Abstract
Epidemiological studies demonstrate that alcohol consumption is associated with an increased risk of colorectal cancer (CRC). In addition to promoting carcinogenesis, alcohol may also accelerate the progression of existing CRC. We hypothesized that alcohol may enhance the aggressiveness of CRC. In this study, we investigated the effect of alcohol on the migration/invasion and metastasis of CRC. Alcohol increased the migration/invasion of colorectal cancer cells (DLD1, HCT116, HT29 and SW480) in a concentration-dependent manner. Among these colon cancer cell lines, HCT116 cells were most responsive while HT29 cells were the least responsive to ethanol-stimulated cell migration/invasion. These in vitro results were supported by animal studies which demonstrated that ethanol enhanced the metastasis of colorectal cancer cells to the liver and lung. Monocyte chemoattractant protein-1 (MCP-1) is a chemokine that plays an important role in regulating tumor microenvironment and metastasis. Alcohol increased the expression of MCP-1 and its receptor CCR2 at both protein and mRNA levels. The pattern of alcohol-induced alterations in MCP-1 expression was consistent with its effect on migration/invasion; HCT116 cells displayed the highest up-regulation of MCP-1/CCR2 in response to alcohol exposure. An antagonist of CCR2 blocked alcohol-stimulated migration. Alcohol caused an initial cytosolic accumulation of β-catenin and its subsequent nuclear translocation by inhibiting GSK3β activity. Alcohol stimulated the activity of MCP-1 gene promoter in a β-catenin-dependent manner. Furthermore, knock-down of MCP-1/CCR2 or β-catenin was sufficient to inhibit alcohol-induced cell migration/invasion. Together, these results suggested that alcohol may promote the metastasis of CRC through modulating GSK3β/β-catenin/MCP-1 pathway.
Keywords: Chemokines, ethanol, metastasis, tumor promotion, Wnt signaling
Introduction
Chronic alcohol consumption is implicated as an etiological factor in the genesis of many types of cancer, including the respiratory tract, gastrointestinal tract, liver and breast [1-5]. Alcohol and its major metabolite, acetaldehyde, are listed as Group 1 human carcinogens by the International Agency for Research on Cancer (IARC). Colorectal cancer (CRC) is the second most common cause of cancer death in developed countries among men (after lung cancer) and the third most common among women [6;7]. Epidemiological study demonstrated that heavy and/or chronic alcohol consumption increases the risk of colon cancer [8-13]. In addition to acting as a risk factor for carcinogenesis, epidemiological studies also indicate that alcohol consumption is often associated with advanced and aggressive tumors [14-16]. Metastasis of CRC is the main cause of CRC mortality. Recent studies demonstrate an increase in incidents of CRC in younger adults (20-40 years) with advanced stages and lower survival rate [17-21]. Heavily drinking is a serious problem among young adults in the U.S. Therefore, it is important to determine whether alcohol promotes the aggressiveness of CRC and understand the underlying mechanisms. In this study, we investigated the effect of alcohol on CRC migration/invasion and metastasis using both in vitro and in vivo models.
Chemokines are a family of secreted cytokines which consists of more than 50 types. Chemokines bind to G-protein-coupled chemokine receptors and are involved in myriad physiological and pathological processes. Chemokines play an important role in tumorigenesis and cancer progression, such as tumor cell growth/survival, angiogenesis and metastasis [22;23]. Monocyte chemoattractant protein 1 (MCP-1), also known as chemokine (C-C motif) ligand 2 (CCL2), is one of the critical chemokines involved in all stages of tumor development, including tumor initiation and metastasis. Serum levels of MCP-1 are positively correlated with tumor stage and grade in breast and bladder cancer patients [24-26]. Recent studies suggest that MCP-1 and its receptor play an important role in colon carcinogenesis and CSC progression [27-29]. In this study, we further investigated the role of MCP-1 in alcohol-mediated CSC aggressiveness and the mechanism underlying alcohol-induced MCP-1 activation.
Materials and Methods
Materials
MTT assay kit was purchased from Roche Molecular Biochemicals (Indianapolis, IN). Transwells were purchased from Becton Dickinson Labware (Franklin lakes, NJ). Matrigel Invasion Chambers were purchased from BD Biosciences (Bedford, MA). Alcohol (200 Proof) was obtained from Fisher Scientific (Pittsburgh, PA). MCP-1 Human ELISA Kit was purchased from Invitrogen Corporation (Carlsbad, CA). Anti-human MCP-1 antibody and CCR2 antagonist (CCR2-I) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Recombinant Human MCP-1/CCL2 was purchased from Biolegend (San Diego, CA). Anti-β-catenin, anti-non phospho β-catenin (Ser33/37/Thr41), anti-phospho-GSK3β and anti-GSK3β antibodies were purchased from Cell Signaling Technology Inc. (Beverly, MA). β-catenin siRNA, MCP-1 siRNA and CCR2 siRNA were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-Ref-1 antibody was provided by Dr. Xianglin Shi (University of Kentucky, Lexington, KY). NE-PER Nuclear and Cytoplasmic Extraction Kit was purchased from Thermo Scientific (Rockford, IL). All other chemicals were obtained from Sigma-Aldrich (St. Louis, MO).
Cell culture and treatments
Human colorectal cancer cell lines: DLD-1, HCT116, HT29 and SW480 were provided by Dr. Zhuo Zhang (University of Kentucky, Lexington, KY). All of these cell lines were cultured in DMEM medium containing 10% fetal bovine serum and 1% antibiotic-antimycotic (Invitrogen Corporation, Carlsbad, CA) at 37°C with 5% CO2. For alcohol exposure, a method utilizing sealed containers was used to maintain alcohol concentrations in the culture medium [30]. With this method, alcohol concentrations in the culture medium can be accurately maintained. For most experiments, a physiologically relevant concentration of alcohol (43.4 mM or 200 mg/dl) was used. To block CCR2 signaling, CCR2 antagonist (CCR-I, 20 nM) was added into cells 2 hours prior to ethanol or MCP-1 exposure.
Cell invasion and migration
Cell invasion was assayed using Matrigel Invasion Chambers (BD Biosciences). Briefly, cells were placed on the upper compartment of invasion chambers and treated with alcohol or MCP-1 in the presence or absence of CCR2 antagonist (CCR2-I). Culture medium containing 10% FBS was added into the lower compartment of invasion chambers and served as chemoattractants for the cells. Cells were maintained in the invasion chambers overnight. The invaded cells were fixed in 3.7% paraformaldehyde and stained with 0.5% crystal violet in 2% ethanol. Membranes were washed and the dye was eluted with 10% acetic acid. Absorbance was measured at 595 nm using a microtiterplate reader (Beckman coulter).
Cell migration was analyzed using a Transwell Migration System (Costar). Briefly, cells were plated into upper chambers (Transwells with 8.0 μm pore size) in serum free medium and treated with alcohol or MCP-1 in the presence or absence of CCR2 antagonist (CCR2-I). The lower compartment of the chamber contained regular medium containing 10% FBS. The chambers were cultured at 37°C in 5% CO2 for 12 hours. Migrated cells were fixed and stained with 0.5% crystal violet, followed by dye elution and absorbance measurement as described above.
MTT assay
The number of viable cells in culture was determined by the MTT assay. Briefly, the cells were plated into 96-well plates and exposed to alcohol or MCP-1 for indicated times. After the treatment, 10 μl of MTT reagent was added into each well and the plates were incubated at 37°C for 4 hours. The cultures were solubilized and spectrophotometric absorbance was measured at 595 nm using a microtiterplate reader (Beckman coulter).
Cell fractionation and immunoblotting
After treatments, cells were washed twice in ice-cold PBS, lysed in RIPA buffer (15 mM NaCl, 50 mM Tris, 1% NP-40, 0.5% sodium deoxycholate, 2 mM EGTA, 1 mM sodium orthovanadate, 1 mM phenylmethanesulfonyl fluoride, 5 μg/ml aprotinin and 2 μg/ml leupeptin). For preparation of nuclear and cytoplasmic extracts, the NE-PER Nuclear and Cytoplasmic Extraction Kit (Thermo Scientific) were used to prepare nuclear and cytoplasmic extracts, respectively. Samples were separated by centrifugation at 10,000 rpm for 10 min at 4°C, and then resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The separated proteins were transferred to nitrocellulose membranes. The membranes were probed with the indicated primary antibodies, followed by the appropriate horseradish peroxidase-conjugated secondary antibodies, and developed by enhanced chemiluminescence.
Quantification of MCP-1 and CCR2 mRNA
The expression of MCP-1 and CCR2 mRNAs were analyzed by quantitative real-time PCR or RT-PCR. Briefly, after ethanol treatment, total RNA was extracted using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. cDNA was then synthesized from 1 microgram RNA, respectively, using the Reverse Transcription System (New England Biolabs) according to the manufacturer's instructions. Real-time PCR was performed using the LightCycler SYBR Green I Master kit (Roche Applied Science, Mannheim, Germany) on Roche LightCycler Real-Time PCR system (Roche). The data were analyzed using 2^-ddCt method. The relative expression levels of mRNA were normalized to the housekeeping gene GAPDH and compared with control groups. MCP-1 or CCR2 cDNA was amplified using DreamTag Green PCR Master Mix (Themo Scientific) according to the manufacturer's instructions. The following reverse (R) and forward (F) Primers were used: GAPDHF: 5'-CTCTCTGCTCCTCCTGTTCGAC-3', GAPDHR: 5'-TGAGCGATGTGGCTCGGCT-3', MCP-1F: 5’-TCATAGCAGCCACCTT C ATTC-3’ and MCP-1R: 5’-CATGGAATCCTGAACCCACTT-3’, CCR2F: 5’-AGAGGCA TAGGGCAGTGAGA, CCR2R: 3’-ACTCCTGGACCTCCACACAC. Each experiment was replicated at least 3 times.
siRNA and cell transfection
Transient transfection of β-catenin siRNA (β-Cat si), MCP-1 siRNA (MCP si) or CCR2 siRNA (CCR si) (San Cruz Biotech) was performed using a Neon Transfection System (Invitrogen Corporation, Carlsbad, CA) according to the manufacturer's protocol. Briefly, HCT116 cells were electroporated and incubated with control (scramble) siRNA (con si), β-Cat si, MCP si or CCR2 si. Forty eight hours after transfection, cells were exposed to alcohol (200 mg/dl) and assayed for cell migration.
Xenografts and metastasis analysis
All procedures were performed in accordance with the guidelines set by the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee. Each of the Balb/c nude mice (10 weeks old) received subcutaneous inoculation of HCT116 cells (106 in 100 μl PBS). Mice were divided into two groups and fed with standard chow diet ad libitum. The mice in the alcohol-exposed group were given 2% alcohol in their drinking water for a 12 hour-period during the night starting at 8:00 pm, and then replaced with water without alcohol at 8:00 am for the remaining 12 hours each day for 4 weeks. The mice in the control group were provided with regular drinking water only. The blood alcohol concentration (BEC) was determined using an Analox AM1 Alcohol Analyzer (Analox Instruments, Lunenburg, MA) as previously described [31]. This paradigm of alcohol exposure resulted in blood alcohol concentration of 40-50 mg/dl which is equivalent to modest alcohol consumption in humans. Metastases in the liver and lung were analyzed as previously described [31]. The initial experiment (n = 5 for EtOH group; n = 6 for control group) examined the effect of alcohol on lung metastasis. One mouse in the EtOH group died leaving only 11 animals (5 for EtOH and 6 for control). The subsequent experiment (n = 6 for EtOH group; n = 6 for control group) examined both livers and lungs.
Statistical analysis
Differences among treatment groups were tested using analysis of variance (ANOVA). Differences in which p was less than 0.05 were considered statistically significant. In cases where significant differences were detected, specific post hoc comparisons between treatment groups were examined with Student-Newman-Keuls tests. The prevalence of liver/lung metastasis between control and alcohol-treated groups was determined by the Fisher exact test.
Results
Alcohol increases migration/invasion and metastasis of colorectal cancer cells
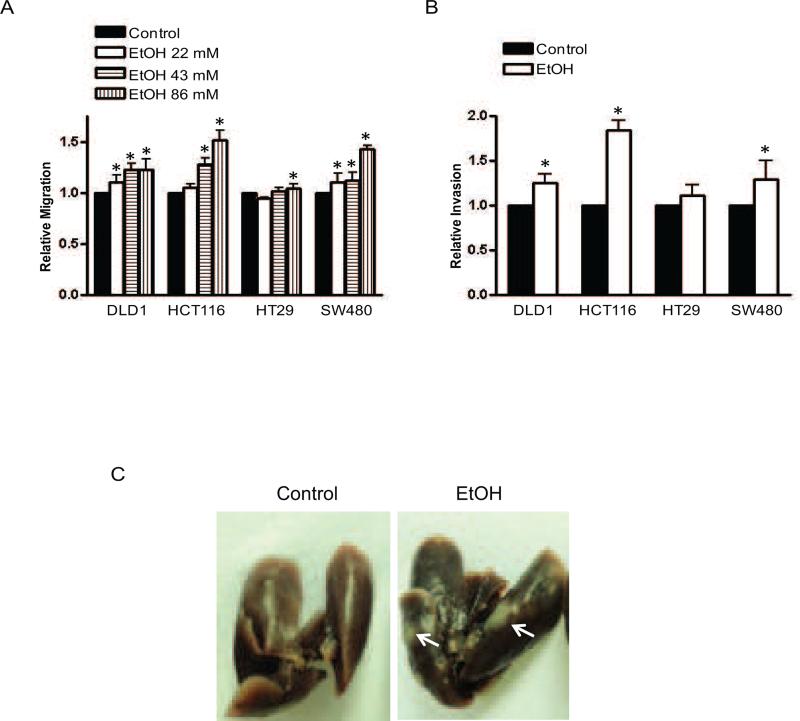
We first examined the effect of alcohol (0, 43.4 or 86.8 mM) on the migration of four CRC lines (DLD1, HCT116, HT29 and SW480). Generally, alcohol increased the migration of all these CRC lines, although the sensitivity to alcohol varied (Fig. 1A). In HCT116 cells, alcohol stimulated cell migration in a concentration dependent manner. Alcohol also enhanced the invasion of DLD1, HCT116 and SW480 cells, but not HT29 cells (Fig. 1B). Among these cells, HCT116 cells were most responsive to alcohol-stimulated migration/invasion while HT29 cells were the least. MTT assay indicated that alcohol at lower concentrations (21.7-43.4 mM or 100-200 mg/dl) had little effect on cell viability, but slightly reduced cell viability at a higher concentration (86.8 mM or 400 mg/dl) (data not shown). The in vitro data suggested that alcohol increased the aggressiveness of CRC. To determine whether alcohol indeed increased CRC aggressiveness in a physiologically relevant condition, we used a xenograft mouse model to investigate CRC metastasis. We initially examined CRC metastasis in the lung (6 mice in control and 5 mice in alcohol-exposed group). In the second set of experiments (6 mice in control and 6 mice in alcohol-exposed group), we studied CRC metastasis in both liver and lung. The data were presented in Table 1 and Fig. 1C. Five out of six mice in the alcohol-exposed group showed liver metastasis in comparison to the control in which only one out of 6 mice had liver metastasis (Table 1). Nine out eleven mice in alcohol-exposed groups exhibited lung metastasis in comparison to the control in which three out of twelve mice had lung metastasis. Therefore, alcohol exposure for 4 weeks significantly increased CRC metastasis in the liver and lung.
Figure 1.
Effect of alcohol on the migration/invasion and metastasis of colorectal cancer cells. Colorectal cancer cells (DLD1, HCT116, HT29 and SW480) were exposed to alcohol (0, 21.7, 43.4 and 86.8 mM) overnight and assayed for migration (A) and invasion (B) as described in the Materials and Methods. Each data point was the mean ± SEM of three independent experiments and presented relative to the control values. * denotes significant difference from control groups. C: HCT116 cells (106/100 μl PBS) were inoculated into nude Balb/c mice. Animals were exposed to alcohol in drinking water as described in the Materials and Methods. After 4 weeks of alcohol exposure, mice were sacrificed and analyzed for tumor metastasis. The image shows lung metastases.
Table 1.
Effect of alcohol on the metastasis of colorectal cancer cells.
| Mice | Metastasis to Liver | |
|---|---|---|
| Control | 6 | 1 |
| EtOH | 6 | 5 |
| p=0.04 |
| Mice | Metastasis to Lung | |
|---|---|---|
| Control | 12 | 3 |
| EtOH | 11 | 9 |
| p=0.01 |
HCT116 cells (106/100 μl PBS) were inoculated into nude Balb/c mice (12 in control and 11 in alcohol-exposed group). Animals were exposed to alcohol in drinking water as described in the Materials and Methods. After 4 weeks of alcohol exposure, mice were sacrificed and analyzed for tumor metastasis. The difference between control and alcohol-exposed group was determined by Fisher exact test.
Alcohol increases MCP-1 expression and function
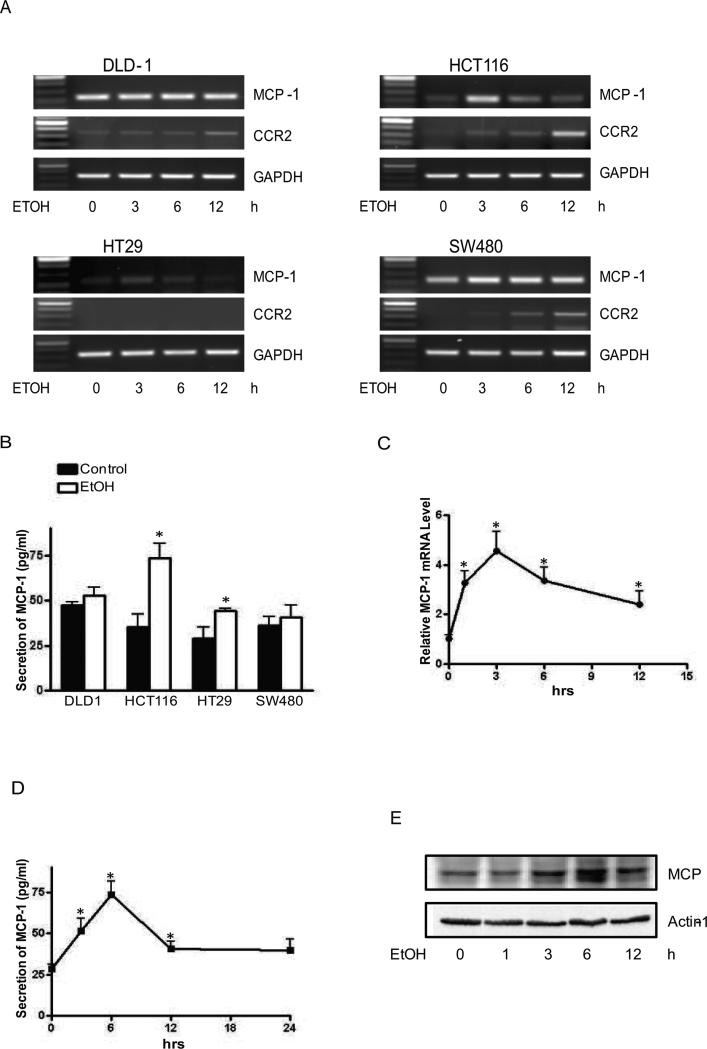
MCP-1 is implicated in the tumorigenesis and the aggressiveness of CRC [27;32], [28]. Since alcohol increased the aggressiveness of CRC, we sought to determine whether alcohol affected MCP-1 expression in CRCs. DLD1, HCT116, HT29 and SW480 cells were exposed to alcohol (43.4 mM or 200 mg/dl) for 0, 3, 6 or 12 hours and mRNA levels of MCP-1 were measured by RT-PCR. All cells expressed MCP-1 and responded to alcohol exposure by increasing MCP-1 expression. Among these cells, HCT116 cells were more sensitive to alcohol and displayed the highest increase of MCP-1 expression following alcohol exposure (Fig. 2A). Alcohol also increased the expression of MCP-1 receptor, CCR2 in DLD-1, HCT116 and SW480 cells; but the time course of alcohol-induced CCR2 up-regulation lagged behind its effect on MCP-1. Alcohol increased MCP-1 secretion in the condition medium collected from HCT116 and HT29 cells (Fig. 2B). Time course study indicated that alcohol-induced increase in MCP-1 mRNA started at 1 hour following alcohol exposure and peaked at 3 hours in HCT116 cells (Fig. 2C). ELISA study indicated that alcohol-enhanced MCP-1 secretion was observed at 3 hours and peaked at 6 hours following ethanol exposure (Fig. 2D). This observation was confirmed by immunoblotting analysis which also showed an upregulation of MCP-1 protein following alcohol exposure (Fig. 2E). Together, these data indicated that alcohol increased MCP-1 expression.
Figure 2.
Effect of alcohol on MCP-1 expression and secretion. A: Colorectal cancer cells (DLD1, HCT116, HT29 and SW480) were exposed to alcohol (43.4 mM or 200 mg/ml) for indicated time (0, 3, 6 and 12 hours). mRNA levels of MCP-1 and CCR2 were analyzed by RT-PCR and GAPDH mRNA was shown as a loading control. B: Colorectal cancer cells were exposed to alcohol (43.4 mM) for 6 hours and conditioned medium was collected and assayed for secreted MCP-1 by ELISA. C: HCT116 cells were exposed to alcohol (43.4 mM) for indicated times (0, 1, 3, 6 and 12 hours). mRNA level of MCP-1 was measured by real-time PCR. The alteration in expression was presented relative to the control level. D: HCT116 cells were exposed to alcohol (43.4 mM) for indicated times and secreted MCP-1 in conditioned medium was assayed as described above. E: HCT116 cells were exposed to alcohol (43.4 mM) for indicated times and the expression of MCP-1 was analyzed by immunoblotting. The expression of actin was shown as a loading control. Each data point was the mean ± SEM of three independent experiments and presented relative to the control values. * denotes significant difference from controls.
MCP-1 increases the migration/invasion of colorectal cancer cells
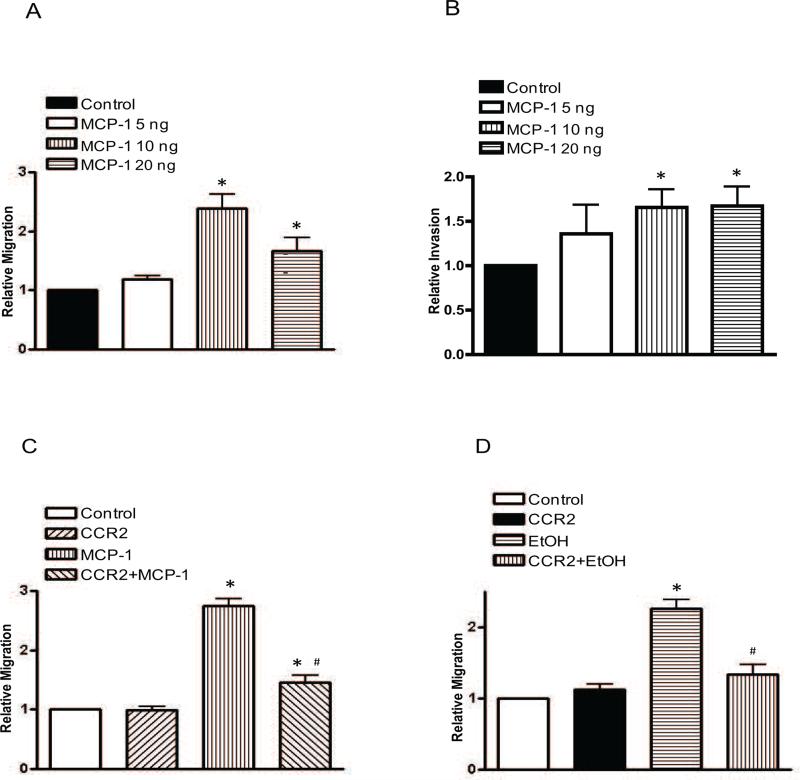
Since HCT116 cells were sensitive to alcohol, the subsequent experiments focused on this cell line. To establish the role of MCP-1 in CRC aggressiveness, we first examined the effect of MCP-1 on the migration/invasion of HCT116. In this experiment, HCT116 cells were treated with MCP-1 (0, 5, 10 or 20 ng/ml) overnight and evaluated for their migration/invasion potential. As shown in Fig. 3, MCP-1 at 10-20 ng/ml significantly increased the migration/invasion of HTC116 cells. However, we did not observe a dose-dependent effect of MCP-1 at this concentration range. MCP-1 treatment did not change the number/viability of HCT116 cells (data not shown). MCP-1 interacts with its receptor, CCR2, to execute its biological function and a recent study indicates that reduced metastasis and extravasation are observed in CCR2 deficient mice [28]. We therefore sought to determine whether blocking CCR2 signaling can inhibit MCP-1 or alcohol-stimulated migration/invasion. To inhibit MCP-1/CCR2 signaling, a CCR2 antagonist (CCR2-I) was added to the cultures 2 hours prior to MCP-1 or alcohol exposure. As shown in Figs 3C and D, CCR2-I significantly inhibited MCP-1- and alcohol-stimulated migration of HCT116 cells. Together, these data suggested that alcohol-stimulated migration/invasion may be mediated by MCP-1/CCR2 signaling.
Figure 3.
Effect of MCP-1 on the migration/invasion of colorectal cancer cells. A and B: HCT 116 cells were treated with MCP-1 (0, 5, 10 or 20 ng/ml) for overnight and assayed for cell migration (A) and invasion (B). C: HCT116 cells were pretreated either with DMSO (control) or CCR2 antagonist (20 μM) for 2 hours and then exposed to MCP-1 (10 ng/ml) overnight. After that, cell migration was assayed as described above. D: HCT116 cells were pretreated either with DMSO (control) or CCR2 antagonist (20 μM) for 2 hours and exposed to alcohol (43.4 mM) overnight, then assayed for cell migration. Each data point was the mean ± SEM of three independent experiments and presented relative to the controls. * denotes significant difference from control groups. # denotes significant difference from MCP-1- or alcohol-treated groups.
Alcohol increases MCP-1 expression through regulating GSK3β/β-catenin pathway
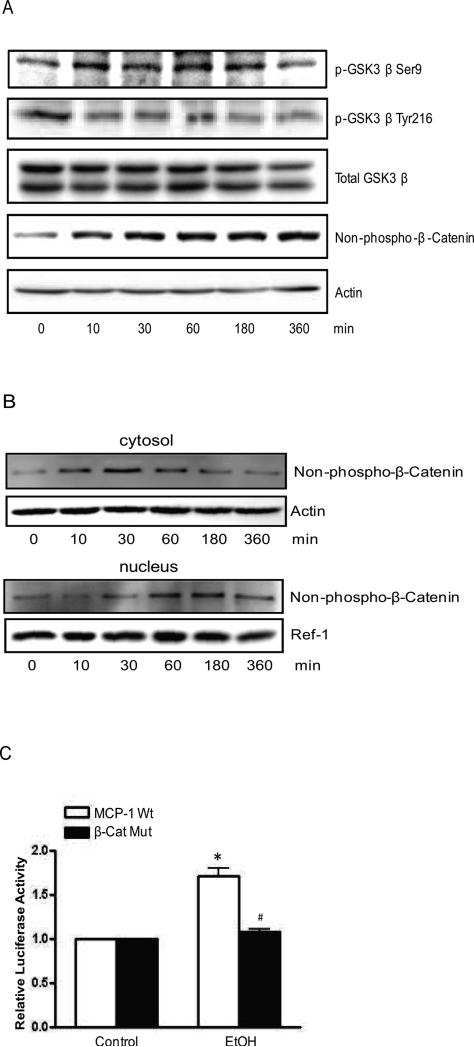
There are multiple signaling pathways that may participate in the regulation of MCP-1 expression; these include ERK, JNK, PKC and PI3K/Akt/GSK3β [33-35]. Several transcription regulators, such as NFκB, AP-1 and β-catenin are involved in this regulation [24;36]. The activity of GSK-3β is positively regulated by its phosphorylation of at Tyr216 [pGSK3β (Tyr216)] and negatively by its phosphorylation at serine 9 [pGSK3β (Ser9)]. We showed that alcohol inhibited GSK3β activity by stimulating pGSK3β (Ser9) and inhibiting pGSK3β Tyr216 (Fig. 4A). GSK-3β negatively regulates β-catenin levels; the activation of GSK-3β phosphorylates β-catenin at Ser33, Ser37 and Tyr41 which leads to the degradation of β-catenin. In contrast, inhibition of GSK-3β stabilizes β-catenin, causing the accumulation of cytosolic β-catenin and subsequent nuclear translocation. Next, we examined the expression level of β-catenin. An antibody detecting only non-phosphorylated β-catenin was used in this study. Alcohol increased protein levels of non-phosphorylated β-catenin (Fig. 4A). Alcohol induced an initial increase of β-catenin in the cytoplasm; which started at 10 min and lasted for 1 hour (Fig. 4B). At 30 min after alcohol exposure, β-catenin level in the nuclear fraction increased, indicating that the β-catenin accumulated in the cytoplasm then translocated to the nucleus (Fig. 4B). The results suggested that alcohol inhibited GSK3β activity and stabilized β-catenin, causing its translocation to the nucleus.
Figure 4.
Effect of alcohol on GSK3β and β-catenin. A: HCT116 cells were exposed to alcohol (200 mg/dl) for 10 min, 30 min, 1 hour, 3 hours and 6 hours. The expression of GSK3β, phosphorylated GSK-3β at serine 9 or tyrosine 216, β-catenin and non-phosphorylated β-catenin was analyzed by immunoblotting as described in the Materials and Methods. The expression of actin was used as a loading control. B: cytoplasmic and nuclear fractions were prepared and non-phosphorylated β-catenin (Ser33/37/Thr41) (Cell Signaling Technology Inc) was analyzed by immunoblotting as described in the Methods and Materials. The expression of actin or Ref-1 was used as a loading control for cytosolic or nuclear protein, respectively. C: HCT116 cells were transfected with luciferase reporter carrying either wild-type MCP-1 gene promoter (MCP-1 Wt) or β-catenin-mutated promoter (β-cat Mut) for 48 hours. Cells were exposed to alcohol (43.4 mM) for 6 hours, and then assayed for luciferase activity. Each data point was the mean ± SEM of three independent experiments and presented relative to the controls. * denotes significant difference from control groups. # denotes significant difference from MCP-1 Wt groups treated with alcohol.
There is a β-catenin binding site (5’-CTTTGTA-3’) on the enhancer region of human MCP-1 gene which is located 1399 bp upstream of the transcription initiation site [24]. To investigate the role of β-catenin in alcohol-stimulated MCP-1 gene transcription, we constructed a luciferase vector containing a mutated β-catenin binding site (CTTTGGC) on the enhancer region of human MCP-1 gene. HCT116 cells were transfected with luciferase vectors containing either the wild type MCP-1 gene promoter or MCP-1 gene promoter with mutated β-catenin binding site on. Forty eight hours after the transfection, cells were exposed with alcohol for 6 hours and assayed for luciferase activity. As shown in Fig. 4C, alcohol stimulation of luciferase activity was observed in the cells carrying wild type MCP-1 gene promoter, but not in cells transfected with a mutated promoter (Fig. 4C). These results indicated that β-catenin played an important role in alcohol-induced MCP-1 gene transcription.
Gene silencing of MCP-1/CCR2 or β-catenin attenuates alcohol-stimulated cell migration
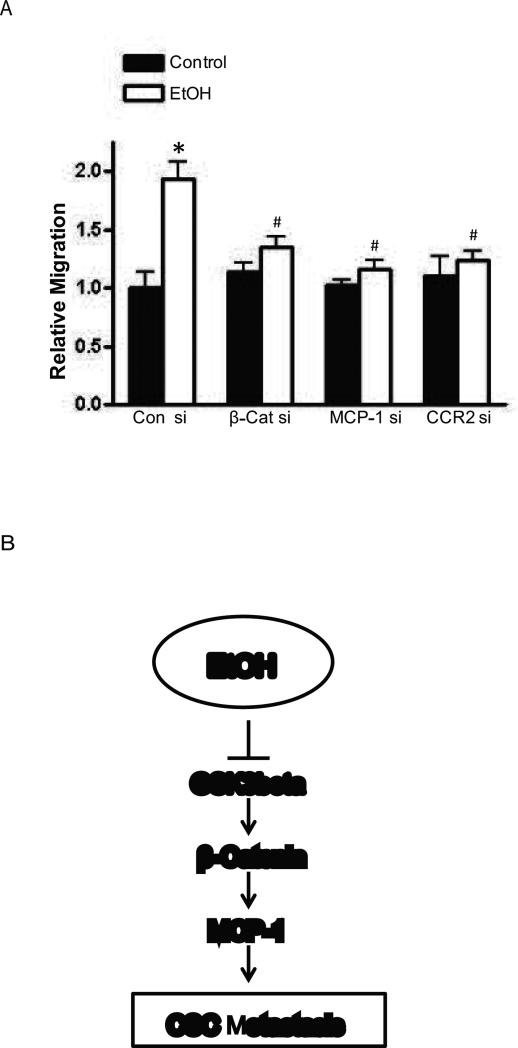
To establish the roles of MCP-1/CCR2 and β-catenin in alcohol-stimulated migration, we knocked down the expression of MCP-1/CCR2 and β-catenin in HCT116 cells with respective siRNAs. Downregulation of MCP-1/CCR2 and β-catenin was verified by immunoblotting analysis (Data not shown). Forty eight hours after the transfection of siRNA, cells were exposed to alcohol and assayed for cell migration. As shown in Fig. 5, alcohol-stimulated migration of HCT116 cells was blocked by the treatment of siRNAs for MCP-1/CCR2 or β-catenin. These results indicated that MCP-1/CCR2 and β-catenin signaling played an important role in alcohol-stimulated migration of colorectal cancer cells.
Figure 5.
Effect of siRNAs for MCP-1/CCR2 and β-catenin on alcohol-stimulated cell migration. A: HCT116 cells were transfected with the scramble siRNA (con si), β-catenin siRNA (β-Cat si), MCP-1 siRNA (MCP-1 si) or CCR2 siRNA (CCR2 si), for 48 hours, and exposed to alcohol (43.4 mM) overnight. After that, cell migration was assayed. Each data point was the mean ± SEM of three independent experiments and presented relative to the controls. * denotes significant difference from the control without alcohol exposure. # denotes significant difference from the control with alcohol exposure. B: Schematic of the signaling pathway that is involved in alcohol-induced CSC metastasis. Alcohol inactivates GSK3β, resulting in nuclear translocation of β-catenin. β-catenin activates MCP-1 gene transcription by stimulating MCP-1 gene promoter. High expression of MCP-1 may enhance CSC metastasis.
Discussion
We demonstrate here that alcohol promotes metastasis and migration/invasion of CRCs in vitro and in vivo, suggesting that alcohol may promote the aggressiveness of existing CRC in addition to increasing the risk of CRC carcinogenesis. Alcohol upregulates the expression of MCP-1 through modulating GSK3β/β-catenin pathway and blocking MCP-1-mediated signaling effectively mitigates alcohol-enhanced migration/invasion of CRCs, suggesting MCP-1 may mediate alcohol-promoted progression of CRC (Fig. 5B).
MCP-1 has been implicated in various aspects of tumorigenesis and cancer progression, such as angiogenesis, tumor growth and metastasis [25;27;29;31]. MCP-1 plays an important role in intestine inflammation and tumorigenesis/progression of colon cancer [27;32;37]. In this study, alcohol increases the migration of all CRCs tested (DLD1, HCT116, HT29 and SW480) (Fig. 1). Alcohol also enhances the invasion of these cells except HT29 cells. Among these cells, HCT116 cells are most responsive to alcohol-enhanced cell migration/invasion while HT29 cells were the least. This is generally consistent with the profile of alcohol-induced upregulation of MCP-1/CCR2; alcohol causes the most drastic increase of MCP-1/CCR2 expression in HCT116 cells. It appears that HT29 cells do not express CCR2.
Alcohol increases MCP-1/CCR2 expression at both mRNA and protein levels; it also stimulates its secretion. We have previously demonstrated that alcohol enhanced MCP-1 production in breast cancer cells which in turn promoted mammary tumor growth and angiogenesis [31]. Therefore, alcohol-mediated activation of MCP-1/CCR2 signaling pathway may not be specific for CRCs. Since MCP-1/CCR2 is important for intestine inflammatory response, alcohol stimulation of MCP-1/CCR2 may be implicated in some intestinal abnormalities associated with heavy alcohol consumption. For example, alcohol consumption is positively associated with inflammatory bowel disease (IBD) [38] and MCP-1 is a known mediator for IBD [27;39]. It is therefore possible that alcohol may promote IBD by activating the MCP-1/CCR2 pathway. In addition, patients with IBD have an increased risk of developing colon cancer. Alcohol-mediated alteration of MCP-1/CCR2 may be implicated the gut inflammation and the carcinogenesis of colon cancer.
Our in vitro data indicate that alcohol stimulates the activity of MCP-1 gene promoter, suggesting that alcohol may directly affect the transcription of MCP-1 gene (Fig. 4). Several transcription factors, such as NFκB, AP-1 and Sp1, are involved in the regulation of MCP-1 gene transcription [36;40;41]. In addition, β-catenin is also shown to regulate MCP-1 gene transcription and there is a β-catenin binding site on human MCP-1 promoter located at 1399 bp upstream of the transcription initiation site [24]. We demonstrate here that alcohol exposure increases the accumulation of β-catenin in the cytoplasm and its subsequent translocation to the nucleus. Alcohol activates MCP-1 gene promoter containing wild type β-catenin binding site, but fails to activate MCP-1 gene promoter containing mutated β-catenin binding site (Fig. 5). Additionally, downregulation of β-catenin or MCP-1 by siRNA effectively inhibits alcohol-induced cell migration. Since NFκB is also a critical transcription factor regulating MCP-1 transcription, we examined the effect of alcohol on MCP-1 gene promoter containing NFκB binding sites. Alcohol does not affect the activity of MCP-1 gene promoter containing either wild type or mutated NFκB binding sites (data not shown). In some cases, NFκβ is reported to negatively regulate MCP-1 expression. For example, NFκβ is shown to repress MCP-1 expression in response to hypoxia and alcohol can induce hypoxia [42-44]. Therefore NFκB may not be involved in alcohol stimulation of MCP-1 gene transcription. Together, these data indicate that β-catenin/MCP-1 pathway is critical for alcohol promotion of CRC aggressiveness (Fig. 5B).
It appears that alcohol-induced upregulation of β-catenin is mediated by GSK3β which regulates β-catenin degradation. GSK3β is constitutively active in cells and forms a complex with adenomatous polyposis coli (APC) and scaffold protein Axin in the absence of Wingless/Wnt signal. Phosphorylation of APC by GSK3β provides a docking site for β-catenin binding. GSK3β phosphorylates β-catenin leading to its degradation by ubiquitin-proteasome pathway [45]. GSK3β is a well-known target of alcohol [46]. We show here that alcohol inhibits GSK3β activity by stimulating pGSK3β (Ser9) and suppressing pGSK3β (Tyr216), which may cause cytosolic accumulation of β-catenin and its subsequent translocation to the nucleus (Fig. 4).
This is the first study to show GSK3β/β-catenin is involved in MCP-1 gene transcription in colorectal cancers. Dysregulation of Wnt/β-catenin signaling has been frequently associated with gastrointestinal cancers and are implicated in colorectal tumorigenesis as well as growth and maintenance of colon cancer stem cells [47-50]. Abnormal expression of many Wnt/β-catenin targeted genes, such as c-myc, c-jun, claudin-1, MMP7 and VEGF, has been identified in colon cancers [51-55]. However, little is known about Wnt/β-catenin signaling and its regulation of the chemokine system in the intestine. A previous study shows that β-catenin/TCF-4 upregulates MCP-1 expression in response to losing of E-cadherin in advanced breast cancer cells [24]. GSK3β is reported to regulate MCP-1 expression in macrophage-like cells [35]. Here, we demonstrate that GSK3β/β-catenin signaling is involved in the regulation of MCP-1 expression in colon cancer in response to alcohol exposure. MCP-1 plays an important role in gastrointestinal inflammation and involved in colorectal tumorigenesis [27;56]. Dysregulation of Wnt/β-catenin signaling is a hall mark of colon cancer. More than 90% of colorectal cancers have a mutation in the components of Wnt/β-catenin signaling pathway [50]. In addition, dysregulation of GSK3β/β-catenin is associated with poor prognosis in colon cancer patients [57]. Our study not only provides a new insight into the mechanisms of alcohol-induced promotion of colon cancer, but also helps to understand how MCP-1 is regulated during colorectal carcinogenesis/progression.
Acknowledgements
This research is supported by grants from the National Institutes of Health (NIH) (AA017226 and AA015407).
Abbreviations
- AMVD
- CCL2
chemokine (C-C motif) ligand 2
- CCR2
CC chemokine receptor 2
- CCR2A
CCR2 antagonist
- CRC
Colorectal cancer
- MCP-1
Monocyte chemoattractant protein-1
Reference List
- 1.Bagnardi V, Blangiardo M, La VC, Corrao G. Alcohol consumption and the risk of cancer: a meta-analysis. Alcohol Res Health. 2001;25:263–70. [PMC free article] [PubMed] [Google Scholar]
- 2.Boffetta P, Hashibe M. Alcohol and cancer. Lancet Oncol. 2006;7:149–56. doi: 10.1016/S1470-2045(06)70577-0. [DOI] [PubMed] [Google Scholar]
- 3.Corrao G, Bagnardi V, Zambon A, La VC. A meta-analysis of alcohol consumption and the risk of 15 diseases. Prev Med. 2004;38:613–9. doi: 10.1016/j.ypmed.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 4.Seitz HK, Becker P. Alcohol metabolism and cancer risk. Alcohol Res Health. 2007;30:38–7. [PMC free article] [PubMed] [Google Scholar]
- 5.Baan R, Straif K, Grosse Y, Secretan B, El GF, Bouvard V, Altieri A, Cogliano V. Carcinogenicity of alcoholic beverages. Lancet Oncol. 2007;8:292–3. doi: 10.1016/s1470-2045(07)70099-2. [DOI] [PubMed] [Google Scholar]
- 6.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 7.Curado MP, Edwards B, Shin HR, et al. Cancer incidence in five continents. IX. IARC Sci Publ; 2009. pp. 1–837. [Google Scholar]
- 8.Poynter JN, Haile RW, Siegmund KD, Campbell PT, Figueiredo JC, Limburg P, Young J, Le ML, Potter JD, Cotterchio M, Casey G, Hopper JL, Jenkins MA, Thibodeau SN, Newcomb PA, Baron JA. Associations between smoking, alcohol consumption, and colorectal cancer, overall and by tumor microsatellite instability status. Cancer Epidemiol Biomarkers Prev. 2009;18:2745–50. doi: 10.1158/1055-9965.EPI-09-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bongaerts BW, van den Brandt PA, Goldbohm RA, de Goeij AF, Weijenberg MP. Alcohol consumption, type of alcoholic beverage and risk of colorectal cancer at specific subsites. Int J Cancer. 2008;123:2411–7. doi: 10.1002/ijc.23774. [DOI] [PubMed] [Google Scholar]
- 10.Tsong WH, Koh WP, Yuan JM, Wang R, Sun CL, Yu MC. Cigarettes and alcohol in relation to colorectal cancer: the Singapore Chinese Health Study. Br J Cancer. 2007;96:821–7. doi: 10.1038/sj.bjc.6603623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akhter M, Kuriyama S, Nakaya N, Shimazu T, Ohmori K, Nishino Y, Tsubono Y, Fukao A, Tsuji I. Alcohol consumption is associated with an increased risk of distal colon and rectal cancer in Japanese men: the Miyagi Cohort Study. Eur J Cancer. 2007;43:383–90. doi: 10.1016/j.ejca.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 12.Wakai K, Kojima M, Tamakoshi K, Watanabe Y, Hayakawa N, Suzuki K, Hashimoto S, Kawado M, Tokudome S, Suzuki S, Ozasa K, Toyoshima H, Ito Y, Tamakoshi A. Alcohol consumption and colorectal cancer risk: findings from the JACC Study. J Epidemiol. 2005;15(Suppl 2):S173–S179. doi: 10.2188/jea.15.S173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Su LJ, Arab L. Alcohol consumption and risk of colon cancer: evidence from the national health and nutrition examination survey I epidemiologic follow-up study. Nutr Cancer. 2004;50:111–9. doi: 10.1207/s15327914nc5002_1. [DOI] [PubMed] [Google Scholar]
- 14.Severi T, van MH, Verslype C, van Pelt JF. Tumor initiation and progression in hepatocellular carcinoma: risk factors, classification, and therapeutic targets. Acta Pharmacol Sin. 2010;31:1409–20. doi: 10.1038/aps.2010.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaeth PA, Satariano WA. Alcohol consumption and breast cancer stage at diagnosis. Alcohol Clin Exp Res. 1998;22:928–34. [PubMed] [Google Scholar]
- 16.Weiss HA, Brinton LA, Brogan D, Coates RJ, Gammon MD, Malone KE, Schoenberg JB, Swanson CA. Epidemiology of in situ and invasive breast cancer in women aged under 45. Br J Cancer. 1996;73:1298–305. doi: 10.1038/bjc.1996.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Behbehani A, Sakwa M, Ehrlichman R, Maguire P, Friedman S, Steele GD, Jr., Wilson RE. Colorectal carcinoma in patients under age 40. Ann Surg. 1985;202:610–4. doi: 10.1097/00000658-198511000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Connell JB, Maggard MA, Liu JH, Etzioni DA, Livingston EH, Ko CY. Rates of colon and rectal cancers are increasing in young adults. Am Surg. 2003;69:866–72. [PubMed] [Google Scholar]
- 19.Ahnen DJ, Wade SW, Jones WF, Sifri R, Mendoza SJ, Greenamyer J, Guiffre S, Axilbund J, Spiegel A, You YN. The increasing incidence of young-onset colorectal cancer: a call to action. Mayo Clin Proc. 2014;89:216–24. doi: 10.1016/j.mayocp.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Singh KE, Taylor TH, Pan CG, Stamos MJ, Zell JA. Colorectal Cancer Incidence Among Young Adults in California. J Adolesc Young Adult Oncol. 2014;3:176–84. doi: 10.1089/jayao.2014.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varma JR, Sample L. Colorectal cancer in patients aged less than 40 years. J Am Board Fam Pract. 1990;3:54–9. [PubMed] [Google Scholar]
- 22.Arya M, Patel HR, Williamson M. Chemokines: key players in cancer. Curr Med Res Opin. 2003;19:557–64. doi: 10.1185/030079903125002216. [DOI] [PubMed] [Google Scholar]
- 23.Balkwill F. Chemokine biology in cancer. Semin Immunol. 2003;15:49–55. doi: 10.1016/s1044-5323(02)00127-6. [DOI] [PubMed] [Google Scholar]
- 24.Mestdagt M, Polette M, Buttice G, Noel A, Ueda A, Foidart JM, Gilles C. Transactivation of MCP-1/CCL2 by beta-catenin/TCF-4 in human breast cancer cells. Int J Cancer. 2006;118:35–42. doi: 10.1002/ijc.21291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–5. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshidome H, Kohno H, Shida T, Kimura F, Shimizu H, Ohtsuka M, Nakatani Y, Miyazaki M. Significance of monocyte chemoattractant protein-1 in angiogenesis and survival in colorectal liver metastases. Int J Oncol. 2009;34:923–30. doi: 10.3892/ijo_00000218. [DOI] [PubMed] [Google Scholar]
- 27.McClellan JL, Davis JM, Steiner JL, Enos RT, Jung SH, Carson JA, Pena MM, Carnevale KA, Berger FG, Murphy EA. Linking tumor-associated macrophages, inflammation, and intestinal tumorigenesis: role of MCP-1. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1087–G1095. doi: 10.1152/ajpgi.00252.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolf MJ, Hoos A, Bauer J, Boettcher S, Knust M, Weber A, Simonavicius N, Schneider C, Lang M, Sturzl M, Croner RS, Konrad A, Manz MG, Moch H, Aguzzi A, van LG, Pasparakis M, Prinz M, Borsig L, Heikenwalder M. Endothelial CCR2 signaling induced by colon carcinoma cells enables extravasation via the JAK2-Stat5 and p38MAPK pathway. Cancer Cell. 2012;22:91–105. doi: 10.1016/j.ccr.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 29.Zhao L, Lim SY, Gordon-Weeks AN, Tapmeier TT, Im JH, Cao Y, Beech J, Allen D, Smart S, Muschel RJ. Recruitment of a myeloid cell subset (CD11b/Gr1 mid) via CCL2/CCR2 promotes the development of colorectal cancer liver metastasis. Hepatology. 2013;57:829–39. doi: 10.1002/hep.26094. [DOI] [PubMed] [Google Scholar]
- 30.Xu M, Bower KA, Chen G, Shi X, Dong Z, Ke Z, Luo J. Ethanol enhances the interaction of breast cancer cells over-expressing ErbB2 with fibronectin. Alcohol Clin Exp Res. 2010;34:751–60. doi: 10.1111/j.1530-0277.2010.01147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang S, Xu M, Li F, Wang X, Bower KA, Frank JA, Lu Y, Chen G, Zhang Z, Ke Z, Shi X, Luo J. Ethanol promotes mammary tumor growth and angiogenesis: the involvement of chemoattractant factor MCP-1. Breast Cancer Res Treat. 2012;133:1037–48. doi: 10.1007/s10549-011-1902-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Torres S, Bartolome RA, Mendes M, Barderas R, Fernandez-Acenero MJ, Pelaez-Garcia A, Pena C, Lopez-Lucendo M, Villar-Vazquez R, de Herreros AG, Bonilla F, Casal JI. Proteome profiling of cancer-associated fibroblasts identifies novel proinflammatory signatures and prognostic markers for colorectal cancer. Clin Cancer Res. 2013;19:6006–19. doi: 10.1158/1078-0432.CCR-13-1130. [DOI] [PubMed] [Google Scholar]
- 33.Arndt PG, Suzuki N, Avdi NJ, Malcolm KC, Worthen GS. Lipopolysaccharide-induced c-Jun NH2-terminal kinase activation in human neutrophils: role of phosphatidylinositol 3-Kinase and Syk-mediated pathways. J Biol Chem. 2004;279:10883–91. doi: 10.1074/jbc.M309901200. [DOI] [PubMed] [Google Scholar]
- 34.Ni CW, Wang DL, Lien SC, Cheng JJ, Chao YJ, Hsieh HJ. Activation of PKC-epsilon and ERK1/2 participates in shear-induced endothelial MCP-1 expression that is repressed by nitric oxide. J Cell Physiol. 2003;195:428–34. doi: 10.1002/jcp.10259. [DOI] [PubMed] [Google Scholar]
- 35.Park DW, Lee HK, Jeong TW, Kim JS, Bae YS, Chin BR, Baek SH. The JAK2-Akt-glycogen synthase kinase-3beta signaling pathway is involved in toll-like receptor 2-induced monocyte chemoattractant protein-1 regulation. Mol Med Rep. 2012;5:1063–7. doi: 10.3892/mmr.2012.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ueda A, Okuda K, Ohno S, Shirai A, Igarashi T, Matsunaga K, Fukushima J, Kawamoto S, Ishigatsubo Y, Okubo T. NF-kappa B and Sp1 regulate transcription of the human monocyte chemoattractant protein-1 gene. J Immunol. 1994;153:2052–63. [PubMed] [Google Scholar]
- 37.Kawada M, Seno H, Kanda K, Nakanishi Y, Akitake R, Komekado H, Kawada K, Sakai Y, Mizoguchi E, Chiba T. Chitinase 3-like 1 promotes macrophage recruitment and angiogenesis in colorectal cancer. Oncogene. 2012;31:3111–23. doi: 10.1038/onc.2011.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swanson GR, Sedghi S, Farhadi A, Keshavarzian A. Pattern of alcohol consumption and its effect on gastrointestinal symptoms in inflammatory bowel disease. Alcohol. 2010;44:223–8. doi: 10.1016/j.alcohol.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grimm MC, Elsbury SK, Pavli P, Doe WF. Enhanced expression and production of monocyte chemoattractant protein-1 in inflammatory bowel disease mucosa. J Leukoc Biol. 1996;59:804–12. doi: 10.1002/jlb.59.6.804. [DOI] [PubMed] [Google Scholar]
- 40.Ueda A, Ishigatsubo Y, Okubo T, Yoshimura T. Transcriptional regulation of the human monocyte chemoattractant protein-1 gene. Cooperation of two NF-kappaB sites and NF-kappaB/Rel subunit specificity. J Biol Chem. 1997;272:31092–9. doi: 10.1074/jbc.272.49.31092. [DOI] [PubMed] [Google Scholar]
- 41.Yadav A, Saini V, Arora S. MCP-1: chemoattractant with a role beyond immunity: a review. Clin Chim Acta. 2010;411:1570–9. doi: 10.1016/j.cca.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 42.Arteel GE, Iimuro Y, Yin M, Raleigh JA, Thurman RG. Chronic enteral ethanol treatment causes hypoxia in rat liver tissue in vivo. Hepatology. 1997;25:920–6. doi: 10.1002/hep.510250422. [DOI] [PubMed] [Google Scholar]
- 43.McKim SE, Uesugi T, Raleigh JA, McClain CJ, Arteel GE. Chronic intragastric alcohol exposure causes hypoxia and oxidative stress in the rat pancreas. Arch Biochem Biophys. 2003;417:34–43. doi: 10.1016/s0003-9861(03)00349-7. [DOI] [PubMed] [Google Scholar]
- 44.Safronova O, Pluemsampant S, Nakahama K, Morita I. Regulation of chemokine gene expression by hypoxia via cooperative activation of NF-kappaB and histone deacetylase. Int J Biochem Cell Biol. 2009;41:2270–80. doi: 10.1016/j.biocel.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 45.Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997;16:3797–804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luo J. GSK3beta in ethanol neurotoxicity. Mol Neurobiol. 2009;40:108–21. doi: 10.1007/s12035-009-8075-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kanwar SS, Yu Y, Nautiyal J, Patel BB, Majumdar AP. The Wnt/beta-catenin pathway regulates growth and maintenance of colonospheres. Mol Cancer. 2010;9:212. doi: 10.1186/1476-4598-9-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kolligs FT, Bommer G, Goke B. Wnt/beta-catenin/tcf signaling: a critical pathway in gastrointestinal tumorigenesis. Digestion. 2002;66:131–44. doi: 10.1159/000066755. [DOI] [PubMed] [Google Scholar]
- 49.Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–90. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 50.White BD, Chien AJ, Dawson DW. Dysregulation of Wnt/beta-catenin signaling in gastrointestinal cancers. Gastroenterology. 2012;142:219–32. doi: 10.1053/j.gastro.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brabletz T, Jung A, Dag S, Hlubek F, Kirchner T. beta-catenin regulates the expression of the matrix metalloproteinase-7 in human colorectal cancer. Am J Pathol. 1999;155:1033–8. doi: 10.1016/s0002-9440(10)65204-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–12. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 53.Mann B, Gelos M, Siedow A, Hanski ML, Gratchev A, Ilyas M, Bodmer WF, Moyer MP, Riecken EO, Buhr HJ, Hanski C. Target genes of beta-catenin-T cell-factor/lymphoid-enhancer-factor signaling in human colorectal carcinomas. Proc Natl Acad Sci U S A. 1999;96:1603–8. doi: 10.1073/pnas.96.4.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miwa N, Furuse M, Tsukita S, Niikawa N, Nakamura Y, Furukawa Y. Involvement of claudin-1 in the beta-catenin/Tcf signaling pathway and its frequent upregulation in human colorectal cancers. Oncol Res. 2001;12:469–76. doi: 10.3727/096504001108747477. [DOI] [PubMed] [Google Scholar]
- 55.Zhang X, Gaspard JP, Chung DC. Regulation of vascular endothelial growth factor by the Wnt and K-ras pathways in colonic neoplasia. Cancer Res. 2001;61:6050–4. [PubMed] [Google Scholar]
- 56.McClellan JL, Davis JM, Steiner JL, Day SD, Steck SE, Carmichael MD, Murphy EA. Intestinal inflammatory cytokine response in relation to tumorigenesis in the Apc(Min/+) mouse. Cytokine. 2012;57:113–9. doi: 10.1016/j.cyto.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Salim T, Sjolander A, Sand-Dejmek J. Nuclear expression of Glycogen synthase kinase-3beta and lack of membranous beta-catenin is correlated with poor survival in colon cancer. Int J Cancer. 2013 doi: 10.1002/ijc.28074. [DOI] [PubMed] [Google Scholar]