Abstract
Alcohol consumption can be enhanced or moderated by sensitivity to its aversive and appetitive properties, including positive social outcomes. These differences emerge post-pubertally, suggesting a potential role of gonadal hormones. To determine the role of gonadal hormones in sensitivity to the social impairing and social context-related attenuations in the aversive effects of ethanol, prepubertal male and female rats were gonadectomized (GX) or sham (SH) operated on postnatal day (P)25, or left non-manipulated (NM). In adulthood (P70), rats were restrained for 90 minutes prior to challenge with 0.0 or 1.0 g/kg ethanol and social interaction (SI) testing. At P77, groups of 4 same-sex littermates from the same surgical condition were given access to a supersaccharin (SS) solution (3% sucrose, 0.125% saccharin), followed by an intraperitoneal injection of ethanol (0.0, 0.50, 1.0, 1.5 g/kg). Intakes of SS were examined 24 hours later for expression of conditioned taste aversions. Acute stress prior to SI testing increased frequency of play fighting in both sexes, whereas there were no GX effects on this measure, social investigation nor contact. GX, however, decreased baseline social preference (a social anxiety-like effect) in males, while inducing anxiolytic-like increases in baseline social preference in females. The social drinking test revealed that females developed ethanol conditioned taste aversions at a lower dose relative to males, regardless of surgical condition. These findings suggest a potential role for gonadal hormones in moderating social-anxiety like behaviors but not sensitivity to the social impairing effects of ethanol or ethanol’s aversive consequences in a social context.
Keywords: Ethanol, gonadal hormones, social interactions, stress, aversion, sex differences
1. Introduction
Men drink more frequently and more per occasion than females (WHO, 2014) and are twice as likely as women to be diagnosed with alcohol dependence or use disorders. Women however, have shorter latencies between onset of drinking and the development of alcohol use disorders (Greenfield et al., 2010). Interestingly, these sex differences in alcohol intake emerge post-pubertally with the development of sexually-dimorphic, adult-typical behaviors (Forbes and Dahl, 2010; Sisk and Foster, 2004). Age-and sex-related differences in ethanol sensitivity appear critical for understanding risk factors related to the development of alcohol use disorders, with for instance increased sensitivity to the rewarding or aversive properties of ethanol likely serving to promote or limit intake, respectively. Although some of these sex differences may be attributable to cultural and psychosocial factors (Foster et al., 2013; Wilsnack et al., 2000), there are also adult-typical sex differences in alcohol-related drug pharmacokinetics (Gandhi et al., 2004), immune responses (Kovacs and Messingham, 2002) and neural sensitivity (Devaud et al., 2003), suggesting that biological influences are likely as well.
Adult-typical patterns of alcohol intake and sensitivity emerge post-pubertally during adolescence in both males and females – i.e., after the reinstatement of activity in the hypothalamic-pituitary-gonadal axis (HPG) axis that initiates puberty and ultimately leads to sexual maturation (Reiter and Grumbach, 1982). Many of the key neural, physiological and behavioral characteristics seen during the adolescent transition are conserved across mammalian species (e.g. see Spear, 2000), hence supporting the use of animal models for study of age-, sex – and hormone-related differences that are difficult to study empirically in developing humans. For instance, not only human adolescents (Johnston et al., 2013), but also their rodent counterparts (see Spear and Varlinskaya, 2000 for reference and review) exhibit two- to three- fold greater alcohol intakes per occasion than do adults. Studies with adolescent rats have shown them to be less sensitive than adults to many of the sedative, motor impairing, aversive, and hangover effects of ethanol that presumably serve as feedback cues to moderate drinking (Brasser and Spear, 2002; Doremus et al., 2003; Varlinskaya and Spear, 2004; White et al., 2002). The approach of adulthood is not only associated with the dissipation of adolescent-typical ethanol sensitivities and intakes, but also the emergence of sex differences in these measures. In rats, characteristic sex differences include significantly greater ethanol intake and preference, as well as decreased sensitivity to the development of conditioned taste aversions in adult females relative to males (Vetter-O’Hagen et al., 2009a), although the latter effect varies with the number of pairings and the social context (Morales et al., 2014a).
Indeed, social context is a particularly important factor in studies with ethanol. Increased social facilitation is one of the many positive expectancies associated with alcohol consumption in humans, an effect particularly pronounced in adolescents (Christiansen et al., 1982; Kuntsche et al., 2005; Lee et al., 2007; Park and Grant, 2005; Read et al., 2004). In rodent studies as well, adolescents are uniquely sensitive to the social facilitating effects of ethanol (Varlinskaya and Spear, 2006) while conversely being relatively insensitive to the social impairing effects of ethanol that emerge at higher doses of ethanol (Varlinskaya and Spear, 2006). Both of these effects decline post-pubertally and are absent by adulthood (Varlinskaya and Spear, 2002). Adolescents are also especially susceptible to increased ethanol consumption for its perceived anxiolytic expectancies, especially in social situations (Wiers et al., 1997). Using a measure of social preference/avoidance in rats that compare the relative number of approaches to versus movement away from a partner as an index of social anxiety-like behavior, acute restraint stress immediately prior to social interaction testing was found to decrease social preference, with this anxiety-like effect reversed by ethanol in adolescents but not adults (Varlinskaya and Spear, 2012).
The emergence of sex differences in stress reactivity and ethanol sensitivity is thought to be moderated by pubertal rises in gonadal hormones. Anxiety disorders increase in frequency and severity during reproductive years and show a clear sex bias towards females (Reardon et al., 2009). These traits are affected by hormones, with females reporting greater levels of anxiety and panic states during premenstrual and postpartum-associated decreases in estrogens (Seeman, 1997). Given that sex differences typically emerge during adolescence and reach their full expression in adulthood, the potential role of pubertal rises in gonadal hormones has significant implications for understanding developmental periods of increased risk for the emergence of alcohol use disorders. Hence, we have used a simple animal model of adolescence in the rat to explore the role of gonadal hormones during puberty on the emergence of adult-and sex-typical ethanol responses, reasoning that gonadal hormones should play at least as important a role in these behaviors in rodents as in humans with their much greater social and environmental complexity.
In our prior work in this area, we have observed that the ethanol intake of gonadectomized (GX) males was similar to that of intact females, regardless of whether GX occurred pre-pubertally or in adulthood; GX in females, in contrast, had little impact on ethanol intake (Vetter-O’Hagen and Spear, 2011). The feminization of ethanol intake in males by GX was attenuated by testosterone, suggesting an activational role for testosterone in contributing to sex differences in ethanol intake (Vetter-O’Hagen et al., 2009b). A recent study by Torres et al., (2014) reported that GX in females eliminated ethanol conditioned place preference, suggesting a potential role for estradiol in mediating the rewarding properties of ethanol, although the effects of GX on ethanol intake was not explored. In contrast, in our work to date, we have been unable to detect notable effects of GX on various alcohol sensitivities thought to influence intake and hence potentially contribute to sex-dependent differences in ethanol intake (Morales et al., 2014b; Vetter-O’ Hagen and Spear, 2012), although the effects of stressors and social context on GX/ethanol interactions have yet to be explored. The purpose of this study, therefore, was to investigate sex differences in the role of pre-pubertal GX on interactions between ethanol and social stimuli via examining sensitivity to: a) the social impairing effects of ethanol following acute restraint stress; as well as b) the aversive effects of ethanol when conditioning/testing occurs within a social context.
2. Materials and methods
2.1 Subjects
Male and female Sprague-Dawley rats bred and reared in our colony at Binghamton University were used as experimental subjects (n = 288) and social partners (n = 288). All animals were housed in a temperature-controlled (22 °C) vivarium maintained on a 12:12 h light-dark cycle (lights on at 0700h) with ad libitum access to food (Purina Rat Chow, Lowell, MA) and water. Litters were culled to 8–10, maintaining relatively equal sex ratios whenever possible. On P21, pups were weaned and pair-housed with a littermate of the same sex assigned to the same surgical condition. At all times, animals were treated in accordance with guidelines for animal care established by the National Institute of Health under protocols approved by the Binghamton University Institutional Animal Care and Use Committee.
2.2 Design
The impact of pre-pubertal gonadectomy on social impairing effects of ethanol following acute stress in adult males (n=144) and females (n=144) was examined using a 3 surgical condition (GX, sham-operated [SH], non-manipulated [NM]) × 2 stress condition (acute restraint stress, no stress) × 2 dose challenge (saline, 1.0 g/kg ethanol) factorial design, with 12 subjects from each sex placed into each experimental condition. The same animals were later used to assess ethanol’s aversive effects in a social drinking context using a conditioned taste aversion (CTA) paradigm. For this phase of the experiment, a 3 surgical condition (GX, SH, NM) × 4 ethanol dose (0.0, 0.5, 1.0, 1.5 g/kg) factorial design was used, with 12 subjects from each sex placed into each experimental condition. Animals from the earlier social test conditions defined by the 2 (restraint stress vs. no stress) × 2 (saline, 1.0 g/kg ethanol) design were re-assigned to the 4 dose conditions of the test, with prior condition counterbalanced.
2.3 Surgery
On P25, animals were anesthetized using isoflurane (3.5 % initially) and maintained at surgical levels of anesthesia throughout the surgery via nose cone supplementation (3% repeated as necessary). For castration of males, each testis was removed, a suture made in each tunic and in the inguinal ring (to prevent possible herniation), and the incision closed with Vetbond tissue adhesive (3M, St. Paul, MN). For ovariectomies, an incision was made on the dorsal side of the animal, caudal to the last rib and through the skin perpendicular to the midline. On each side of this incision, an opening was made in the muscle wall via blunt dissection, with the oviduct on each side sutured proximal to the ovary, the ovary excised, and the muscle wall and entry incision sutured. For SH surgeries, animals were anesthetized and an incision was made; however, reproductive tissue was not manipulated nor were the gonads removed. At the time of surgery and then again later in the day, animals received a subcutaneous injection of the anti-inflammatory agent, carprofen (5 mg/kg); this two-injection procedure was repeated the following day. Following surgery, animals were given a subcutaneous injection of saline (1.50 cc/animal) and were returned to their home cages, with a wire-mesh divider used to separate each housing pair for a recovery period of approximately 72 hours. On the fourth day, the divider was removed, and subjects were left undisturbed until testing in early adulthood (i.e., P69).
2.4 Drugs
For both social interaction and CTA testing, ethanol was administered intraperitoneally (i.p.) as a 12.6% v/v solution in 0.9% saline. Saline was administered as an equivalent volume to the highest ethanol dose used in that test procedure. All solutions were administered at room temperature.
2.5 Social interaction (SI) testing
Each social testing apparatus was made of clear Plexiglas (Binghamton Plate Glass, Binghamton, NY) and measured 45 × 30 × 30 cm. The apparatus was divided on the long axis into two equal sized compartments by a clear Plexiglas wall containing an aperture (9 × 7 cm) that allowed movement between the compartments. Two adjacent apparati were separated by an opaque wall that allowed testing of two experimental animals and their corresponding social partners, with little to no interference between the pairs. A white noise generator was located in the room to attenuate any extraneous sound. Before each test, the walls/floors were wiped clean with a 6.0% hydrogen peroxide solution, allowed to dry and fresh shavings were added.
Social test sessions were recorded by an overhead camera (Sony Handycam) and analyzed at a later date. The behaviors of the experimental animal were scored in terms of frequency of play (pouncing or playful nape attack; chasing and pinning), social investigation (sniffing any body part of the partner), and contact (crawling over or under the partner and social grooming). Number of cross-overs toward and away from the partner were also determined and summed as an index of activity in the social test situation. An index of social motivation was also determined from these data using a social preference/avoidance coefficient [coefficient % = [(# of compartment crossovers toward the partner) − (# of compartment crossovers away from the partner)] / [(# of crossovers towards the partner) + (# crossovers away from the partner) × 100]. Positive coefficients indicated social preference, with negative coefficients reflecting social avoidance.
One day before SI testing (P69), experimental animals were weighed and individually habituated to the testing apparatus for 30 min before being returned to their home cages. The following day (P70), experimental animals were weighed and marked with a vertical line to differentiate them from their social partners. For the pre-test condition, cage mates were randomly assigned to the no stress or acute restraint stress condition. Animals in the no stress group were left undisturbed in their home cage prior to testing. Subjects in the restraint stress group were restrained for 90 min in a round slotted Plexiglas cylinder (20.5 cm length × 7.0 cm diameter for females and 23.0 length × 8.0 cm diameter for males), with a plunger used to gently but snuggly restrain the animal. Immediately thereafter (or upon removal from the home cage for the no stress group), each cage mate was injected with either saline or ethanol and placed individually into the test apparatus for 30 min. Following this period, an unfamiliar, non-manipulated social partner (matched by weight and age) was placed in the apparatus for a 10 min test session. Immediately after the test session, blood samples were collected from the experimental animals via tail nick for later analysis of blood ethanol concentrations (BECs). Animals were returned to their home cage until further testing one week later (P77).
2.6 Social CTA test procedure
On P77, four littermates of the same age, sex and surgical condition were weighed, marked with distinct patterns to differentiate them from each other and habituated together to the testing environment (i.e. white opaque plastic cage, [50.8 × 40.64 × 20.32cm) for 30 min before being returned to their home cages. On conditioning day (P78), littermates were again weighed, marked, and placed into the testing environment for 30 min with access to two bottles of a novel sweetened supersaccharin solution (3.0% sucrose and 0.125 % saccharin in water). Immediately thereafter, bottles were removed and each animal was injected intraperitoneally with one of the four ethanol challenge doses, with all 4 doses represented in each test squad of littermates. After injection, animals were returned to their home cages and left undisturbed for 24 hours. On test day (P79), littermates were again weighed, marked and placed into the testing environment for 30 min with access to two bottles of the sweetened supersaccharin solution. Pre and post-test bottle weights were recorded on conditioning and test days to determine total consumption of the 4 test animals in the social drinking situation. To parse consumption among individual animals, conditioning and test sessions were recorded by an overhead camera (Sony Handycam) and analyzed at a later date for total time (in seconds) each animal spent drinking. Videos were analyzed be experimenters blind to the experimental conditions (≥ 95% interexperimenter agreement). Immediately after the test session, animals were decapitated and blood was collected for assay of testosterone, estradiol, progesterone and CORT. See figure 1 for timeline).
Figure 1.
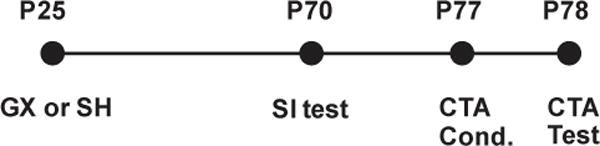
Timeline of behavioral tests. At P25, animals received GX or SH surgery followed by social interaction (SI) test on P70. One week later (P77), animals underwent consecutive CTA conditioning (Cond.) day and test day.
2.7 Blood Analysis
For blood ethanol concentrations (BECs), whole blood samples were stored and maintained at −80 °C until time of assay. Analyses were determined by head-space gas chromatography, using a Hewlett Packard (HP) 5890 series II Gas Chromatograph, a HP 7694E Headspace Sampler, and HP Chemstation software (see Vetter O’Hagan and Spear, 2012).
Plasma collected from whole blood was stored and maintained at −80 °C until time of assay. Testosterone, estradiol and progesterone levels were used to confirm removal of the gonadal tissue in males and females, respectively. For analysis of these hormones, plasma samples were thawed and assessed via radioimmunoassay (RIA) using 125I double antibody kits from MP Biomedicals (Solon, OH) and procedures in standard use in our laboratory (see Vetter-O’Hagan and Spear, 2012). Levels of the stress-related hormone, CORT were also assessed by RIA using competitive binding tritium-based kits obtained from MP Biomedicals, INC (see Vettter O’Hagan and Spear, 2012).
2.8 Data analysis
Social interaction data were analyzed using a 2 sex × 3 surgical condition (GX, SH, NM) × 2 stress condition (restraint stress, no stress) × 2 dose challenge (saline, 1.0 g/kg ethanol) factorial analysis of variance (ANOVA). Data were checked for outliers and any experimental animal with BECs ±2 standard deviations from the mean were removed prior to statistical analysis of the social interaction data. A total of 5 animals were removed from this analysis with no more than 2 animals excluded per group, leaving a final sample size of 10–12/group. Due to significant sex differences in these data, patterns of interactions among surgical manipulation, ethanol and the stressor were examined separately by sex. Prior to the ANOVAs, Levene’s tests showed violations in homogeneity of variance (HV) for play fighting (in males and females), social investigation (females only) and contact (males and females). HV in play fighting was improved by transformation, and was analyzed using a log10(n+1) transformation and social investigation was improved by X2 transformation. Contact data could not be improved and were analyzed without transformation, with data interpretation qualified accordingly. Significant effects and interactions in these and other analyses in the study were explored further using Fisher’s post-hoc test.
CTA intake data for each animal were converted from time spent drinking into ml [(time spent drinking per rat/time spent drinking by group) × total ml consumed by the group]. Preliminary analyses of the CTA data revealed no residual effects of the differential manipulation associated with the social interaction testing that was conducted one week before CTA training/testing (i.e. the ethanol administration and/or acute stressor challenge). Due to significant sex differences across groups in conditioning day intake, data were analyzed using separate ANOVAs by sex using a 3 surgical condition (GX, SH, NM) × 4 ethanol dose (0.0, 0.5, 1.0, 1.5 g/kg) factorial ANOVA. Before analysis, test day data were transformed into percent baseline (conditioning day) intake using the formula: ((test day ml/conditioning day ml)*100). Experimental animals with ≤ 1.0 ml intake on conditioning day were removed prior to statistical analysis. Across all groups, 20 animals were excluded for this reason, resulting in final sample sizes of 10–12/group. Significant effects and interactions were explored further using Fisher’s post-hoc tests.
Body weight and BECs were analyzed by factorial ANOVAs and hormone levels were analyzed for main effects of surgical condition. Testosterone data were analyzed using nonparametric statistics (Kruskal-Wallis) due to the marked non-homogeneity of variance in these data.
3. Results
3.1 Body weight
The ANOVA of adult body weights at P70 revealed only a main effect of surgical condition in both males [F(2, 132) = 6.83, p < .01] and females [F(2, 135) = 235.42, p < .001]. Similar findings emerged at P77, [F(2, 130) = 10.26, p < .001] and [F(2, 118) = 197.56, p < .001], respectively. Gonadectomy in males slightly but significantly reduced body weights compared to NM and SH groups, whereas this manipulation conversely increased body weight in females (Table 1).
Table 1.
Body weights in adulthood prior to social interaction testing and social drinking context.
| Sex | Surgical Condition | P70 | P77 |
|---|---|---|---|
| Males | NM | 404.6 ± 5.2 | 426.4 ± 5.6 |
| SH | 392.3 ± 4.5 | 410.6 ± 5.1 | |
| GX | 376.8 ± 4.5↓ | 393.2 ± 4.7↓ | |
|
| |||
| Females | NM | 245.3 ± 3.1 | 255.6 ± 3.6 |
| SH | 240.8 ± 2.7 | 247.6 ± 2.8 | |
| GX | 330.1 ± 3.7↑ | 341.5 ± 4.2↑ | |
Down arrow (↓) indicate significant GX body weight decrease relative to sex matched NM and SH controls; up arrow (↑) indicates significant increase from both control groups, all p < .01.
3.2 Blood ethanol concentrations (BECs)
Initial analysis of the BEC data at P70 revealed significantly higher BEC’s for males compared to females [F(1,259) = 12.78, p < .05]. The ANOVA of BECs in males revealed a main effect of stress condition in males with acute restraint stress decreasing BECs (regardless of surgical condition), F(2,64) = 5.88, p < .05. In contrast, female BECs differed only as a function of surgical condition [F(2, 66) = 3.49, p < .05], with GX females having higher BECs compared to NM and SH females (see Table 2). Analysis of the BEC data in conjunction with the social interaction data using Pearson’s product moment correlation coefficients revealed significant negative correlations of BECs and frequency of social play fighting (r = −.56, p < .05), social investigation (r = −.39, p < .05) and social contact (r = −.42, p < .05). However, BECs and the social preference/avoidance coefficient were not significantly correlated (r = −0.06, p > .05).
Table 2.
Blood ethanol concentrations (mg/dl) ± SEM.
| Sex | Surgical Condition | No Restraint | Restraint |
|---|---|---|---|
| Males | NM | 81.4 ± 11.5 | 67.9 ± 18.1+ |
| SH | 71.7 ± 7.3 | 75.2 ± 13.6+ | |
| GX | 86.1 ± 15.2 | 68.6 ± 14.9+ | |
|
| |||
| Females | NM | 66.5 ± 13.4 | 59.1 ± 11.5 |
| SH | 67.4 ± 11.0 | 62.8 ± 12.4 | |
| GX | 76.4 ± 17.9* | 69.3 ± 16.0* | |
= significant stress effects (data collapsed across all surgical conditions;
= significant effects of surgical conditions (data collapsed across all stress conditions).
3.3 Hormone levels
As expected, surgical condition influenced gonadal hormone levels in both males and females. A non-parametric Kruskal-Wallis test revealed that gonadectomy in males significantly reduced testosterone levels, (H(2) = 79.01, p < .00001. In females, GX significantly reduced estradiol [F(2, 138) = 6.76, p < .001] and progesterone levels [F(2, 138) = 49.29, p < .001]. Progesterone levels were not affected by the surgical manipulation in males (Table 3).
Table 3.
Hormone levels ± SEM.
| Sex | Surgical Condition | Testosterone (ng/ml) | Progesterone (ng/ml) | CORT (ng/ml) |
|---|---|---|---|---|
| Males | NM | 1.91 ± 0.15 | 9.61 ± 1.10 | 222.50 ± 15.35 |
| SH | 4.31 ± 2.21 | 8.55 ± 0.84 | 198.13 ± 18.62 | |
| GX | 0.00 ± 0.00* | 8.98 ± 0.96 | 292.26 ± 22.26* | |
|
| ||||
| Females | Surgical Condition | Estradiol (pg/ml) | Progesterone (ng/ml) | CORT (ng/ml) |
|
| ||||
| NM | 110.11 ± 3.50 | 52.86 ± 4.02 | 400.79 ± 39.34 | |
| SH | 115.54 ± 4.76 | 51.76 ± 4.13 | 315.67 ± 33.94 | |
| GX | 96.50 ± 3.19* | 11.11 ± 0.88* | 343.26 ± 22.27 | |
Asterisks (*) indicate significant hormone level differences relative to the corresponding NM and SH controls, p < .05.
Analysis of the CORT data collected immediately following the CTA test day revealed a main effect of surgical condition in males [F(2,130) = 6.30, p < .01], with gonadectomy increasing CORT levels relative to NM and SH males (see Table 3). These data in males were not affected by any of the conditions of the previous SI test. In females, CORT levels did not vary as a function of surgical condition or challenge dose. However, among females, past history of acute restraint stress prior to the SI test was associated with elevated CORT levels immediately following the CTA testing in the social context (prior acute restraint on P70: 423.47 ng/dl ± 25.38; no stress on P70: 297.5 ng/ml ± 22.98).
3.4 Social impairing effects of ethanol following acute stress in adulthood
No effects of gonadectomy on social play were evident in either males or females (see Figure 2). Analysis of social play in males revealed main significant effect of stress condition [F(1,131) = 5.40, p < .05] and ethanol challenge [F(1,131) = 97.54, p < .001]. Acute restraint stress increased social play behaviors in males while the reverse was true for acute ethanol challenge (see inserts to Figure 2). Similar significant main effects for stress condition [F(1,127) = 4.60, p < .05] and ethanol challenge [F(1,127) = 0.63, p < .001] were seen in females (see Figure 2).
Figure 2.
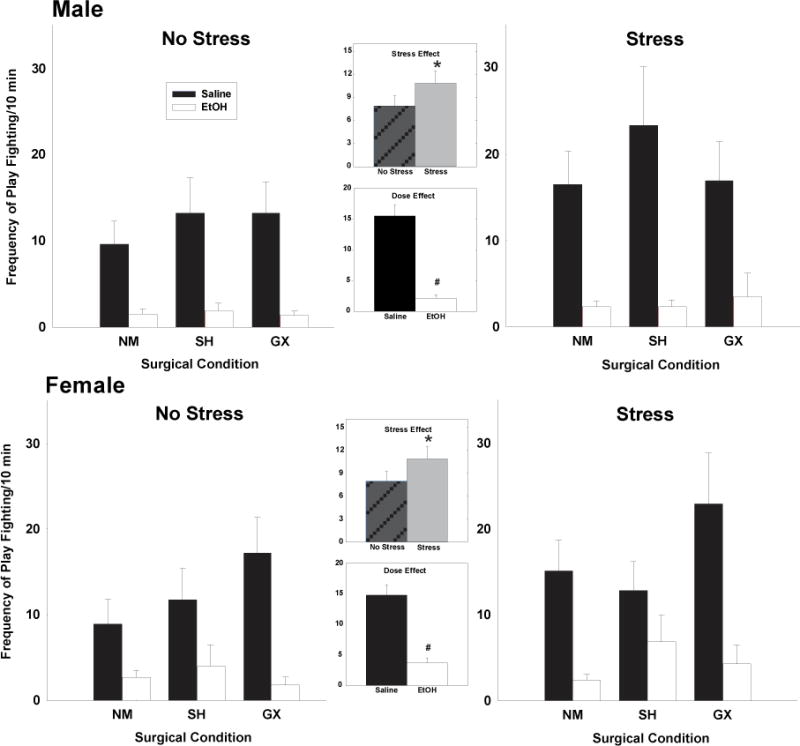
Effects of surgical and pre-test conditions on play fighting in males and females. Inserts display the main effects of stress condition and ethanol dose. Asterisks (*) indicate significant difference from no stress control (p < .05); hashtags (#) indicate significant ethanol dose difference from saline controls (p < .001).
Social investigation and social contact differed significantly only as a function of ethanol challenge in both males and females, with acute ethanol challenge decreasing frequencies of social investigation [F(1,131) = 37.73, p < .001]; [F(1,127) = 15.48, p < .001] and social contact [F(1,131) = 33.80, p < .001]; [F(1,127) = 22.51, p < .001] (data not shown).
In males, analysis of the social preference/avoidance coefficient revealed a main effect of surgical condition [F(2,131) = 4.66, p < .01] and a significant 3-way interaction of surgical condition, stress and ethanol, [F(2,131) = 3.96, p < .05]. Generally, GX males had lower levels of social preference than NM and SH males. As can be seen in figure 3, this GX effect was significant in saline challenged males under both stress conditions when compared to NM males, and in non-stressed males relative to their SH counterparts as well. Upon acute ethanol challenge, a GX-associated decrease in social preference relative to SH and NM animals was only seen in stressed animals. Among females, GX effects differed only as a function of stressor condition (a significant surgical condition × stress condition interaction: [F(2,127) = 4.28, p < .05], with no main or interaction effects involving acute ethanol challenge. In marked contrast to males, non-stressed GX females had greater social preference scores than their NM counterparts, with no effects of GX seen in stressed females. Among NM females, the acute stressor increased social preference, an effect that was not evident in SH and GX females.
Figure 3.
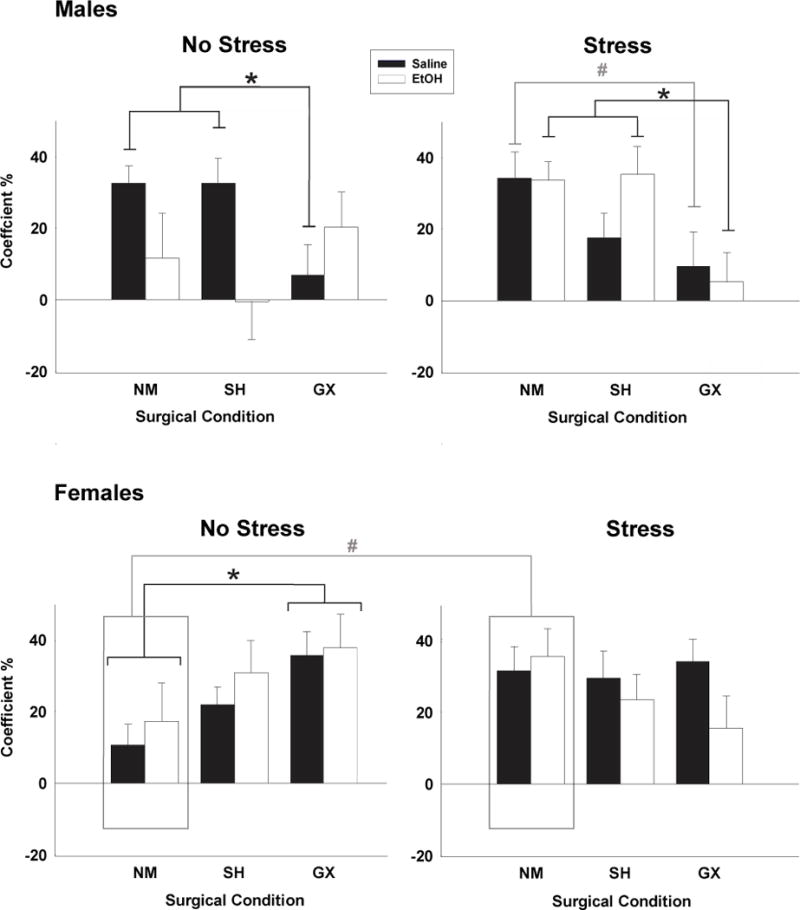
Effect of surgical condition on preference coefficient in males and females. Asterisks (*) indicate a significant difference between GX males and NM and SH controls in the no stress saline condition and the stress ethanol condition (p < .05); hashtags (#) indicate a significant difference in GX males compared to NM males in the stress ethanol condition (p < .001). Females displayed a main effect of stress condition from NM females as indicated by boxed bars (p < .05). (@) indicates significant differences in GX females from NM females in the no stress condition, collapsed across dose (p < .05).
Total number of crosses (used as an index of locomotor activity) during the social testing period did not vary as a function of surgical condition or acute restraint stress in males or females. However, there was a main effect of ethanol challenge, with acute challenge significantly decreasing locomotor activity in males [F(1,131) = 41.18, p < .001: saline: 29.9±1.2; EtOH: 19.6±1.0] and females [F(1,127) = 30.69, p < .001: saline: 31.9±1.4; EtOH: 22.1±1.0].
3.5 Social conditioned taste aversion
In males, baseline intake of the supersaccharin solution on conditioning day differed as a function of surgical condition [F(2,126) = 3.54, p < .05], with GX animals exhibiting significantly greater levels of intake (5.2 ml ± 0.3) than NM (4.1 ml ± 0.3) and SH (4.0 ml ± 0.3) males. Analysis of test day intake in the males as a percentage of baseline intake revealed only a dose effect [F(3,126) = 13.56, p < .001] with significant decreases in supersaccharin intake relative to control animals emerging following the 1.0 and 1.5 g/kg ethanol doses (see Figure 4). In females, analysis of baseline intake on conditioning day revealed no significant differences. On test day, again only a significant dose effect was evident [F(3,118) = 9.50, p < .001], with females showing lower supersaccharin consumption at all doses of ethanol relative to saline. These data are shown in Figure 4.
Figure 4.
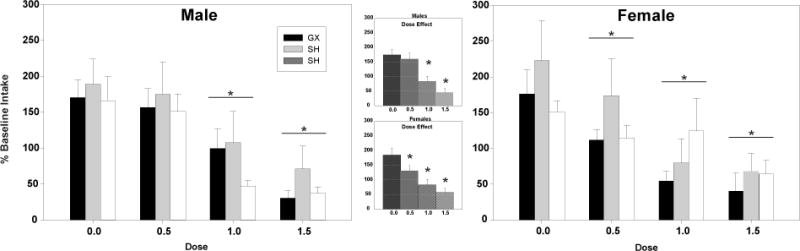
Effect of surgical condition and conditioning dose on conditioned taste aversions. Data shown as percent from baseline intake. Asterisks (*) indicate a main effect of dose in males and females relative to saline controls (p < .001). Inserts indicate main effect of dose collapsed across surgical conditions.
4. Discussion
In our prior work, we have observed few effects of pre-pubertal or adult GX on ethanol responsiveness in either male or female rats (see Varlinskaya et al., 2013). Given that males and females differ notably in their responses to stressors (Kirschbaum et al., 1992; Kudielka and Kirschbaum, 2005; Shors et al., 2001; Solomon and Herman, 2009) and the impact of a social setting on ethanol sensitivity (Douglas et al., 2004; Morales et al., 2014b; Varlinskaya et al., 2015), the present study assessed whether pre-pubertal GX would influence ethanol/stress interactions, or responsiveness to ethanol in a social setting. Again, we found little impact of GX on ethanol sensitivity in animals of either sex under these circumstances. Although ineffective in altering ethanol sensitivity per se, GX exerted pronounced sex-specific baseline effects on social preference/avoidance. Other notable sex differences were evident, with the acute stressor increasing social preference in non-manipulated females (but not males), and females developing ethanol CTA at a lower dose than males under these social test circumstances.
The present study revealed sex-dependent GX effects on social anxiety but not on ethanol responsiveness following acute challenge. Among males, GX increased social anxiety-like behaviors (indexed via decreases in the social preference/avoidance coefficient) regardless of stressor condition, whereas GX conversely exerted an anxiolytic-like effect in non-stressed females, increasing their social preference. These sex-specific GX effects are unlikely to be related to alterations in general activity, as analysis of the total cross-over data yielded no main effect or interaction(s) involving these variables. The current preference/avoidance coefficient data are reminiscent of those reported by Morales et al. (2014) in males and females gonadectomized pre-pubertally and tested socially in late adolescence (P49). Thus the increase in social-anxiety like behavior seen after GX in males and decrease evident in GX females are not only apparent post-pubertally but extend well into adulthood. These sex-specific GX effects implicate the presence or absence of testosterone and estradiol in attenuating social anxiety in males and facilitating social anxiety in females, respectively. Gonadal hormones also have comparable influences on physiological stress reactivity, with testosterone inhibiting and estrogens enhancing HPA axis reactivity (Lund et al., 2004; Viau et al., 2005). Overall, females show more rapid and robust CORT/HPA axis responses following stress challenge and increased latency to return to baseline (Kudielka and Kirschbaum, 2005), characteristics often associated with affective disorders such as depression (Barden, 2004; Young and Ribeiro, 2006) and social phobia (Condren et al., 2002).
Indeed, human studies have found that during adolescence (when gonadal hormones are rising in both males and females), females show increasing symptoms of generalized anxiety disorders over time whereas males show developmental decreases in symptoms of generalized anxiety disorders (William et al., 2008) and overanxious symptoms (Cohen et al., 1993). Moreover, adolescent females are more likely to be diagnosed with an anxiety disorder, have significantly more anxiety symptoms, and develop anxiety disorders at a faster rate than do males, even after controlling for various psychosocial factors (Lewinsohn et al., 1998). Although the adolescent period represents a heightened risk phase, hormonal fluctuations during estrous/menstrual cycle, pregnancy and menopause also affect the prevalence of affective disorders (Altemus, 2006). Thus effects of gonadal hormones on anxious states likely reflect an activational rather than a pubertally-associated organizational impact of these hormones. Investigation of the effects of GX and hormone replacement would address this possibility directly.
In contrast to the pronounced sex-specific effects of GX on baseline social anxiety, GX had no impact on baseline social activity or following ethanol challenge in the presence or absence of acute stress. The literature on GX and social interaction is varied, with some citing pronounced decreases in social activity following pre but not post-pubertal gonadectomy in males (Primus and Kellogg, 1990) and others citing altered microstructure of social behaviors in adult males and females regardless of surgical timing (Vetter-O’ Hagen and Spear, 2012). Less is known about the effects of GX and social interaction following ethanol challenge. Previous work in our lab has shown that the dose of ethanol (1.0 g/kg) used in this study suppresses social behavior (e.g. play fighting) (Varlinskaya and Spear, 2006) and is on the threshold for increasing social anxiety, sometimes significantly suppressing social preference (Varlinskaya and Spear, 2012) and other times not (Varlinskaya et al., 2014). The former but not the latter effect was evident in the current study. It is possible that use of a broader dose-response curve may have revealed GX-related alterations in ethanol effects on social behavior, although a similar lack of GX effects on social behavior (albeit in the absence of stress challenge) was seen when assessing low, moderate and high doses of ethanol in late adolescence (Morales et al., 2014b).
Although GX had no impact on the social consequences of ethanol following acute restraint stress, the stress challenge itself altered social behavior. This stress-induced increase in social play was evident in both males and females and was not dependent on gonadal status. In contrast, a notable sex difference of stressor exposure was observed in the social preference data, with the acute stressor increasing social preference in non-manipulated females (but not SH and GX females), a stress effect that was not evident in males. Although unexpected, this stress-induced increase is reminiscent of other evidence in females showing stress-induced decreases in anxiety-like behaviors in non-social anxiety tasks such as the open field (Wilson et al., 2004) and elevated plus maze (Doremus-Fitzwater et al., 2009; Verma et al., 2010). In the current study, stressed animals were not only restrained but socially isolated during the restraint period, whereas subjects in the non-stress condition were not isolated from cage mates prior to social interaction. Hence, the stress-induced increase in social play seen in both males and females could reflect either pre-test social isolation or the restraint per se. Repeated social deprivation has been previously reported to increase social play of both male and female adults (Doremus-Fitzwater et al., 2009).
The preferential increases in social play but not social investigation or contact implicate alternative systems in mediating stress and drug reward interactions on these social behaviors. Drugs such as morphine that affect the opioid system, selectively increase social play behaviors not but social behaviors unrelated to play (e.g. social grooming or contact) (Vanderschuren et al., 1995b). Studies examining the role of specific opioid receptor subtypes in regulating social behaviors implicate ɥ opioid receptor (MOR) activation in increasing social play behaviors while ƙ opioid receptor (KOR) activation antagonizes the effects of MOR (Vanderschuren et al., 1995a) and produce states of dysphoria (Wee and Koob, 2010). Given that acute restraint stress can increase endogenous opioid activity (Bruchas et al., 2010) it is possible that the acute stress challenge in the current study may have enhanced the rewarding aspects of social play through MOR activation. Yet, although gonadal hormones do exert significant modulatory effects on central opioid systems, some studies have found changes in brain KOR but not MOR affinity following ovariectomy and ovariectomy with hormone replacement (Gordon and Soliman, 1996). Likewise, no differences in mu-opioid mRNA expression in the preoptic area or arcuate nucleus were reported following ovariectomy and ovariectomy with estradiol replacement (Petersen and LaFlamme, 1997).
Blood ethanol concentrations were significantly increased in GX females compared to control females given a comparable dose. Previous studies have not found significant differences in BECs (Vetter-O’ Hagen and Spear, 2012) or brain ethanol concentrations (Morales et al., 2014b) between GX and control females. However, drug bioavailability and distribution can be influenced by the presence of estrogen. Estrous cycles in gonadally intact females were not assessed in the current study and hence it is possible that cycle timing among the non-manipulated and sham females at blood collection could have contributed to the significant effects of GX observed here. BECs were significantly correlated with some but not all social behaviors. Social play, investigation and contact were negatively correlated with BECs. However, BECs were not significantly correlated with social preference data, the measure which demonstrated robust sex-dependent GX effects on baseline social anxiety-like behaviors.
In the conditioned taste aversion test, GX had no effect on sensitivity to the aversive properties of ethanol when male and females were conditioned and tested in a social context. A similar lack of effect of GX was evident in females when conditioned and tested alone (Morales and Spear, 2013). Males showed GX-dependent increases in acquisition of conditioned taste aversions (Chambers et al., 1981; Morales and Spear, 2013), effects not seen in the socially conditioned males in this study. Although the results of the current study indicate that circulating levels of gonadal hormones are not responsible for the acquisition of sex-dependent differences in sensitivity to the aversive effects of ethanol, it is possible that GX-dependent effects in males and females might have emerged over multiple testing sessions or in rates of extinction, as these parameters have been found to be significantly influenced by gonadal hormones (Chambers, 1980; Chambers et al., 1981; Morales and Spear, 2013; Weinberg et al., 1982; Yuan and Chambers, 1999).
Despite the lack of GX effects on ethanol conditioned taste aversions, gonadally independent sex differences were apparent. Females showed significantly enhanced sensitivity to the aversive effects of ethanol than males, showing CTA at all doses whereas males exhibited CTA after only moderate and high doses of ethanol. It is unclear why females would be more sensitive to ethanol under these test circumstances than males given that previous work in our laboratories and others has found females to be as sensitive or even more sensitive than males to the production of conditioned taste aversions to ethanol (Morales et al., 2014), LiCl (Chambers et al., 1981) and cocaine (Busse et al., 2005). The possibility that social testing conditions influenced this enhanced sensitivity is unlikely as our lab has reported decreased sensitivity in adult females tested in a similar social context (Morales et al., 2014a). A more likely explanation is differences in testing parameters. Animals in the Morales study were not manipulated prior to CTA training (in contrast to the surgical manipulations during adolescence and prior SI testing in adulthood in the present study). Although statistical analysis revealed no effect of prior stress challenge on CTA testing in the present study, the CORT samples taken immediately after the CTA test session showed evidence of increased HPA axis reactivity in females who had been previously stressed. Therefore, prior stress may have primed neural/physiological responses during CTA testing.
Taken together, these results show that the presence or absence of gonadal hormones has a significant impact on baseline levels of social anxiety-like behaviors in males and females, with the presence of gonadal hormones increasing social anxiety-like responding in females while decreasing it in males. This supports known human data on sex differences in the onset, frequency and severity of pathological anxiety disorders which begin in reproductive years and are exacerbated by hormonal fluctuations in females (Reardon et al., 2009; Seeman, 1997). Although mood/affective disorders are highly correlated with problem drinking, we did not find conclusive evidence to implicate gonadal hormones in moderating ethanol sensitivities that could contribute to increased ethanol intake. Acute stress also did little to alter the social consequences of ethanol, despite clinical and preclinical literature suggesting otherwise (Lynch et al., 2002). Given the lack of effect of gonadal status on the social impairing effects of ethanol, further investigation into alternative neural and physiological systems underlying sex-dependent effects is needed.
Highlights.
GX decreased social preference in stressed and non-stressed males
GX increased baseline social preference in females
Acute restraint stress increased social play in males and females
Females developed conditioned taste aversions at a lower dose than males
Acknowledgments
The work presented in this manuscript was funded by R01-AA017355 to Linda P. Spear.
Abbreviations
- CTA
conditioned taste aversion
- GX
gonadectomy
- NM
non-manipulated
- SH
sham
- SS
supersaccharin solution
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barden N. Implication of the hypothalamic–pituitary–adrenal axis in the physiopathology of depression. Journal of Psychiatry and Neuroscience. 2004;29(3):185. [PMC free article] [PubMed] [Google Scholar]
- Brasser SM, Spear NE. Physiological and behavioral effects of acute ethanol hangover in juvenile, adolescent, and adult rats. Behavioral neuroscience. 2002;116(2):305. doi: 10.1037//0735-7044.116.2.305. [DOI] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Chavkin C. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain research. 2010;1314:44–55. doi: 10.1016/j.brainres.2009.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse GD, Freeman KB, Riley AL. The interaction of sex and route of drug administration in cocaine-induced conditioned taste aversions. Pharmacology Biochemistry and Behavior. 2005;81(4):814–820. doi: 10.1016/j.pbb.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Chambers KC. Progesterone, estradiol, testosterone and dihydrotestosterone: Effects on rate of extinction of a conditioned taste aversion in rats. Physiology & behavior. 1980;24(6):1061–1065. doi: 10.1016/0031-9384(80)90048-7. [DOI] [PubMed] [Google Scholar]
- Chambers KC, Sengstake CB, Yoder RL, Thornton JE. Sexually dimorphic acquisition of a conditioned taste aversion in rats: effects of gonadectomy, testosterone replacement and water deprivation. Physiology & behavior. 1981;27(1):83–88. doi: 10.1016/0031-9384(81)90303-6. [DOI] [PubMed] [Google Scholar]
- Christiansen BA, Goldman MS, Inn A. Development of alcohol-related expectancies in adolescents: Separating pharmacological from social-learning influences. Journal of consulting and Clinical Psychology. 1982;50(3):336. doi: 10.1037//0022-006x.50.3.336. [DOI] [PubMed] [Google Scholar]
- Cohen P, Cohen J, Kasen S, Velez CN, Hartmark C, Johnson J, Rojas M, Brook J, Streuning EL. An Epidemiological Study of Disorders in Late Childhood and Adolescence—I. Age‐and Gender‐Specific Prevalence. Journal of Child Psychology and Psychiatry. 1993;34(6):851–867. doi: 10.1111/j.1469-7610.1993.tb01094.x. [DOI] [PubMed] [Google Scholar]
- Condren RM, O’Neill A, Ryan MCM, Barrett P, Thakore JH. HPA axis response to a psychological stressor in generalised social phobia. Psychoneuroendocrinology. 2002;27(6):693–703. doi: 10.1016/s0306-4530(01)00070-1. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Alele P, Chadda R. Sex differences in the central nervous system actions of ethanol. Critical Reviews in Neurobiology. 2003;15(1) doi: 10.1615/critrevneurobiol.v15.i1.20. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Social and non-social anxiety in adolescent and adult rats after repeated restraint. Physiology & behavior. 2009;97(3):484–494. doi: 10.1016/j.physbeh.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Varlinskaya EI, Spear LP. Anxiogenic effects during withdrawal from acute ethanol in adolescent and adult rats. Pharmacology Biochemistry and Behavior. 2003;75(2):411–418. doi: 10.1016/s0091-3057(03)00134-5. [DOI] [PubMed] [Google Scholar]
- Douglas LA, Varlinskaya EI, Spear LP. Rewarding properties of social interactions in adolescent and adult male and female rats: impact of social versus isolate housing of subjects and partners. Developmental Psychobiology. 2004;45(3):153–162. doi: 10.1002/dev.20025. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Dahl RE. Pubertal development and behavior: hormonal activation of social and motivational tendencies. Brain and cognition. 2010;72(1):66–72. doi: 10.1016/j.bandc.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster DW, Young CM, Steers M-LN, Quist MC, Bryan JL, Neighbors C. Tears in your beer: Gender differences in coping drinking motives, depressive symptoms and drinking. International journal of mental health and addiction. 2013:1–17. doi: 10.1007/s11469-014-9504-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi M, Aweeka F, Greenblatt RM, Blaschke TF. Sex differences in pharmacokinetics and pharmacodynamics. Annu Rev Pharmacol Toxicol. 2004;44:499–523. doi: 10.1146/annurev.pharmtox.44.101802.121453. [DOI] [PubMed] [Google Scholar]
- Gordon FT, Soliman MR. The effects of estradiol and progesterone on pain sensitivity and brain opioid receptors in ovariectomized rats. Hormones and behavior. 1996;30(3):244–250. doi: 10.1006/hbeh.1996.0029. [DOI] [PubMed] [Google Scholar]
- Greenfield SF, Back SE, Lawson K, Brady KT. Substance abuse in women. Psychiatric Clinics of North America. 2010;33(2):339–355. doi: 10.1016/j.psc.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national survey results on drug use, 1975–2012: Volume II, College Students and Adults. Age. 2013:19–50. [Google Scholar]
- Kirschbaum C, Wust S, Hellhammer D. Consistent sex differences in cortisol responses to psychological stress. Psychosomatic medicine. 1992;54(6):648–657. doi: 10.1097/00006842-199211000-00004. [DOI] [PubMed] [Google Scholar]
- Kovacs EJ, Messingham KA. Influence of alcohol and gender on immune response. Alcohol Research and Health. 2002;26(4):257–263. [PMC free article] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biological psychology. 2005;69(1):113–132. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Kuntsche E, Knibbe R, Gmel G, Engels R. Why do young people drink? A review of drinking motives. Clinical psychology review. 2005;25(7):841–861. doi: 10.1016/j.cpr.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Lee CM, Geisner IM, Lewis MA, Neighbors C, Larimer ME. Social motives and the interaction between descriptive and injunctive norms in college student drinking. Journal of studies on alcohol and drugs. 2007;68(5):714. doi: 10.15288/jsad.2007.68.714. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Gotlib IH, Lewinsohn M, Seeley JR, Allen NB. Gender differences in anxiety disorders and anxiety symptoms in adolescents. Journal of abnormal psychology. 1998;107(1):109. doi: 10.1037//0021-843x.107.1.109. [DOI] [PubMed] [Google Scholar]
- Lund T, Munson D, Haldy M, Handa R. Androgen Inhibits, While Oestrogen Enhances, Restraint-Induced Activation of Neuropeptide Neurones in the Paraventricular Nucleus of the Hypothalamus. Journal of neuroendocrinology. 2004;16(3):272–278. doi: 10.1111/j.0953-8194.2004.01167.x. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology. 2002;164(2):121–137. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- Morales M, Schatz KC, Anderson RI, Spear LP, Varlinskaya EI. Conditioned taste aversion to ethanol in a social context: Impact of age and sex. Behavioural brain research. 2014a;261:323–327. doi: 10.1016/j.bbr.2013.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M, Spear LP. Differences in sensitivity to ethanol-induced conditioned taste aversions emerge after pre-or post-pubertal gonadectomy in male and female rats. Behavioural brain research. 2013;240:69–75. doi: 10.1016/j.bbr.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M, Varlinskaya EI, Spear LP. Pre-pubertal gonadectomy and the social consequences of acute ethanol in adolescent male and female rats. Hormones and behavior. 2014b doi: 10.1016/j.yhbeh.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CL, Grant C. Determinants of positive and negative consequences of alcohol consumption in college students: Alcohol use, gender, and psychological characteristics. Addictive behaviors. 2005;30(4):755–765. doi: 10.1016/j.addbeh.2004.08.021. [DOI] [PubMed] [Google Scholar]
- Petersen SL, LaFlamme KD. Progesterone increases levels of μ-opioid receptor mRNA in the preoptic area and arcuate nucleus of ovariectomized, estradiol-treated female rats. Molecular brain research. 1997;52(1):32–37. doi: 10.1016/s0169-328x(97)00194-0. [DOI] [PubMed] [Google Scholar]
- Primus RJ, Kellogg CK. Gonadal hormones during puberty organize environment-related social interaction in the male rat. Hormones and behavior. 1990;24(3):311–323. doi: 10.1016/0018-506x(90)90012-m. [DOI] [PubMed] [Google Scholar]
- Read JP, Wood MD, Lejuez C, Palfai TP, Slack M. Gender, alcohol consumption, and differing alcohol expectancy dimensions in college drinkers. Experimental and clinical psychopharmacology. 2004;12(4):298. doi: 10.1037/1064-1297.12.4.298. [DOI] [PubMed] [Google Scholar]
- Reardon LE, Leen-Feldner EW, Hayward C. A critical review of the empirical literature on the relation between anxiety and puberty. Clinical psychology review. 2009;29(1):1–23. doi: 10.1016/j.cpr.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter E, Grumbach M. Neuroendocrine control mechanisms and the onset of puberty. Annual Review of Physiology. 1982;44(1):595–613. doi: 10.1146/annurev.ph.44.030182.003115. [DOI] [PubMed] [Google Scholar]
- Seeman MV. Psychopathology in women and men: focus on female hormones. American Journal of Psychiatry. 1997;154(12):1641–1647. doi: 10.1176/ajp.154.12.1641. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Chua C, Falduto J. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. The Journal of neuroscience. 2001;21(16):6292–6297. doi: 10.1523/JNEUROSCI.21-16-06292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nature neuroscience. 2004;7(10):1040–1047. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- Solomon MB, Herman JP. Sex differences in psychopathology: of gonads, adrenals and mental illness. Physiology & behavior. 2009;97(2):250–258. doi: 10.1016/j.physbeh.2009.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Niesink RJ, Spruijt BM, Van Ree JM. µ-and κ-opioid receptor-meiated opioid effects on social play in juvenile rats. European journal of pharmacology. 1995a;276(3):257–266. doi: 10.1016/0014-2999(95)00040-r. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Spruijt BM, Van Ree JM, Niesink RJM. Effects of morphine on different aspects of social play in juvenile rats. Psychopharmacology. 1995b;117(2):225–231. doi: 10.1007/BF02245191. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Acute effects of ethanol on social behavior of adolescent and adult rats: role of familiarity of the test situation. Alcoholism: Clinical and Experimental Research. 2002;26(10):1502–1511. doi: 10.1097/01.ALC.0000034033.95701.E3. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Acute Ethanol Withdrawal (Hangover) and Social Behavior in Adolescent and Adult Male and Female Sprague†Dawley Rats. Alcoholism: Clinical and Experimental Research. 2004;28(1):40–50. doi: 10.1097/01.ALC.0000108655.51087.DF. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Differences in the social consequences of ethanol emerge during the course of adolescence in rats: Social facilitation, social inhibition, and anxiolysis. Developmental Psychobiology. 2006;48(2):146–161. doi: 10.1002/dev.20124. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Increases in anxiety-like behavior induced by acute stress are reversed by ethanol in adolescent but not adult rats. Pharmacology Biochemistry and Behavior. 2012;100(3):440–450. doi: 10.1016/j.pbb.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Truxell E, Spear LP. Chronic intermittent ethanol exposure during adolescence: effects on social behavior and ethanol sensitivity in adulthood. Alcohol. 2014 doi: 10.1016/j.alcohol.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Truxell EM, Spear LP. Ethanol Intake Under Social Circumstances or Alone in Sprague-Dawley Rats: Impact of Age, Sex, Social Activity, and Social Anxiety-Like Behavior. Alcoholism: Clinical and Experimental Research. 2015;39(1):117–125. doi: 10.1111/acer.12604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma P, Hellemans KG, Choi FY, Yu W, Weinberg J. Circadian phase and sex effects on depressive/anxiety-like behaviors and HPA axis responses to acute stress. Physiology & behavior. 2010;99(3):276–285. doi: 10.1016/j.physbeh.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter-O’ Hagen CS, Spear LP. The effects of gonadectomy on sex-and age-typical responses to novelty and ethanol-induced social inhibition in adult male and female Sprague-Dawley rats. Behavioural brain research. 2012;227(1):224–232. doi: 10.1016/j.bbr.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter-O’Hagen C, Varlinskaya E, Spear L. Sex differences in ethanol intake and sensitivity to aversive effects during adolescence and adulthood. Alcohol and Alcoholism. 2009a;7 doi: 10.1093/alcalc/agp048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter-O’Hagen CS, Sanders KW, Spear LP. Evidence for suppressant effects of testosterone on sex-typical ethanol intake in male Sprague-Dawley rats. Behavioural brain research. 2009b;224(2):403–407. doi: 10.1016/j.bbr.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter-O’Hagen CS, Spear LP. The Effects of Gonadectomy on Age and Sex Typical Patterns of Ethanol Consumption in Sprague Dawley Rats. Alcoholism: Clinical and Experimental Research. 2011;35(11):2039–2049. doi: 10.1111/j.1530-0277.2011.01555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viau V, Bingham B, Davis J, Lee P, Wong M. Gender and puberty interact on the stress-induced activation of parvocellular neurosecretory neurons and corticotropin-releasing hormone messenger ribonucleic acid expression in the rat. Endocrinology. 2005;146(1):137–146. doi: 10.1210/en.2004-0846. [DOI] [PubMed] [Google Scholar]
- Wee S, Koob GF. The role of the dynorphin–κ opioid system in the reinforcing effects of drugs of abuse. Psychopharmacology. 2010;210(2):121–135. doi: 10.1007/s00213-010-1825-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg J, Gunnar MR, Brett LP, Gonzalez CA, Levine S. Sex differences in biobehavioral responses to conflict in a taste aversion paradigm. Physiology & behavior. 1982;29(2):201–210. doi: 10.1016/0031-9384(82)90004-x. [DOI] [PubMed] [Google Scholar]
- White AM, Truesdale MC, Bae JG, Ahmad S, Wilson WA, Best PJ, Swartzwelder HS. Differential effects of ethanol on motor coordination in adolescent and adult rats. Pharmacology Biochemistry and Behavior. 2002;73(3):673–677. doi: 10.1016/s0091-3057(02)00860-2. [DOI] [PubMed] [Google Scholar]
- Wiers RW, Hoogeveen KJ, Sergeant JA, Gunning WB. High - and low - dose alcohol -related expectancies and the differential associations with drinking in male and female adolescents and young adults. Addiction. 1997;92(7):871–888. [PubMed] [Google Scholar]
- William H, Raaijmakers Q, Muris P, Meeus W. Developmental trajectories of adolescent anxiety disorder symptoms: A 5-year prospective community study. Journal of the American Academy of Child & Adolescent Psychiatry. 2008;47(5):556–564. doi: 10.1097/CHI.0b013e3181676583. [DOI] [PubMed] [Google Scholar]
- Wilsnack RW, Vogeltanz ND, Wilsnack SC, Harris TR. Gender differences in alcohol consumption and adverse drinking consequences: cross†cultural patterns. Addiction. 2000;95(2):251–265. doi: 10.1046/j.1360-0443.2000.95225112.x. [DOI] [PubMed] [Google Scholar]
- Wilson MA, Burghardt PR, Ford KA, Wilkinson MB, Primeaux SD. Anxiolytic effects of diazepam and ethanol in two behavioral models: comparison of males and females. Pharmacology Biochemistry and Behavior. 2004;78(3):445–458. doi: 10.1016/j.pbb.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Young EA, Ribeiro SC. Sex differences in the ACTH response to 24H metyrapone in depression. Brain research. 2006;1126(1):148–155. doi: 10.1016/j.brainres.2006.05.053. [DOI] [PubMed] [Google Scholar]
- Yuan DL, Chambers KC. Estradiol accelerates extinction of a conditioned taste aversion in female and male rats. Hormones and behavior. 1999;36(1):1–16. doi: 10.1006/hbeh.1999.1520. [DOI] [PubMed] [Google Scholar]


