Abstract
Individuals with borderline personality disorder (BPD) display an impoverished sense of self and representations of self and others that shift between positive and negative poles. However, little research has investigated the nature of representational disturbance in BPD. The present study takes a multi-modal approach. A card sort task was employed to investigate complexity, integration and valence of self-representation in BPD. Impairment in maintenance of self and other representations was assessed using a personality representational maintenance task. Finally, functional magnetic resonance imaging (fMRI) was used to assess whether individuals with BPD show neural abnormalities related specifically to the self and what brain areas may be related to poor representational maintenance. Individuals with BPD sorted self-aspects suggesting more complexity of self-representation, but also less integration and more negative valence overall. On the representational maintenance task, individuals with BPD showed less consistency in their representations of self and others over the 3-hour period, but only for abstract, personality-based representations. Performance on this measure mediated between-group brain activation in several areas supporting social cognition. We found no evidence for social cognitive disturbance specific to the self. Additionally, the BPD group showed main effects, insensitive to condition, of hyperactivation in the medial prefrontal cortex, temporal parietal junction, several regions of the frontal pole, the precuneus and middle temporal gyrus, all areas crucial social cognition. In contrast, controls evidenced greater activation in visual, sensory, motor and mirror neuron regions. These findings are discussed in relation to research regarding hypermentalization and the overlap between self- and other-disturbance.
Keywords: Self, Identity Diffusion, Perspective-Taking, Self-reflection, fMRI
Self-disturbance in borderline personality disorder (BPD) is important to the diagnosis and prognosis of the disorder. Having a strong predictive power for a positive BPD diagnosis, prominent self-disturbance factors have emerged from a number of factor-analytic studies (Clarkin, Hull, & Hurt, 1993; Spitzer, Endicott, & Gibbon, 1979). Longitudinally, self-disturbance is a strong predictor of self-injury (Yen et al., 2004), and among hospitalized adolescents, is a good predictor of continued BPD symptoms into adulthood (Garnet, Levy, Mattanah, Edell, & McGlashan, 1994). Though long considered a core feature of BPD, self-disturbance has been neglected relative to other aspects of the disorder. Theorists have proposed that the self in BPD is characterized by all-negative and all-positive splits in self-representation (Kernberg, 1967), lack of differentiation between self and others (Masterson, 2013), black-and-white thinking regarding self (Beck, Freeman, & Davis, 2004), and an impoverished self-representation. Over the past decade, within social psychology and social neuroscience, the study of the self and its relationship to social cognition in general has been rigorously studied. At the same time, abnormal social cognition in BPD, particularly related to cognition about others, has become a major focus. Informed by each of these realms of study, using a multimodal methodology including fMRI, performance-based tasks and self-report, we aimed to better understand various aspects of self and social cognition in BPD.
Self and Other representations and BPD
Erikson (1968) placed the struggle to carve out a coherent, consistent identity as the primary task of adolescence. Building on Erickson’s work, Kernberg (1967) observed that patients with BPD often vacillate between extreme positive and negative representations of self and others. Theorists across clinical orientations have described self- and other-disturbance as crucial to BPD (e.g., Bateman & Fonagy, 2004; Clarkin, Yeomans, & Kernberg, 2006; Heard & Linehan, 1993). Recently, Bender and Skodol (2007) argued that there is a consensus among disparate theories that self-other representational disturbance is a core symptom of the disorder. Likewise, the DSM-5 Personality Disorders Workgroup (Skodol et al., 2011) proposed that all personality disorders are defined by disturbed thinking about self and others, and that BPD, in particular, is characterized by fragile self-representation, impoverished and/or unstable self-structure, self-loathing and difficulties with self-other differentiation.
The suggestion that individuals with BPD experience difficulty in integrating positive and negative aspects of self and others is among the most prominent theories of self in BPD. Kernberg (1967) originally proposed BPD is characterized by splitting positive and negative parts of self and others. Many others have suggested similar difficulties (e.g., Masterson, 2013). Beck and colleagues, for instance, described this aspect of BPD experience as black-and-white, or dichotomous thinking (Beck et al., 2004). Some research has investigated splitting and dichotomous thinking regarding perceptions of strangers in BPD (Coifman, Berenson, Rafaeli, & Downey, 2012). Limited evidence has supported poorly integrated representations of self (Semerari et al., 2005). Perhaps related, individuals with BPD also have a predominantly negative self-representation. Individuals with BPD often feel inherently unacceptable, evil, and/or helpless (Westen & Cohen, 1993). Theorists (e.g., Kernberg, 1967) argue that such negative self-appraisals are the consequence of difficulties in integrating self-representations.
Aside from abnormalities in the valence and structural arrangement of representations, theorists have routinely proposed that a major aspect of self-disturbance in BPD is impoverished identity, defined by poor understanding and maintenance of personal qualities. Individuals with BPD are thought to have a severe impairment in creating and maintaining representations of self and others (Bender & Skodol, 2007). Relatedly, research supports that individuals with BPD have difficulty with self-other differentiation, defining the psychological boundaries between oneself and another (e.g., Beeney et al., 2015; de Bonis, De Boeck, Lida-Pulik, & Feline, 1995). Linehan (1993) rooted the cause of impoverished identity in routine parental invalidation of a child’s feelings and perceptions, which causes the child to rely on others for a definition of internal reality, inhibiting self-development. Fonagy and Bateman (2004) have described a similar process in which the lack of emotional attunement in parents towards children, particularly poor mirroring of child affect, leads to inability for the child to develop clear representations of self and others. Within Fonagy and Bateman’s model, self and other-representational abilities are connected, as is mentalization, the social cognitive ability to understand the actions of self and others in terms of intentional mental states. Many theorists link self- and other-representational disturbance, implicitly or explicitly (Bender & Skodol, 2007). These theories are consonant with recent neural findings of extensive overlap in neural networks for processing all three forms of thought (e.g., Legrand & Ruby, 2009).
Is the self special, or just a form of social cognition?
The singularity or non-singularity of the self has implications for self and social cognitive disturbance in BPD, particularly whether difficulties with self-disturbance and social cognition are independent or intertwined. Evidence exists both for and against specific cognitive processes for self-representation. The self-reference effect, a memory effect in which information processed through the self is better recalled, provides evidence of a special cognitive and neural status for the self (Rogers et al., 1977). Recall of self-processed information is associated with greater ventral medial PFC (vmPFC) activation during encoding (Macrae, Moran, Heatherton, Banfield, & Kelley, 2004). The vmPFC is routinely found to differentiate self in relation to others (e.g., Kelley et al., 2002; Lomardo et al., 2010). In a study of individuals with autism (Lombardo, Chakrabarti, Bullmore, Sadek, et al., 2010), another population characterized by self-disturbance, researchers found individuals with autism showed less differentiation in activation in the vmPFC when mentalizing about self versus other, compared to controls. Likewise, researchers have found that the temporal-parietal junction (TPJ) is involved in control of self- and other-representations, and thus may allow improved distinction of self and other (Uddin, Molnar-Szakacs, Zaidel, & Iacoboni, 2006). However, recent research has highlighted that overlapping neural networks are activated when reflecting on the self or others, suggesting a non-specialized role of the self. Two-large scale neural networks, a lower-level mirror neuron system, processing embodied simulation representations, and a higher-level cortical midline structures, processing inference-based and abstract representations (Ripoll, Snyder, Steele, & Siever, 2013), are thought to be involved in mentalizing about both self and others (e.g., Lombardo, Chakrabarti, Bullmore, Wheelwright, et al., 2010; Uddin, Iacoboni, Lange, & Keenan, 2007). In addition, though preferential activation for self in the mPFC and TPJ is plausible, Legrand and Ruby (2009) provide a review of empirical studies suggesting these areas are just as frequently active for other-versus-self contrasts. Further, these authors postulated that neural circuit, including the mPFC, TPJ, precuneus and temporal poles – are active in all tasks in which inferences are made on information retrieved from memory.
To our knowledge, no neuroimaging study has focused on aspects of self in BPD, yet researchers have examined differences in the neural networks that process social cognition. When the brain is at rest, the mPFC, medial temporal lobe, precuneus and the inferior parietal cortex exhibit higher metabolism, forming the default mode network (DMN). A large research base has connected the DMN with self-reflection, mentalization/theory-of-mind, and inner speech, among related constructs (Wolf et al., 2011). Wolf and colleagues (2011) found increased functional connectivity within this network among individuals with BPD in the left frontopolar cortex and left insula and decreased functional connectivity in the cuneus. The mPFC/frontopolar cortex, in particular, is known to be involved in processing self-relevant information and other social stimuli, and has additionally been found to be hyperactived in BPD during social exclusion (Ruocco et al., 2010). Interestingly, though numerous studies have identified decreased frontal activation in BPD, hyperactivation in the medial PFC and other areas related to mentalization/theory of mind, has been found in BPD using tasks assessing social cognition (e.g., Beblo et al., 2006; Ruocco et al., 2010). Domsalla and colleagues (Domsalla et al., 2013) found hyperactivation of the dorsal medial PFC in a BPD group in a social rejection study. This activation was a main effect of group, meaning this hyperactivation was present across all social conditions (exclusion, inclusion, or the control condition). These authors suggested that individuals with BPD hypermentalize in social situations; that is, they over-interpret or overattribute mental states to others (Domsalla et al., 2013; Sharp et al., 2011).
Additional studies demonstrate abnormal neural activation in BPD in regions that contribute to self-processing. A meta-analysis of fMRI studies of negative emotionality (Ruocco, Amirthavasagam, Choi-Kain, & McMain, 2013), some of which included social stimuli, showed increased activation in the insula and posterior cingulate cortex among individuals with BPD, and decreased activation in the amygdala, subgenual ACC and dorsolateral PFC. Dziobek and colleagues (2011) found decreased activation among individuals with BPD in the left superior sulcus and gyrus (STS/STG) during an empathy task. Similarly, Mier and colleagues (Mier et al., 2012) found reduced activation in the STS/STG and inferior frontal gyrus and increased activation in the amygdala while making attributions of intentions from affective facial stimuli. The STS/STG has strong connections to both the amygdala and prefrontal areas, a network which is involved in numerous social cognitive processes (Uddin et al., 2007).
Current Study
We used a multi-modal framework. Methods included a self-aspects card sort task, self-report, a self- and other-representation maintenance task, and an fMRI self- and other-reflection task. Given the theoretical literature, and limited research, we hypothesized that individuals with BPD would identify self-aspects with greater complexity, but less integration of negative and positive traits, as well as more negative self-appraisals. We also hypothesized that individuals with BPD would show a poorer ability in maintaining representations of self- and others, specific to personality, rather than physical traits. Given theory and neuroscience research suggesting overlapping neural mechanisms of self- and other-representation and other forms of social cognition, we hypothesized individuals with BPD would not show functional neural abnormalities specific to self, other or perspective taking conditions, but would evidence hyperactivation in brain areas related to social cognition, irrespective of condition. This hypothesis is consistent with a number of studies in BPD which find lack of group x condition interactions using social tasks (e.g., Domsalla et al., 2013; Mier et al., 2012). We did, however, expect group differences in maintaining representations of self and others would mediate activation in the mirror-neuron and CMS networks (Uddin et al., 2007).
Method
Participants
Participants were 38 (BPD = 17, Controls = 21) right-handed females between the ages of 18 and 60. Demographic characteristics are detailed in Table 1. In the BPD group, seven (41%) participants had mood disorders, six (35%) had anxiety disorders, four (24%) had Post-Traumatic Stress Disorder, four (24%) had alcohol-related disorders, and three (18%) had somatoform or eating disorders. In the control group, 3 (14%) participants had lifetime MDD diagnoses and 2 (10%) met for past alcohol abuse. BPD participants were recruited from a community mental health clinic at The Pennsylvania State University. Control participants were community residents. Among all participants, individuals were excluded who were left-handed, had a significant medical illness or who met lifetime diagnostic criteria for psychotic disorders, bipolar I, delirium, dementia, traumatic brain injury, and/or mental retardation. HC participants were excluded with current DSM-IV-TR Axis I or II diagnoses, suicidal or self-injurious behaviors, or more than two cluster-B personality disorder criteria.
Table 1.
Sample Characteristics
| Characteristic | BPD (N=17) | HC (N=21) | p-value |
|---|---|---|---|
| Age (years) | |||
| M | 35.51 | 33.33 | .667 |
| SD | 10.84 | 9.81 | |
| Education N (%) | .670 | ||
| High school | 6 (35%) | 5 (23%) | |
| Some college | 3 (14%) | 11 (50%) | |
| College graduate | 7 (41%) | 4 (18%) | |
| Postgraduate | 1 (6%) | 2 (9%) | |
| Marital status N (%) | .642 | ||
| Single | 7 (42%) | 11 (52%) | |
| Married/cohabiting | 4 (17%) | 7 (33%) | |
| Divorced | 6 (26%) | 3 (14%) | |
| Ethnicity (%) | .578 | ||
| Caucasian | 10 (59%) | 15 (72%) | |
| African American | 5 (22%) | 3 (14%) | |
| Latino/Latina | 1 (4%) | 1 (5%) | |
Note. No significant between-group differences were present for demographic variables.
Participants were evaluated using the Structural Clinical Interview for DSM-IV (First, Spitzer, & Williams, 1997) and the International Personality Disorder Examination (Loranger, Janca, & Sartorius, 1997). Doctoral-level therapists, trained to reliability, conducted the clinical evaluations under the supervision of a licensed psychologist. Final diagnoses were established at an evaluation conference using the LEAD standard (Spitzer, 1983). This method involves using all available clinical data to establish a “best-estimate” diagnosis (Pilkonis, Heape, Ruddy, & Serrao, 1991). Second raters viewed video of diagnostic interviews on a subset of cases (n=15, BPD = 9, HC = 6). Kappas (κ) for Axis I diagnoses ranged from .67 to 1.0 and Kappas (κ) for personality disorder diagnoses ranged from .74 to 1.0 (κ = .89 for BPD diagnosis). These ranges are considered substantial agreement to almost perfect. Intraclass correlation coefficients were .94 for number of BPD criteria and .98 for BPD dimensional scores. BPD dimensional scores were calculated as the sum of all ratings for consensus-rated IPDE BPD criteria. Clinician-rated BPD dimensional scores were correlated with self-report and performance-based data in table 3. Participants were not excluded for medication use. For individuals with BPD, 10 participants took medications. Of these, 7 participants took antidepressants, 4 took antipsychotics, 5 took mood stabilizers and 3 took anxiolytics. Two control participants took antidepressants.
Table 3.
Correlations among self measures and BPD
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Representational Maintenance | 1.00 | ||||||||||
| 2 | Self-Differentiation Total | 0.41* | 1.00 | |||||||||
| 3 | Emotional Reactivity | 0.26 | 0.84** | 1.00 | ||||||||
| 4 | “I” Position | 0.49** | 0.78** | 0.65* | 1.00 | |||||||
| 5 | Emotional Cutoff | 0.38* | 0.67** | 0.35* | 0.23 | 1.00 | ||||||
| 6 | Fusion With Others | −0.01 | 0.71** | 0.51** | 0.42* | 0.37* | 1.00 | |||||
| 7 | Overlap | 0.24 | 0.25 | 0.30 | 0.08 | 0.17 | 0.21 | 1.00 | ||||
| 8 | Number of Aspects | −0.16 | −0.49** | −0.41* | −0.33* | −0.32* | −0.43** | 0.16 | 1.00 | |||
| 9 | Evaluative Integration | −0.09 | −0.29 | −0.30 | −0.14 | −0.17 | −0.27 | −0.66 | −0.10 | 1.00 | ||
| 10 | Proportion of Cards Positive | 0.51** | 0.65** | 0.59** | 0.45** | 0.60** | .22 | .35* | −0.32* | −0.50** | 1.00 | |
| 11 | BPD Dimensional Score | −0.47** | −0.64** | −0.55** | −0.34* | −0.61** | −0.35* | −0.32* | 0.32* | 0.37* | −0.52** | 1.00 |
n = 38; for all Compartmentalization/Integration correlations, n=34
Representational Maintenance = correlation of ratings of personality traits across a 3 hour period. Self Differentiation Total, Emotional Reactivity, “I” Position, Emotional Cutoff, and Fusion with others = Differentiation of Self Inventory. Number of Aspects, Compartmentalization, Proportion of Cards Positive = Self-Aspects Card Sort Task. BPD dimensional scores from clinician rated IPDE.
High score = high self-differentiation, low emotion reactivity, high “I” position, low emotional cutoff, low fusion with others, high overlap (less complexity), greater # of aspects, more compartmentalization (less integration), greater proportion of cards positive.
On the day of the scan, participants were consented and provided a number of self-report measures to complete. The first self-report measure was self- and other-representational trait measure. Participants then completed the fMRI task. Following scanning, participants completed self-report measures, the self-aspects card sort task, and completed the post-test of the self-other representational trait measure at 3-hours past the time of the first administration.
Self-Aspects Card Sort Task
Participants were provided a deck of 40 trait cards (20 positive and 20 negative; see Showers, Abramson, & Hogan, 1998) and asked to sort the cards into different groups, with each group describing a distinct aspect of self. Extensive research has investigated aspects of self, related to a personality-trait sorting task. A number of authors (e.g., Brown & Rafaeli, 2007; Locke, 2003) have provided evidence that self-complexity is best assessed by computing separate indices for 1) the number of self-aspects created in a sort (NASPECTS) and 2) the overlap of content among these self-aspects (OL). Studies suggest that both NASPECTS (Rothermund & Meiniger, 2004) and OL (Cohen, Spiegler, Young, Hankin, & Abela, 2014; Constantino et al., 2006) are associated with increased depressed mood in response to stressors and have incremental validity over other measures in predicting self-related variables (Luo, Watkins, & Lam, 2009). Evaluative integration (Showers & Kling, 1996), is assessed by the phi statistic, which ranges from 0 to 1 (1 describing a self-concept that is perfectly organized by valence, and 0 a perfectly random sort with respect to valence). Research suggests that high phi scores are associated with vulnerability of self-esteem to negative events (Ziegler-Hill & Showers, 2007) and with mood disorder symptoms (Power, de Jong, & Lloyd, 2002). The phi statistic also shows good temporal stability (Showers et al., 1998). A fourth metric is proportion of all cards sorted that are positive, assessing valence of self-representation.
Self- and Other-Representation Maintenance
Consistency of self-representation is the tendency to maintain a similar self-representation across situations and at different times. Researchers have frequently used lists of traits to evaluate aspects of self-representational consistency. Greater consistency over time and across situations has been linked to well-being (e.g., Sheldon, Ryan, Tawsthorne, & Ilardi, 1997), and higher self-esteem (e.g., Campbell, 1990). Individuals with greater self-consistency are also less susceptible to the negative influence of others (Morse & Gergen, 1970). We examined self-consistency over time rather than across relationships or situations, because such an approach is less susceptible to presentation bias, and not dependent on culture (English & Chen, 2007). In addition, BPD is frequently characterized by a difficulty in maintaining representations of self and others, which is better addressed by assessing self at different time points. Participants rated how well 37 different traits described their own personality and the personality of a close friend. Traits used here and within the fMRI task (consisting of different sets) were selected from previous research (Anderson, 1968), and for high degree of use in the English language. An additional 23 traits related to physical features of themselves and other were also used. Participants rated all traits twice, separated by 3 hours.
Self-Other Differentiation
The Differentiation of Self Inventory (DSI; Skowron & Friedlander, 1998) is a 43-item self-report measure assessing four aspects of differentiation in current relationships. “I” Position reflects the degree to which a person has a defined sense of self (e.g., ‘I usually do not change my behavior simply to please another person’). Fusion with Others reflects emotional overinvolvement (e.g., ‘When my spouse or partner is away long, I feel like I am missing a part of me’). Emotional Reactivity reflects reactivity to environmental stimuli (‘If someone is upset with me, I can’t seem to let it go easily’). Emotional Cutoff reflects fears of engulfment, and efforts to distance oneself (e.g., ‘When one of my relationships becomes very intense, I feel the urge to run away from it’). Higher scores indicate more differentiation. Research has found the measure to reliable and valid (Skowron & Friedlander, 1998).
fMRI Task
The task was based on D’Argembeau and colleagues (2007) and consists of evaluating personality traits. Participants were asked to identify a friend with whom they were close. Evaluation targets were arranged in a 2×2 factorial design with the following conditions: a) first-person, self (Are you kind?), b) first-person, other (Is Julie kind?), c) third-person, self (According to Julie, are you kind?), and d) third-person, other (According to Julie, is she kind?). Each block consisted of four 5-sec trials, in which four questions within the same condition were presented. Traits for each trial were pulled randomly from a list of 160 personality traits, with 40 traits presented per condition. All blocks were separated by a 7–12 second inter-trial interval.
fMRI data collection and analysis
Functional data were collected with a 3T Siemens Tim Trio scanner, with 4 runs of 127 T2* images (TR: 2500 ms, TE: 25ms, 36 interleaved slices, 3.44mm in-plane resolution, 3.4-mm slice thickness, no gap). Data were preprocessed using AFNI (Cox, 1996) and FSL 5.0.2 software (Jenkinson, Beckmann, Behrens, Woolrich, & Smith, 2012). Specific preprocessing steps were: 1) removal of transient signal spikes caused by large head movements or MRI artifacts (3dDespike); 2) slice-timing correction (slicetimer); 3) motion correction using sinc interpolation (mcflirt); 4) removal of non-brain voxels (bet); 5) co-registration of the functional scan to the structural scan; 6) spline-based warping of functional scans to the MNI stereotaxic space using both 12-parameter linear and cubic spline nonlinear transforms based on the structural scan (flirt and fnirt); 7) nonlinear spatial smoothing using a 5mm full width at half maximum kernel (susan); and 8) grand median intensity rescaling (3dcalc). As part of the warp to MNI space, data were resampled to 3mm cubic voxels for further analysis. We included 6 motion parameters in the single-subject analyses, as well as spike regressors that censored volumes where change in global intensity was above the 75th %ile + 1.5*IQR cutoff.
Neural activation for each subject was calculated using general linear model (GLM) analyses. Contrast estimates, as well as their standard errors, were used as the inputs for the mixed-effects group analyses computed using FSL’s FLAME software. In addition, we used a small-volume correction to probe areas related to self–representation in past research. As articulated above, a central question of our study was whether BPD is characterized by a specific problem with self-representation, so small volume correction was an important step to reduce the risk of Type II errors. To generate a mask containing only voxels relevant to self and other representations, we performed a meta-analysis using Neurosynth (Yarkoni, Poldrack, Nichols, Van Essen, & Wager, 2011). We generated a reverse inference map based on a union of the terms “self,” “self-reflection,” and “perspective”. This software performs a meta-analysis derived from studies applicable to the search terms used. The meta-analysis generated a mask included large clusters in brain regions (e.g., mPFC, bilateral TPJ, temporal poles, precuneus, inferior frontal gyri), determined a priori to be involved in self- and other-processing. Relevant voxels were included by thresholding the map at p < .05, FDR corrected, voxel extent = 20. To allow for anatomical variability across subjects, the mask was dilated once, resulting in 7115 candidate voxels. Using this mask for cluster correction simulations, clusters of 19 or more voxels were significant at cluster p < .05, voxel p < .005.
To interrogate a potential psychological basis of the substantial group differences in task-related neural activating, we ran a voxelwise mediation analysis (Preacher & Hayes, 2008) to test whether task-related differences in activation across groups were mediated by personality representation maintenance. We chose personality representation maintenance as a mediator because it represents a performance-based index (making it less susceptible to demand characteristics or self-insight) and has been well researched. In addition, the difficulty in activating a consistent representation of oneself and others is believed to be a central aspect of BPD. Mediation was conducted using the BRAVO Toolbox (Verstynen & Weinstein, 2011), which applies the approach of Preacher and Hayes (2008) to statistics for each voxel. Group (BPD v. control) served as the IV, personality representation maintenance the mediator, and brain activation the DV. To focus on brain areas in which some group difference was evident, we constrained analyses to voxels that were significantly different between groups at p < .1 in the main effect group map. Mediation significance tests were based on the product of the group → consistency and consistency → representation activation coefficients, and p-values were calculated using bias-corrected nonparametric bootstrapping (Verstynen & Weinstein, 2011).
Results
Card-Sort Indices
Table 2 details group differences on card-sort indices. As expected, participants with BPD created sorts with less integrated self-concepts compared to healthy controls. Also as predicted, individuals with BPD had a higher proportion of negative traits contained in their sorts. BPD participants generated more self-aspects on average compared to healthy participants.
Table 2.
Group Differences in self-report and Self-Aspects Card Sort Measures.
| Measure | BPD | HC | Cohen’s d | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| t | p-value | M | SD | M | SD | ||
|
|
|
||||||
| DSI Total | −5.29 | <.001 | 3.30 | 0.47 | 4.05 | 0.39 | 1.73 |
| DSI - Emotional Reactivity | −4.14 | <.001 | 2.98 | 0.72 | 3.87 | 0.58 | 1.35 |
| DSI – “I” Position | −2.60 | .014 | 3.74 | 0.89 | 4.41 | 0.68 | .84 |
| DSI – Emotional Cutoff | −4.47 | <.001 | 3.79 | 0.78 | 4.81 | 0.61 | 1.46 |
| DSI – Fusion with Others | −2.50 | .017 | 2.70 | 0.66 | 3.13 | 0.36 | .81 |
| Overlap | −1.51 | .139 | 0.20 | 0.14 | 0.29 | 0.19 | .50 |
| Number of Aspects | 2.24 | .031 | 8.18 | 4.84 | 5.62 | 1.83 | .70 |
| Evaluative Integration (Phi) | 2.15 | .039 | 0.82 | 0.18 | 0.66 | 0.25 | .75 |
| Proportion positive | −4.38 | <.001 | 0.59 | 0.14 | 0.80 | 0.16 | 1.44 |
Note. DSI = Differentiation of Self Inventory. Remaining measures from Self-Aspects Card Sort measure.
Maintenance of self- and other-representations
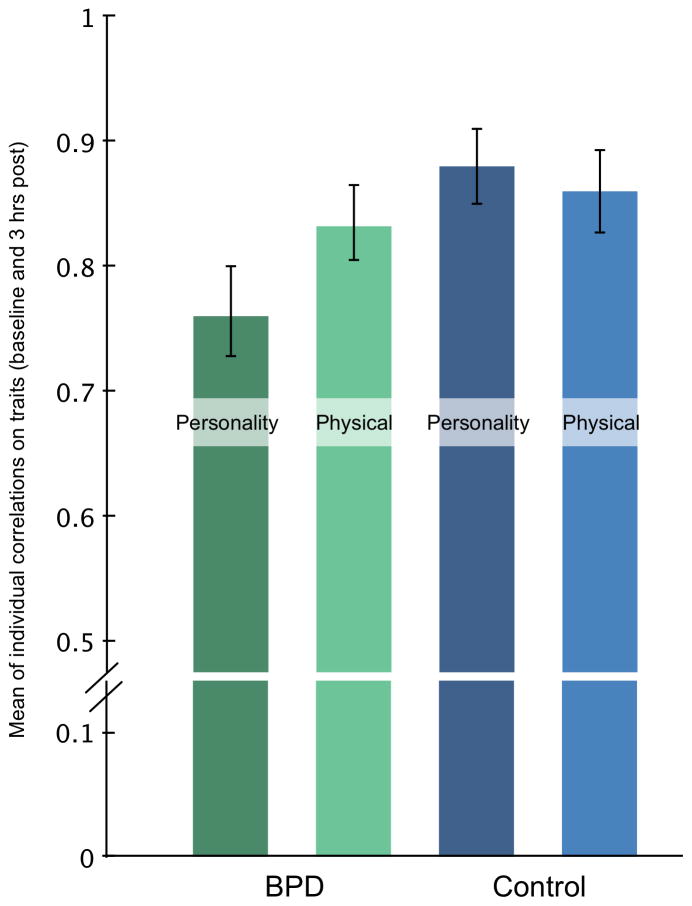
Maintenance of self-representation was assessed by within-person correlations of participant ratings with their ratings 3 hours later. A 2×2×2 mixed-design ANOVA, with group, self-other target, and personality versus physical traits as factors examined the influence of these conditions on maintenance of trait ratings. We omitted one BPD participant who had low consistency (r=.16). The within-subjects main effect of trait type (physical v. personality), F(1, 35) = 6.24, p = .028, η2 = .13 and the between-subjects group effect were both significant, F(1, 35) = 5.62, p = .023, η2 = .14. These main effects were qualified by a two-way interaction between group and trait type (physical versus personality), F(1, 34) = 6.74, p = .028, η2 = .16. Comparison of estimated marginal means revealed BPD participants were less consistent in rating personality traits, M = .78, SD = .03, 95% CI = .72–.82, but not physical traits M = .83, 95% CI = .77–.88, compared to control personality traits, M = .88, SD = .02, 95% CI = .83–.93, or physical traits M = .89, SD = .03, 95% CI =.83–.94 (see figure 1).
Figure 1.
Means and standard errors for the self and other representational maintenance task. Individual with BPD had lower consistency specific to personality traits over three hours.
Self-report
Table 2 details group differences on measures of self-representation. As expected, examining the DSI scale, the BPD group evidenced less differentiation between themselves and others overall, and differed on all subscales of the measure in the expected direction. Table 3 details correlations between self-report and performance based measures.
fMRI task and neural analyses
Reaction times for the fMRI task were different based on condition F(3, 108) = 2.72, p=.048, but there was no group or group x condition interaction. Pairwise tests revealed participants answered first-self (M = 1770.87ms, SE = 76.90) and first-other (M = 1760.70ms, SE = 76.90) conditions faster than third-other (M = 1886.30ms, SE = 101.60) conditions (ps < .05). In order to determine the effects of group, target (self vs. other) and perspective (third vs. first) on brain activation, a 2 × 2 × 2 random-effects ANOVA was run. To control for familywise error, we used cluster correction based on Monte Carlo simulations estimated in AFNI 3dClustSim based on the residual smoothness of the data. We chose a corrected cluster threshold of p < .05, with a voxel-wise threshold p < .001, resulting in a minimum cluster size of 30 voxels. At this threshold, no significant clusters remained for the group x target x perspective interaction. In addition, no cluster survived this threshold for the group x target or group x perspective interactions. There were, however, significant main effects for group, target, and perspective, which are presented in Table 3. Because there was no significant activation for any interaction, we interpreted the main effects.
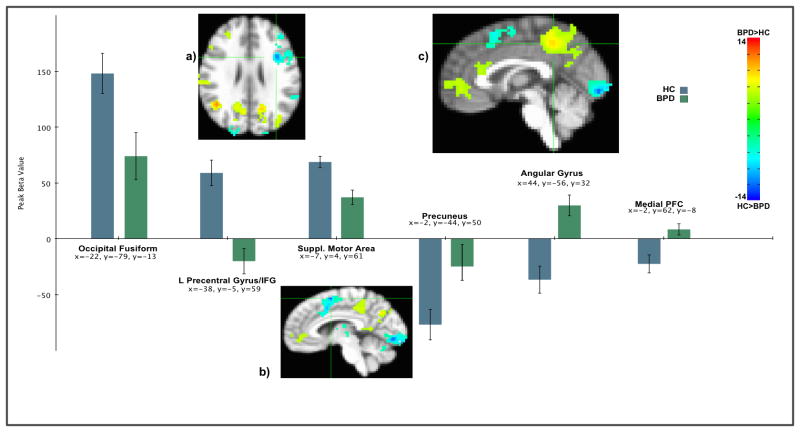
The third-person > first-person contrast yielded greater activation in the lingual gyrus, occipital cortex, precuneus, and angular gyrus. Contrasting self-representation > other-representation, there was greater activation the medial prefrontal cortex (mPFC), extending to the rostral and dorsal anterior cingulate cortex (rACC and dACC); a cluster including the right inferior parietal lobule (IFP) and temporoparietal junction (TPJ); a cluster centered on the precuneus; and a smaller cluster in the caudate. In terms of the main effect of group, those with BPD had greater activation in numerous areas including the precuneus, mPFC, and a cluster including the right angular gyrus and TPJ. The control group had greater activation in occipital areas, precentral gyrus, supramarginal gyrus, superior parietal lobule, IFG, SMA and left TPJ. Cluster peaks from selected brain areas are presented in figure 2.
Figure 2.
Peak activation from selected clusters from BPD>control ANOVA contrast. More positive betas for the control group relative to BPD are in light blue to blue. More positive betas for the BPD group are light green to red. Areas shown are a) left Precentral/inferior frontal gyrus, b) supplemental motor area, and c) precuenous. Maps thresholded at z=4.89. Color bar refers to z-scores.
To rule out the possibility that we failed to detect significant group-task interactions because of our stringent whole-brain statistical threshold, we used a small-volume correction to within regions involved in self-representation according to a meta-analysis of relevant studies (described above). Nevertheless, we did not find any significant activation for any of the interactions at this more liberal statistical threshold, using the more focused approach described in the methods section. As a consequence, results from the whole-brain ANOVA were retained.
Mediation
Greater BOLD signal in the lingual gyrus, other visual areas, the IFG, ACC, and supramarginal gyrus in the HC control group was mediated by greater consistency in representing oneself and others. Greater activation for the BPD group in the temporal pole and other temporal areas, precuneus, mPFC, insula and parahippocampal gyrus (see table 5), regions thought to support reflecting on self and others, was mediated by their poorer consistency.
Table 5.
Brain areas in which group difference activation is mediated by personality representation maintenance scores
| Brain region | MNI-Coordinates | ab coefficient | ab standard dev | z-score | Cluster size | |||
|---|---|---|---|---|---|---|---|---|
| H | x | y | z | |||||
| Lingual Gyrus | L/R | −1.5 | −70.5 | −1.5 | .16 | .05 | 12.32 | 1967 |
| Precuneus | L/R | 4.5 | −52.5 | 40.5 | −.03 | .02 | −9.50 | 373 |
| Supramarginal Gyrus/Postcentral Gyrus | L | −13.5 | −37.5 | 64.5 | .15 | .04 | 9.87 | 357 |
| Medial Prefrontal Cortex | R | 13.5 | 52.5 | 1.5 | −.12 | .07 | −9.08 | 159 |
| Inferior Frontal Gyrus | L | −49.5 | 16.5 | 4.5 | −.15 | .04 | −10.53 | 121 |
| Frontal Pole | R | 34.5 | 55.5 | −10.5 | .11 | .09 | 9.61 | 109 |
| Parahippocampal Gyrus | L | −22.5 | −31.5 | −19.5 | −.13 | .05 | −10.38 | 100 |
| Lateral Occipital Cortex | L | −22.5 | −79.5 | 49.5 | .13 | .03 | 9.27 | 99 |
| Insula, Orbital Fontal Cortex | L | −34.5 | 16.5 | −10.5 | −.13 | .03 | −8.25 | 95 |
| Superior Temporal Gyrus | L | −61.5 | −10.5 | 7.5 | .14 | .04 | 8.44 | 91 |
| Inferior Frontal Gyrus | R | 43.5 | 10.5 | 19.5 | −.15 | .05 | −7.42 | 84 |
| Temporal Pole | R | 52.5 | 1.5 | −13.5 | −.13 | .03 | −7.71 | 82 |
| Postcentral Gyrus | L | −25.5 | −34.5 | 64.5 | .15 | .03 | 7.39 | 74 |
| Dorsal Anterior Cingulate Gyrus | L/R | 1.5 | −1.5 | 34.5 | .13 | .03 | 7.99 | 66 |
| Postcentral Gyrus | R | 31.5 | −34.5 | 46.5 | .12 | .03 | 7.39 | 66 |
Note. Theshold p < .001, k=30, corrected. All indirect effects represent full mediation. After controlling for ab paths, no group -> BOLD associations were significant at p <.05. Better maintenance of representations among the control group was associated with greater activation in the lingual gyrus and other occipital areas, mirror neuron regions (IFG, Supramarginal gyrus, Postcentral Gyrus, dACC), and the frontal pole. Poor maintenance of representations among the BPD group was associated with greater activation in regions for abstract aspects of mentalization, including the precuneus, mPFC, temporal pole and insula, and the parrahippocampal gyrus.
Relationship to Depressive Symptomology and Medication Use
In order to examine the effect of depressive symptoms and medication use on brain activation, we isolated each of the clusters from the main effect of group from our ANOVA analysis and extracted mean activation from each of these ROIs for each participant. Using multilevel models, we ran a 2×2×2 ANOVA (group x self-other x first-third) for each ROI, entering BDI-II (Beck, Steer, Ball, & Ranieri, 1996) summary scores and medication use (yes/no) as potential covariates. Group effects in all ROIs remained significant after adjusting for these covariates. We duplicated this analysis on ROIs from the mediation analysis with the same result and found that group-related mediated effects were robust to these covariates.
Discussion
We sought to investigate the nature of self- and other-representational disturbance in BPD using a number of methods. We hypothesized that a) individuals with BPD, compared to controls, would have more complex, but less integrated and more negative self-representations, b) individuals would show poorer maintenance of self- and other-representations for personality, but not physical traits, and poor self-other differentiation, and c) that, on a neural level, individuals with BPD would evidence neural abnormalities, but not specifically to conditions of self or other. Additionally, based on hypothesis C, we expected that self- and other-representation maintenance, across self and others, would mediate activation in regions comprising mirror-neuron and CMS neural networks. With our card-sort task, we found that individuals with BPD sorted more self-aspects, but each sort tended to be more compartmentalized by valence, rather than integrated, and more negative as a whole. BPD patients also evidenced less maintenance of self and other personality representations over a 3-hour period. In addition, using fMRI, we found the BPD group displayed differences in brain regions related to social cognition and self-reflection, though did not evidence specific abnormalities for any single condition. Mediation analyses revealed that greater activation in visual, sensory, motor, and mirror neuron regions in the control group were mediated, in part, by better personality representation maintenance. In contrast, greater activation by the BPD group in the regions crucial to mentalization and self-other representation were mediated by relative inconsistency in self and other personality representations.
Across levels of analysis, our results paint a convergent pattern of self and social cognition in BPD. In this study, complexity mixed with relatively less organization appears to characterize the social cognition of individuals with BPD. Participants with BPD identified more self-aspects during the card-sort task, and yet this additional complexity was mixed with less integration of self-aspects, less well-defined boundaries between self and other (self-report), and poorer maintenance of personality representations of oneself and others (representation maintenance task). The neural results are consistent with previous reports of hyperactivation in areas related to mentalization (e.g., mPFC, precuneus) and other social cognition in BPD (e.g., Domsalla et al., 2013; Ruocco et al., 2010). Interestingly, the neural hyperactivation displayed by the BPD group was not modulated by condition, but rather was a main effect of group. Previous reports signal similar insensitivity to task conditions in BPD (e.g, Domsalla et al., 2013). In addition, BPD group hyperactivation was associated with a poorer stability in social representation over time, a measure found by previous research to be associated with well-being, self-esteem and ability to resist merging emotionally and cognitively with others (e.g., Morse & Gergen, 1970). Others have speculated that hyperactivation, particularly in the mPFC, among individuals with BPD is a neural marker of hypermentalization, a tendency to form more complex interpretations and attributions of other’s behavior than seems warranted by data (Sharp et al., 2011). Given the current pattern of greater activation and poorer performance, a similar, more complex/abstract approach to reflecting on self and others may explain difficulties individuals with BPD have with self and other representation. Further research, using non-social contrasts and behavioral measures of hypermentalization are needed to confirm such a proposal.
We asked whether there is evidence of a selective impairment related to self in BPD. Because we used exclusively social conditions in our fMRI task, rather than a non-social control condition, the main effects of group are more difficult to interpret. However, task-based group differences (main effects not modulated by condition) between BPD participants and controls were in brain areas central to social cognition. The BPD group displayed hyperactivation in three of four areas (mPFC, TPJ, and precuneus) named by Legrand and Ruby as central to social evaluation. In addition, the main effects of self versus other and third versus first conditions were robust, and consistent with previous reports, insuring the task worked as anticipated. The dearth of group x condition findings, even at liberal thresholds, underscores the need to further investigate the lack of specificity of self-disturbance in BPD, particularly because another of our findings supports this idea (the BPD group was less consistent in personality-based representations of both self and others).
Card Sort Indices
Previous research, generally using projective measures, has found that individuals with BPD have complex representations of others, make illogical and malevolent attributions toward others and tend to see others in terms of need-gratification (e.g., Blatt & Lerner, 1983; Westen, Lohr, Silk, Gold, & Kerber, 1990). Our results extend these findings by focusing on self-representation in BPD. Individuals with BPD differed from controls on three of four indices on the self-aspects card sort – Number of Aspects, Compartmentalization and Proportion Positive. The groups did not differ on Overlap (a measure of integration of content across self-aspects). Given that theorists describe poor integration along valence, rather than content, per se (Bender & Skodol, 2007), this finding is not surprising. Interestingly, the BPD group sorted more self-aspects on average. Healthy self-complexity has been previously defined as a high number of self-aspects, coupled with high content integration. The BPD group evidenced complexity in self-representation as expressed through more self-aspects, but showed poorer integration across valence. Donahue and colleagues (Donahue, Robins, Roberts, & John, 1993) have differentiated healthy self-complexity from fragmented self-aspects, present when there is a lack of integration evident across aspects. The latter is associated with BPD-like outcomes: low self-esteem, loneliness, dissociation and depression. Though individuals with BPD generated more self-aspects, these aspects were more fragmented in terms of valence.
Maintaining representation of self and other
Bender and Skodol (Bender & Skodol, 2007) described a core difficulty of BPD as the ability to create and maintain integrated self- and other-representations (see figure 1). We asked participants to rate themselves and a close other on personality and physical traits at two time points separated by three hours. Participants with BPD were less consistent in their ratings of personality traits, but not physical traits. Thus, this group difference is specific in that individuals with BPD are less reliable in generating abstract representations of self and others, but comparable in generating concrete representations. Interestingly, this effect was found after only a short time period and with no emotional perturbation. The mechanism of this greater slippage in representations is unclear. However, with our mediation analysis, we found several brain regions that were mediated by representational maintenance. Increased activation by the control group in areas that are part of the cortical midline system (dACC) and mirror-neuron system (Supramarginal gyrus, inferior frontal gyrus, postcentral gyrus) was mediated by greater representational consistency. These networks crucially interact for the creation and maintenance of both physical and abstract and evaluative representations of self and others. Poorer representational maintenance in the BPD group mediated greater activation in the core regions of the default mode network (mPFC, Precuneus, parahippocampal gyrus). Given previous reports have found greater activation in BPD in the mPFC and other regions in BPD during social tasks (e.g., Domsalla et al., 2013), it may be that individuals with BPD exhibit hyperactivity in this network during social tasks, which impairs social cognition.
Self-report
As expected, individuals with BPD and healthy controls differed on all scales of the DSI - “I” Position, Emotional Reactivity, Fusion With Others and Emotional Cuttoff - supporting the hypothesis that individuals with BPD have more difficulty with self and other differentiation. Some of the scales of the DSI appear to directly target BPD symptoms. However, the emotional reactivity scale, for instance, generally measures reactivity in social contexts (e.g., ‘I often wonder what kind of impression I create’), suggesting more of a difficulty in disentangling emotionally and cognitively from others compared to the DSM symptom of emotional instability. Given the results of this measure, individuals with BPD see themselves as having more difficulty disentangling form others emotionally, have greater difficulty maintaining a strong sense of self in the presence of others (‘I’ Position), feel a need to separate from others emotionally (‘Emotional Cutoff’) and have difficulty achieving psychological distance from close others (‘Fusion with Others’). These findings are consistent with previous research (e.g, de Bonis et al., 1995).
Neural results of social reflection
Because our ANOVA did not find significant group x condition differences, we interpreted the main effects of each factor, self v. other, first v. third, and group. Consistent with previous reports of brain regions supporting self-representation (Legrand & Ruby, 2009), we found, across groups, that making trait judgments about oneself versus close others resulted in greater activation in the medial prefrontal cortex (mPFC), anterior cingulate cortex (ACC), right inferior parietal lobule (IFP), right temporoparietal junction (TPJ), precuneus, and caudate. The mPFC is activated when thinking about thoughts and intentions, and other social cognitive activities (Gilbert et al., 2006). The IFP and TPJ have been shown to be involved in self-other discrimination (Kelley et al., 2002; Uddin et al., 2007), while the precuneus is involved in self-consciousness and self-awareness (Cavanna & Trimble, 2006). The main effect of taking a third-person versus first-person perspective was also consistent with previous findings. Taking the perspective of a friend activated areas of the lingual gyrus, other occipital areas, precuneus, and angular gyrus. The lingual gyrus is likely involved in accessing a mental picture of another (Jackson, Meltzoff, & Decety, 2006), whereas the precuneus and angular gyrus are active in perspective taking, and other social cognitive tasks (Ruby & Decety, 2003).
While differences in brain activation were evident between BPD and control groups throughout the task, group differences in brain activation were not evident when comparing groups by condition. During social-representation as a whole, the BPD group evidenced greater activation in areas known to support self- and other-representation including the precuneus, mPFC, and a cluster including the Angular Gyrus and rTPJ. In addition, the BPD group showed greater activation in three clusters located in the frontal pole, a region supporting internal abstract evaluation. The control group had greater activation in areas related to visual processing, sensory and motor processing (precentral gyrus, supramarginal gyrus), retrieval of episodic memories (superior parietal lobule), mirror neuron regions (IFG, SMA) and regions for discerning intentions of others (lTPJ). This pattern suggests the possibility that controls were more reliant on integration of streams of information from multiple domains during the task. At the same time, the BPD group had relatively less recruitment of sensory, motor, episodic memory and mirror neuron regions. This pattern of activation may suggest excessive attempts to understand self and other that are, however, less grounded in concrete representations based on visual, episodic, sensory and mental state information. Because of the limitations of our fMRI design, these suggestions need to be verified with further research.
Strengths and Limitations
The current study had a number of strengths. First, it examined an important aspect of BPD that has been written about extensively, but largely neglected by researchers. Second, we utilized methods from social-personality psychology and technical and statistical advances from social neuroscience to better understand self-disturbance in BPD. Third, we assessed aspects of the self at phenomenological, neural, and behavioral levels, allowing for a multimethod examination of social cognition. A number of limitations were also notable. Participants were taking medications and enrolled in psychotherapy, both of which alter brain function and behavior. However, the use of unmedicated samples, who are naïve to treatment is problematic, too, in that these samples are often non-representative (Zanarini et al., 2010). Similar to other fMRI studies involving BPD patients, the diversity of medications is a complicating factor but also guards against the systematic affects of a particular class of medications (Silbersweig et al., 2007). In addition, we allowed participants with current alcohol use disorders into the study and did not provide tests of insobriety on the day of the scan. A final limitation is that we did not include an active task with which we could compare neural activation related to self- and other-representation, but instead relied on baseline fixation activation. Group differences may not just be related to self- and other-representation, but also activation related to being “on-task”.
Conclusion
This study was a novel investigation of disrupted representations of oneself and others in BPD. We found that individuals with BPD had poor temporal consistency in self and other representations, reported poor differentiation between self and others, and had more complex, though unintegrated, and negative self-representations. In addition, BPD participants evidenced hyperactivation in a network supporting self- and other-representation during the representation task as a whole. As with our behavioral results, we found little evidence of specific self-representation neural processing abnormalities in BPD. The present results suggest that individuals with BPD may utilize more complex strategies for representing self and others that are less well organized.
Table 4.
ANOVA main effects, whole brain analyses
| Main effect of self-representation>other-representation
| ||||||
|---|---|---|---|---|---|---|
| Self>Other (n=38) | MNI-Coordinates | z-score | Cluster size | |||
| Brain region | H | x | Y | z | ||
| Anterior Cingulate Gyrus/Medial Prefrontal | L/R | −4.5 | 34.5 | 16.5 | 4.05 | 441 |
| Temporoparietal Junction | R | 52.5 | −19.5 | 10.5 | 3.73 | 157 |
| Precuneus | L/R | −1.5 | −40.5 | 52.5 | 3.95 | 136 |
| Caudate | L/R | −4.5 | 13.5 | 7.5 | 3.57 | 65 |
| Main effect of third person> first person perspective
| ||||||
|---|---|---|---|---|---|---|
| Third> First (n=38) | MNI-Coordinates | z-score | Cluster size | |||
| Brain region | H | x | Y | z | ||
| Lingual Gyrus | L/R | −7.5 | −82.5 | 1.5 | 8.5 | 560 |
| Precuneus | L/R | −4.5 | −58.5 | 37.5 | 4.02 | 142 |
| Lateral Occipital Cortex | R | 37 | 15 | 36 | 3.53 | 55 |
| Angular Gyrus | L | −40.5 | −55.5 | 31.5 | 3.96 | 47 |
| Main effect of group
| ||||||
|---|---|---|---|---|---|---|
| BPD>HC (n=38) | MNI-Coordinates | z-score | Cluster size | |||
| Brain region | H | x | Y | z | ||
| Angular Gyrus extending to Temporal-Parietal Junction | R | 43.5 | −55.5 | 31.5 | 13.7 | 653 |
| Precuneus | L | −1.5 | −43.5 | 49.5 | 9.9 | 564 |
| Frontal Pole | R | 46.5 | 37.5 | 19.5 | 12.6 | 226 |
| Medial Prefrontal Cortex | L/R | 1.5 | 46.5 | 4.5 | 6.9 | 121 |
| Frontal Pole | R | 16.5 | 64.5 | −4.5 | 10.0 | 108 |
| Frontal Pole | R | 22.5 | 34.5 | 34.5 | 7.5 | 102 |
| Middle Temporal Gyrus | R | 55.5 | 1.5 | 16.5 | 7.9 | 86 |
| HC>BPD | ||||||
| Brain region | H | x | Y | z | z-score | Cluster size |
|
| ||||||
| Occipital fusiform gyrus | L/R | −22.5 | −79.5 | −13.5 | 17.4 | 1594 |
| Lateral Occipital | L/R | −31.5 | −76.5 | 19.5 | 16.8 | 1279 |
| Precentral Gyrus extending to Inferior Frontal Gyrus | L | −37.5 | −4.5 | 58.5 | 16.7 | 906 |
| Supplementary Motor Cortex | L/R | −7.5 | 4.5 | 61.5 | 15.1 | 258 |
| Superior Parietal Lobule | L | −28.5 | 1.5 | 46.5 | 15.4 | 164 |
| Precentral Gyrus | R | 46.5 | 1.5 | 46.5 | 11.8 | 148 |
| Supramarginal Gyrus | L | −46.5 | −46.5 | 16.5 | 8.7 | 110 |
| Superior Parietal Lobule | R | 31.5 | −40.5 | 52.5 | 11.5 | 79 |
| Temporo-Parietal Junction | L | −64.5 | −43.5 | 22.5 | 10.7 | 76 |
Note. Theshold p < .001, k=30, corrected.
Acknowledgments
This research was supported by grants to Kenneth N. Levy from the Pennsylvania State University Social Science Research Institute, International Psychoanalytic Association, and American Psychoanalytic Association and dissertation grants to Joseph E. Beeney from the National Institute of Health (R36 MH086285) and the Pennsylvania State University Research and Graduate Studies Office Dissertation Support Grant from the College of the Liberal Arts. We also acknowledge funding provided by the Pennsylvania State University Social, Life and Engineering Science Imaging Center. We would like to thank Samantha Bernecker, Tracy Clouthier, Lori N. Scott, Ph.D., Ann B. Stonebraker, M.S., Christina M. Temes, M.S. and Rachel H. Wasserman, Ph.D., for their assistance in conducting interview assessments. In addition, we wish to acknowledge the technical assistance of Wesley Scala, Zachary Infantolino and Tyler Richardson. Finally, we would like to thank Sharon E. Nelson and Shannon McCarrick for their assistance in recruiting participants.
Footnotes
The authors declare no competing financial interests.
Contributor Information
Joseph E. Beeney, University of Pittsburgh
Michael N. Hallquist, University of Pittsburgh
William D. Ellison, Brown University
Kenneth N. Levy, The Pennsylvania State University
References
- Anderson NH. Likableness ratings of 555 personality-trait words. Journal of Personality and Social Psychology. 1968;9(3):272–279. doi: 10.1037/h0025907. [DOI] [PubMed] [Google Scholar]
- Bateman A, Fonagy P. Psychotherapy for borderline personality disorder: Mentalization-based treatment. Oxford University Press; USA: 2004. [Google Scholar]
- Beblo T, Driessen M, Mertens M, Wingenfeld K, Piefke M, Rullkoetter N, Woermann FG. Functional MRI correlates of the recall of unresolved life events in borderline personality disorder. Psychological Medicine. 2006;36(March):845–856. doi: 10.1017/S0033291706007227. [DOI] [PubMed] [Google Scholar]
- Beck A, Freeman A, Davis D. Cognitive therapy of personality disorders. 2. 2004. [PubMed] [Google Scholar]
- Beck A, Steer R, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67(3):588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Beeney JE, Stepp SD, Hallquist MN, Scott LN, Wright AGC, Ellison WD, Pilkonis PA. Attachment and Social Cognition in Borderline Personality Disorder: Specificity in Relation to Antisocial and Avoidant Personality Disorders. Personality Disorders: Theory, Research, and Treatment. 2015 doi: 10.1037/per0000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender DS, Skodol AE. Borderline personality as a self-other representational disturbance. J Pers Disord. 2007;21(5):500–517. doi: 10.1521/pedi.2007.21.5.500. [DOI] [PubMed] [Google Scholar]
- Blatt SJ, Lerner H. The psychological assessment of object representation. Journal of Personality Assessment. 1983;47(1):7–28. doi: 10.1207/s15327752jpa4701_2. [DOI] [PubMed] [Google Scholar]
- Brown G, Rafaeli E. Components of Self-Complexity as Buffers for Depressed Mood. Journal of Cognitive Psychotherapy. 2007 doi: 10.1891/088983907782638761. [DOI] [Google Scholar]
- Campbell JD. Self-esteem and clarity of the self-concept. Journal of Personality and Social Psychology. 1990;59(3):538–549. doi: 10.1037/0022-3514.59.3.538. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129(Pt 3):564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Clarkin JF, Hull JW, Hurt SW. Factor structure of borderline personality disorder criteria. Journal of Personality Disorders. 1993;7(2):137–143. [Google Scholar]
- Clarkin JF, Yeomans FE, Kernberg OF. Psychotherapy for borderline personality: Focusing on object relations. American Psychiatric Pub; 2006. [Google Scholar]
- Coifman KG, Berenson KR, Rafaeli E, Downey G. From negative to positive and back again: Polarized affective and relational experience in borderline personality disorder. J Abnorm Psychol. 2012;121(3):668–679. doi: 10.1037/a0028502. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- D’Argembeau A, Ruby P, Collette F, Degueldre C, Balteau E, Luxen A, Salmon E. Distinct regions of the medial prefrontal cortex are associated with self-referential processing and perspective taking. J Cogn Neurosci. 2007;19(6):935–944. doi: 10.1162/jocn.2007.19.6.935. [DOI] [PubMed] [Google Scholar]
- De Bonis M, De Boeck P, Lida-Pulik H, Feline A. Identity disturbances and self-other differentiation in schizophrenics, borderlines, and normal controls. Comprehensive Psychiatry. 1995;36(5):362–366. doi: 10.1016/S0010-440X(95)90117-5. [DOI] [PubMed] [Google Scholar]
- Domsalla M, Koppe G, Niedtfeld I, Vollstädt-Klein S, Schmahl C, Bohus M, Lis S. Cerebral processing of social rejection in patients with borderline personality disorder. Social Cognitive and Affective Neuroscience. 2013 doi: 10.1093/scan/nst176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue EM, Robins RW, Roberts BW, John OP. The divided self: concurrent and longitudinal effects of psychological adjustment and social roles on self-concept differentiation. J Pers Soc Psychol. 1993;64(5):834–846. doi: 10.1037//0022-3514.64.5.834. [DOI] [PubMed] [Google Scholar]
- Dziobek I, Preissler S, Grozdanovic Z, Heuser I, Heekeren HR, Roepke S. Neuronal correlates of altered empathy and social cognition in borderline personality disorder. Neuroimage. 2011;57(2):539–548. doi: 10.1016/j.neuroimage.2011.05.005. [DOI] [PubMed] [Google Scholar]
- English T, Chen S. Culture and self-concept stability: consistency across and within contexts among Asian Americans and European Americans. Journal of Personality and Social Psychology. 2007;93(3):478–490. doi: 10.1037/0022-3514.93.3.478. [DOI] [PubMed] [Google Scholar]
- Erikson EH. Identity, youth, and crisis. New York: W. W. Norton; 1968. [Google Scholar]
- First MB, Spitzer RL, Williams JB. Structured clinical interview for DSM-IV axis I disorders SCID-I: clinician version, administration booklet. American Psychiatric Pub; 1997. [Google Scholar]
- Garnet KE, Levy KN, Mattanah JJ, Edell WS, McGlashan TH. Borderline personality disorder in adolescents: ubiquitous or specific? Am J Psychiatry. 1994;151(9):1380–1382. doi: 10.1176/ajp.151.9.1380. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Spengler S, Simons JS, Steele JD, Lawrie SM, Frith CD, Burgess PW. Functional specialization within rostral prefrontal cortex (area 10): a meta-analysis. J Cogn Neurosci. 2006;18(6):932–948. doi: 10.1162/jocn.2006.18.6.932. [DOI] [PubMed] [Google Scholar]
- Heard HL, Linehan MM. Problems of self and borderline personality disorder: A dialectical behavioral analysis. The Self in Emotional Distress: Cognitive and Psychodynamic Perspectives. 1993:301–333. [Google Scholar]
- Jackson PL, Meltzoff AN, Decety J. Neural circuits involved in imitation and perspective-taking. Neuroimage. 2006;31(1):429–439. doi: 10.1016/j.neuroimage.2005.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62(2):782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. J Cogn Neurosci. 2002;14(5):785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Kernberg OF. Borderline personality organization. J Am Psychoanal Assoc. 1967;15(3):641–685. doi: 10.1177/000306516701500309. [DOI] [PubMed] [Google Scholar]
- Legrand D, Ruby P. What is self-specific? Theoretical investigation and critical review of neuroimaging results. Psychol Rev. 2009;116(1):252–282. doi: 10.1037/a0014172. [DOI] [PubMed] [Google Scholar]
- Locke KD. H as a measure of complexity of social information processing. Pers Soc Psychol Rev. 2003;7(3):268–280. doi: 10.1207/S15327957PSPR0703_05. [DOI] [PubMed] [Google Scholar]
- Lombardo MV, Chakrabarti B, Bullmore ET, Wheelwright SJ, Sadek Sa, Suckling J, Baron-Cohen S. Shared neural circuits for mentalizing about the self and others. Journal of Cognitive Neuroscience. 2010;22:1623–1635. doi: 10.1162/jocn.2009.21287. [DOI] [PubMed] [Google Scholar]
- Lombardo MV, Chakrabarti B, Bullmore ET, Sadek Sa, Pasco G, Wheelwright SJ, Baron-Cohen S. Atypical neural self-representation in autism. Brain. 2010;133:611–624. doi: 10.1093/brain/awp306. [DOI] [PubMed] [Google Scholar]
- Loranger AW, Janca A, Sartorius N. Assessment and diagnosis of personality disorders: The ICD-10 international personality disorder examination (IPDE) Cambridge Univ Pr; 1997. [Google Scholar]
- Macrae CN, Moran JM, Heatherton TF, Banfield JF, Kelley WM. Medial prefrontal activity predicts memory for self. Cereb Cortex. 2004;14(6):647–654. doi: 10.1093/cercor/bhh025. [DOI] [PubMed] [Google Scholar]
- Masterson JF. The real self: A developmental, self and object relations approach. Routledge; 2013. [PubMed] [Google Scholar]
- Mier D, Lis S, Esslinger C, Sauer C, Hagenhoff M, Ulferts J, Kirsch P. Neuronal correlates of social cognition in borderline personality disorder. Soc Cogn Affect Neurosci. 2012 doi: 10.1093/scan/nss028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse S, Gergen KJ. Social comparison, self-consistency, and the concept of self. Journal of Personality and Social Psychology. 1970;16:148–156. doi: 10.1037/h0029862. [DOI] [PubMed] [Google Scholar]
- Pilkonis PA, Heape CL, Ruddy J, Serrao P. Validity in the diagnosis of personality disorders: The use of the LEAD standard. Psychological Assessment: A Journal of Consulting and Clinical Psychology. 1991;3(1):46. [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods. 2008;40(3):879–891. doi: 10.3758/BRM.40.3.879. [DOI] [PubMed] [Google Scholar]
- Ripoll LH, Snyder R, Steele H, Siever LJ. The neurobiology of empathy in borderline personality disorder. Curr Psychiatry Rep. 2013;15(3):344. doi: 10.1007/s11920-012-0344-1. [DOI] [PubMed] [Google Scholar]
- Ruby P, Decety J. What you believe versus what you think they believe: a neuroimaging study of conceptual perspective-taking. European Journal of Neuroscience. 2003;17(11):2475–2480. doi: 10.1046/j.1460-9568.2003.02673.x. [DOI] [PubMed] [Google Scholar]
- Ruocco AC, Amirthavasagam S, Choi-Kain LW, McMain SF. Neural correlates of negative emotionality in borderline personality disorder: An activation-likelihood-estimation meta-Analysis. Biological Psychiatry. 2013;73:153–160. doi: 10.1016/j.biopsych.2012.07.014. [DOI] [PubMed] [Google Scholar]
- Ruocco AC, Medaglia JD, Tinker JR, Ayaz H, Forman EM, Newman CF, Chute DL. Medial prefrontal cortex hyperactivation during social exclusion in borderline personality disorder. Psychiatry Res. 2010;181(3):233–236. doi: 10.1016/j.pscychresns.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Semerari A, Carcione A, Dimaggio G, Nicoló G, Pedone R, Procacci M. Metarepresentative functions in borderline personality disorder. J Pers Disord. 2005;19(6):690–710. doi: 10.1521/pedi.2005.19.6.690. [DOI] [PubMed] [Google Scholar]
- Sharp C, Pane H, Ha C, Venta A, Patel AB, Sturek J, Fonagy P. Theory of mind and emotion regulation difficulties in adolescents with borderline traits. J Am Acad Child Adolesc Psychiatry. 2011;50(6):563–573. e1. doi: 10.1016/j.jaac.2011.01.017. [DOI] [PubMed] [Google Scholar]
- Showers C, Kling KC. Organization of self-knowledge: implications for recovery from sad mood. J Pers Soc Psychol. 1996;70(3):578–590. doi: 10.1037//0022-3514.70.3.578. [DOI] [PubMed] [Google Scholar]
- Silbersweig D, Clarkin JF, Goldstein M, Kernberg OF, Tuescher O, Levy KN, Stern E. Failure of frontolimbic inhibitory function in the context of negative emotion in borderline personality disorder. Am J Psychiatry. 2007;164(12):1832–1841. doi: 10.1176/appi.ajp.2007.06010126. [DOI] [PubMed] [Google Scholar]
- Skodol AE, Clark LA, Bender DS, Krueger RF, Morey LC, Verheul R, Oldham JM. Proposed changes in personality and personality disorder assessment and diagnosis for DSM-5 Part I: Description and rationale. Personal Disord. 2011;2(1):4–22. doi: 10.1037/a0021891. [DOI] [PubMed] [Google Scholar]
- Skowron EA, Friedlander ML. The Differentiation of Self Inventory: Development and initial validation. Journal of Counseling Psychology. 1998;45(3):235. [Google Scholar]
- Spitzer RL. Psychiatric diagnosis: are clinicians still necessary? Compr Psychiatry. 1983;24(5):399–411. doi: 10.1016/0010-440x(83)90032-9. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Endicott J, Gibbon M. Crossing the border into borderline personality and borderline schizophrenia. The development of criteria. Arch Gen Psychiatry. 1979;36(1):17–24. doi: 10.1001/archpsyc.1979.01780010023001. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Iacoboni M, Lange C, Keenan JP. The self and social cognition: the role of cortical midline structures and mirror neurons. Trends Cogn Sci. 2007;11(4):153–157. doi: 10.1016/j.tics.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Molnar-Szakacs I, Zaidel E, Iacoboni M. rTMS to the right inferior parietal lobule disrupts self-other discrimination. Social Cognitive and Affective Neuroscience. 2006;1:65–71. doi: 10.1093/scan/nsl003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westen D, Cohen RP. The self in borderline personality disorder: a psychodynamic perspective. The Self in Emotional Distress: Cognitive and Psychodynamic Perspectives. 1993:334–368. [Google Scholar]
- Westen D, Lohr N, Silk KR, Gold L, Kerber K. Object relations and social cognition in borderlines, major depressives, and normals: A Thematic Apperception Test analysis. Psychological Assessment: A Journal of Consulting and Clinical Psychology. 1990;2(4):355. [Google Scholar]
- Wolf RC, Sambataro F, Vasic N, Schmid M, Thomann PA, Bienentreu SD, Wolf ND. Aberrant connectivity of resting-state networks in borderline personality disorder. J Psychiatry Neurosci. 2011;36(6):402–411. doi: 10.1503/jpn.100150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods. 2011;8(8):665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen S, Shea MT, Sanislow CA, Grilo CM, Skodol AE, Gunderson JG, Morey LC. Borderline personality disorder criteria associated with prospectively observed suicidal behavior. Am J Psychiatry. 2004;161(7):1296–1298. doi: 10.1176/appi.ajp.161.7.1296. [DOI] [PubMed] [Google Scholar]
- Zanarini MC, Stanley B, Black DW, Markowitz JC, Goodman M, Pilkonis P, Bohus M. Methodological considerations for treatment trials for persons with borderline personality disorder. Annals of Clinical Psychiatry. 2010;22(2):75–83. [PMC free article] [PubMed] [Google Scholar]