Abstract
Insulin resistance (IR) is a metabolic disorder characterized by impaired glucose uptake in response to insulin. The current paradigm for insulin signaling centers upon the insulin receptor (InsR) and its substrate IRS1; the latter is believed to be the chief conduit for post-receptor signaling. We recently demonstrated that GIV, a Guanidine Exchange Factor (GEF) for the trimeric G protein, Gαi, is a major hierarchical conduit for the metabolic insulin response. By virtue of its ability to directly bind the InsR, IRS1 and PI3K, GIV enhances the InsR-IRS1-Akt-AS160 (RabGAP) signaling cascade and cellular glucose uptake via its GEF function. Phosphoinhibition of GIV-GEF by the fatty-acid/PKCθ pathway inhibits the cascade and impairs glucose uptake. Here we show that GIV directly and constitutively binds the exocyst complex subunit Exo-70 and also associates with GLUT4-storage vesicles (GSVs) exclusively upon insulin stimulation. Without GIV or its GEF function, membrane association of Exo-70 as well as exocytosis of GSVs in response to insulin are impaired. Thus, GIV is an essential upstream component within the insulin signaling cascade that couples components within the InsR and G-Protein signaling cascade to those within the exocytic pathway. These findings suggest a role of GIV in coordinating the signaling and trafficking events of metabolic insulin response.
Keywords: GIV, PI3-Kinase, Akt, GLUT4, Exo-70, Exocytosis
INTRODUCTION
Insulin signaling begins with the binding of the hormone insulin to its receptors (InsR, IGF1R), which triggers receptor autophosphorylation, and subsequent tyrosine phosphorylation of insulin receptor substrate 1 (IRS1), amongst others. This leads to the recruitment and activation of Src-Homology-2 (SH2) proteins such as p85α(PI3K) and downstream activation of Akt [1]. Akt triggers the translocation of the 12-transmembrane glucose transporter 4 (GLUT4) to the plasma membrane (PM) by phosphoinhibiting the Rab GTPase activating protein (GAP) AS160 [2]. Among the many adaptors that relay signals within the insulin cascade, IRS1 is widely believed to serve as the major node for orchestrating metabolic insulin signaling [1].
Besides IRS1, metabolic insulin signaling relies also on the activation of heterotrimeric G proteins, another major hub in eukaryotic signal transduction. InsRs are functionally coupled to the pertussis-toxin sensitive Gαi/o proteins, e.g., insulin can trigger their activation, localization and phosphorylation (reviewed in [3]). Activation of Gi augments insulin sensitivity [4, 5], enhances tyrosine phosphorylation of both InsR and IRS1 [6] and triggers efficient translocation of GLUT4 to the PM [4, 7, 8]. Although numerous clues consistently point to a critical role of Gi activation in the insulin response, who/what couples and activates Gi downstream of InsR, and how such activation may cross-talk with IRS1-dependent insulin signaling and trigger downstream metabolic events remained unknown until recently.
Recently, we demonstrated [3] that a multi-modular signal transducer GIV, is a major hierarchical conduit for the metabolic insulin response with distinctive features. GIV is a Guanidine Exchange Factor (GEF) for the trimeric G protein, Gαi1/2/3 [9] which contains a SH2-like domain that directly binds InsR [10]. GIV is also a substrate of InsR which phosphorylates GIV at Y1764 [11]. GIV directly and constitutively binds the N-terminus of IRS1 [3] and is a bona-fide enhancer of the PI3K-Akt pathway downstream of multiple RTKs, including InsR [11]. We showed that by virtue of its ability to directly bind the InsR, IRS1 and PI3K, GIV enhances the InsR-IRS1 signaling cascade and glucose uptake via its GEF function. Site-directed mutagenesis or phosphoinhibition of GIV-GEF by the fatty-acid/PKCθ pathway triggers IR, and that insulin sensitizers reverse phosphoinhibition of GIV and reinstate insulin sensitivity. Using cell-permeable TAT-GIV peptides we showed that turning GIV-GEF “ON” serves as a therapeutic approach for exogenous manipulation of physiologic insulin response and for the reversal of IR in skeletal muscles [3]. Based on these findings it was concluded that GIV is an essential upstream component that couples InsR to G-Protein signaling to enhance the metabolic insulin response, and impairment of such coupling triggers IR.
Although these findings reveal the importance of GIV as a key determinant of insulin sensitivity in physiology and that its phosphoregulation by PKCθ triggers IR, what remains unknown is how GIV may link these upstream signaling events within the insulin pathway to the downstream membrane trafficking events that ultimately coordinate glucose uptake by GLUT4 transporters. Here we show that GIV associates with two additional key components within the metabolic insulin response: 1) GLUT4 storage vesicles (GSVs) and 2) exocyst complex subunit Exo-70. Findings provide insights into how GIV may link signaling events within the insulin cascade to the membrane trafficking components that regulate the exocytosis of GSVs.
MATERIALS AND METHODS
Detailed methods are presented in Supplemental Information.
Cell culture, transfection, imunoblotting, immunofluorescence and protein-protein interaction assays
These assays were carried out exactly as described before [13, 14]. All Odyssey images were processed using Image J software (NIH) and assembled for presentation using Photoshop and Illustrator software (Adobe).
Assay for measuring cell surface GLUT4
The efficacy of GLUT4 translocation to the PM was analyzed by carrying out cell-surface immunofluorescence staining under non-permeabilizing conditions on cells expressing the extensively characterized GLUT4 chimera, HA-GLUT4-GFP [15].
Proximity Ligation Assay (PLA)
In situ interaction of endogenous GIV with either HA-GLUT4-GFP or Exo70-HA was detected using a proximity ligation assay kit Duolink (Olink Biosciences).
Data Analysis and Statistics
All experiments were repeated at least three times, and results were presented either as one representative experiment or as average ± SD or SEM. Statistical significance was assessed with two-tailed Student’s t test.
RESULTS
Activation of Gαi by GIV-GEF is required for GLUT4 exocytosis
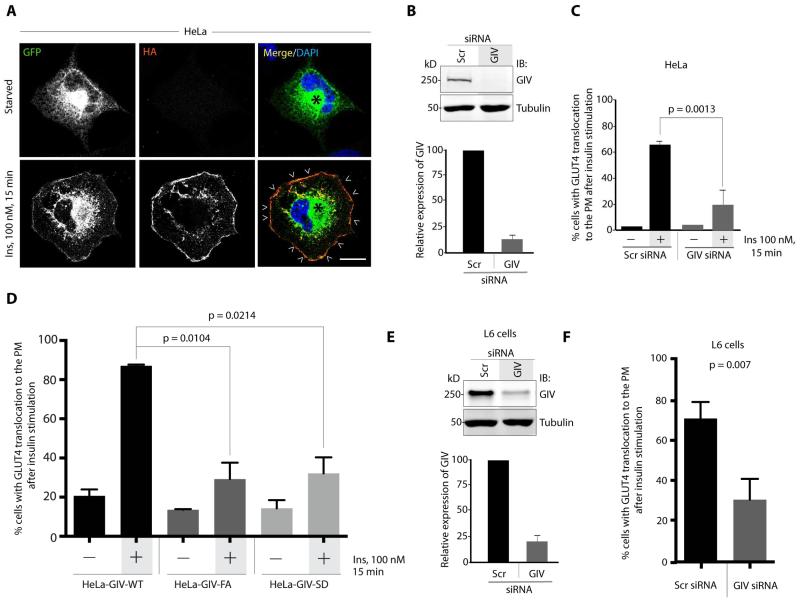
Because exocytosis of GLUT4 is enhanced by both the activation of G proteins [7] and the PI3K-Akt pathway [1], we asked if GIV and its GEF function regulates GLUT4 exocytosis and glucose uptake in response to insulin. We monitored insulin-triggered GLUT4 translocation to the PM using the well-characterized HA-GLUT4-GFP chimera in HeLa cells by confocal fluorescence microscopy, a reliable model system that has been widely used to study the trafficking of GLUT4 containing vesicles (GSVs; detected by GFP) [15]. In starved cells, GSVs are visualized in the cytosol, and insulin stimulation triggers their redistribution to the PM, where the exocytosed pool of the GLUT4 chimera is measured by indirect immunofluorescence of the HA tag (Figure 1A). Depletion of GIV (by ~80-85%; Figure 1B-C) reduced the efficiency of GLUT4 exocytosis ~3-fold, a defect that was rescued by stably expressed siRNA-resistant wild type GIV (GIV-WT), but not the GEF-deficient F1685A mutant of GIV (GIV-FA) which can neither bind, nor activate Gαi [9] (Figure 1D). These findings indicate that GIV is required for GLUT4 exocytosis and that its GEF domain is essential.
Figure 1. GIV’s GEF function is essential for GLUT4 exocytosis.
(A) Starved or insulin-stimulated HeLa cells expressing HA-GLUT4-GFP were fixed, stained with HA mAb and analyzed by confocal microscopy. Exocytosed GLUT4 at the PM (arrowheads) was detected by surface labeling with HA (red), whereas total GLUT4 was detected by GFP signal. Bar = 10 μm. (B) Top: Lysates of HeLa cells treated either with control (Scr) or with GIV siRNA were analyzed for GIV and tubulin by immunoblotting (IB). Bottom: Bar graph displays efficiency of GIV depletion. (C) Control (Scr) and GIV-depleted (GIV siRNA) HeLa cells were analyzed for insulin-triggered exocytosis of HA-GLUT4-GFP by confocal microcoscopy as in A. Bar graph displays % cells with exocytosed GLUT4. Error bars represent mean ± S.D. n = 3. (D) HeLa cells stably expressing siRNA-resistant GIV-WT, GIV-FA or GIV-SD were depleted of endogenous GIV by siRNA, serum starved and subsequently analyzed for exocytosis of HA-GLUT4-GFP after insulin stimulation as in A. Bar graph displays % cells with exocytosed GLUT4. Error bars represent mean ± S.D. n = 3. (E) Top: Lysates of L6 cells treated either with control (Scr) or with GIV siRNA were analyzed for GIV and tubulin by immunoblotting (IB). Bottom: Bar graph displays efficiency of GIV depletion. (F) Control (Scr) and GIV-depleted (GIV siRNA) L6 cells were analyzed for insulin-triggered exocytosis of HA-GLUT4-GFP by confocal microcoscopy as in A. Bar graph displays % cells with exocytosed GLUT4. Error bars represent mean ± S.D. n = 3.
Next we asked if phosphoinhibition of GIV’s GEF at Ser1689 by PKCθ [12] also inhibits GLUT4 exocytosis. Compared to controls, cells expressing the constitutively phosphoinhibited S1689D mutant of GIV (GIV-SD) were inefficient in GLUT4 exocytosis (Figure 1D), thereby pinpointing the inhibitory action of PKCθ on GIV-GEF as a key event that inhibits insulin-triggered exocytosis of this transporter. These findings are consistent with the fact that insulin-stimulated glucose uptake, as determined by a well-established fluorometric assay [16] was also impaired in both HeLa cells depleted of GIV or those expressing the GEF-deficient mutants (GIV-FA and GIV-SD) compared to control cells expressing GIV-WT [3]. Taken together, findings indicate that GIV’s GEF function is required for efficient GLUT4 exocytosis and glucose uptake and that its phosphoinhibition by PKCθ impairs both.
To translate the above findings into insulin-resistance models and interrogate the GIV-GEF-dependent pathway for its pathophysiologic relevance, next we switched to L6 rat skeletal myotubes. Our rationale for this choice was guided by the fact that although both adipocytes and skeletal muscles are sites for IR, full-length GIV is expressed more abundantly in skeletal muscles than in mature adipocytes [17]. Findings in L6 myotubes concurred with those in HeLa cells, in that GIV was essential for insulin-triggered GLUT4 exocytosis because GIV-depleted myotubes (~80% depletion; Figure 1E) impaired exocytosis of GLUT4 by 70%; Figure 1F; S1).
GIV associates with GSVs
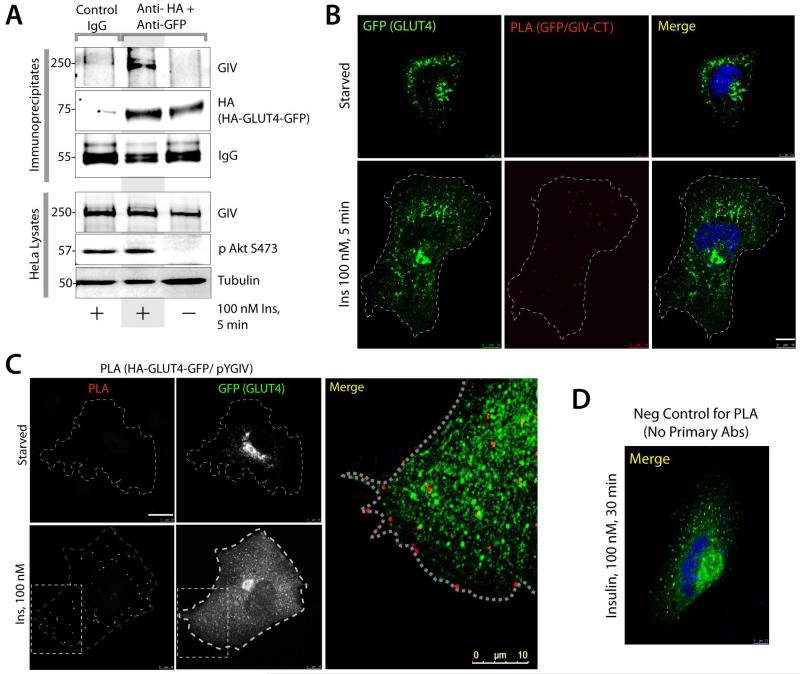
Because exocytosis of GLUT4 requires the recruitment of signaling proteins, like Akt and exocyst complexes onto GCVs and rapid remodeling of cortical actin [18], next we asked if GIV, a bona-fide Akt enhancer [11] and an actin remodeler [19] also associates with GSVs. Immunofluorescence studies showed partial colocalization between active GIV [identified using anti-pY1764-GIV, a site phosphorylated by InsRβ [11]] and GLUT4 at the PM exclusively after insulin stimulation (arrowheads, Figure S2). Immunoisolation of GSVs (in the absence of detergent) from cells transfected with HA-GLUT4-GFP showed that GIV associates with GSVs exclusively after insulin stimulation (Figure 2A). To confirm if GIV and GSVs interact at the PM, we performed proximity ligation assays (PLA) [20] to detect in situ GIV-GCV complexes in Cos7 cells transfected with HA-GLUT4-GFP. PLA signals were detected between HA-GLUT4-GFP and endogenous total GIV (Figure 2B) or active GIV[pY1764] (Figure 2C-D) in the cell periphery exclusively after ligand stimulation indicating that they interact [i.e., the maximum distance between the two is ≤ 30-40 nm [20]]. These results demonstrate that GIV can associates with GSVs in a ligand-dependent manner.
Figure 2. GIV associates with GSVs.
(A) Full length GIV is detected in immunoisolated GSVs exclusively after insulin stimulation. Immunoisolation of GSVs was carried out on homogenates (no detergent) of serum starved or insulin treated HeLa cells expressing HA-GLUT4-GFP using a mix of anti-HA and anti-GFP antibodies. Homogenates and immunoisolates were analyzed for GIV and HA-GLUT4-GFP by immunoblotting (IB). (B) GIV interacts with GSVs after insulin stimulation. Serum starved HeLa cells expressing HA-GLUT4-GFP were stimulated or not with insulin prior to fixation. Fixed cells were analyzed for interaction with GIV upon insulin stimulation by in situ PLA using rabbit GIV-CT and mouse anti-GFP. GFP signal shows GLUT4 vesicles. Red dots = interaction. Scale bar = 10 μm. (C) Serum starved and insulin stimulated HeLa cells expressing HA-GLUT4-GFP were analyzed for interaction between active GIV (pYGIV) and GSVs by in situ PLA using rabbit anti-pY1764-GIV and mouse anti-HA. GFP signal shows GLUT4 vesicles. Red dots = interaction. Bar = 10 μm. (D) HeLa cells incubated only with secondary antibodies during PLA do not show any false positive signals. HeLa cells expressing HA-GLUT4-GFP were incubated with anti-mouse and anti-rabbit secondary PLA antibodies as negative control. GFP signal shows GLUT4 vesicles in the cell periphery. Unlike Figure 2B-C, no red signal is seen. Scale bar = 7.5 μm.
GIV directly and constitutively binds exocyst complex subunit Exo-70
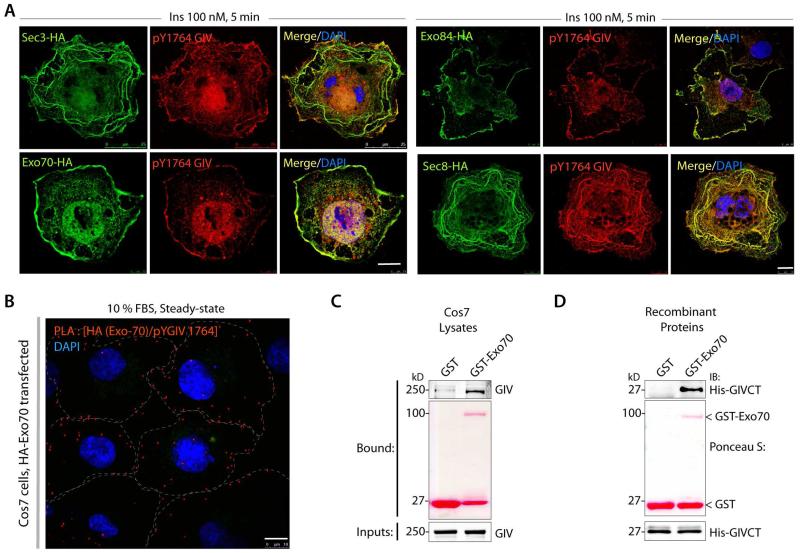
As to how GIV may associate with GSVs and assist in their exocytosis, we noted that a high-throughput mass spectrometry study [21] identified GIV as one of the unconfirmed interacting partners of exocyst complex protein, which are key players mediating exocytosis of GCVs [22]. Immunofluorescence studies revealed that upon insulin stimulation active GIV[pY1764] colocalized at the PM with most exocyst components tested, e.g., Sec3, Exo70, Exo84 and Sec8 (Figure 3A). PLA studies in Cos7 cells transfected with HA-Exo70 revealed that active GIV interacts with Exo70 (Figure 3B; S3A-B), a core subunit that is critical for the functional assembly of the exocyst complex [22]. This interaction was constitutive because PLA signals were equal in serum-starved cells after insulin stimulation and at steady-state. These results indicated that GIV may associate with exocyts complex, and perhaps Exo-70 subunit specifically, and that such interaction is constitutive (i.e., independent of ligand stimulation).
Figure 3. GIV colocalizes with the exocyst complex and directly binds the subunit Exo-70.
(A) Tyrosine phosphorylated GIV colocalizes with several subunits of the exocyst complex. Serum starved Cos7 cells expressing various subunits of the exocyst complex, as indicated were stimulated with insulin, fixed and subsequently stained for pY1764-GIV (red), HA (green) and DAPI/DNA (blue). Bar = 10 μm. (B) Cos7 cells expressing Exo70-HA were grown in 10% FBS (steady-state; right) and analyzed for interaction between GIV and Exo-70 by in situ PLA using rabbit anti-pY1764-GIV and mouse anti-HA. Red dots = interaction. Bar = 10 μm. (C-D) Pulldown assays were carried out using either Cos7 cell lysates (C) or recombinant His-GIV-CT (aa 1660-1870) (D) as source of GIV and GST or GST-Exo70 immobilized on glutathione beads. Inputs and bound proteins were analyzed by immunoblotting (IB) for GIV (left) or for His (His-GIV-CT, right). Equal loading of GST proteins was confirmed by Ponceau S stain.
Next we asked if GIV specifically binds the Exo-70 subunit of exocyst complex. Pulldown assays using either Cos7 lysates as the source of GIV and bacterially expressed and purified GST-tagged Exo-70 subunit showed that full length GIV can indeed bind Exo-70 (Figure 3C). Pulldown assays using recombinant GIV-CT and Exo-70 proteins confirmed that GIV’s C-terminus (aa 1660-1870) binds Exo70, demonstrating that the GIV:Exo-70 interaction we observe is direct, and that the C-terminal ~210 aa are sufficient for such interaction (Figure 3D).
GIV and its GEF function is required for the localization of Exo-70 to the plasma membrane
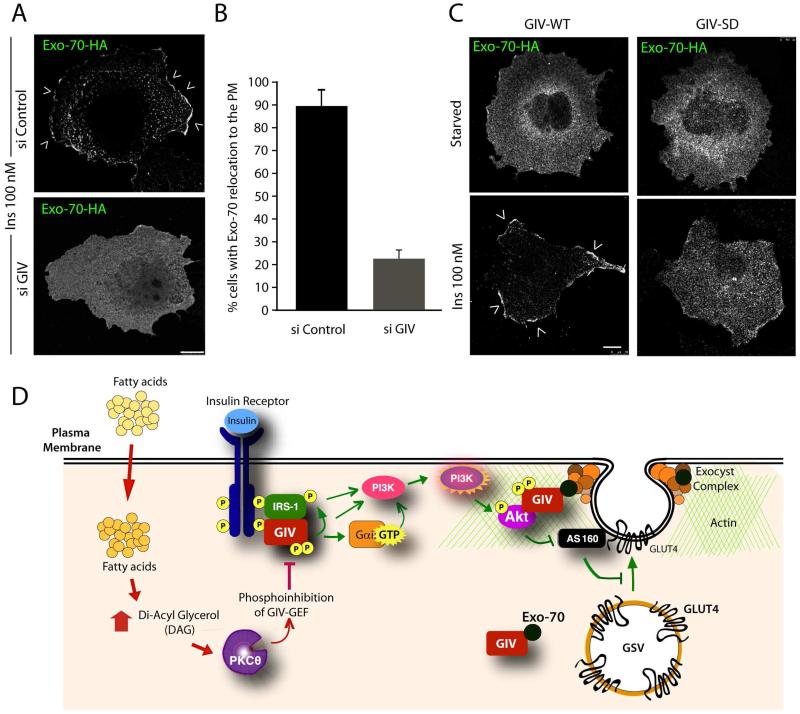
Because protein-protein interactions affect protein targeting/localization, next we asked if impaired glucose uptake we observed in the absence of GIV or a functional GEF motif in GIV [3] is also accompanied by defects in insulin-triggered association of Exo70 with the PM. To study this, we carried out immunofluorescence studies to monitor the relocation of HA-tagged Exo-70 from cytosol to the PM in Cos7 cells responding to insulin. These studies revealed that insulin stimulation of cells that are depleted of GIV or those that express the phosphoinhibited GIV-SD mutant, which are defective in GLUT4 exocytosis (Figure 1C-D) and glucose uptake [3], also have inefficient translocation of HA-Exo70 from the cytosol to the PM compared to controls (Figure 3A-C; S4). These results indicate that modulation of insulin signals by GIV’s GEF function [3] also affects the ligand-dependent association of Exo70 with the PM. Based on these results we propose that constitutive GIV-Exo70 complexes remain in cytosol in starved cells and associate with GCVs at the PM exclusively after insulin stimulation (see legend and working model; Figure 3D). Thus, GIV functionally interacts with several key downstream components of rapid insulin response (Akt, actin, GSVs, Exo70) and GIV’s GEF function is required for ensuring efficiency at various steps of GLUT4 exocytosis and glucose uptake in response to insulin.
DISCUSSION
The fundamental discovery in this work is that GIV and its GEF function plays a major role in linking signaling to membrane trafficking events, which ultimately coordinate efficient glucose uptake in response to insulin. Previous work [3] has established that physiologic insulin response is triggered by activation of GIV by tyrosine phosphorylation, GIV’s GEF function is turned “on” and Gαi is activated, metabolic insulin signaling is initiated through the InsR/IRS1/PI3K/Akt signaling axis, culminating in efficient uptake of glucose. In patients with IR, circulating free fatty-acids trigger the accumulation of diacyl glycerol (DAG) and activation of PKCθ in skeletal muscle, which in turn phosphorylates GIV’s GEF motif at S1689 and selectively turns “off” the GEF function. Consequently, Gαi remains inactive and a majority of the key elements of metabolic insulin signaling are suppressed, thereby inhibiting glucose uptake and resultant hyperglycemia. By reporting novel interactions of GIV in this work we have now provided a more complete picture as to how GIV-dependent insulin signaling events are relayed to the exocytic machinery via that ultimately coordinates GSV exocytosis and glucose uptake.
GIV’s GEF function integrates both trafficking and signaling components within the insulin response cascade
We previously demonstrated [3] that activation of Gαi by GIV impacts many tiers within the insulin cascade and that phosphoinhibition of GIV’s GEF function antagonizes them all. At the level of the receptor, activation of Gαi via GIV’s GEF motif is required for maximal autophosphorylation and activation of InsRβ and recruitment and phosphoactivation of its major substrate, IRS1, two upstream events in insulin signaling. At the immediate post-receptor level, we demonstrated that GIV directly and constitutively binds IRS1 [3] and enhances the recruitment of IRS1 to the ligand-activated receptors at the PM, triggers robust tyrosine phosphorylation at Y632 and Y941 on IRS1 and enhances the formation of IRS1-p85α(PI3K) complexes. All of these are key events implicated in metabolic insulin signaling via IRS1. Further downstream, the PI3K-Akt signaling pathway was maximally enhanced and the Rab-GAP AS160 was maximally phosphoinhibited only in the presence of an intact GIV-GEF. These downstream events are known to coordinately trigger docking of GSVs at the PM via activation of Rab proteins and their subsequent tethering to the PM in response to insulin [23]. Here we report two new interactions of GIV that may help GIV integrate signaling with membrane trafficking during GSV exocytosis. First, we found that GIV associates with GSVs, much like other proteins, e.g., Akt and AS160 that are also known to localize to that compartment and regulate GSV exocytosis [reviewed in [18, 24]]. Such association is not surprising because GSVs represent a specialized recycling endosomal system [25], and GIV has previously been shown to be associated with recycling endosomes [26]. Second, we demonstrate that GIV’s C-terminus directly binds the Exo-70 subunit and regulates its recruitment to the PM after insulin stimulation. Previous work has established that Exo-70 is recruited to the PM via activation of the small G protein TC10 [22] and that its primary role is to help tether GSVs to the PM for subsequent SNARE-mediated fusion [27]. It is possible that GIV’s ability to associate with GSVs and with Exo-70 serves to tether GSVs to the exocyst complexes at the PM. Because the actin cytoskeleton has also been described as a tether for GSVs [28], it is possible that the previously characterized role of GIV as a remodeler of the cortical actin cytoskeleton further aids in GSV exocytosis [13, 19]. We conclude that GIV interacts with and enhances key signaling and membrane trafficking events within the insulin response cascade, and in doing so, it exemplifies a molecular basis for the observed engagement between these events during insulin-triggered glucose uptake into cells.
In conclusion, we have defined activation of Gαi by GIV’s GEF function as a central node that coordinately enhances the physiologic insulin response by coordinating signaling events with membrane trafficking events. Because the GIV-node also serves as the point of convergence for the antagonistic actions of fatty acids and insulin sensitizers [3], selective modulation of this node emerges as a promising and precise strategy to regulate metabolic insulin response and glucose uptake in tissues responsive to insulin.
Supplementary Material
Highlights.
GIV-GEF is essential for insulin-triggered exocytosis of GLUT4-storage vesicles
GIV associates with GLUT4-storage vesicles exclusively after insulin stimulation.
GIV directly binds Exo-70 and enhances its recruitment to the plasma membrane.
GIV couples signaling and trafficking and coordinates metabolic insulin response.
Figure 4. GIV and its GEF function are required for the relocation of Exo-70 to the PM.
(A) Control (si Control) or GIV-depleted (si GIV) expressing Exo-70-HA were serum starved and subsequently stimulated with insulin for 10 min prior to fixation. Fixed cells were stained with HA (green; shown here in grayscale). Arrowheads = PM. Bar = 10 μm. (B) Bar graph displays the % cells in A in which Exo-70 was translocated to the PM. p = 0.0001. (C) Cos7 cells coexpressing Exo-70-HA and either GIV-WT-FLAG or GIV-SD-FLAG were serum starved and insulin-stimulated and then stained with HA (green; shown here in grayscale). FLAG (red) and DNA/DAPI (blue) are displayed in Figure S4. Arrowheads = PM. Bar = 10 μm. (D) Schematic summarizing our proposed model for how GIV interacts with several key upstream and downstream components of rapid insulin response (Akt, actin, GCVs, exo70) that are essential for efficient GLUT4 exocytosis and glucose uptake in response to insulin. Previous work [3] has shown that physiologic insulin response (pathways indicated in green on the right) requires insulin triggered formation of InsR-GIV complexes, followed by tyrosine phosphorylation of GIV (pY1764), and subsequent activation of Gαi via GIV’s GEF function. Consequently, metabolic insulin signaling is enhanced through the InsR/IRS1/PI3K/Akt pathway, which culminates in phosphoinhibition of RabGAP AS160. Findings in this work indicate that GIV associates constitutively with Exo-70 subunit of the exocyst complex, and that it associates also with GSVs exclusively after ligand stimulation. In doing so, GIV anatomically couples the signaling components (InsR, IRS1, Akt and PI3K) to the membrane trafficking components (AS160, GSVs, the exocyst complex) that regulate GSV exocytosis for rapid uptake of glucose. In IR (pathways shown in red on the left), circulating free fatty-acids trigger the accumulation of diacyl glycerol (DAG) and PKCθ is activated. PKCθ phosphorylates GIV at S1689 and turns “off” its GEF function. Consequently, Gαi remains inactive and the InsR/IRS1/PI3K/Akt/AS160 signaling cascade is suppressed, Exo-70 fails to translocate to the PM, and GSV exocytosis and glucose uptake are impaired.
ACKNOWLEDGMENTS
This work was funded by NIH (R01CA160911) and the Burroughs Wellcome Fund (CAMS award) to P.G. I.L-S was supported by a fellowship from the American Heart Association (AHA #14POST20050025) and G.S.M by the Doris Duke Charitable Foundation (DDCF Grant #: 2013073 to P.G.).
Abbreviations
- IR
Insulin resistance
- InsR
Insulin receptor
- GEF
Guanine-nucleotide Exchange Factor
- GSVs
GLUT4-storage vesicles
- IRS1
insulin receptor substrate 1
- Exo-70
Exocyst complex subunit of 70 kDa
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action, Nature reviews. Molecular cell biology. 2006;7:85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- [2].Miinea CP, Sano H, Kane S, Sano E, Fukuda M, Peranen J, Lane WS, Lienhard GE. AS160, the Akt substrate regulating GLUT4 translocation, has a functional Rab GTPase-activating protein domain. The Biochemical journal. 2005;391:87–93. doi: 10.1042/BJ20050887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ma G, Lopez-Sanchez I, Aznar N, Kalogriopoulos N, Midde K, Ciaraldi TP, Henry HR, Ghosh P. Activation of G proteins by GIV-GEF is a Pivot Point for Insulin Resistance and Sensitivity. Revised and Resubmitted, Mol. Biol of Cell. 2015 doi: 10.1091/mbc.E15-08-0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Song X, Zheng X, Malbon CC, Wang H. Galpha i2 enhances in vivo activation of and insulin signaling to GLUT4. The Journal of biological chemistry. 2001;276:34651–34658. doi: 10.1074/jbc.M105894200. [DOI] [PubMed] [Google Scholar]
- [5].Chen JF, Guo JH, Moxham CM, Wang HY, Malbon CC. Conditional, tissue-specific expression of Q205L G alpha i2 in vivo mimics insulin action. J Mol Med (Berl) 1997;75:283–289. doi: 10.1007/s001090050113. [DOI] [PubMed] [Google Scholar]
- [6].Moxham CM, Malbon CC. Insulin action impaired by deficiency of the G-protein subunit G ialpha2. Nature. 1996;379:840–844. doi: 10.1038/379840a0. [DOI] [PubMed] [Google Scholar]
- [7].Ciaraldi TP, Maisel A. Role of guanine nucleotide regulatory proteins in insulin stimulation of glucose transport in rat adipocytes. Influence of bacterial toxins, The Biochemical journal. 1989;264:389–396. doi: 10.1042/bj2640389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kanoh Y, Ishizuka T, Morita H, Ishizawa M, Miura A, Kajita K, Kimura M, Suzuki T, Sakuma H, Yasuda K. Effect of pertussis toxin on insulin-induced signal transduction in rat adipocytes and soleus muscles. Cellular signalling. 2000;12:223–232. doi: 10.1016/s0898-6568(99)00081-9. [DOI] [PubMed] [Google Scholar]
- [9].Garcia-Marcos M, Ghosh P, Farquhar MG. GIV is a nonreceptor GEF for G alpha i with a unique motif that regulates Akt signaling. Proc Natl Acad Sci U S A. 2009;106:3178–3183. doi: 10.1073/pnas.0900294106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lin C, Ear J, Midde K, Lopez-Sanchez I, Aznar N, Garcia-Marcos M, Kufareva I, Abagyan R, Ghosh P. Structural basis for activation of trimeric Gi proteins by multiple growth factor receptors via GIV/Girdin. Molecular biology of the cell. 2014 doi: 10.1091/mbc.E14-05-0978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lin C, Ear J, Pavlova Y, Mittal Y, Kufareva I, Ghassemian M, Abagyan R, Garcia-Marcos M, Ghosh P. Tyrosine phosphorylation of the Galpha-interacting protein GIV promotes activation of phosphoinositide 3-kinase during cell migration. Sci Signal. 2011;4:ra64. doi: 10.1126/scisignal.2002049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lopez-Sanchez I, Garcia-Marcos M, Mittal Y, Aznar N, Farquhar MG, Ghosh P. Protein kinase C-theta (PKCtheta) phosphorylates and inhibits the guanine exchange factor, GIV/Girdin. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:5510–5515. doi: 10.1073/pnas.1303392110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ghosh P, Beas AO, Bornheimer SJ, Garcia-Marcos M, Forry EP, Johannson C, Ear J, Jung BH, Cabrera B, Carethers JM, Farquhar MG. A G{alpha}i-GIV molecular complex binds epidermal growth factor receptor and determines whether cells migrate or proliferate. Molecular biology of the cell. 2010;21:2338–2354. doi: 10.1091/mbc.E10-01-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ghosh P, Garcia-Marcos M, Bornheimer SJ, Farquhar MG. Activation of Galphai3 triggers cell migration via regulation of GIV. The Journal of cell biology. 2008;182:381–393. doi: 10.1083/jcb.200712066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Dawson K, Aviles-Hernandez A, Cushman SW, Malide D. Insulin-regulated trafficking of dual-labeled glucose transporter 4 in primary rat adipose cells. Biochemical and biophysical research communications. 2001;287:445–454. doi: 10.1006/bbrc.2001.5620. [DOI] [PubMed] [Google Scholar]
- [16].Yamamoto N, Sato T, Kawasaki K, Murosaki S, Yamamoto Y. A nonradioisotope, enzymatic assay for 2-deoxyglucose uptake in L6 skeletal muscle cells cultured in a 96-well microplate. Analytical biochemistry. 2006;351:139–145. doi: 10.1016/j.ab.2005.12.011. [DOI] [PubMed] [Google Scholar]
- [17].Uhlen M, Oksvold P, Fagerberg L, Lundberg E, Jonasson K, Forsberg M, Zwahlen M, Kampf C, Wester K, Hober S, Wernerus H, Bjorling L, Ponten F. Towards a knowledge-based Human Protein Atlas. Nature biotechnology. 2010;28:1248–1250. doi: 10.1038/nbt1210-1248. [DOI] [PubMed] [Google Scholar]
- [18].Kupriyanova TA, Kandror KV. Akt-2 binds to Glut4-containing vesicles and phosphorylates their component proteins in response to insulin. The Journal of biological chemistry. 1999;274:1458–1464. doi: 10.1074/jbc.274.3.1458. [DOI] [PubMed] [Google Scholar]
- [19].Enomoto A, Murakami H, Asai N, Morone N, Watanabe T, Kawai K, Murakumo Y, Usukura J, Kaibuchi K, Takahashi M. Akt/PKB regulates actin organization and cell motility via Girdin/APE. Developmental cell. 2005;9:389–402. doi: 10.1016/j.devcel.2005.08.001. [DOI] [PubMed] [Google Scholar]
- [20].Soderberg O, Gullberg M, Jarvius M, Ridderstrale K, Leuchowius KJ, Jarvius J, Wester K, Hydbring P, Bahram F, Larsson LG, Landegren U. Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nature methods. 2006;3:995–1000. doi: 10.1038/nmeth947. [DOI] [PubMed] [Google Scholar]
- [21].Camargo LM, Collura V, Rain JC, Mizuguchi K, Hermjakob H, Kerrien S, Bonnert TP, Whiting PJ, Brandon NJ. Disrupted in Schizophrenia 1 Interactome: evidence for the close connectivity of risk genes and a potential synaptic basis for schizophrenia. Molecular psychiatry. 2007;12:74–86. doi: 10.1038/sj.mp.4001880. [DOI] [PubMed] [Google Scholar]
- [22].Inoue M, Chang L, Hwang J, Chiang SH, Saltiel AR. The exocyst complex is required for targeting of Glut4 to the plasma membrane by insulin. Nature. 2003;422:629–633. doi: 10.1038/nature01533. [DOI] [PubMed] [Google Scholar]
- [23].Bai L, Wang Y, Fan J, Chen Y, Ji W, Qu A, Xu P, James DE, Xu T. Dissecting multiple steps of GLUT4 trafficking and identifying the sites of insulin action. Cell Metab. 2007;5:47–57. doi: 10.1016/j.cmet.2006.11.013. [DOI] [PubMed] [Google Scholar]
- [24].Larance M, Ramm G, James DE. The GLUT4 code. Mol Endocrinol. 2008;22:226–233. doi: 10.1210/me.2007-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kandror KV, Pilch PF. Multiple endosomal recycling pathways in rat adipose cells. The Biochemical journal. 1998;331(Pt 3):829–835. doi: 10.1042/bj3310829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Simpson F, Martin S, Evans TM, Kerr M, James DE, Parton RG, Teasdale RD, Wicking C. A novel hook-related protein family and the characterization of hook-related protein 1. Traffic. 2005;6:442–458. doi: 10.1111/j.1600-0854.2005.00289.x. [DOI] [PubMed] [Google Scholar]
- [27].Bao Y, Lopez JA, James DE, Hunziker W. Snapin interacts with the Exo70 subunit of the exocyst and modulates GLUT4 trafficking. The Journal of biological chemistry. 2008;283:324–331. doi: 10.1074/jbc.M706873200. [DOI] [PubMed] [Google Scholar]
- [28].Stockli J, Fazakerley DJ, James DE. GLUT4 exocytosis. J Cell Sci. 2011;124:4147–4159. doi: 10.1242/jcs.097063. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.