Abstract
Drug seeking is maintained by encounters with drug-associated cues, and disrupting retrieval of these drug-cue associations would reduce the risk of relapse. Retrieval of cocaine-associated memories is dependent on β-adrenergic receptor (β-AR) activation, and blockade of these receptors induces a persistent retrieval deficit. Whether retrieval of cocaine-associated memory is mediated by a specific β-AR subtype, however, remains unclear. Using a cocaine conditioned place preference (CPP) procedure, we examined whether retrieval of a cocaine CPP memory is mediated collectively by β1- and β2-ARs, or by one of these β-AR subtypes alone. We show that co-blockade of β1- and β2-ARs abolished CPP expression on that and subsequent drug-free CPP tests, resulting in a long-lasting retrieval deficit that prevented subsequent cocaine-induced reinstatement. To dissociate the necessity of either β1- or β2-ARs alone, we administered subtype-specific antagonists prior to retrieval. Administration of a β1-AR antagonist before the initial CPP trial dose-dependently reduced expression of a CPP on that and subsequent drug-free trials as compared to vehicle administration. In contrast, administration of a β2-AR antagonist had no effect on initial CPP expression, although the highest dose reduced subsequent CPP expression. Importantly, either β1- or β2-AR blockade prior to an initial retrieval trial prevented subsequent cocaine-induced reinstatement. Our findings indicate that the β1-AR subtype mediates retrieval of a cocaine CPP, and that acutely blocking either β1- or β2-ARs can prevent subsequent cocaine-induced reinstatement. Thus, β-AR antagonists, particularly β1-ARs antagonists, could serve as adjuncts for addiction therapies to prevent retrieval of drug-associated memories and provide protection against relapse.
Keywords: noradrenergic beta-receptor; norepinephrine; betaxolol; ICI 118, 551; memory retrieval; cocaine-induced reinstatement; relapse; drug abuse
1. Introduction
Drug-seeking behavior is maintained by encounters with drug-associated cues, as these cues can evoke craving, relapse, or withdrawal [1–3]. Drug-associated cue presentation results in the retrieval of memories associated with drug experience. Previously, we have shown that administration of non-specific β-adrenergic receptor (β-AR) antagonists persistently disrupts retrieval of a cocaine-associated memory and prevents subsequent cocaine-induced reinstatement [4]. Furthermore, we have demonstrated that non-specific β-AR blockade in the prelimbic medial prefrontal cortex (PL-mPFC) or dorsal hippocampus (dHipp) also persistently blocks retrieval of a cocaine-associated memory and prevents subsequent cocaine-induced reinstatement [5,6]. Whether retrieval is mediated by a specific β-AR subtype, however, remains unclear.
The most abundant and widely studied β-AR subtypes in the brain are the β1-AR and β2-AR. Activation of either subtype results in stimulation of adenylyl cyclase [7] but differences in receptor efficacy and distribution have been found. For example, the non-selective β-AR agonist, isoproterenol, stimulates greater β2-AR-mediated adenylyl cyclase activity than that mediated by β1-AR, despite isoproterenol having equal affinity for these β-AR subtypes [8]. However, β1-ARs have been found in much higher concentration than β2-ARs within forebrain structures such as the cerebral cortex, caudate, hippocampus and amygdala [9]. Additionally, subtype-specific activation or blockade of β1- or β2-ARs has been shown to have differential effects in several memory tasks [10–13]. Importantly, systemic β2-AR blockade prior to a test for cocaine conditioned place preference (CPP) did not affect expression of the CPP, suggesting that these receptors do not mediate retrieval of cocaine-associated memory [10]. However, additional tests were not conducted to determine whether subsequent CPP expression was affected. Thus, whether one or both β-AR subtypes mediate drug-associated memory retrieval, and whether blockade of either subtype persistently disrupts cocaine-associated memory retrieval remains to be determined.
Here we assessed the necessity of conjoint or individual activation of β1-ARs and β2-ARs for retrieval of a cocaine-associated memory using the CPP procedure. Following conditioning, we examined the effects of β1-AR and/or β2-AR blockade on CPP memory retrieval by systemically administering the specific β1-AR antagonist betaxolol and/or the specific β2-AR antagonist ICI 118,551 prior to the initial CPP test trial. Rats were then exposed to daily drug-free CPP tests to determine whether blocking either β-AR resulted in a long-lasting retrieval deficit. We also determined whether specific β-AR subtype blockade would provide long-lasting protection against cocaine-induced reinstatement. Our results demonstrate that selective blockade of β1-ARs induces a persistent retrieval deficit.
2. Materials and methods
2.1 Subjects
Male Long-Evans rats (Harlan Laboratories, Madison, WI) weighing 250–275 g were individually housed in clear plastic cages. Rats were maintained on a 14 h light/10 h dark cycle (lights on at 7am) and had unlimited access to both water and standard laboratory rat chow (Harlan Laboratories). Rats were weighed and handled daily. All experimental protocols were approved by the Institutional Animal Care and Use Committee at the University of Wisconsin-Milwaukee in accordance with National Institutes of Health guidelines.
2.2 Place preference apparatus
A three-chamber apparatus was used for testing and conditioning in which two larger chambers (13″ × 9″ × 11.5″) were separated by a center chamber (6″ × 7″ × 11.5″) as described previously [4]. One of the larger chambers had white walls and mesh wire flooring, whereas the other had a black wall and a gold-grated flooring. The center chamber had aluminum flooring. During conditioning, rats were isolated within the conditioning chambers. During baseline and post-conditioning CPP tests, rats had access to the entire apparatus. Time was recorded during CPP tests with four infrared photobeams located in the conditioning chambers.
2.3 Drugs
Cocaine HCl (National Institute on Drug Abuse) was dissolved in sterile 0.9% saline at a concentration of 10 mg/ml, and administered at a dose of 10 mg/kg (i.p.) for all experiments. The β1-AR antagonist betaxolol and the β2-AR antagonist ICI 118,551 were purchased from Tocris Bioscience (Minneapolis, MN), and were dissolved in sterile 0.9% saline. Betaxolol and ICI 118,551 are commonly used to dissociate β1- and β2-ARs [14–16] and doses were chosen based on previous studies [10,17].
2.4 Conditioning and testing
Behavioral testing and conditioning were conducted as previously described [4]. Briefly, baseline preferences were determined by placing the rats in the center chamber of a 3-chamber apparatus with free access to all chambers for 15 min. Overall, rats showed no preference for either of the larger conditioning chambers during this baseline trial, although less time was spent in the smaller center chamber. ANOVA revealed a significant effect of chamber (F2,164 = 216.92, p<0.001), and post hoc tests revealed that rats spent less time in the center chamber than either of the larger chambers (p<0.05) and an equivalent amount of time in the larger chambers (p>0.05). Following this baseline test, an unbiased conditioning procedure was used in which rats were randomly assigned to receive cocaine or saline in one of the two distinct conditioning chambers in a pseudorandom and counterbalanced fashion over 8 d. Injections of saline or cocaine were given on alternating days immediately before 20-min conditioning sessions during which the rats were confined to their respective chambers. Following conditioning, rats were tested repeatedly with daily CPP trials in which they had free access to the entire apparatus for 15 min. A CPP was determined when rats spent significantly more time in the previously cocaine-paired than the saline-paired chamber. Rats received additional drug-free trials until they no longer expressed a CPP for at least two consecutive days, demonstrating extinction of the CPP.
We first investigated the effect of co-injecting 20 mg/kg of betaxolol with 8 mg/kg of ICI 118,551 (Bet20+ICI8; n = 12) or 1 ml/kg of saline vehicle (n = 12) 30 minutes prior to an initial CPP trial on CPP expression across trials. To determine the effects of β-AR subtype antagonists on CPP memory retrieval, rats were administered (i.p.) one of the following drugs 30 min prior to the initial CPP trial: 3 mg/kg of betaxolol (Bet3; n = 12), 20 mg/kg of betaxolol (Bet20; n = 12), 4 mg/kg of ICI 118,551 (ICI4; n = 12), 8 mg/kg of ICI 118,551 (ICI8; n = 12), or 1 ml/kg of saline vehicle (n = 12). Following the initial CPP trial, rats were given daily drug-free trials to determine whether betaxolol or ICI 118,551 treatment induced a persistent effect.
2.5 Reinstatement
To examine whether betaxolol, ICI 118,551, or co-injection of betaxolol with ICI 118,551 treatment during initial CPP trials would prevent later cocaine-induced reinstatement after extinction, the day after the final CPP trial for each treatment group rats were given a priming injection of cocaine (5 mg/kg, i.p.) 15 min before an additional CPP trial to test for cocaine-induced reinstatement.
2.6 Data analysis
Cocaine seeking was analyzed across CPP trials by comparing time spent in the cocaine-paired, saline-paired, and center chambers using a repeated measures ANOVA. Following a significant effect of chamber, Tukey’s Honestly Significant Difference (HSD) post hoc tests were used to compare the amount of time spent in the previously cocaine-paired vs. saline-paired chambers for single or across multiple CPP trials.
To further assess group differences between experimental and control animals, Pearson’s chi-squared test of independence was used to assess the proportion of animals expressing high preferences (>90 sec more in the cocaine-paired chamber than the saline-paired chamber) throughout the first three days of tests.
3. Results
3.1 Concomitant β1- and β2-AR blockade impairs retrieval of a CPP and protects against subsequent reinstatement
We first examined the necessity of both β1- and β2-AR blockade on retrieval of a CPP. Rats were pretested, conditioned, and subjected to daily CPP trials. Thirty min prior to the first CPP trial, rats were injected with either saline or concurrently with the β1- and β2-AR antagonists (Bet20+ICI8). Whereas the saline-treated animals demonstrated a CPP for the previously cocaine-paired chamber throughout all trials, the Bet20+ICI8 animals did not (Figure 1). Repeated measures ANOVA revealed a significant effect of chamber for the saline-treated (F2,22=58.17, p<0.0001) and Bet20+ICI8 rats (F2,22=8.77, p=0.001). Post-hoc analyses confirmed that the saline-treated rats spent significantly more time in the previously cocaine-paired chamber than in the saline-paired chamber throughout all trials (ps<0.01). Bet20+ICI8-treated rats, however, spent a similar amount of time in the cocaine- and saline-paired chambers throughout all trials (p>0.05). Across groups, the proportion of animals that met high preference levels differed significantly between Bet20+ICI8 (0.39) and saline (0.83) treatment (X22,N=72=14.29, p<0.01) as Bet20+ICI8-treated animals were less likely to show a CPP than saline-treated animals. Thus, concomitant blockade of both β1- and β2-ARs persistently disrupted retrieval of a CPP across trials, similar to previously reported results using a non-specific β-AR antagonist propranolol [4].
Figure 1.
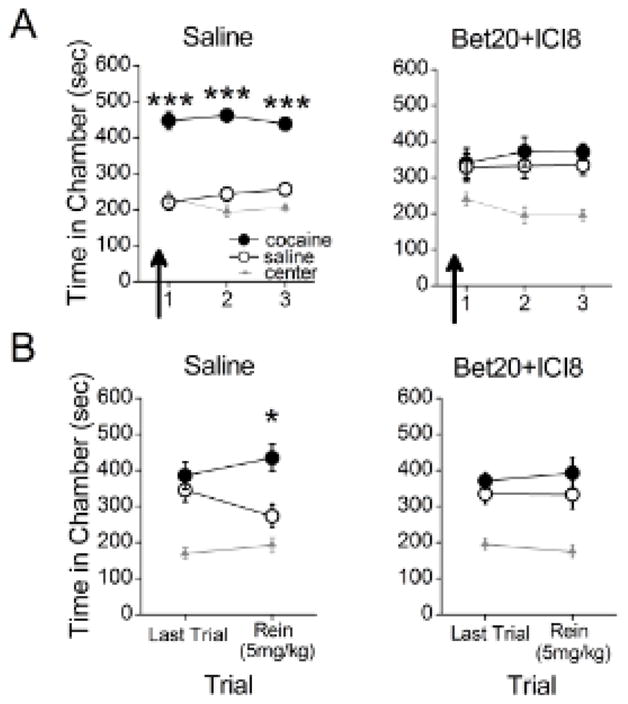
Concomitant β1- and β2-AR blockade disrupts CPP retrieval and prevents subsequent cocaine-induced reinstatement. (A) Administration of Bet20+ICI8, but not saline, prevented rats from expressing a CPP during that trial and subsequent drug-free trials (n = 12 per group). (B) Rats did not express a CPP during a final CPP trial. The following day, rats previously treated with saline, but not Bet20+ICI8, expressed reinstatement of the CPP following a priming injection of cocaine. *p<0.05, ***p<0.001
Next we determined whether the retrieval deficit induced by Bet20+ICI8 would prevent subsequent reinstatement of a CPP. Rats continued to receive additional drug-free trials to ensure extinction of the CPP (Figure S1; Figure 1B). On the final trial, ANOVA revealed a significant effect of chamber for the saline (F2,33=14.69, p<0.001) and Bet20+ICI8 group (F2,33=18.46, p<0.001). Post-hoc analyses revealed that rats in all groups spent a similar amount of time in the cocaine- and saline-paired chambers (ps>0.05), and less time in the center chamber (ps<0.05). Thus, the CPP was extinguished in all groups. To test for reinstatement, rats were injected with cocaine (5 mg/kg, i.p.) 15 min before the next CPP trial. For the reinstatement test, ANOVA revealed a significant effect of chamber for the saline (F2,33=15.92, p<0.001) and Bet20+ICI8 groups (F2,33=10.42, p<0.001). Post-hoc analyses revealed that saline-treated rats spent significantly more time in the previously cocaine-paired chamber than in the saline-paired chamber (p=0.002), whereas Bet20+ICI8-treated rats did not (p>0.05; see Figure 1B). Thus, concomitant blockade of both β1- and β2-ARs disrupted retrieval of a CPP and prevented cocaine-induced reinstatement in the absence of further treatment.
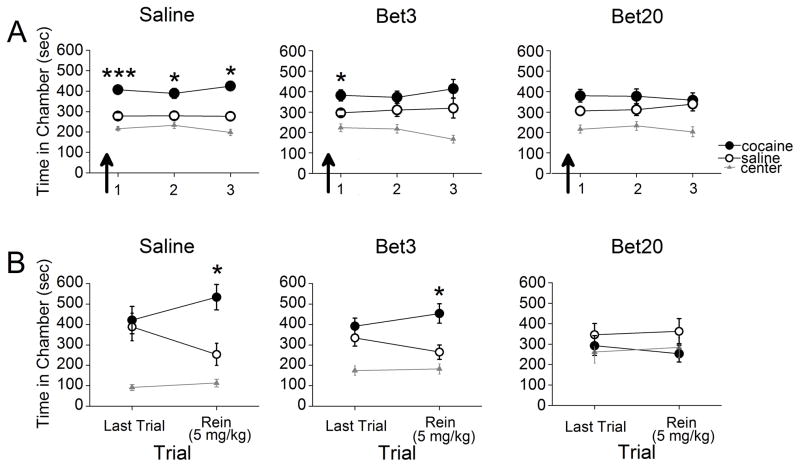
3.2 β1-AR blockade dose-dependently impairs retrieval of a CPP and protects against subsequent reinstatement
To dissociate the role of β1-ARs from β2-ARs, we first used a β1-AR specific antagonist to determine the necessity of this receptor for the retrieval of a cocaine-associated CPP. Rats were pretested, conditioned, and subjected to daily CPP trials. Thirty min prior to the first CPP trial, rats were injected with either saline or one of two doses of betaxolol (Bet3 or Bet20). While the saline-treated rats demonstrated a CPP for the previously cocaine-paired chamber throughout all trials, the Bet3-treated rats only expressed a CPP on the initial trial and the Bet20-treated rats did not (Figure 2). Repeated measures ANOVA revealed a significant effect of chamber for the saline (F2,22=32.99, p<0.001), Bet3 (F2,22=9.05, p=0.001), and Bet20 groups (F2,22=6.78, p=0.005). Post-hoc analyses confirmed that the saline-treated rats spent significantly more time in the previously cocaine-paired chamber than in the saline-paired chamber throughout all trials (ps<0.001). Bet3-treated rats spent significantly more time in the previously cocaine-paired chamber than in the saline-paired chamber during the first trial (p=0.017), and a similar amount of time in each chamber on subsequent trials (ps>0.05). Across groups, the proportion of animals that showed high preference differed significantly between Bet3 (0.44) and saline (0.75) treatment (X22,N=72=6.98, p<0.01) as Bet3-treated animals were less likely to show a CPP than saline-treated animals.
Figure 2.
β1-AR blockade disrupts CPP retrieval and prevents subsequent cocaine-induced reinstatement. (A) Administration of Bet20, but not Bet3 or saline, prevented rats from expressing a CPP during that trial and subsequent drug-free trials (n=12 per group). (B) Rats did not express a CPP during a final CPP trial. The following day, rats previously treated with saline or Bet3, but not Bet20, expressed reinstatement of the CPP following a priming injection of cocaine. *p<0.05.
Bet20-treated rats spent a similar amount of time in the cocaine- and saline-paired chambers throughout all trials (ps>0.05; Figure 2A). Across groups, the proportion of animals that showed high preferences differed significantly between Bet20 (0.44) and saline (0.75) treatment (X22,N=72=6.98, p<0.01) as Bet20-treated animals were less likely to show a CPP than saline-treated animals. Thus, a single 20 mg/kg dose, but not a 3 mg/kg dose, of betaxolol reduced initial expression of a CPP, whereas both doses reduced expression of a CPP long after treatment.
We next determined whether the retrieval deficit induced by betaxolol would prevent cocaine-induced reinstatement of a CPP. Rats continued to receive additional drug-free trials to ensure extinction of the CPP (Figure 2B). On the final trial, ANOVA revealed a significant effect of chamber for the saline (F2,33=10.73, p<0.001) and Bet3 (F2,33=10.10, p<0.001) groups, but not for the Bet20 group (F2,33=.692, p>0.05; Figure 1B). Post-hoc analyses revealed that rats in all groups spent a similar amount of time in the cocaine- and saline-paired chambers (ps>0.05), and less time in the center chamber (ps<0.05). Thus, the CPP was extinguished in all groups. To test for reinstatement, rats were injected with cocaine (5 mg/kg, i.p.) 15 min before the next CPP trial. For the reinstatement trial, ANOVA revealed a significant effect of chamber for the saline (F2,33=19.22, p<0.001) and Bet3 groups (F2,33=13.97, p<0.001), but not for the Bet20 group (F2,33=1.25, p>0.05). Post-hoc analyses revealed that saline- and Bet3-treated rats spent significantly more time in the previously cocaine-paired chamber than in the saline-paired chamber (ps<0.001), whereas Bet20-treated rats did not (Figure 2B). Thus, a high dose, but not a low dose, of betaxolol disrupted retrieval of a CPP and prevented cocaine-induced reinstatement in the absence of further betaxolol treatment.
3.3 β2-AR blockade does not affect retrieval of a CPP, but protects against subsequent reinstatement
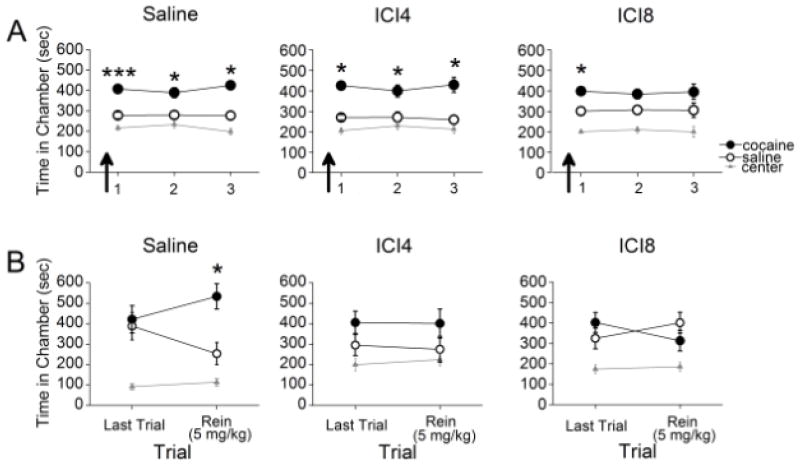
Lastly, we examined the necessity of β2-AR activation for the retrieval of a cocaine-associated CPP. Rats were pretested, conditioned, and subjected to daily CPP trials. Thirty min prior to the first CPP trial, rats were injected with either saline or one of two doses of the selective β2-AR antagonist ICI 118,551 (ICI4 or ICI8). While the saline- and ICI4-treated rats demonstrated a CPP for the previously cocaine-paired chamber throughout all trials, the ICI8-treated rats only expressed a CPP on the initial trial (Figure 3A). Repeated measures ANOVA revealed a significant effect of chamber for the saline (F2,22=32.99, p<0.001), ICI4 (F2,22=24.92, p<0.001), and ICI8 group (F2,22=29.313, p<0.001). Post-hoc analyses confirmed that the saline group spent significantly more time in the previously cocaine-paired chamber than in the saline-paired chamber throughout all trials (ps<0.001). Both the ICI4- and ICI8-treated rats spent significantly more time in the previously cocaine-paired chamber than in the saline-paired chamber during the initial trial (ps<0.001). However, during the second and third trial, only the ICI4-treated rats spent significantly more time in the previously cocaine-paired chamber than in the saline-paired chamber (ps<0.002) whereas the ICI8-treated rats did not (p>0.05). Across groups, the proportion of animals that showed high preferences did not differ significantly between ICI4 (0.69) and saline (0.75) treatment (X22,N=80=0.53, p>0.05) or ICIC8 (0.61) and saline (0.75) treatment (X22,N=72=1.59, p>0.05). Thus, ICI4-, ICI8-, and saline-treated animals were equally likely to show a CPP. Overall, a single 4 mg/kg or 8 mg/kg treatment of ICI 118,551 did not affect initial expression of a CPP, but a single 8 mg/kg treatment of ICI 118,551 reduced expression of a CPP long after treatment.
Figure 3.
β2-AR blockade does not disrupt CPP retrieval, but prevents subsequent cocaine-induced reinstatement. (A) Administration of saline, ICI4, or ICI8 did not affect expression of a CPP. On subsequent drug-free trials, saline and ICI4 groups continued to express a CPP whereas the ICI8 group did not (n=12 per group). (B) Rats did not express a CPP during a final CPP trial. The following day, rats previously treated with saline, but not ICI4 or ICI8, expressed reinstatement of the CPP following a priming injection of cocaine. *p<0.05, ***p<0.001
We next tested whether β2-AR blockade would prevent subsequent cocaine-induced reinstatement of a CPP. Rats continued to receive additional drug-free CPP trials to ensure extinction of the CPP. On the final trial, ANOVA revealed a significant effect of chamber for the saline (F2,33=10.73, p<0.001), ICI4 (F2,33=4.78, p=0.015), and ICI8 group (F2,33=7.43, p=0.002). Post-hoc analyses revealed that all rats spent a similar amount of time in the cocaine- and saline-paired chambers (ps>0.05), and less time in the center chamber (ps<0.05; Figure 3B). Thus, the CPP was extinguished in all groups. To test for reinstatement, rats were injected with cocaine (5 mg/kg, i.p.) 15 min before the next CPP trial. For the reinstatement test, ANOVA revealed a significant effect of chamber for the saline- (F2,33=19.22, p<0.001) and ICI8-treated rats (F2,33=5.96, p=0.006), but not for the ICI4-treated rats (F2,33=2.43, p>0.05). Post-hoc analyses revealed that the saline-treated group spent more time in the cocaine- than in the saline-paired chamber (p=0.001), whereas both ICI-treated groups did not (ps>0.05; see Figure 3B). These results show that β2-AR blockade prevents subsequent cocaine-induced reinstatement in the absence of further treatment.
4. Discussion
We investigated the necessity of β1- and β2-AR subtypes for the retrieval of a cocaine CPP, and found that blockade of β1-ARs, but not β2-ARs, impaired retrieval of a cocaine CPP in a dose-dependent manner. First, we found that co-injection of β1- and β2-AR blockers induced a persistent retrieval deficit and prevented subsequent reinstatement to a priming injection of cocaine in a similar manner to the non-selective β-AR antagonist propranolol [4]. Next, we showed that specific β1-AR blockade induced a persistent retrieval deficit similar to that induced by a non-specific β-AR blocker [4]. Moreover, β1-AR blockade prevented subsequent cocaine-induced reinstatement. In contrast, β2-AR blockade did not affect initial retrieval of a cocaine CPP, although a higher dose disrupted drug seeking on subsequent drug-free trials. However, β2-AR blockade prior to retrieval prevented subsequent reinstatement to a priming injection of cocaine. Our results are the first to reveal that selective β1-AR blockade induces a lasting disruption in retrieval of a cocaine CPP. Furthermore, we found that β2-AR blockade had no effect on initial CPP expression but impaired subsequent expression in the absence of further treatment, suggesting that β2-AR blockade disrupts reconsolidation.
Our findings are supported by previous studies using mice lacking dopamine β-hydroxylase (DBH), the enzyme responsible for the synthesis of norepinephrine from dopamine. Although DBH-knockout mice were able to express a CPP to one of three conditioning doses of cocaine in one study [18], this was not true in another [19]. Moreover, DBH-knockout mice were unable to express a CPP after conditioning with five different doses of morphine [20]. When norepinephrine was restored in these mice, drug seeking was rescued indicating that retrieval was dependent on norepinephrine. This effect was shown to be specific to drug memories, since the mice lacking dopamine β-hydroxylase were able to express a food-induced CPP. Moreover, memory retrieval in both appetitive and aversive learning paradigms has been demonstrated to depend specifically on β-AR activation [21,22,4], and evidence suggests that the β1-AR subtype mediates retrieval in a contextual fear paradigm [22]. We now show that β1-ARs mediate cocaine-associated memory retrieval and extend these findings to demonstrate that retrieval disruption by β1-AR blockade results in a persistent retrieval deficit.
The necessity of β1-ARs, but not β2-ARs, for cocaine-associated memory retrieval agrees with results from previous studies. Blocking β1-ARs, but not β2-ARs, has been shown to reduce expression of shock avoidance learning [11] and contextual fear conditioning [23,22] in a manner similar to general β-AR antagonists. In contrast, β2-ARs, but not β1-ARs, have been shown to mediate reconsolidation of a cocaine CPP [10]. Importantly, retrieval and reconsolidation of a cocaine CPP are mediated by separate and distinct neuronal loci [5,6]. In particular, we have shown that bilateral infusions of non-selective β-AR blockers into PL-mPFC or dHipp disrupt retrieval, but not reconsolidation, of a CPP [5,6]. In contrast, bilateral infusions of non-selective β-AR blockers [5] or selective β2-AR blockers [10] into the basolateral amygdala (BLA) disrupt reconsolidation, but not retrieval, of a cocaine CPP. Additionally, previous work found that systemic ICI administration did not alter retrieval of CPP, but did attenuate the retrieval-induced BLA c-Fos response when administered prior to CPP retrieval [10]. This observation supports our findings that β2-ARs do not mediate retrieval, but that β-ARs (specifically β2-ARs) in the BLA mediate reconsolidation of a cocaine associated memory [5, 10].
In both PL-mPFC and dHipp, β1-ARs account for roughly 80% of the total β-ARs [9] which could account for the dependence on β1-ARs for retrieval of a cocaine CPP. In contrast, the BLA has been reported to express 65% β1-ARs and 35% β2-ARs [9]. In areas such as the BLA where β2-ARs are more highly concentrated, these receptors likely have a more predominant role in β-AR-mediated processes. Notably, the non-selective β-AR agonist, isoproterenol, stimulates adenylyl cyclase to a greater extent through β2-ARs than β1-ARs [8], although both β-ARs stimulate cAMP signaling [24,25]. Thus, the greater stimulation of adenylyl cyclase through β2-ARs rather than β1-ARs in regions such as the BLA could explain the selective role of β2-ARs in reconsolidation of a cocaine CPP. These data suggest that receptor distribution in regions mediating retrieval and reconsolidation is likely a determinant of which β-AR subtype primarily mediates these memory processes.
We previously reported that non-specific β-AR antagonism persistently disrupts memory retrieval and subsequent cocaine-induced reinstatement of a CPP in rodents [5,6]. Similarly, following β-AR blockade with propranolol, human subjects showed a sustained reduction in the retrieval of emotional words [26] and visual memories [27]. These effects were long lasting, and may also prevent memory reinstatement [27]. Thus, converging evidence from rodents and humans support the conclusion that pharmacological antagonism of β-ARs can persistently induce memory deficits in retrieval. Our results are the first to reveal that β1-ARs specifically mediate cocaine-associated memory retrieval.
The presentation of drug-associated stimuli leads to drug seeking and relapse in addicts [2]. Use of pharmacological interventions to disrupt the retrieval of drug-associated memories, therefore, could improve exposure therapies for cocaine abuse that have largely been unsuccessful without the use of pharmacological adjuncts [28]. Although the use of β-AR blockers as adjuncts remains untested, clinical studies with human addicts have demonstrated that treatment with the non-specific β-AR blocker propranolol alone enhances treatment retention [29]. Use of specific β-AR subtype blockers has not yet been investigated, but β1-AR blockade may have therapeutic potential as this treatment prevents anxiety-like behavior during withdrawal [30] and stress-induced reinstatement following extinction [17]. Our findings reveal that β1-AR blockade during memory retrieval also prevents subsequent cocaine-induced reinstatement of a CPP. Thus, targeting β1-ARs during therapy in patients with drug abuse liability could reduce the probability of relapse due to stress or exposure to drug-associated cues during or even long after treatment.
Supplementary Material
Highlights.
β-adrenergic receptors mediate retrieval of cocaine-associated memory
Blocking the β1 receptor subtype induces a persistent deficit in retrieval
Blocking either β1 or β2 subtypes prevents subsequent reinstatement
Acknowledgments
This research was supported by R01 DA038042 and a grant from the University of Wisconsin—Milwaukee Graduate School to DM.
Footnotes
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ehrman RN, Robbins SJ, Childress AR, O’Brien CP. Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology (Berl) 1992;107:523–529. doi: 10.1007/BF02245266. [DOI] [PubMed] [Google Scholar]
- 2.Foltin RW, Haney M. Conditioned effects of environmental stimuli paired with smoked cocaine in humans. Psychopharmacology (Berl) 2000;149:24–33. doi: 10.1007/s002139900340. [DOI] [PubMed] [Google Scholar]
- 3.O’Brien CP, Testa T, O’Brien TJ, Brady JP, Wells B. Conditioned narcotic withdrawal in humans. Science. 1977;195:1000–1002. doi: 10.1126/science.841320. [DOI] [PubMed] [Google Scholar]
- 4.Otis JM, Mueller D. Inhibition of β-adrenergic receptors induces a persistent deficit in retrieval of a cocaine-associated memory providing protection against reinstatement. Neuropsychopharmacology. 2011;36:1912–1920. doi: 10.1038/npp.2011.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Otis JM, Dashew KB, Mueller D. Neurobiological dissociation of retrieval and reconsolidation of cocaine-associated memory. J Neurosci. 2013;33:1271–1281a. doi: 10.1523/JNEUROSCI.3463-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Otis JM, Fitzgerald MK, Mueller D. Inhibition of hippocampal β-adrenergic receptors impairs retrieval but not reconsolidation of cocaine-associated memory and prevents subsequent reinstatement. Neuropsychopharmacology. 2014;39:303–310. doi: 10.1038/npp.2013.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levy FO, Zhu X, Kaumann AJ, Birnbaumer L. Efficacy of beta 1-adrenergic receptors is lower than that of beta 2-adrenergic receptors. Proc Natl Acad Sci U S A. 1993;90:10798–10802. doi: 10.1073/pnas.90.22.10798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Green SA, Holt BD, Liggett SB. Beta 1- and beta 2-adrenergic receptors display subtype-selective coupling to Gs. Mol Pharmacol. 1992;41:889–893. [PubMed] [Google Scholar]
- 9.Rainbow TC, Parsons B, Wolfe BB. Quantitative autoradiography of beta 1- and beta 2-adrenergic receptors in rat brain. Proc Natl Acad Sci U S A. 1984;81:1585–1589. doi: 10.1073/pnas.81.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernardi RE, Ryabinin AE, Berger SP, Lattal KM. Post-retrieval disruption of a cocaine conditioned place preference by systemic and intrabasolateral amygdala beta2- and alpha1-adrenergic antagonists. Learn Mem. 2009;16:777–789. doi: 10.1101/lm.1648509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flexner JB, Flexner LB, Church AC, Rainbow TC, Brunswick DJ. Blockade of beta 1- but not of beta 2-adrenergic receptors replicates propranolol’s suppression of the cerebral spread of an engram in mice. Proc Natl Acad Sci U S A. 1985;82:7458–7461. doi: 10.1073/pnas.82.21.7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramos BP, Colgan L, Nou E, Ovadia S, Wilson SR, Arnsten AF. The beta-1 adrenergic antagonist, betaxolol, improves working memory performance in rats and monkeys. Biol Psychiatry. 2005;58:894–900. doi: 10.1016/j.biopsych.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 13.Ramos BP, Colgan LA, Nou E, Arnsten AF. Beta2 adrenergic agonist, clenbuterol, enhances working memory performance in aging animals. Neurobiol Aging. 2008;29:1060–1069. doi: 10.1016/j.neurobiolaging.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abraham PA, Xing G, Zhang L, Yu EZ, Post R, Gamble EH, Li H. beta1- and beta2-adrenoceptor induced synaptic facilitation in rat basolateral amygdala. Brain Res. 2008;1209:65–73. doi: 10.1016/j.brainres.2008.02.082. [DOI] [PubMed] [Google Scholar]
- 15.Leri F, Flores J, Rodaros D, Stewart J. Blockade of stress-induced but not cocaine-induced reinstatement by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the central nucleus of the amygdala. J Neurosci. 2002;22:5713–5718. doi: 10.1523/JNEUROSCI.22-13-05713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mantsch JR, Weyer A, Vranjkovic O, Beyer CE, Baker DA, Caretta H. Involvement of noradrenergic neurotransmission in the stress- but not cocaine-induced reinstatement of extinguished cocaine-induced conditioned place preference in mice: role for β-2 adrenergic receptors. Neuropsychopharmacology. 2010;35:2165–2178. doi: 10.1038/npp.2010.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vranjkovic O, Hang S, Baker DA, Mantsch JR. β-adrenergic receptor mediation of stress-induced reinstatement of extinguished cocaine-induced conditioned place preference in mice: roles for β1 and β2 adrenergic receptors. J Pharmacol Exp Ther. 2012;342:541–551. doi: 10.1124/jpet.112.193615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schank JR, Ventura R, Puglisi-Allegra S, Alcaro A, Cole CD, Liles LC, et al. Dopamine β-hydroxylase knockout mice have alterations in dopamine signaling and are hypersensitive to cocaine. Neuropsychopharmacology. 2006;31:2221–2230. doi: 10.1038/sj.npp.1301000. [DOI] [PubMed] [Google Scholar]
- 19.Jasmin L, Narasaiah M, Tien D. Noradrenaline is necessary for the hedonic properties of addictive drugs. Vascul Pharmacol. 2006;45:243–250. doi: 10.1016/j.vph.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 20.Olson VG, Heusner CL, Bland RJ, During MJ, Weinshenker D, Palmiter RD. Role of noradrenergic signaling by the nucleus tractus solitarius in mediating opiate reward. Science. 2006;311:1017–1020. doi: 10.1126/science.1119311. [DOI] [PubMed] [Google Scholar]
- 21.Devauges V, Sara SJ. Memory retrieval enhancement by locus coeruleus stimulation: evidence for mediation by beta-receptors. Behav Brain Res. 1991;43:93–97. doi: 10.1016/s0166-4328(05)80056-7. [DOI] [PubMed] [Google Scholar]
- 22.Murchison CF, Zhang XY, Zhang WP, Ouyang M, Lee A, Thomas SA. A distinct role for norepinephrine in memory retrieval. Cell. 2004;117:131–143. doi: 10.1016/s0092-8674(04)00259-4. [DOI] [PubMed] [Google Scholar]
- 23.Murchison CF, Schutsky K, Jin SH, Thomas SA. Norepinephrine and ss(1)-adrenergic signaling facilitate activation of hippocampal CA1 pyramidal neurons during contextual memory retrieval. Neuroscience. 2011;18:109–116. doi: 10.1016/j.neuroscience.2011.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garnier V, Zini R, Sapena R, Tillement JP. A match between binding to beta-adrenoceptors and stimulation of adenylyl cyclase parameters of (−)isoproterenol and salbutamol on rat brain. Pharmacol Res. 1997;35:303–312. doi: 10.1006/phrs.1997.0141. [DOI] [PubMed] [Google Scholar]
- 25.Barthel F, Loeffler JP. Beta 2-adrenoceptors stimulate c-fos transcription through multiple cyclic AMP- and Ca(2+)-responsive elements in cerebellar granular neurons. J Neurochem. 1995;64:41–51. doi: 10.1046/j.1471-4159.1995.64010041.x. [DOI] [PubMed] [Google Scholar]
- 26.Kroes MC, Strange BA, Dolan RJ. Beta-adrenergic blockade during memory retrieval in humans evokes a sustained reduction of declarative emotional memory enhancement. J Neurosci. 2010;30:3959–3963. doi: 10.1523/JNEUROSCI.5469-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kroes MC, Tona KD, Muller N, Den Ouden HE, Van Wingen GA, Fernandez G. Beta-adrenergic blockade affects the neural network of extinction learning and prevents the return of fear in humans. Paper presented at the Society for Neuroscience; 2012; New Orleans, LA. [Google Scholar]
- 28.Conklin CA, Tiffany ST. Applying extinction research and theory to cue-exposure addiction treatments. Addiction. 2002;97:155–167. doi: 10.1046/j.1360-0443.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- 29.Kampman KM, Dackis C, Lynch KG, Pettinati H, Tirado C, Gariti P, et al. A double-blind, placebo-controlled trial of amantadine, propranolol, and their combination for the treatment of cocaine dependence in patients with severe cocaine withdrawal symptoms. Drug Alcohol Depend. 2006;85:129–137. doi: 10.1016/j.drugalcdep.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 30.Rudoy CA, Van Bockstaele EJ. Betaxolol, a selective beta(1)-adrenergic receptor antagonist, diminishes anxiety-like behavior during early withdrawal from chronic cocaine administration in rats. Prog Neuropsychopharmacol Biol Psych. 2007;31:1119–1129. doi: 10.1016/j.pnpbp.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.