133].
Growth factors and exercise can activate a 110-kDa lipid kinase, PI3K subgroup Iα [134,135]. Another class of PI3K, p110γ, is activated by G-protein coupled receptors (GPCR) due to biomechanical stress and neuro-hormonal mediators [136,137]. Both of these pathways can lead to Akt phosphorylation and causing inhibition of GSK3β signaling, which eventually leads to protein synthesis and transcriptional activation causing cardiac hypertrophy [129].
Over-expression of Akt has been shown to be maladaptive for the heart. This ill-effect seems to be rescued by PI3K, suggesting the importance of PI3K in cardiovascular treatments [138]. Akt over-expression seems to enlarge the cell size and increase the contractility of cardiomyocytes in Akt transgenic mouse model [139].
Constitutively active Akt can increase the angiogenesis in heart. Initially this can contribute to adaptive cardiac hypertrophy. At a later stage this can lead to cardiac hypertrophy and heart failure [140]. However, this is a dilemma since exercise is known to increase the cardiovascular health through increased Akt activity [141]. There must be a fine balance between the healthy and maladaptive Akt activation levels and duration which is yet to be investigated.
Vascular remodeling
Vascular remodeling process consists of changes of cell growth, cell death, cell migration and production or degradation of extracellular matrix [142]. Akt plays a role in the pathogenesis of vascular remodeling. Akt substrate GSK3β is a crucial protein in smooth muscle proliferation where inhibition of GSK3β by Akt-induced phosphorylation increases smooth muscle proliferation [143]. Increased cell survival is also achieved through Akt signaling [144]. In restenosis and atherosclerosis, increased proliferation of vascular smooth muscle cells is mediated through Akt pathway [145]. Increased proliferation and survival of vascular smooth muscle cells contribute to medial thickening. Akt also affects the activity of adventitia in the vasculature. After arterial injury, Akt activity is significantly higher in the adventitia and contributes the increased proliferation of adventitial fibroblasts [146].
Several mitogens such as platelet-derived growth factor (PDGF), transforming growth factor-beta (TGFβ), endothelin and thrombospondin-1 stimulate Akt phosphorylation causing the polyubiquitination and proteasomal degradation of cAMP response element binding protein (CREB) in pulmonary artery smooth muscle cells and resulting in medial smooth muscle cell proliferation, hypertrophy and extracellular matrix production [147]. In addition, mTORC2 also induces the activation and phosphorylation of Akt which leads to mTORC1 and S6K pathway in pulmonary vascular remodeling [148–150]. Akt1 rather than Akt2 is the important signaling molecule for the development and progression of pulmonary vascular remodeling and pulmonary hypertension [151].
Akt inhibitors
Several Akt inhibitors are currently being developed and tested as anti-tumor agents. But only few of them have been tested in cardiovascular diseases. There are several strategies to inhibit the activation of Akt by its upstream signaling. Akt upstream inhibitors, allosteric inhibitors, pseudo-substrates, pleckstrin homology (PH) domain inhibitors and ATP binding pocket inhibitors are some of the major inhibitors.
Upstream inhibitors
Many upstream molecules of Akt pathway can be inhibited to reduce their downstream activation of Akt. Wortamanin, PX-866, and LY294002 are well-known potent pan-PI3K inhibitors [152–154]. HS-173/PIK75, TGX-221, CZC24832 and CAL-101 are selective PI3K inhibitors for PI3K p110 catalytic subunits α, β, γ and δ respectively [155–158]. PDK inhibitors OS-03012, BX-795, BX-912 and PHT-427 have been used successfully to reduce proliferation and increase cell apoptosis in various cancer models [159–161]. Rapamycin is one of the first successful mTORC inhibitors discovered [162]. Rapamycin forms a complex with FK-binding protein 12 (FKBP12) and inhibits mTOR. Several rapamycin derivatives including temsirolimus, everolimus and deforolimus have been developed to be more potent mTORC1 inhibitors [163]. Pan-mTOR inhibitors AZD8055, KU-0063794, and PP242 can be more effective alternative since they inhibit both up-stream mTORC2 and the downstream mTORC1 [164–166].
Dehydroepiandrosterone (DHEA), a widely used steroid, is another Akt upstream inhibitor tested for treatment of vascular remodeling by inhibiting smooth muscle proliferation and activating vascular smooth muscle cell apoptosis. DHEA inhibits Akt pathway through an as-yet unidentified GPCR. That leads to increased GSK3 activity causing suppression of smooth muscle proliferation and activation of vascular smooth muscle cell apoptosis. In addition, DHEA directly acts on Kv (4-aminopyridine-sensitive [4-AP]) and BKCa (iberiotox-insensitive [IbTx]) channels, causing reduction of smooth muscle [Ca2+], cell proliferation, and vascular remodeling [167,168].
DNA-PK inhibitors NU7441and KU0060648 can be used to inhibit Akt activation [169–171]. These inhibitors have been studied as anti-cancer agents.
Allosteric inhibitors
New strategies are needed to lower the toxic off-target effect of pan-kinase inhibitors. Allosteric inhibitors block the activity of the target kinases by altering the protein conformation through interacting with regions distal to the ATP binding site [172]. Triciribine suppresses the phosphorylation level and kinase activity of Akt. Triciribine can selectively inhibit all Akt isoforms without inhibiting known upstream activators, PDK1 and PI 3-Kinase. MK-2206 is an allosteric inhibitor of Akt, which is now in clinical trials as a cancer drug. However the specific mechanism of action of this is not known. MK-2206 inhibits all isoforms of Akt. IC50 of MK-2206 is 8nM for Akt1, 12nM for Akt2, and 65nM for Akt3 [173,174].
Pseudo-substrates
A 14-mer AKTide-2T peptide with a sequence of ARKRERTYSFGHHA has been identified to be able to bind to the substrate binding domain of Akt1 with a Ki of 12 mM. A hybrid of this sequence and a region of FOXO3 (sequence: VELDPEFEPRARERTYSFGH) has improved its affinity with a Ki of 1.1 mM. Replacing the tyrosine and serine residue to alanine (sequence: VELDPEFEPRARERAYSFGH and VELDPEFEPRARERTYAFGH) has improved their inhibitory effect to Ki of 95 nM and 110 nM, respectively [175].
Pleckstrin homology domain inhibitors
Perifosine is a new class of Akt inhibitors which target the PH domain. It is a synthetic alkylphospholipid which is effective in controlling Akt-mediated cell proliferation [176]. Tirucallic acid and PITENINs (antagonists of PIP3/PH domain binding) are novel classes of PH domain inhibitors [177,178]. However their inhibitory concentration is in µM range, making them less potent for pharmacological interventions.
ATP binding pocket inhibitors
Several small molecules which compete for the ATP binding pocket have been found as potent inhibitors of Akt. Analogs of H89, which are inhibitors of PKA, have been developed as a Akt inhibitors [179]. A-443654 is another example of indazole-pyridines used as an ATP binding pocket inhibitor. However, inhibition of Akt phosphorylation by this inhibitor can create a feedback loop where phosphorylated Akt can increase later [162].GSK690693 is a pan Akt inhibitor which competitively inhibits all isoforms of Akt with IC50 of 2 nM for Akt1, 13 nM for Akt2, and 9 nM for Akt3 [180]. New isoform-selective ATP binding pocket inhibitors are being experimented. For example, A-674563 is a potent and selective Akt1 inhibitor with an IC50 of 14 nM. A-674563 also inhibits activity of PKA and CDK2 with IC50 of 16 and 46 nM, respectively. CCT128930 inhibits Akt2 selectively with IC50 of 6 nM, 28-fold greater selectivity for Akt2 than PKA and other Akt isoforms [181].
Future perspectives
As more evidence of complexity of Akt signal transduction pathway is discovered, it is important to consider these complexities in future research and retrospective analysis of Akt research data. The initial research on Akt with regards to cancer or cardiovascular diseases largely neglected isoform-specific and phosphorylation site-specific effects of Akt. It is important to understand the roles of these different mechanisms in disease context. As more and more cross-talk between Akt and other signal transduction pathways becomes evident, more research should focus on the system biology of these pathways [182]. Moreover, cardiovascular system consists of several types of tissues in a unique arrangement. Interactions between these cell types play an integral role in health and disease. There is growing evidence of Akt signaling in cardiovascular system to be influenced by the neighboring cell types in tissues [183]. Simulation of these conditions will be possible with co-culture of cells other than mono-cultures. Further, novel research areas like long-noncoding RNAs seems to have a control in the signal transduction pathways. Akt and non-coding RNA associations are also evident now [184]. In addition, Akt is affected by epigenetic control and also acts as a mediator for epigenetic control of disease [185,186]. Another ignored area in Akt studies is the non-canonical pathway of its activation. Knowledge and research in these pathways might be able to explain phenomenon which cannot be explained by the classical Akt pathway. Finally, most of the research on Akt is in the field of cancer, though there are some similarities between certain pathological conditions in cardiovascular disease and the hallmarks of cancer. More studies should be done to understand Akt in cardiovascular diseases. There is an urgent need to develop potent tissue specific inhibitors and activators of Akt pathway for research and therapeutic purposes.
Figure 4.
The downstream signals of Akt. Akt stimulates cell growth and proliferation through mTORC1. Akt phosphorylates BAD, epherin receptor, MEKK3, eNOS and MDM2, and up-regulates the activities of these proteins. Direct phosphorylation of caspase-9, YAP, MLK3, GSK3 and FoxO by Akt results in inhibitions of their enzymatic activities. The action of Akt on its downstream targets determines its function in cardiovascular processes such as cell survival, growth, proliferation, angiogenesis, vasorelaxation, and cell metabolism.
Acknowledgement
This work was supported by NIH grants HL088261 and HL115078 to YS and Flight Attendants Medical Research Institute grant 113018_CIA to YS, and by the Department of Veterans Affairs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–1934. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- 2.Bellacosa A, Franke TF, Gonzalez-Portal ME, Datta K, Taguchi T, Gardner J, Cheng JQ, Testa JR, Tsichlis PN. Structure, expression and chromosomal mapping of c-akt: relationship to v-akt and its implications. Oncogene. 1993;8:745–754. [PubMed] [Google Scholar]
- 3.Murthy SS, Tosolini A, Taguchi T, Testa JR. Mapping of AKT3, encoding a member of the Akt/protein kinase B family, to human and rodent chromosomes by fluorescence in situ hybridization. Cytogenet Cell Genet. 2000;88:38–40. doi: 10.1159/000015481. [DOI] [PubMed] [Google Scholar]
- 4.Franke TF, Tartof KD, Tsichlis PN. The SH2-like Akt homology (AH) domain of c-akt is present in multiple copies in the genome of vertebrate and invertebrate eucaryotes. Cloning and characterization of the Drosophila melanogaster c-akt homolog Dakt1. Oncogene. 1994;9:141–148. [PubMed] [Google Scholar]
- 5.GeneCards. V-Akt Murine Thymoma Viral Oncogene Homolog 1. 2015 http://www.genecards.org/cgi-bin/carddisp.pl?gene=AKT1#. [Google Scholar]
- 6.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 7.Bellacosa A, Testa JR, Staal SP, Tsichlis PN. A retroviral oncogene akt, encoding a serine-threonine kinase containing an SH2-like region. Science. 1991;254:274–277. doi: 10.1126/science.254.5029.274. [DOI] [PubMed] [Google Scholar]
- 8.Jones PF, Jakubowicz T, Pitossi FJ, Maurer F, Hemmings BA. Molecular cloning and identification of a serine/threonine protein kinase of the second-messenger subfamily. Proc Natl Acad Sci U S A. 1991;88:4171–4175. doi: 10.1073/pnas.88.10.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coffer PJ, Woodgett JR. Molecular cloning and characterisation of a novel putative protein-serine kinase related to the cAMP-dependent and protein kinase C families. Eur J Biochem. 1991;201:475–481. doi: 10.1111/j.1432-1033.1991.tb16305.x. [DOI] [PubMed] [Google Scholar]
- 10.Bellacosa A, Testa JR, Moore R, Larue L. A portrait of AKT kinases: human cancer and animal models depict a family with strong individualities. Cancer Biol Ther. 2004;3:268–275. doi: 10.4161/cbt.3.3.703. [DOI] [PubMed] [Google Scholar]
- 11.Laine J, Kunstle G, Obata T, Noguchi M. Differential regulation of Akt kinase isoforms by the members of the TCL1 oncogene family. J Biol Chem. 2002;277:3743–3751. doi: 10.1074/jbc.M107069200. [DOI] [PubMed] [Google Scholar]
- 12.Cheng JQ, Altomare DA, Klein MA, Lee WC, Kruh GD, Lissy NA, Testa JR. Transforming activity and mitosis-related expression of the AKT2 oncogene: evidence suggesting a link between cell cycle regulation and oncogenesis. Oncogene. 1997;14:2793–2801. doi: 10.1038/sj.onc.1201121. [DOI] [PubMed] [Google Scholar]
- 13.Calera MR, Pilch PF. Induction of Akt-2 correlates with differentiation in Sol8 muscle cells. Biochem Biophys Res Commun. 1998;251:835–841. doi: 10.1006/bbrc.1998.9566. [DOI] [PubMed] [Google Scholar]
- 14.Miwa W, Yasuda J, Murakami Y, Yashima K, Sugano K, Sekine T, Kono A, Egawa S, Yamaguchi K, Hayashizaki Y, Sekiya T. Isolation of DNA sequences amplified at VPH-D-15-00060-R1 chromosome 19q13.1-q13.2 including the AKT2 locus in human pancreatic cancer. Biochem Biophys Res Commun. 1996;225:968–974. doi: 10.1006/bbrc.1996.1280. [DOI] [PubMed] [Google Scholar]
- 15.Nakatani K, Thompson DA, Barthel A, Sakaue H, Liu W, Weigel RJ, Roth RA. Up-regulation of Akt3 in estrogen receptor-deficient breast cancers and androgen-independent prostate cancer lines. J Biol Chem. 1999;274:21528–21532. doi: 10.1074/jbc.274.31.21528. [DOI] [PubMed] [Google Scholar]
- 16.Dillon RL, Muller WJ. Distinct biological roles for the akt family in mammary tumor progression. Cancer Res. 2010;70:4260–4264. doi: 10.1158/0008-5472.CAN-10-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen YH, Zhang L, Ren P, Nguyen MT, Zou S, Wu D, Wang XL, Coselli JS, LeMaire SA. AKT2 confers protection against aortic aneurysms and dissections. Circ Res. 2013;112:618–632. doi: 10.1161/CIRCRESAHA.112.300735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J Biol Chem. 2001;276:38349–38352. doi: 10.1074/jbc.C100462200. [DOI] [PubMed] [Google Scholar]
- 19.Easton RM, Cho H, Roovers K, Shineman DW, Mizrahi M, Forman MS, Lee VM, Szabolcs M, de Jong R, Oltersdorf T, Ludwig T, Efstratiadis A, Birnbaum MJ. Role for Akt3/protein kinase Bgamma in attainment of normal brain size. Mol Cell Biol. 2005;25:1869–1878. doi: 10.1128/MCB.25.5.1869-1878.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phung TL, Du W, Xue Q, Ayyaswamy S, Gerald D, Antonello Z, Nhek S, Perruzzi CA, Acevedo I, Ramanna-Valmiki R, Rodriguez-Waitkus P, Enayati L, Hochman ML, Lev D, Geeganage S, Benjamin LE. Akt1 and akt3 exert opposing roles in the regulation of vascular tumor growth. Cancer Res. 2015;75:40–50. doi: 10.1158/0008-5472.CAN-13-2961. [DOI] [PubMed] [Google Scholar]
- 21.Lee MY, Luciano AK, Ackah E, Rodriguez-Vita J, Bancroft TA, Eichmann A, Simons M, Kyriakides TR, Morales-Ruiz M, Sessa WC. Endothelial Akt1 mediates angiogenesis by phosphorylating multiple angiogenic substrates. Proc Natl Acad Sci U S A. 2014;111:12865–12870. doi: 10.1073/pnas.1408472111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morrow JK, Du-Cuny L, Chen L, Meuillet EJ, Mash EA, Powis G, Zhang S. Recent development of anticancer therapeutics targeting Akt. Recent Pat Anticancer Drug Discov. 2011;6:146–159. doi: 10.2174/157489211793980079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar CC, Madison V. AKT crystal structure and AKT-specific inhibitors. Oncogene. 2005;24:7493–7501. doi: 10.1038/sj.onc.1209087. [DOI] [PubMed] [Google Scholar]
- 24.Burgering BM, Coffer PJ. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature. 1995;376:599–602. doi: 10.1038/376599a0. [DOI] [PubMed] [Google Scholar]
- 25.Abid MR, Guo S, Minami T, Spokes KC, Ueki K, Skurk C, Walsh K, Aird WC. Vascular endothelial growth factor activates PI3K/Akt/forkhead signaling in endothelial cells. Arterioscler Thromb Vasc Biol. 2004;24:294–300. doi: 10.1161/01.ATV.0000110502.10593.06. [DOI] [PubMed] [Google Scholar]
- 26.Andjelkovic M, Jakubowicz T, Cron P, Ming XF, Han JW, Hemmings BA. Activation and phosphorylation of a pleckstrin homology domain containing protein kinase (RAC-PK/PKB) promoted by serum and protein phosphatase inhibitors. Proc Natl Acad Sci U S A. 1996;93:5699–5704. doi: 10.1073/pnas.93.12.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ushio-Fukai M, Alexander RW, Akers M, Yin Q, Fujio Y, Walsh K, Griendling KK. Reactive oxygen species mediate the activation of Akt/protein kinase B by angiotensin II in vascular smooth muscle cells. J Biol Chem. 1999;274:22699–22704. doi: 10.1074/jbc.274.32.22699. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi T, Taniguchi T, Konishi H, Kikkawa U, Ishikawa Y, Yokoyama M. Activation of Akt/protein kinase B after stimulation with angiotensin II in vascular smooth muscle cells. Am J Physiol. 1999;276:H1927–H1934. doi: 10.1152/ajpheart.1999.276.6.H1927. [DOI] [PubMed] [Google Scholar]
- 29.Dihlmann S, Kloor M, Fallsehr C, Doeberitz M. Regulation of AKT1 expression by beta-catenin/Tcf/Lef signaling in colorectal cancer cells. Carcinogenesis. 2005;26:1503–1512. doi: 10.1093/carcin/bgi120. [DOI] [PubMed] [Google Scholar]
- 30.Park S, Kim D, Kaneko S, Szewczyk KM, Nicosia SV, Yu H, Jove R, Cheng JQ. Molecular cloning and characterization of the human AKT1 promoter uncovers its up-regulation by the Src/Stat3 pathway. J Biol Chem. 2005;280:38932–38941. doi: 10.1074/jbc.M504011200. [DOI] [PubMed] [Google Scholar]
- 31.Huls G, Koornstra JJ, Kleibeuker JH. Non-steroidal anti-inflammatory drugs and molecular carcinogenesis of colorectal carcinomas. Lancet. 2003;362:230–232. doi: 10.1016/s0140-6736(03)13915-3. [DOI] [PubMed] [Google Scholar]
- 32.Agarwal NK, Qu C, Kunkalla K, Kunkulla K, Liu Y, Vega F. Transcriptional regulation of serine/threonine protein kinase (AKT) genes by glioma-associated oncogene homolog 1. The Journal of biological chemistry. 2013;288:15390–15401. doi: 10.1074/jbc.M112.425249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alessi DR. Discovery of PDK1, one of the missing links in insulin signal transduction. Colworth Medal Lecture. Biochem Soc Trans. 2001;29:1–14. doi: 10.1042/0300-5127:0290001. [DOI] [PubMed] [Google Scholar]
- 34.Vincent EE, Elder DJ, Thomas EC, Phillips L, Morgan C, Pawade J, Sohail M, May MT, Hetzel MR, Tavare JM. Akt phosphorylation on Thr308 but not on Ser473 correlates with Akt protein kinase activity in human non-small cell lung cancer. Br J Cancer. 2011;104:1755–1761. doi: 10.1038/bjc.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khor TO, Gul YA, Ithnin H, Seow HF. Positive correlation between overexpression of phospho-BAD with phosphorylated Akt at serine 473 but not threonine 308 in colorectal carcinoma. Cancer Lett. 2004;210:139–150. doi: 10.1016/j.canlet.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 36.Cicenas J. The potential role of Akt phosphorylation in human cancers. Int J Biol Markers. 2008;23:1–9. doi: 10.1177/172460080802300101. [DOI] [PubMed] [Google Scholar]
- 37.Al-Saad S, Donnem T, Al-Shibli K, Persson M, Bremnes RM, Busund LT. Diverse prognostic roles of Akt isoforms, PTEN and PI3K in tumor epithelial cells and stromal compartment in non-small cell lung cancer. Anticancer Res. 2009;29:4175–4183. [PubMed] [Google Scholar]
- 38.Peterson RT, Schreiber SL. Kinase phosphorylation: Keeping it all in the family. Curr Biol. 1999;9:R521–R524. doi: 10.1016/s0960-9822(99)80326-1. [DOI] [PubMed] [Google Scholar]
- 39.Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 40.Yang J, Cron P, Thompson V, Good VM, Hess D, Hemmings BA, Barford D. Molecular mechanism for the regulation of protein kinase B/Akt by hydrophobic motif phosphorylation. Mol Cell. 2002;9:1227–1240. doi: 10.1016/s1097-2765(02)00550-6. [DOI] [PubMed] [Google Scholar]
- 41.Frech M, Andjelkovic M, Ingley E, Reddy KK, Falck JR, Hemmings BA. High affinity binding of inositol phosphates and phosphoinositides to the pleckstrin homology domain of RAC/protein kinase B and their influence on kinase activity. J Biol Chem. 1997;272:8474–8481. doi: 10.1074/jbc.272.13.8474. [DOI] [PubMed] [Google Scholar]
- 42.James SR, Downes CP, Gigg R, Grove SJ, Holmes AB, Alessi DR. Specific binding of the Akt-1 protein kinase to phosphatidylinositol 3,4,5-trisphosphate without subsequent activation. Biochem J. 1996;315(Pt 3):709–713. doi: 10.1042/bj3150709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Andjelkovic M, Alessi DR, Meier R, Fernandez A, Lamb NJ, Frech M, Cron P, Cohen P, Lucocq JM, Hemmings BA. Role of translocation in the activation and function of protein kinase B. J Biol Chem. 1997;272:31515–31524. doi: 10.1074/jbc.272.50.31515. [DOI] [PubMed] [Google Scholar]
- 44.Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 45.Stokoe D, Stephens LR, Copeland T, Gaffney PR, Reese CB, Painter GF, Holmes AB, McCormick F, Hawkins PT. Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science. 1997;277:567–570. doi: 10.1126/science.277.5325.567. [DOI] [PubMed] [Google Scholar]
- 46.Hemmings BA. PtdIns(3,4,5)P3 gets its message across. Science. 1997;277:534. doi: 10.1126/science.277.5325.534. [DOI] [PubMed] [Google Scholar]
- 47.Wymann MP, Zvelebil M, Laffargue M. Phosphoinositide 3-kinase signalling--which way to target? Trends Pharmacol Sci. 2003;24:366–376. doi: 10.1016/S0165-6147(03)00163-9. [DOI] [PubMed] [Google Scholar]
- 48.Simpson L, Parsons R. PTEN: life as a tumor suppressor. Exp Cell Res. 2001;264:29–41. doi: 10.1006/excr.2000.5130. [DOI] [PubMed] [Google Scholar]
- 49.Balendran A, Casamayor A, Deak M, Paterson A, Gaffney P, Currie R, Downes CP, Alessi DR. PDK1 acquires PDK2 activity in the presence of a synthetic peptide derived from the carboxyl terminus of PRK2. Curr Biol. 1999;9:393–404. doi: 10.1016/s0960-9822(99)80186-9. [DOI] [PubMed] [Google Scholar]
- 50.Williams MR, Arthur JS, Balendran A, van der Kaay J, Poli V, Cohen P, Alessi DR. The role of 3-phosphoinositide-dependent protein kinase 1 in activating AGC kinases defined in embryonic stem cells. Curr Biol. 2000;10:439–448. doi: 10.1016/s0960-9822(00)00441-3. [DOI] [PubMed] [Google Scholar]
- 51.Hill MM, Andjelkovic M, Brazil DP, Ferrari S, Fabbro D, Hemmings BA. Insulin-stimulated protein kinase B phosphorylation on Ser-473 is independent of its activity and occurs through a staurosporine-insensitive kinase. J Biol Chem. 2001;276:25643–25646. doi: 10.1074/jbc.C100174200. [DOI] [PubMed] [Google Scholar]
- 52.Moschella PC, McKillop J, Pleasant DL, Harston RK, Balasubramanian S, Kuppuswamy D. mTOR complex 2 mediates Akt phosphorylation that requires PKCepsilon in adult cardiac muscle cells. Cell Signal. 2013;25:1904–1912. doi: 10.1016/j.cellsig.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morton CR, Du HJ, Xiao HM, Maisch B, Zimmermann M. Inhibition of nociceptive responses of lumbar dorsal horn neurones by remote noxious afferent stimulation in the cat. Pain. 1988;34:75–83. doi: 10.1016/0304-3959(88)90184-4. [DOI] [PubMed] [Google Scholar]
- 54.Chen R, Kim O, Yang J, Sato K, Eisenmann KM, McCarthy J, Chen H, Qiu Y. Regulation of Akt/PKB activation by tyrosine phosphorylation. J Biol Chem. 2001;276:31858–31862. doi: 10.1074/jbc.C100271200. [DOI] [PubMed] [Google Scholar]
- 55.Zheng Y, Peng M, Wang Z, Asara JM, Tyner AL. Protein tyrosine kinase 6 directly phosphorylates AKT and promotes AKT activation in response to epidermal growth factor. Mol Cell Biol. 2010;30:4280–4292. doi: 10.1128/MCB.00024-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guo JP, Coppola D, Cheng JQ. IKBKE protein activates Akt independent of phosphatidylinositol 3-kinase/PDK1/mTORC2 and the pleckstrin homology domain to sustain malignant transformation. J Biol Chem. 2011;286:37389–37398. doi: 10.1074/jbc.M111.287433. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 57.Joung SM, Park ZY, Rani S, Takeuchi O, Akira S, Lee JY. Akt contributes to activation of the TRIF-dependent signaling pathways of TLRs by interacting with TANK-binding kinase 1. J Immunol. 2011;186:499–507. doi: 10.4049/jimmunol.0903534. [DOI] [PubMed] [Google Scholar]
- 58.Bozulic L, Surucu B, Hynx D, Hemmings BA. PKBalpha/Akt1 acts downstream of DNA-PK in the DNA double-strand break response and promotes survival. Mol Cell. 2008;30:203–213. doi: 10.1016/j.molcel.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 59.Peters RT, Liao SM, Maniatis T. IKKepsilon is part of a novel PMA-inducible IkappaB kinase complex. Mol Cell. 2000;5:513–522. doi: 10.1016/s1097-2765(00)80445-1. [DOI] [PubMed] [Google Scholar]
- 60.Tenoever BR, Ng SL, Chua MA, McWhirter SM, Garcia-Sastre A, Maniatis T. Multiple functions of the IKK-related kinase IKKepsilon in interferon-mediated antiviral immunity. Science. 2007;315:1274–1278. doi: 10.1126/science.1136567. [DOI] [PubMed] [Google Scholar]
- 61.Woronicz JD, Gao X, Cao Z, Rothe M, Goeddel DV. IkappaB kinase-beta: NF-kappaB activation and complex formation with IkappaB kinase-alpha and NIK. Science. 1997;278:866–869. doi: 10.1126/science.278.5339.866. [DOI] [PubMed] [Google Scholar]
- 62.Cenni V, Sirri A, Riccio M, Lattanzi G, Santi S, de Pol A, Maraldi NM, Marmiroli S. Targeting of the Akt/PKB kinase to the actin skeleton. Cell Mol Life Sci. 2003;60:2710–2720. doi: 10.1007/s00018-003-3349-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sato S, Fujita N, Tsuruo T. Modulation of Akt kinase activity by binding to Hsp90. Proc Natl Acad Sci U S A. 2000;97:10832–10837. doi: 10.1073/pnas.170276797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu R, Kausar H, Johnson P, Montoya-Durango DE, Merchant M, Rane MJ. Hsp27 regulates Akt activation and polymorphonuclear leukocyte apoptosis by scaffolding MK2 to Akt signal complex. J Biol Chem. 2007;282:21598–21608. doi: 10.1074/jbc.M611316200. [DOI] [PubMed] [Google Scholar]
- 65.Maira SM, Galetic I, Brazil DP, Kaech S, Ingley E, Thelen M, Hemmings BA. Carboxyl-terminal modulator protein (CTMP), a negative regulator of PKB/Akt and v-Akt at the plasma membrane. Science. 2001;294:374–380. doi: 10.1126/science.1062030. [DOI] [PubMed] [Google Scholar]
- 66.Du K, Herzig S, Kulkarni RN, Montminy M. TRB3: a tribbles homolog that inhibits Akt/PKB activation by insulin in liver. Science. 2003;300:1574–1577. doi: 10.1126/science.1079817. [DOI] [PubMed] [Google Scholar]
- 67.Alessi DR, Caudwell FB, Andjelkovic M, Hemmings BA, Cohen P. Molecular basis for the substrate specificity of protein kinase B. comparison with MAPKAP kinase-1 and p70 S6 kinase. FEBS Lett. 1996;399:333–338. doi: 10.1016/s0014-5793(96)01370-1. [DOI] [PubMed] [Google Scholar]
- 68.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 70.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 71.Ogawara Y, Kishishita S, Obata T, Isazawa Y, Suzuki T, Tanaka K, Masuyama N, Gotoh Y. Akt enhances Mdm2-mediated ubiquitination and degradation of p53. J Biol Chem. 2002;277:21843–21850. doi: 10.1074/jbc.M109745200. [DOI] [PubMed] [Google Scholar]
- 72.Gottlieb TM, Leal JF, Seger R, Taya Y, Oren M. Cross-talk between Akt, p53 and Mdm2: possible implications for the regulation of apoptosis. Oncogene. 2002;21:1299–1303. doi: 10.1038/sj.onc.1205181. [DOI] [PubMed] [Google Scholar]
- 73.Mayo LD, Donner DB. A phosphatidylinositol 3-kinase/Akt pathway promotes translocation of Mdm2 from the cytoplasm to the nucleus. Proc Natl Acad Sci U S A. 2001;98:11598–11603. doi: 10.1073/pnas.181181198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou BP, Liao Y, Xia W, Zou Y, Spohn B, Hung MC. HER-2/neu induces p53 ubiquitination via Akt-mediated MDM2 phosphorylation. Nat Cell Biol. 2001;3:973–982. doi: 10.1038/ncb1101-973. [DOI] [PubMed] [Google Scholar]
- 75.Basu S, Totty NF, Irwin MS, Sudol M, Downward J. Akt phosphorylates the Yes-associated protein YAP, to induce interaction with 14-3-3 and attenuation of p73-mediated apoptosis. Mol Cell. 2003;11:11–23. doi: 10.1016/s1097-2765(02)00776-1. [DOI] [PubMed] [Google Scholar]
- 76.Zhang X, Tang N, Hadden TJ, Rishi AK. Akt, FoxO and regulation of apoptosis. Biochim Biophys Acta. 2011;1813:1978–1986. doi: 10.1016/j.bbamcr.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 77.Barthwal MK, Sathyanarayana P, Kundu CN, Rana B, Pradeep A, Sharma C, Woodgett JR, Rana A. Negative regulation of mixed lineage kinase 3 by protein kinase B/AKT leads to cell survival. J Biol Chem. 2003;278:3897–3902. doi: 10.1074/jbc.M211598200. [DOI] [PubMed] [Google Scholar]
- 78.Zhang R, Luo D, Miao R, Bai L, Ge Q, Sessa WC, Min W. Hsp90-Akt phosphorylates ASK1 and inhibits ASK1-mediated apoptosis. Oncogene. 2005;24:3954–3963. doi: 10.1038/sj.onc.1208548. [DOI] [PubMed] [Google Scholar]
- 79.Sugishita Y, Leifer DW, Agani F, Watanabe M, Fisher SA. Hypoxia-responsive signaling regulates the apoptosis-dependent remodeling of the embryonic avian cardiac outflow tract. Dev Biol. 2004;273:285–296. doi: 10.1016/j.ydbio.2004.05.036. [DOI] [PubMed] [Google Scholar]
- 80.Cai SL, Tee AR, Short JD, Bergeron JM, Kim J, Shen J, Guo R, Johnson CL, Kiguchi K, Walker CL. Activity of TSC2 is inhibited by AKT-mediated phosphorylation and membrane partitioning. J Cell Biol. 2006;173:279–289. doi: 10.1083/jcb.200507119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nascimento EB, Snel M, Guigas B, van der Zon GC, Kriek J, Maassen JA, Jazet IM, Diamant M, Ouwens DM. Phosphorylation of PRAS40 on Thr246 by PKB/AKT facilitates efficient phosphorylation of Ser183 by mTORC1. Cell Signal. 2010;22:961–967. doi: 10.1016/j.cellsig.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 82.Wang H, Zhang Q, Wen Q, Zheng Y, Lazarovici P, Jiang H, Lin J, Zheng W. Proline-rich Akt substrate of 40kDa (PRAS40): a novel downstream target of PI3k/Akt signaling pathway. Cell Signal. 2012;24:17–24. doi: 10.1016/j.cellsig.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 83.Wiza C, Nascimento EB, Ouwens DM. Role of PRAS40 in Akt and mTOR signaling in health and disease. Am J Physiol Endocrinol Metab. 2012;302:E1453–E1460. doi: 10.1152/ajpendo.00660.2011. [DOI] [PubMed] [Google Scholar]
- 84.Murakami M, Ichisaka T, Maeda M, Oshiro N, Hara K, Edenhofer F, Kiyama H, Yonezawa K, Yamanaka S. mTOR is essential for growth and proliferation in early mouse embryos and embryonic stem cells. Mol Cell Biol. 2004;24:6710–6718. doi: 10.1128/MCB.24.15.6710-6718.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Demirkan G, Yu K, Boylan JM, Salomon AR, Gruppuso PA. Phosphoproteomic profiling of in vivo signaling in liver by the mammalian target of rapamycin complex 1 (mTORC1) PLoS One. 2011;6:e21729. doi: 10.1371/journal.pone.0021729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhu J, Blenis J, Yuan J. Activation of PI3K/Akt and MAPK pathways regulates Myc-mediated transcription by phosphorylating and promoting the degradation of Mad1. Proc Natl Acad Sci U S A. 2008;105:6584–6589. doi: 10.1073/pnas.0802785105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Amati B, Alevizopoulos K, Vlach J. Myc and the cell cycle. Front Biosci. 1998;3:d250–d268. doi: 10.2741/a239. [DOI] [PubMed] [Google Scholar]
- 89.Liang J, Slingerland JM. Multiple roles of the PI3K/PKB (Akt) pathway in cell cycle progression. Cell Cycle. 2003;2:339–345. [PubMed] [Google Scholar]
- 90.Laughner E, Taghavi P, Chiles K, Mahon PC, Semenza GL. HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1alpha (HIF-1 alpha) synthesis: novel mechanism for HIF-1-mediated vascular endothelial growth factor expression. Mol Cell Biol. 2001;21:3995–4004. doi: 10.1128/MCB.21.12.3995-4004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ackah E, Yu J, Zoellner S, Iwakiri Y, Skurk C, Shibata R, Ouchi N, Easton RM, Galasso G, Birnbaum MJ, Walsh K, Sessa WC. Akt1/protein kinase Balpha is critical for ischemic and VEGF-mediated angiogenesis. J Clin Invest. 2005;115:2119–2127. doi: 10.1172/JCI24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Peterson RT, Desai BN, Hardwick JS, Schreiber SL. Protein phosphatase 2A interacts with the 70-kDa S6 kinase and is activated by inhibition of FKBP12-rapamycinassociated protein. Proc Natl Acad Sci U S A. 1999;96:4438–4442. doi: 10.1073/pnas.96.8.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fukuda R, Hirota K, Fan F, Jung YD, Ellis LM, Semenza GL. Insulin-like growth factor 1 induces hypoxia-inducible factor 1-mediated vascular endothelial growth factor expression, which is dependent on MAP kinase and phosphatidylinositol 3-kinase signaling in colon cancer cells. J Biol Chem. 2002;277:38205–38211. doi: 10.1074/jbc.M203781200. [DOI] [PubMed] [Google Scholar]
- 94.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 95.Phung TL, Ziv K, Dabydeen D, Eyiah-Mensah G, Riveros M, Perruzzi C, Sun J, Monahan-Earley RA, Shiojima I, Nagy JA, Lin MI, Walsh K, Dvorak AM, Briscoe DM, Neeman M, Sessa WC, Dvorak HF, Benjamin LE. Pathological angiogenesis is induced by sustained Akt signaling and inhibited by rapamycin. Cancer Cell. 2006;10:159–170. doi: 10.1016/j.ccr.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kureishi Y, Luo Z, Shiojima I, Bialik A, Fulton D, Lefer DJ, Sessa WC, Walsh K. The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat Med. 2000;6:1004–1010. doi: 10.1038/79510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Morales-Ruiz M, Fulton D, Sowa G, Languino LR, Fujio Y, Walsh K, Sessa WC. Vascular endothelial growth factor-stimulated actin reorganization and migration of endothelial cells is regulated via the serine/threonine kinase Akt. Circ Res. 2000;86:892–896. doi: 10.1161/01.res.86.8.892. [DOI] [PubMed] [Google Scholar]
- 98.Dimmeler S, Dernbach E, Zeiher AM. Phosphorylation of the endothelial nitric oxide synthase at ser-1177 is required for VEGF-induced endothelial cell migration. FEBS Lett. 2000;477:258–262. doi: 10.1016/s0014-5793(00)01657-4. [DOI] [PubMed] [Google Scholar]
- 99.Primo L, di Blasio L, Roca C, Droetto S, Piva R, Schaffhausen B, Bussolino F. Essential role of PDK1 in regulating endothelial cell migration. J Cell Biol. 2007;176:1035–1047. doi: 10.1083/jcb.200607053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pitulescu ME, Adams RH. Eph/ephrin molecules--a hub for signaling and endocytosis. Genes Dev. 2010;24:2480–2492. doi: 10.1101/gad.1973910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Miao H, Li DQ, Mukherjee A, Guo H, Petty A, Cutter J, Basilion JP, Sedor J, Wu J, Danielpour D, Sloan AE, Cohen ML, Wang B. EphA2 mediates ligand-dependent inhibition and ligand-independent promotion of cell migration and invasion via a reciprocal regulatory loop with Akt. Cancer Cell. 2009;16:9–20. doi: 10.1016/j.ccr.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Frisch SM, Screaton RA. Anoikis mechanisms. Curr Opin Cell Biol. 2001;13:555–562. doi: 10.1016/s0955-0674(00)00251-9. [DOI] [PubMed] [Google Scholar]
- 103.Fujio Y, Walsh K. Akt mediates cytoprotection of endothelial cells by vascular endothelial growth factor in an anchorage-dependent manner. J Biol Chem. 1999;274:16349–16354. doi: 10.1074/jbc.274.23.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Papapetropoulos A, Fulton D, Mahboubi K, Kalb RG, O'Connor DS, Li F, Altieri DC, Sessa WC. Angiopoietin-1 inhibits endothelial cell apoptosis via the Akt/survivin pathway. J Biol Chem. 2000;275:9102–9105. doi: 10.1074/jbc.275.13.9102. [DOI] [PubMed] [Google Scholar]
- 105.Suhara T, Mano T, Oliveira BE, Walsh K. Phosphatidylinositol 3-kinase/Akt signaling controls endothelial cell sensitivity to Fas-mediated apoptosis via regulation of FLICE-inhibitory protein (FLIP) Circ Res. 2001;89:13–19. doi: 10.1161/hh1301.092506. [DOI] [PubMed] [Google Scholar]
- 106.Gratton JP, Morales-Ruiz M, Kureishi Y, Fulton D, Walsh K, Sessa WC. Akt down-regulation of p38 signaling provides a novel mechanism of vascular endothelial growth factor-mediated cytoprotection in endothelial cells. J Biol Chem. 2001;276:30359–30365. doi: 10.1074/jbc.M009698200. [DOI] [PubMed] [Google Scholar]
- 107.Dimmeler S, Aicher A, Vasa M, Mildner-Rihm C, Adler K, Tiemann M, Rutten H, Fichtlscherer S, Martin H, Zeiher AM. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. J Clin Invest. 2001;108:391–397. doi: 10.1172/JCI13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 109.Mai J, Qiu Q, Lin YQ, Luo NS, Zhang HF, Wen ZZ, Wang JF, YangXin C. Angiotensin II-derived reactive oxygen species promote angiogenesis in human late endothelial progenitor cells through heme oxygenase-1 via ERK1/2 and AKT/PI3K pathways. Inflammation. 2014;37:858–870. doi: 10.1007/s10753-013-9806-9. [DOI] [PubMed] [Google Scholar]
- 110.Fukumura D, Gohongi T, Kadambi A, Izumi Y, Ang J, Yun CO, Buerk DG, Huang PL, Jain RK. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc Natl Acad Sci U S A. 2001;98:2604–2609. doi: 10.1073/pnas.041359198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Brouet A, Sonveaux P, Dessy C, Balligand JL, Feron O. Hsp90 ensures the transition from the early Ca2+-dependent to the late phosphorylation-dependent activation of the endothelial nitric-oxide synthase in vascular endothelial growth factor-exposed endothelial cells. J Biol Chem. 2001;276:32663–32639. doi: 10.1074/jbc.M101371200. [DOI] [PubMed] [Google Scholar]
- 113.Brouet A, Sonveaux P, Dessy C, Moniotte S, Balligand JL, Feron O. Hsp90 and caveolin are key targets for the proangiogenic nitric oxide-mediated effects of statins. Circ Res. 2001;89:866–873. doi: 10.1161/hh2201.100319. [DOI] [PubMed] [Google Scholar]
- 114.Fontana J, Fulton D, Chen Y, Fairchild TA, McCabe TJ, Fujita N, Tsuruo T, Sessa WC. Domain mapping studies reveal that the M domain of hsp90 serves as a molecular scaffold to regulate Akt-dependent phosphorylation of endothelial nitric oxide synthase and NO release. Circ Res. 2002;90:866–873. doi: 10.1161/01.res.0000016837.26733.be. [DOI] [PubMed] [Google Scholar]
- 115.Luo Z, Fujio Y, Kureishi Y, Rudic RD, Daumerie G, Fulton D, Sessa WC, Walsh K. Acute modulation of endothelial Akt/PKB activity alters nitric oxide-dependent vasomotor activity in vivo. J Clin Invest. 2000;106:493–499. doi: 10.1172/JCI9419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Scotland RS, Morales-Ruiz M, Chen Y, Yu J, Rudic RD, Fulton D, Gratton JP, Sessa WC. Functional reconstitution of endothelial nitric oxide synthase reveals the importance of serine 1179 in endothelium-dependent vasomotion. Circ Res. 2002;90:904–910. doi: 10.1161/01.res.0000016506.04193.96. [DOI] [PubMed] [Google Scholar]
- 117.Iaccarino G, Ciccarelli M, Sorriento D, Cipolletta E, Cerullo V, Iovino GL, Paudice A, Elia A, Santulli G, Campanile A, Arcucci O, Pastore L, Salvatore F, Condorelli G, Trimarco B. AKT participates in endothelial dysfunction in hypertension. Circulation. 2004;109:2587–2593. doi: 10.1161/01.CIR.0000129768.35536.FA. [DOI] [PubMed] [Google Scholar]
- 118.Bae SS, Cho H, Mu J, Birnbaum MJ. Isoform-specific regulation of insulin-dependent glucose uptake by Akt/protein kinase B. J Biol Chem. 2003;278:49530–49536. doi: 10.1074/jbc.M306782200. [DOI] [PubMed] [Google Scholar]
- 119.Krycer JR, Sharpe LJ, Luu W, Brown AJ. The Akt-SREBP nexus: cell signaling meets lipid metabolism. Trends Endocrinol Metab. 2010;21:268–276. doi: 10.1016/j.tem.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 120.Nakae J, Park BC, Accili D. Insulin stimulates phosphorylation of the forkhead transcription factor FKHR on serine 253 through a Wortmannin-sensitive pathway. J Biol Chem. 1999;274:15982–15985. doi: 10.1074/jbc.274.23.15982. [DOI] [PubMed] [Google Scholar]
- 121.Cross DA, Alessi DR, Vandenheede JR, McDowell HE, Hundal HS, Cohen P. The inhibition of glycogen synthase kinase-3 by insulin or insulin-like growth factor 1 in the rat skeletal muscle cell line L6 is blocked by wortmannin, but not by rapamycin: evidence that wortmannin blocks activation of the mitogen-activated protein kinase pathway in L6 cells between Ras and Raf. Biochem J. 1994;303(Pt 1):21–26. doi: 10.1042/bj3030021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chavakis E, Dernbach E, Hermann C, Mondorf UF, Zeiher AM, Dimmeler S. Oxidized LDL inhibits vascular endothelial growth factor-induced endothelial cell migration by an inhibitory effect on the Akt/endothelial nitric oxide synthase pathway. Circulation. 2001;103:2102–2107. doi: 10.1161/01.cir.103.16.2102. [DOI] [PubMed] [Google Scholar]
- 123.Allard D, Figg N, Bennett MR, Littlewood TD. Akt regulates the survival of vascular smooth muscle cells via inhibition of FoxO3a and GSK3. J Biol Chem. 2008;283:19739–19747. doi: 10.1074/jbc.M710098200. [DOI] [PubMed] [Google Scholar]
- 124.Jia G, Cheng G, Gangahar DM, Agrawal DK. Insulin-like growth factor-1 and TNF-alpha regulate autophagy through c-jun N-terminal kinase and Akt pathways in human atherosclerotic vascular smooth cells. Immunol Cell Biol. 2006;84:448–454. doi: 10.1111/j.1440-1711.2006.01454.x. [DOI] [PubMed] [Google Scholar]
- 125.Babaev VR, Hebron KE, Wiese CB, Toth CL, Ding L, Zhang Y, May JM, Fazio S, Vickers KC, Linton MF. Macrophage deficiency of Akt2 reduces atherosclerosis in Ldlr null mice. J Lipid Res. 2014;55:2296–2308. doi: 10.1194/jlr.M050633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Goetze S, Eilers F, Bungenstock A, Kintscher U. PPAR activators inhibit endothelial cell migration by targeting Akt. Biochemical and …. 2002 doi: 10.1016/S0006-291X(02)00385-6. [DOI] [PubMed] [Google Scholar]
- 127.Hardt SE, Sadoshima J. Negative regulators of cardiac hypertrophy. Cardiovasc Res. 2004;63:500–509. doi: 10.1016/j.cardiores.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 128.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7:589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 129.Dorn GW, 2nd, Force T. Protein kinase cascades in the regulation of cardiac hypertrophy. J Clin Invest. 2005;115:527–537. doi: 10.1172/JCI24178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Scheuer J, Malhotra A, Hirsch C, Capasso J, Schaible TF. Physiologic cardiac hypertrophy corrects contractile protein abnormalities associated with pathologic hypertrophy in rats. J Clin Invest. 1982;70:1300–1305. doi: 10.1172/JCI110729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Grossman W, Jones D, McLaurin LP. Wall stress and patterns of hypertrophy in the human left ventricle. J Clin Invest. 1975;56:56–64. doi: 10.1172/JCI108079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Seidman JG, Seidman C. The genetic basis for cardiomyopathy: from mutation identification to mechanistic paradigms. Cell. 2001;104:557–567. doi: 10.1016/s0092-8674(01)00242-2. [DOI] [PubMed] [Google Scholar]
- 133.Sano M, Schneider MD. Still stressed out but doing fine: normalization of wall stress is superfluous to maintaining cardiac function in chronic pressure overload. Circulation. 2002;105:8–10. [PubMed] [Google Scholar]
- 134.McMullen JR, Shioi T, Zhang L, Tarnavski O, Sherwood MC, Kang PM, Izumo S. Phosphoinositide 3-kinase(p110alpha) plays a critical role for the induction of physiological, but not pathological, cardiac hypertrophy. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:12355–12360. doi: 10.1073/pnas.1934654100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Oudit GY, Sun H, Kerfant BG, Crackower MA, Penninger JM, Backx PH. The role of phosphoinositide-3 kinase and PTEN in cardiovascular physiology and disease. J Mol Cell Cardiol. 2004;37:449–471. doi: 10.1016/j.yjmcc.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 136.Patrucco E, Notte A, Barberis L, Selvetella G, Maffei A, Brancaccio M, Marengo S, Russo G, Azzolino O, Rybalkin SD, Silengo L, Altruda F, Wetzker R, Wymann MP, Lembo G, Hirsch E. PI3Kgamma modulates the cardiac response to chronic pressure overload by distinct kinase-dependent and -independent effects. Cell. 2004;118:375–387. doi: 10.1016/j.cell.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 137.Crackower MA, Oudit GY, Kozieradzki I, Sarao R, Sun H, Sasaki T, Hirsch E, Suzuki A, Shioi T, Irie-Sasaki J, Sah R, Cheng H-YMY, Rybin VO, Lembo G, Fratta L, Oliveira-dos-Santos AJ, Benovic JL, Kahn CR, Izumo S, Steinberg SF, Wymann MP, Backx PH, Penninger JM. Regulation of myocardial contractility and cell size by distinct PI3K-PTEN signaling pathways. Cell. 2002;110:737–749. doi: 10.1016/s0092-8674(02)00969-8. [DOI] [PubMed] [Google Scholar]
- 138.Nagoshi T, Matsui T, Aoyama T, Leri A, Anversa P, Li L, Ogawa W, del Monte F, Gwathmey JK, Grazette L, Hemmings BA, Kass DA, Champion HC, Rosenzweig A. PI3K rescues the detrimental effects of chronic Akt activation in the heart during ischemia/reperfusion injury. J Clin Invest. 2005;115:2128–2138. doi: 10.1172/JCI23073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Condorelli G, Drusco A, Stassi G, Bellacosa A, Roncarati R, Iaccarino G, Russo MA, Gu Y, Dalton N, Chung C, Latronico MV, Napoli C, Sadoshima J, Croce CM, Ross J., Jr Akt induces enhanced myocardial contractility and cell size in vivo in transgenic mice. Proc Natl Acad Sci U S A. 2002;99:12333–12338. doi: 10.1073/pnas.172376399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Shiojima I, Sato K, Izumiya Y, Schiekofer S, Ito M, Liao R, Colucci WS, Walsh K. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J Clin Invest. 2005;115:2108–2118. doi: 10.1172/JCI24682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cheng SM, Ho TJ, Yang AL, Chen IJ, Kao CL, Wu FN, Lin JA, Kuo CH, Ou HC, Huang CY, Lee SD. Exercise training enhances cardiac IGFI-R/PI3K/Akt and Bcl-2 family associated pro-survival pathways in streptozotocin-induced diabetic rats. Int J Cardiol. 2013;167:478–485. doi: 10.1016/j.ijcard.2012.01.031. [DOI] [PubMed] [Google Scholar]
- 142.Epstein FH, Gibbons GH, Dzau VJ. The emerging concept of vascular remodeling. New England Journal of …. 1994 doi: 10.1056/NEJM199405193302008. [DOI] [PubMed] [Google Scholar]
- 143.Park KW, Yang HM, Youn SW, Yang HJ. Constitutively active glycogen synthase kinase-3β gene transfer sustains apoptosis, inhibits proliferation of vascular smooth muscle cells, and reduces neointima …. …. 2003 doi: 10.1161/01.ATV.0000081633.53390.B4. [DOI] [PubMed] [Google Scholar]
- 144.Gerber HP, McMurtrey A, Kowalski J, Yan M. Vascular Endothelial Growth Factor Regulates Endothelial Cell Survival through the Phosphatidylinositol 3′-Kinase/Akt Signal Transduction Pathway …. Journal of Biological …. 1998 doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
- 145.Stabile E, Zhou YF, Saji M, Castagna M, Shou M, Kinnaird TD, Baffour R, Ringel MD, Epstein SE, Fuchs S. Akt controls vascular smooth muscle cell proliferation in vitro and in vivo by delaying G1/S exit. Circ Res. 2003;93:1059–1065. doi: 10.1161/01.RES.0000105086.31909.1B. [DOI] [PubMed] [Google Scholar]
- 146.Havelka GE, Kibbe MR. The vascular adventitia: its role in the arterial injury response. Vasc Endovascular Surg. 2011;45:381–390. doi: 10.1177/1538574411407698. [DOI] [PubMed] [Google Scholar]
- 147.Garat CV, Crossno JT, Sullivan TM, Reusch JE, Klemm DJ. Inhibition of phosphatidylinositol 3-kinase/Akt signaling attenuates hypoxia-induced pulmonary artery remodeling and suppresses CREB depletion in arterial smooth muscle cells. Journal of cardiovascular pharmacology. 2013;62:539–548. doi: 10.1097/FJC.0000000000000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Krymskaya VP, Snow J, Cesarone G, Khavin I, Goncharov DA, Lim PN, Veasey SC, Ihida-Stansbury K, Jones PL, Goncharova EA. mTOR is required for pulmonary arterial vascular smooth muscle cell proliferation under chronic hypoxia. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2011;25:1922–1933. doi: 10.1096/fj.10-175018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Houssaini A, Abid S, Mouraret N, Wan F, Rideau D, Saker M, Marcos E, Tissot C-MM, Dubois-Randé J-LL, Amsellem V, Adnot S. Rapamycin reverses pulmonary artery smooth muscle cell proliferation in pulmonary hypertension. American journal of respiratory cell and molecular biology. 2013;48:568–577. doi: 10.1165/rcmb.2012-0429OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Goncharov DA, Kudryashova TV, Ziai H, Ihida-Stansbury K, DeLisser H, Krymskaya VP, Tuder RM, Kawut SM, Goncharova EA. Mammalian target of rapamycin complex 2 (mTORC2) coordinates pulmonary artery smooth muscle cell metabolism, proliferation, and survival in pulmonary arterial hypertension. Circulation. 2014;129:864–874. doi: 10.1161/CIRCULATIONAHA.113.004581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Haiyang T, Jiwang C, Dustin RF, Shanshan S, Justin RS, Abigail RD, Stefan O, Richard DY, Marcelo GB, Richard DM, Joe GNG, Roberto FM, Ayako M, Jason XJY. Deficiency of Akt1, but not Akt2, Attenuates the Development of Pulmonary Hypertension. American journal of physiology. Lung cellular and molecular physiology. 2014 doi: 10.1152/ajplung.00242.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ihle NT, Williams R, Chow S, Chew W, Berggren MI, Paine-Murrieta G, Minion DJ, Halter RJ, Wipf P, Abraham R, Kirkpatrick L, Powis G. Molecular pharmacology and antitumor activity of PX-866, a novel inhibitor of phosphoinositide-3-kinase signaling. Mol Cancer Ther. 2004;3:763–772. [PubMed] [Google Scholar]
- 153.Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- 154.Powis G, Bonjouklian R, Berggren MM, Gallegos A, Abraham R, Ashendel C, Zalkow L, Matter WF, Dodge J, Grindey G, et al. Wortmannin, a potent and selective inhibitor of phosphatidylinositol-3-kinase. Cancer Res. 1994;54:2419–2423. [PubMed] [Google Scholar]
- 155.Ikeda H, Hideshima T, Fulciniti M, Perrone G, Miura N, Yasui H, Okawa Y, Kiziltepe T, Santo L, Vallet S, Cristea D, Calabrese E, Gorgun G, Raje NS, Richardson P, Munshi NC, Lannutti BJ, Puri KD, Giese NA, Anderson KC. PI3K/p110{delta} is a novel therapeutic target in multiple myeloma. Blood. 2010;116:1460–1468. doi: 10.1182/blood-2009-06-222943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bergamini G, Bell K, Shimamura S, Werner T, Cansfield A, Muller K, Perrin J, Rau C, Ellard K, Hopf C, Doce C, Leggate D, Mangano R, Mathieson T, O'Mahony A, Plavec I, Rharbaoui F, Reinhard F, Savitski MM, Ramsden N, Hirsch E, Drewes G, Rausch O, Bantscheff M, Neubauer G. A selective inhibitor reveals PI3Kgamma dependence of T(H)17 cell differentiation. Nat Chem Biol. 2012;8:576–582. doi: 10.1038/nchembio.957. [DOI] [PubMed] [Google Scholar]
- 157.Straub A, Wendel HP, Dietz K, Schiebold D, Peter K, Schoenwaelder SM, Ziemer G. Selective inhibition of the platelet phosphoinositide 3-kinase p110beta as promising new strategy for platelet protection during extracorporeal circulation. Thromb Haemost. 2008;99:609–615. doi: 10.1160/TH07-07-0452. [DOI] [PubMed] [Google Scholar]
- 158.Lee H, Jung KH, Jeong Y, Hong S, Hong SS. HS-173, a novel phosphatidylinositol 3-kinase (PI3K) inhibitor, has anti-tumor activity through promoting apoptosis and inhibiting angiogenesis. Cancer Lett. 2013;328:152–159. doi: 10.1016/j.canlet.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 159.Feldman RI, Wu JM, Polokoff MA, Kochanny MJ, Dinter H, Zhu D, Biroc SL, Alicke B, Bryant J, Yuan S, Buckman BO, Lentz D, Ferrer M, Whitlow M, Adler M, Finster S, Chang Z, Arnaiz DO. Novel small molecule inhibitors of 3-phosphoinositide-dependent kinase-1. J Biol Chem. 2005;280:19867–19874. doi: 10.1074/jbc.M501367200. [DOI] [PubMed] [Google Scholar]
- 160.Meuillet EJ, Zuohe S, Lemos R, Ihle N, Kingston J, Watkins R, Moses SA, Zhang S, Du-Cuny L, Herbst R, Jacoby JJ, Zhou LL, Ahad AM, Mash EA, Kirkpatrick DL, Powis G. Molecular pharmacology and antitumor activity of PHT-427, a novel Akt/phosphatidylinositide-dependent protein kinase 1 pleckstrin homology domain inhibitor. Mol Cancer Ther. 2010;9:706–717. doi: 10.1158/1535-7163.MCT-09-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sargeant AM, Klein RD, Rengel RC, Clinton SK, Kulp SK, Kashida Y, Yamaguchi M, Wang X, Chen CS. Chemopreventive and bioenergetic signaling effects of PDK1/Akt pathway inhibition in a transgenic mouse model of prostate cancer. Toxicol Pathol. 2007;35:549–561. doi: 10.1080/01926230701338966. [DOI] [PubMed] [Google Scholar]
- 162.Al-Batran SE, Ducreux M, Ohtsu A. mTOR as a therapeutic target in patients with gastric cancer. Int J Cancer. 2012;130:491–496. doi: 10.1002/ijc.26396. [DOI] [PubMed] [Google Scholar]
- 163.Zaytseva YY, Valentino JD, Gulhati P, Evers BM. mTOR inhibitors in cancer therapy. Cancer Lett. 2012;319:1–7. doi: 10.1016/j.canlet.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 164.Feldman ME, Apsel B, Uotila A, Loewith R, Knight ZA, Ruggero D, Shokat KM. Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol. 2009;7:e38. doi: 10.1371/journal.pbio.1000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Garcia-Martinez JM, Moran J, Clarke RG, Gray A, Cosulich SC, Chresta CM, Alessi DR. Ku-0063794 is a specific inhibitor of the mammalian target of rapamycin (mTOR) Biochem J. 2009;421:29–42. doi: 10.1042/BJ20090489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chresta CM, Davies BR, Hickson I, Harding T, Cosulich S, Critchlow SE, Vincent JP, Ellston R, Jones D, Sini P, James D, Howard Z, Dudley P, Hughes G, Smith L, Maguire S, Hummersone M, Malagu K, Menear K, Jenkins R, Jacobsen M, Smith GC, Guichard S, Pass M. AZD8055 is a potent, selective, and orally bioavailable ATP-competitive mammalian target of rapamycin kinase inhibitor with in vitro and in vivo antitumor activity. Cancer Res. 2010;70:288–298. doi: 10.1158/0008-5472.CAN-09-1751. [DOI] [PubMed] [Google Scholar]
- 167.Bonnet S, Rochefort G, Sutendra G. The nuclear factor of activated T cells in pulmonary arterial hypertension can be therapeutically targeted. Proceedings of the …. 2007 doi: 10.1073/pnas.0610467104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bonnet S, Paulin R, Sutendra G, Dromparis P, Roy M. Dehydroepiandrosterone reverses systemic vascular remodeling through the inhibition of the Akt/GSK3-β/NFAT axis. Circulation. 2009 doi: 10.1161/CIRCULATIONAHA.109.848911. [DOI] [PubMed] [Google Scholar]
- 169.Munck JM, Batey MA, Zhao Y, Jenkins H, Richardson CJ, Cano C, Tavecchio M, Barbeau J, Bardos J, Cornell L, Griffin RJ, Menear K, Slade A, Thommes P, Martin NM, Newell DR, Smith GC, Curtin NJ. Chemosensitization of cancer cells by KU-0060648, a dual inhibitor of DNA-PK and PI-3K. Mol Cancer Ther. 2012;11:1789–1798. doi: 10.1158/1535-7163.MCT-11-0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Talekar M, Ganta S, Amiji M, Jamieson S, Kendall J, Denny WA, Garg S. Development of PIK-75 nanosuspension formulation with enhanced delivery efficiency and cytotoxicity for targeted anti-cancer therapy. Int J Pharm. 2013;450:278–289. doi: 10.1016/j.ijpharm.2013.04.057. [DOI] [PubMed] [Google Scholar]
- 171.Leahy JJ, Golding BT, Griffin RJ, Hardcastle IR, Richardson C, Rigoreau L, Smith GC. Identification of a highly potent and selective DNA-dependent protein kinase (DNA-PK) inhibitor (NU7441) by screening of chromenone libraries. Bioorg Med Chem Lett. 2004;14:6083–6087. doi: 10.1016/j.bmcl.2004.09.060. [DOI] [PubMed] [Google Scholar]
- 172.Yun J. Allosteric AKT inhibitors as a targeted cancer therapy. Cancer Biol Ther. 2010;9:504–506. doi: 10.4161/cbt.9.7.11356. [DOI] [PubMed] [Google Scholar]
- 173.Hirai H, Sootome H, Nakatsuru Y, Miyama K, Taguchi S, Tsujioka K, Ueno Y, Hatch H, Majumder PK, Pan BS, Kotani H. MK-2206, an allosteric Akt inhibitor, enhances antitumor efficacy by standard chemotherapeutic agents or molecular targeted drugs in vitro and in vivo. Mol Cancer Ther. 2010;9:1956–1967. doi: 10.1158/1535-7163.MCT-09-1012. [DOI] [PubMed] [Google Scholar]
- 174.Wilson JM, Kunnimalaiyaan S, Gamblin TC, Kunnimalaiyaan M. MK2206 inhibits hepatocellular carcinoma cellular proliferation via induction of apoptosis and cell cycle arrest. J Surg Res. 2014;191:280–285. doi: 10.1016/j.jss.2014.05.083. [DOI] [PubMed] [Google Scholar]
- 175.Luo Y, Smith RA, Guan R, Liu X, Klinghofer V, Shen J, Hutchins C, Richardson P, Holzman T, Rosenberg SH, Giranda VL. Pseudosubstrate peptides inhibit Akt and induce cell growth inhibition. Biochemistry. 2004;43:1254–1263. doi: 10.1021/bi034515p. [DOI] [PubMed] [Google Scholar]
- 176.Kondapaka SB, Singh SS, Dasmahapatra GP, Sausville EA, Roy KK. Perifosine, a novel alkylphospholipid, inhibits protein kinase B activation. Mol Cancer Ther. 2003;2:1093–1103. [PubMed] [Google Scholar]
- 177.Miao B, Skidan I, Yang J, Lugovskoy A, Reibarkh M, Long K, Brazell T, Durugkar KA, Maki J, Ramana CV, Schaffhausen B, Wagner G, Torchilin V, Yuan J, Degterev A. Small molecule inhibition of phosphatidylinositol-3,4,5-triphosphate (PIP3) binding to pleckstrin homology domains. Proc Natl Acad Sci U S A. 2010;107:20126–20131. doi: 10.1073/pnas.1004522107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Estrada AC, Syrovets T, Pitterle K, Lunov O, Buchele B, Schimana-Pfeifer J, Schmidt T, Morad SA, Simmet T. Tirucallic acids are novel pleckstrin homology domain-dependent Akt inhibitors inducing apoptosis in prostate cancer cells. Mol Pharmacol. 2010;77:378–387. doi: 10.1124/mol.109.060475. [DOI] [PubMed] [Google Scholar]
- 179.Lochner A, Moolman JA. The many faces of H89: a review. Cardiovasc Drug Rev. 2006;24:261–274. doi: 10.1111/j.1527-3466.2006.00261.x. [DOI] [PubMed] [Google Scholar]
- 180.Heerding DA, Rhodes N, Leber JD, Clark TJ, Keenan RM, Lafrance LV, Li M, Safonov IG, Takata DT, Venslavsky JW, Yamashita DS, Choudhry AE, Copeland RA, Lai Z, Schaber MD, Tummino PJ, Strum SL, Wood ER, Duckett DR, Eberwein D, Knick VB, Lansing TJ, McConnell RT, Zhang S, Minthorn EA, Concha NO, Warren GL, Kumar R. Identification of 4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-1-ethyl-7-{[(3S)-3-piperidinylmethyl]oxy}-1H-imidazo[4,5-c]pyridin-4-yl)-2-methyl-3-butyn-2-ol (GSK690693), a novel inhibitor of AKT kinase. J Med Chem. 2008;51:5663–5679. doi: 10.1021/jm8004527. [DOI] [PubMed] [Google Scholar]
- 181.Yap TA, Walton MI, Hunter LJ, Valenti M, de Haven Brandon A, Eve PD, Ruddle R, Heaton SP, Henley A, Pickard L, Vijayaraghavan G, Caldwell JJ, Thompson NT, Aherne W, Raynaud FI, Eccles SA, Workman P, Collins I, Garrett MD. Preclinical pharmacology, antitumor activity, and development of pharmacodynamic markers for the novel, potent AKT inhibitor CCT128930. Mol Cancer Ther. 2011;10:360–371. doi: 10.1158/1535-7163.MCT-10-0760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Mosca E, Barcella M, Alfieri R, Bevilacqua A, Canti G, Milanesi L. Systems biology of the metabolic network regulated by the Akt pathway. Biotechnol Adv. 2012;30:131–141. doi: 10.1016/j.biotechadv.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 183.Brown DJ, Rzucidlo EM, Merenick BL, Wagner RJ, Martin KA, Powell RJ. Endothelial cell activation of the smooth muscle cell phosphoinositide 3-kinase/Akt pathway promotes differentiation. J Vasc Surg. 2005;41:509–516. doi: 10.1016/j.jvs.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 184.Yang C, Li X, Wang Y, Zhao L, Chen W. Long non-coding RNA UCA1 regulated cell cycle distribution via CREB through PI3-K dependent pathway in bladder carcinoma cells. Gene. 2012;496:8–16. doi: 10.1016/j.gene.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 185.Tsang DP, Cheng AS. Epigenetic regulation of signaling pathways in cancer: role of the histone methyltransferase EZH2. J Gastroenterol Hepatol. 2011;26:19–27. doi: 10.1111/j.1440-1746.2010.06447.x. [DOI] [PubMed] [Google Scholar]
- 186.Zuo T, Liu TM, Lan X, Weng YI, Shen R, Gu F, Huang YW, Liyanarachchi S, Deatherage DE, Hsu PY, Taslim C, Ramaswamy B, Shapiro CL, Lin HJ, Cheng AS, Jin VX, Huang TH. Epigenetic silencing mediated through activated PI3K/AKT signaling in breast cancer. Cancer Res. 2011;71:1752–1762. doi: 10.1158/0008-5472.CAN-10-3573. [DOI] [PMC free article] [PubMed] [Google Scholar]