Abstract
Introduction
Epidemiological studies have linked domperidone use with serious cardiac arrhythmias, including sudden cardiac death, but data on age, dose, and duration of use are limited.
Objectives
The aim of this study was to assess the risk of out-of-hospital sudden cardiac death associated with domperidone use versus proton pump inhibitors (PPIs), metoclopramide, or non-use of all three medications, and to evaluate the risk of sudden cardiac death in relation to age and domperidone dose.
Methods
This was a population-based case-control study nested in a cohort of subjects aged ≥2 years in the Clinical Practice Research Datalink with one or more prescriptions for domperidone, any PPI, or metoclopramide from 2005 to 2011. Out-of-hospital sudden cardiac death was assessed by linkage with Hospital Episode Statistics and death certificates. Controls were matched on age, sex, and medical practice. The risk of sudden cardiac death in domperidone users versus risk in users of PPIs or metoclopramide was evaluated with multivariable conditional logistic regression; case-crossover analysis addressed possible residual confounding.
Results
From the study cohort (n = 681,104), 3239 sudden cardiac death cases were matched to 12,572 controls. The adjusted odds ratio (95 % confidence interval) for sudden cardiac death with current use of domperidone alone was 1.71 (0.92–3.18) versus non-use of study medications, 1.26 (0.68–2.34) versus current PPI use, and 0.40 (0.17–0.94) current metoclopramide use. The adjusted odds ratio (95 % confidence interval) relative to exposure to no study drug for domperidone >30 mg/day (eight cases, five controls) was 3.20 (0.59–17.3) and 1.65 (0.89–3.07) for age ≥61 years (27 cases, 49 controls). The odds ratio (95 % confidence interval) was 3.17 (1.72–5.83) for within-person periods of domperidone use versus non-use in the case-crossover analysis.
Conclusions
Compared with non-use of any study drug, current domperidone use was associated with sudden cardiac death in nested case-control and case-crossover analyses, with a suggestion of higher risk in older persons and users of higher daily doses.
Electronic supplementary material
The online version of this article (doi:10.1007/s40264-015-0338-0) contains supplementary material, which is available to authorized users.
Key Points
| We found an increased risk of sudden cardiac death associated with current use of domperidone compared with non-use of study medications. |
| Certain subgroups of domperidone users, e.g. users of higher daily doses and those older than 60 years of age, may be at higher risk than others. The higher risk is confined to the first 16 days of continuous treatment. |
| The risk of sudden cardiac death appears to be increased in patients receiving metoclopramide and was an unexpected finding. |
Introduction
Domperidone, a peripherally acting dopamine 2-receptor antagonist with both gastrokinetic and antiemetic actions, has been on the market since 1978 [1, 2]. It is used for the treatment of symptoms associated with upper gastrointestinal motility disorders such as diabetic gastroparesis, gastroesophageal reflux, and nausea and vomiting associated with therapy for Parkinson’s disease and cancer [3, 4].
The cardiovascular safety of domperidone came into question in the mid-1980s when the injectable formulation was withdrawn from the market after serious cardiac events, ventricular arrhythmia, cardiac arrest, and sudden cardiac death were reported in cancer patients receiving high doses of domperidone for chemotherapy-related nausea and vomiting [2]. In these case reports, intravenous adult doses ranged from 20 to 200 mg [2]. Since then, information has accumulated about the possible association between oral domperidone and increased risk of ventricular arrhythmia and sudden cardiac death from spontaneous reports in several countries [1, 2]. Results from population-based epidemiologic investigations have shown an increased risk of sudden cardiac death or a combined outcome of sudden cardiac death and serious ventricular arrhythmia in patients currently exposed to domperidone compared with patients not currently exposed in routine clinical practice [5–7]. One study found that exposure to daily doses of domperidone >30 mg (odds ratio [OR] 11.4; 95 % confidence interval [CI] 2.0–65.2) accounted for most of the effect, but this finding was based on only four exposed cases and three exposed controls in the high-dose group [6]. Another study indicated a higher risk for patients older than 60 years of age [7].
The current cohort study was designed to (i) perform an in-depth analysis of the potential dose-response effects and effects of duration of current domperidone exposure on the risk of sudden cardiac death; (ii) control possible confounding by indication by using the comparison with metoclopramide, a therapeutic alternative to domperidone [2] and proton pump inhibitors (PPIS); and (iii) evaluate the influence of possible confounding by personal characteristics on effect estimates using a case-crossover analysis. At the time the study was planned, there was insufficient published evidence to suggest an association of the PPIs or metoclopramide with sudden cardiac death.
Methods
Data Source
The source population was the Clinical Practice Research Datalink (CPRD) GOLD, a longitudinal, primary healthcare, electronic medical record database in the UK that contains data recorded by general practitioners (GPs) as part of their routine clinical practice [8]. The CPRD GOLD covers between 7 and 8 % of the UK population (4.6 million subjects as of 2011), with subjects generally representative of the whole UK population by age and sex [9].
Data recorded in the CPRD GOLD include demographic and lifestyle information; medical diagnoses that are part of the GP’s routine clinical care; and clinical events, including the date of the event, preventive care, specialist referrals and specialty consultation notes, and hospital referrals [10]. Medical diagnoses are recorded using Read codes, which have been shown to be valid for a number of clinical conditions [11]. Electronic prescriptions issued by the GPs are also recorded, including prescription date, formulation, strength, quantity prescribed, and route of administration. Dosing instructions to the patient are contained in free-text notes. Practices in England accounting for more than 40 % of subjects in the database are linked individually and anonymously to hospital episode statistics (HES) data and to death certificates collected by the office for national statistics (ONS) [12]. Linkage to this information was necessary in this study to assess out-of-hospital sudden cardiac death.
Study Design
This was a population-based, nested case-control study using longitudinal data from a single database in the UK for the years 2005 through 2011.
Study Population
The study population was selected from all individuals with permanent registration status in up-to-standard English practices whose data were linkable to HES and ONS data. A cohort of subjects was identified by the first exposure to a study drug (domperidone, any PPI medication, or metoclopramide) after at least 1 continuous year of enrolment in the CPRD GOLD from 1 January 2005 until 31 December 2011. Subjects were at least 2 years of age upon cohort entry. Subjects residing in institutions on or before cohort entry were excluded, as were subjects with a diagnosis of cancer (other than non-melanoma skin cancer) at any time before the cohort entry date.
Subjects were followed until the earliest of the last data collection date at the practice, date of diagnosis of a cancer other than non-melanoma skin cancer, date of subject transfer out of the practice, date the practice lost its up-to-standard status or its link to HES or ONS data, date of record indicating institutional residence, date of death, or 31 December 2011.
Assessment of Outcome
The study outcome was sudden cardiac death occurring out of the hospital, and was defined as an unexpected natural death from circulatory arrest, usually due to a life-threatening ventricular arrhythmia, and that was consistent with an underlying cardiac cause (i.e. no evidence of a non-cardiac underlying process such as pneumonia responsible for the death) [5, 13, 14]. To capture out-of-hospital deaths, any sudden cardiac death that occurred between the admission date of a hospitalization and 30 days after the discharge date was excluded.
All deaths occurring during the observation period were ascertained independently of exposure history by screening the electronic medical records for Read codes indicating death, by identifying patients with death recorded as the reason for transferring out of the practice, and by linkage of the CPRD GOLD with ONS death certificate information to obtain date and place of death, underlying cause of death, and all other causes of death listed on the death certificate. Causes of death are recorded in the ONS data using International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10) diagnosis codes. Deaths identified during the study period were screened for sudden cardiac death using an automated algorithm based on a published, validated computer case definition for sudden cardiac death using specific cause-of-death codes highly predictive of sudden cardiac death [13]. The list of ICD-10 codes in the algorithm, as well as additional details of case ascertainment, are found in the published protocol [15].
We excluded expected cases of death in subjects with evidence of palliative or end-of-life care shortly before the death by applying an algorithm to all suspected cases of sudden cardiac death. Cases were excluded if (i) within 45 days before the date of death, codes were recorded for palliative care or a prescription for end-of-life medication; or (ii) within 14 days before the date of death, codes were recorded for at least two specific medications prescribed for end-of-life care according to the National Health Service guidelines for palliative and end-of-life care for cancer and non-cancer patients (i.e. any morphine, except apomorphine, or a potent opioid formulation plus one or more of cyclizine, haloperidol, levomepromazine, intravenous metoclopramide, midazolam, glycopyrronium, hyoscine hydrobromide, or hyoscine butylbromide) [16].
Final cases of sudden cardiac death were cardiac deaths with an underlying cause-of-death diagnosis code consistent with sudden cardiac death, without an alternative non-cardiac cause of death (such as pneumonia or substance overdose), that occurred in a non-institutional setting and did not have evidence of palliative or end-of-life care shortly before death. The case index date was the date of death as verified by death certificate information.
Exposures
The primary exposure of interest was oral domperidone (tablets and oral solutions). The two comparator gastrointestinal medications were oral metoclopramide and oral PPIs as a group (omeprazole, lansoprazole, esomeprazole, pantoprazole, and rabeprazole). The nested case-control analysis of 28 cases and 52 controls exposed to domperidone had one case and one control with rectal exposure to domperidone. Analyses examining dose of oral domperidone excluded these individuals.
Evaluation of Exposure Categories
Using all available recorded prescription information (prescription dates, quantity prescribed, and prescription duration), we classified the person-time at risk for each individual for each study medication over the entire study period into mutually exclusive continuous intervals of current use: the time from date of prescription to end of calculated duration of exposure (duration of prescription plus 7 days); past use: the 60 days after the end of a current use time window; and non-use: study person-time outside of current use or past-use windows. We then cross-classified all study exposures to create mutually exclusive categories of current use of each medication alone, combined current use if there was exposure to more than one medication (four categories), past use of each medication, and no exposure to any study drug. Exposure evaluation, including assessment of dose and duration of domperidone, was done with the case-control status masked.
Evaluation of Domperidone Dose
We estimated the daily dose for domperidone using recorded values for the total quantity prescribed, number of packs, prescription duration, and numeric daily dose, which is derived from dosage instructions in the CPRD GOLD free text using an algorithm developed by Shah and Martinez [17]. Approximately 73 % of domperidone prescriptions in the CPRD on or after 1 January 2005 have a derived numeric daily dose value recorded. We compared the electronic numeric daily dose values with the free-text dosage instructions manually, and modified the recorded numeric daily dose value or assigned an average value when the free-text instructions were very clear. We conducted a physician survey for all cases and controls with missing dose information for domperidone prescriptions closest to the index date. Questionnaires were sent to the physicians of 44 patients without numeric daily dose recorded and, for verification, to a random sample of 75 patients with numeric daily dose recorded. Current domperidone exposure was categorized into daily dose categories of <30, 30, and >30 mg for analyses.
Evaluation of Domperidone Duration
If the numeric duration field was missing or had values that were not considered reasonable, we derived duration by dividing the quantity prescribed by the numeric daily dose value if this field was available. Duration was evaluated in the physician survey for individuals who had missing duration information after this estimation. If duration for a particular exposure episode was still missing after the physician survey, and the individual had more than one prescription of domperidone, we estimated duration based on the intervals between domperidone prescriptions for that individual. If there was only one prescription for an individual, we assigned a value corresponding to half the average length of time between first and second prescriptions for domperidone among persons with more than one prescription.
Covariates
Covariates evaluated as potential confounding variables were selected based on their possible association with sudden cardiac death and study medication use based on previous literature [6, 7]. Covariates included medical conditions evaluated using all available time up to the index date (history of serious ventricular arrhythmia, autonomic neuropathy, cerebrovascular disease, ischemic heart disease or coronary revascularization procedure, heart failure, cardiomyopathy, valvular heart disease, pulmonary heart disease, other arrhythmias and conduction disorders, hypertension, hypercholesterolemia or hyperlipidemia, severe chronic obstructive pulmonary disease, asthma, epilepsy, renal impairment, hepatic impairment, gastrointestinal conditions, Parkinson’s disease, depression, and schizophrenia); medications used concomitantly with domperidone at the index date (cytochrome P450 [CYP] 3A4 inhibitors, QTc-prolonging drugs, and drugs that may interact with domperidone metabolism); past medication exposures in the previous 365 days (cardiac medications, antihypertensives, antiarrhythmic agents, and gastrointestinal medications other than the study exposures); indicators of healthcare utilization (number of hospital episodes, hospital days, and physician visits) during the 365 days before the index date; and lifestyle variables (body mass index, cigarette smoking, and alcohol use) in all available history before the index date [18]. The codes and procedures to assess the covariates can be found in the publicly available protocol [15].
Statistical Analyses
Analyses were performed using SAS version 9.3 (SAS Institute, Inc., Cary, NC, USA) and Episheet [19]. Descriptive analyses included tabulation of individual characteristics at the cohort entry date and study exposures using frequency distributions to describe categorical data and mean, standard deviation, median, and range to summarise continuous data. Incidence rates of sudden cardiac death during current periods of use of the study medications were calculated as a measure of the absolute risk and were expressed per 1000 person-years with corresponding 95 % CIs.
Nested Case-Control Analysis
Up to four controls were matched to each case on case index date, age, sex, and general practice in which the case was registered. We first examined the effect of each covariate individually on the outcome using conditional logistic regression adjusted only for the matching variables. We next examined the effect of the study exposures on sudden cardiac death using conditional logistic regression adjusted only for the matching variables, with separate models using different reference categories for current domperidone exposure. The reference categories were (i) non-use of either comparator drug class; (ii) current PPI medication use; and (iii) current metoclopramide use. We calculated ORs with 95 % CIs as estimates of the risk ratio. To select variables for the multivariable models, we used a change-in-estimation procedure to determine whether each covariate changed the value of the OR for current domperidone exposure on sudden cardiac death without that covariate by at least 5, 3, and 1 %. For the final multivariable model, we included variables that individually changed the effect estimate by at least 3 % (n = 9) and added additional variables that changed the estimate by 1 % by applying a forward selection procedure. The final model adjusted for 19 covariates.
Sensitivity Analysis (Case-Crossover)
To explore the impact of residual confounding on the effect estimates, we performed a case-crossover analysis using the final cases of sudden cardiac death from the study cohort. The case window was the 40 days up to and including the date of sudden cardiac death, and three control windows of equal length began 6, 12, and 18 months before the index date of sudden cardiac death. The window was selected based on the average duration of domperidone exposure plus 10 days to account for potential carryover effects. A case or control window was considered exposed if a prescription date for domperidone, a PPI medication, or metoclopramide occurred within the time window. ORs were estimated using conditional logistic regression on the self-matched sets, separately for each of the three exposures.
Results
Description of Study Cohort
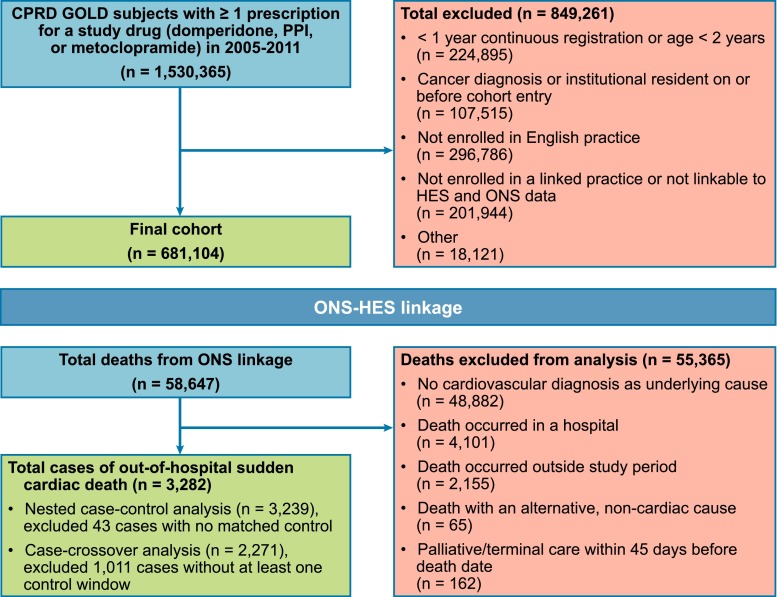
The final study cohort comprised 681,104 subjects (Fig. 1). The initial study medication at cohort entry was a PPI in 89.2 % of subjects, metoclopramide in 6.9 % of subjects, and domperidone in 5.3 % of subjects (Table 1). The median age at cohort entry was 55 years (range 2–110 years), and 57 % were females. Subjects treated with PPIs alone were older (median age 57 years) than subjects treated with domperidone alone (median age 41 years) or metoclopramide alone (median age 39 years). The proportion of females was higher among users of metoclopramide alone (73.4 %) or domperidone alone (69.9 %) than users of PPIs alone (55 %). Subjects were in the database for an average of approximately 8 years before cohort entry, which was similar for users of the three medications.
Fig. 1.
Cohort creation and assessment of deaths. CPRD clinical practice research datalink, HES hospital episode statistics, ONS office for national statistics, PPI proton pump inhibitor
Table 1.
Characteristics of the exposure cohort overall and by current use of study medications at the cohort entry date
| Characteristic | Domperidone only | PPI only | Metoclopramide only | Domperidone + PPI | Domperidone + metoclopramide | PPI + metoclopramide | Domperidone + PPI + metoclopramide | Overall |
|---|---|---|---|---|---|---|---|---|
| Sex [N (%)] | ||||||||
| Male | 9285 (30.1) | 269,107 (45.0) | 11,298 (26.6) | 2017 (38.0) | 14 (17.5) | 1661 (38.3) | 10 (43.5) | 293,392 (43.1) |
| Female | 21,580 (69.9) | 328,880 (55.0) | 31,213 (73.4) | 3286 (62.0) | 66 (82.5) | 2674 (61.7) | 13 (56.5) | 387,712 (56.9) |
| Age at cohort entry, years | ||||||||
| Mean (SD) | 44 (23) | 56 (18) | 43 (20) | 51 (22) | 42 (20) | 54 (20) | 51 (20) | 55 (19) |
| Median | 41 | 57 | 39 | 52 | 41 | 54 | 51 | 55 |
| Range | 2–106 | 2–108 | 2–110 | 2–103 | 9–85 | 2–99 | 15–84 | 2–110 |
| Time in baseline period, years | ||||||||
| Mean (SD) | 8.2 (5.2) | 8.1 (5.4) | 7.7 (5.1) | 7.7 (5.6) | 6.8 (5.1) | 7.7 (5.3) | 4.0 (3.8) | 8.1 (5.4) |
| Median | 7.4 | 7.4 | 6.9 | 6.6 | 5.6 | 6.9 | 2.4 | 7.3 |
| Range | 1.0–24 | 1.0–24 | 1.0–24 | 1.0–23 | 1.0–19 | 1.0–23 | 1.0–16 | 1.0–24 |
PPI proton pump inhibitor, SD standard deviation
Incidence of Sudden Cardiac Death
The final case count for sudden cardiac death was 3282, among 2,122,382 person-years of follow-up (Fig. 1). The overall incidence rate per 1000 person-years for sudden cardiac death was 1.55 (95 % CI 1.50–1.61) (see Table S1 in the electronic supplementary material). During person-time with current use of the study medications with or without exposure to the other study medications, the incidence rate per 1000 person-years was 4.47 (3.59–5.49) for domperidone, 5.17 (4.12–6.41) for metoclopramide, and 2.65 (2.54–2.77) for the PPI medications. The incidence rate per 1000 person-years was 0.87 (0.82–0.92) during person-time with no use of any of the study medications.
Of the 3282 cases of sudden cardiac death, 43 could not be matched with at least one control, resulting in 3239 total cases in the nested case-control analysis. A total of 12,572 controls were matched to these cases. There were 28 cases and 52 controls with current exposure to domperidone alone at the index date (see Table S2 in the electronic supplementary material for exposure status to the study medications at the index date). The results for conditional logistic regression models of each individual covariate on sudden cardiac death without the exposure variables is shown in Table S3 of the electronic supplementary material.
The ORs for sudden cardiac death for the exposure groups adjusted only for the matching variables is shown in Table 2. Multivariable adjustment resulted in attenuation of the OR for all three medication groups: current domperidone use: adjusted OR 1.71 (95 % CI 0.92–3.18); current PPI use: adjusted OR 1.35 (95 % CI 1.21–1.51); and current metoclopramide use: adjusted OR 4.31 (95 % CI 2.33–7.98). Compared with use of PPIs, during periods of current domperidone use, the adjusted OR for sudden cardiac death was 1.26 (95 % CI 0.68–2.34). An inverse association resulted when current domperidone use was compared with current metoclopramide use (adjusted OR 0.40; 95 % CI 0.17–0.94).
Table 2.
Sudden cardiac death by study exposures, nested case-control analysis: results of unadjusted and multivariable conditional logistic regression
| Exposure category (categories are mutually exclusive) | SCD cases [N = 3239] | Controls [N = 12,572] | Matched ORa | 95 % CI | Adjusted ORb | 95 % CI | ||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | |||||
| Reference level: no exposure to any study drug | ||||||||
| Current exposure to domperidonec | 28 | 0.9 | 52 | 0.4 | 3.23 | 2.02–5.16 | 1.71 | 0.92–3.18 |
| Current exposure to PPI | 1935 | 59.7 | 6499 | 51.7 | 1.72 | 1.57–1.89 | 1.35 | 1.21–1.51 |
| Current exposure to metoclopramide | 37 | 1.1 | 44 | 0.3 | 5.15 | 3.29–8.06 | 4.31 | 2.33–7.98 |
| Current combined exposured | 96 | 3.0 | 154 | 1.2 | 3.62 | 2.77–4.73 | 2.68 | 1.87–3.83 |
| Past exposure to any study drug | 341 | 10.5 | 1330 | 10.6 | 1.47 | 1.27–1.70 | 1.20 | 1.01–1.43 |
| No exposure to any study drug | 802 | 24.8 | 4493 | 35.7 | Reference | Reference | ||
| Reference level: current exposure to PPI | ||||||||
| Current exposure to domperidonec | 28 | 0.9 | 52 | 0.4 | 1.88 | 1.18–2.99 | 1.26 | 0.68–2.34 |
| Current exposure to PPI | 1935 | 59.7 | 6499 | 51.7 | Reference | Reference | ||
| Current exposure to metoclopramide | 37 | 1.1 | 44 | 0.3 | 2.99 | 1.92–4.65 | 3.19 | 1.73–5.88 |
| Current combined exposured | 96 | 3.0 | 154 | 1.2 | 2.10 | 1.62–2.73 | 1.98 | 1.40–2.81 |
| Past exposure to any study drug | 341 | 10.5 | 1330 | 10.6 | 0.85 | 0.75–0.97 | 0.89 | 0.75–1.05 |
| No exposure to any study drug | 802 | 24.8 | 4493 | 35.7 | 0.58 | 0.53–0.64 | 0.74 | 0.66–0.83 |
| Reference level: current exposure to metoclopramide | ||||||||
| Current exposure to domperidonec | 28 | 0.9 | 52 | 0.4 | 0.63 | 0.33–1.18 | 0.40 | 0.17–0.94 |
| Current exposure to PPI | 1935 | 59.7 | 6499 | 51.7 | 0.33 | 0.21–0.52 | 0.31 | 0.17–0.58 |
| Current exposure to metoclopramide | 37 | 1.1 | 44 | 0.3 | Reference | Reference | ||
| Current combined exposured | 96 | 3.0 | 154 | 1.2 | 0.70 | 0.42–1.17 | 0.62 | 0.31–1.25 |
| Past exposure to any study drug | 341 | 10.5 | 1330 | 10.6 | 0.29 | 0.18–0.45 | 0.28 | 0.15–0.52 |
| No exposure to any study drug | 802 | 24.8 | 4493 | 35.7 | 0.19 | 0.12–0.30 | 0.23 | 0.13–0.43 |
OR odds ratio, CI confidence interval, PPI proton pump inhibitor, SCD sudden cardiac death, BMI body mass index, GP general practitioner, hERG human Ether-à-go-go-Related Gene
aOR matched for age, sex, and practice. The 12,572 controls were matched to cases as follows: 70 cases with one control each, 54 cases with two controls each, 66 cases with three controls each, and 3049 cases with four controls each. Twenty-eight cases and 52 controls were exposed solely to domperidone at the index date
bOR matched for age, sex, and practice, and adjusted for the following covariates: history of serious ventricular arrhythmia, myocardial infarction, heart failure, valvular heart disease including valve replacement, cardiomyopathy, other arrhythmia or conduction disorder, epilepsy, depression, group 2 QTc-prolonging drugs, drugs that affect hERG, digoxin, diuretics, laxatives, β-blockers, BMI, alcohol use, smoking history, number of GP visits, and number of hospital admissions
cIncludes one case and one control with rectal exposure to domperidone
dCurrent exposure to more than one study drug: domperidone + PPI, domperidone + metoclopramide, PPI + metoclopramide, or domperidone + PPI + metoclopramide
Dose and Duration of Domperidone
There were 246 subjects (92 cases and 154 controls) with current domperidone exposure at the index date, of whom 59 (24 %) had missing daily dose information. There were 15 questionnaires for which the physician could not be reached or refused participation. Surveys were sent to the remaining 44 physicians to obtain dose information, and 37 questionnaires (84 %) were completed and returned; of these, 34 (92 %) contained dose information. We also sampled subjects with dosage information to verify the numeric daily dose information recorded in the CPRD GOLD, and sent 75 questionnaires to practices willing to participate; 47 questionnaires (63 %) were received, with dose being recorded in 44 of these questionnaires (94 %). For 40 of these (91 %; 95 % CI 79–97), the numeric daily dose value derived from the CPRD GOLD algorithm agreed with the value derived from the physician questionnaire. In the remaining four questionnaires, the dose reported by physicians was lower than that recorded in the CPRD GOLD. Using all sources of information, we were able to estimate the average daily dose for 241 of 246 subjects (98 %) exposed to domperidone at the index date. Using only numeric daily dose values recorded in CPRD GOLD would have resulted in missing information for 54 domperidone-exposed subjects (22 %).
Among subjects exposed to domperidone alone at the index date, the OR of sudden cardiac death compared with no exposure was highest for those exposed to more than 30 mg/day (adjusted OR 3.20; 95 % CI 0.59–17.34) (Table 3). The adjusted OR for those exposed to <30 mg/day was 1.96 (95 % CI 0.44–8.76) and 1.48 (95 % CI 0.69–3.19) for 30 mg/day. All estimates had very wide 95 % CIs due to the low number of subjects.
Table 3.
Evaluation of dose of current oral domperidone exposure on risk of sudden cardiac death, nested case-control analysis: results of multivariable conditional logistic regression, reference group non-use of any study drug
| Exposure category (categories are mutually exclusive) | SCD cases | Controls | Matched ORa | 95 % CI | Adjusted ORb | 95 % CI | ||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | |||||
| Current exposure to single oral domperidone alonec (estimated average daily dose) [mg/day] | ||||||||
| <30 | 4 | 0.1 | 10 | 0.1 | 2.32 | 0.72–7.44 | 1.96 | 0.44–8.76 |
| 30 | 15 | 0.5 | 35 | 0.3 | 2.60 | 1.41–4.79 | 1.48 | 0.69–3.19 |
| >30 | 8 | 0.2 | 5 | 0.0 | 9.07 | 2.95–27.88 | 3.20 | 0.59–17.34 |
| No exposure to any study drug | 802 | 24.8 | 4493 | 35.7 | Reference | Reference | ||
OR odds ratio, CI confidence interval, SCD sudden cardiac death, BMI body mass index, GP general practitioner, hERG human Ether-à-go-go-Related Gene
aOR matched for age, sex, and practice
bOR matched for age, sex, and practice and adjusted for the following covariates: history of serious ventricular arrhythmia, myocardial infarction, heart failure, valvular heart disease including valve replacement, cardiomyopathy, other arrhythmia or conduction disorder, epilepsy, depression, group 2 QTc-prolonging drugs, drugs that affect hERG, digoxin, diuretics, laxatives, β-blockers, BMI, alcohol use, smoking history, number of GP visits, and number of hospital admissions
cOral exposure only; analysis excludes one case and one control with rectal exposure and one control for whom dose could not be determined
Compared with no exposure, current exposure to domperidone alone for less than 16 days resulted in a higher OR for sudden cardiac death (adjusted OR 4.06; 95 % CI 1.55–10.67) than exposure for at least 16 days (adjusted OR 0.97; 95 % CI 0.42–2.26) (Table 4).
Table 4.
Evaluation of duration of current domperidone exposure on risk of sudden cardiac death, nested case-control analysis: results of multivariable conditional logistic regression, reference group non-use of any study drug
| Exposure category (categories are mutually exclusive) | SCD cases | Controls | Matched ORa | 95 % CI | Adjusted ORb | 95 % CI | ||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | |||||
| Current exposure to domperidone alone (estimated duration of current exposure episode) [days] | ||||||||
| <16 | 16 | 0.5 | 14 | 0.1 | 6.64 | 3.21–13.71 | 4.06 | 1.55–10.67 |
| 16 or more | 12 | 0.4 | 38 | 0.3 | 1.94 | 1.01–3.73 | 0.97 | 0.42–2.26 |
| No exposure to any study drug | 802 | 24.8 | 4493 | 35.7 | Reference | Reference | ||
OR odds ratio, CI confidence interval, SCD sudden cardiac death, BMI body mass index, GP general practitioner, hERG human Ether-à-go-go-Related Gene
aOR matched for age, sex, and practice
bOR matched for age, sex, and practice and adjusted for the following covariates: history of serious ventricular arrhythmia, myocardial infarction, heart failure, valvular heart disease including valve replacement, cardiomyopathy, other arrhythmia or conduction disorder, epilepsy, depression, group 2 QTc-prolonging drugs, drugs that affect hERG, digoxin, diuretics, laxatives, β-blockers, BMI, alcohol use, smoking history, number of GP visits, and number of hospital admissions
Subgroup Analyses
When stratified by age, the OR for sudden cardiac death with current use of domperidone (27 exposed cases and 49 exposed controls) was highest in the group aged 61 years or more (adjusted OR 1.65; 95 % CI 0.89–3.07). There was only one case of sudden cardiac death with current exposure to domperidone at the index date in the youngest age group (2–60 years) and three exposed controls; therefore, the point estimate in this group was very unstable.
In other subgroup analyses, many estimates were unstable (see Table S4 in the electronic supplementary material), but the results indicated that for current domperidone use, the risk of sudden cardiac death was higher among persons without diagnosed diabetes than with, among persons with concurrent exposure to CYP3A4 inhibitor medications than without, and among persons with concurrent exposure to group 1 QTc-prolonging medications (current or past use) at the index date than without.
Case-Crossover Analysis
A total of 2271 cases had at least one control window, and 1154 cases had three control windows. Results of the individual conditional regression models for each study exposure revealed that within-person periods of current use of domperidone, with or without another study exposure, were associated with an increase in risk of sudden cardiac death (conditional OR 3.17; 95 % CI 1.72–5.83) compared with periods of non-use of any study medication. Periods of current use of metoclopramide, with or without another study exposure, were also associated with an increase in risk of sudden cardiac death (conditional OR 3.08; 95 % CI 1.67–5.66); however, periods of current use of PPIs were not associated with an increase in risk of sudden cardiac death (conditional OR 1.02; 95 % CI 0.87–1.19) (Table 5).
Table 5.
Risk of sudden cardiac death with domperidone exposure: case-crossover analysis, results of individual conditional logistic regression models for each of the three study exposures
| N | Number of control windows exposed | ORa | 95 % CI | ||||
|---|---|---|---|---|---|---|---|
| 3 | 2 | 1 | 0 | ||||
| Total cases in each modelb | 2271 | ||||||
| Domperidone usec | 3.17 | 1.72–5.83 | |||||
| Cases with three control windows | 1154 | ||||||
| Case window exposed | 34 | 8 | 10 | 3 | 13 | ||
| Case window unexposed | 1120 | 3 | 0 | 6 | 1111 | ||
| Cases with two control windows | 661 | ||||||
| Case window exposed | 12 | 6 | 2 | 4 | |||
| Case window unexposed | 649 | 1 | 5 | 643 | |||
| Cases with one control window | 456 | ||||||
| Case window exposed | 12 | 6 | 6 | ||||
| Case window unexposed | 444 | 2 | 442 | ||||
| PPI usec | 1.02 | 0.87–1.19 | |||||
| Cases with three control windows | 1154 | ||||||
| Case window exposed | 625 | 412 | 115 | 57 | 41 | ||
| Case window unexposed | 529 | 48 | 72 | 73 | 336 | ||
| Cases with two control windows | 661 | ||||||
| Case window exposed | 384 | 266 | 86 | 32 | |||
| Case window unexposed | 277 | 40 | 56 | 181 | |||
| Cases with one control window | 456 | ||||||
| Case window exposed | 282 | 230 | 52 | ||||
| Case window unexposed | 174 | 51 | 123 | ||||
| Metoclopramide usec | 3.08 | 1.67–5.66 | |||||
| Cases with three control windows | 1154 | ||||||
| Case window exposed | 20 | 7 | 4 | 0 | 9 | ||
| Case window unexposed | 1134 | 0 | 2 | 7 | 1125 | ||
| Cases with two control windows | 661 | ||||||
| Case window exposed | 13 | 3 | 4 | 6 | |||
| Case window unexposed | 648 | 1 | 5 | 642 | |||
| Cases with one control window | 456 | ||||||
| Case window exposed | 20 | 9 | 11 | ||||
| Case window unexposed | 436 | 2 | 434 | ||||
OR odds ratio, CI confidence interval, PPI proton pump inhibitor
aFrom conditional logistic regression on the matched sets. Separate models were constructed for each of the three study exposures
bOf the 3282 cases of sudden cardiac death in the study, 2271 had at least one control window identified and were included in the case-crossover analysis
cWith or without use of another study medication
Discussion
We found an adjusted OR of 1.71 (95 % CI 0.92–3.18) for out-of-hospital sudden cardiac death associated with current exposure to domperidone relative to no use of any study medication after adjustment for multiple potentially confounding factors. The finding that the OR in the case-crossover analysis was higher than the adjusted OR in the nested case-control analysis suggests that the elevated adjusted effect cannot be explained by unresolved confounding by time-invariant intrinsic factors. One possible explanation is that some within-subject factors could have introduced additional confounding in the opposite direction of the confounders in the nested case-control analysis. Another possibility is the appearance of a new diagnosis shortly before the index date that would have been adjusted for in the case-control analysis but could confound the case-crossover analysis. Alternatively, it could be that the results of the two analyses were not comparable because the case-crossover design captured only intermittent rather than chronic use of the medication, while the nested case-control analysis included both. The analyses stratified by duration of current domperidone exposure provide evidence to support this explanation as the increased risk for sudden cardiac death was concentrated in the first 15 days of domperidone exposure. However, such a pattern is also consistent with, among other possibilities, depletion of susceptibles or a decrease in dose after 15 days because the indication for which the drug was prescribed has abated. One of the indications for domperidone, nausea and vomiting is, typically, a short-term condition, thus the use of domperidone for this indication may be greatest in the first 15 days after prescription. Finally, it is possible that the increased OR for short-term use could reflect protopathic bias. Vomiting and nausea are non-specific symptoms of many syndromes and diseases that can trigger the prescription of domperidone or other gastrointestinal medications. If these symptoms are related to a condition that can suddenly terminate in death, then the prescription would be associated with, but not be the cause of, the outcome. As an example, episodes of severe dizziness with nausea and vomiting can be symptoms of prolonged QT interval, torsades de pointes, or other severe ventricular arrhythmias, which, if not diagnosed and treated, can terminate in sudden cardiac death. Prodromic symptoms of coronary heart disease could mimic those of gastrointestinal disease (i.e. gastroesophageal reflux) and could be the reason for prescribing domperidone or other gastrointestinal medication. However, an increased OR was not observed with the PPI medications.
The current nested case-control analysis included 28 cases with current domperidone exposure, of 3239 sudden cardiac death cases, and 52 controls with current domperidone exposure, of 12,572 controls; results are consistent with previously published population-based case-control studies conducted in databases in The Netherlands and Canada [5–7]. The study by Straus et al. [5] described 9 of 775 cases and 15 of 6297 controls exposed to domperidone but was designed to look at non-cardiac QTc-prolonging drugs as a class, not domperidone alone, and found an adjusted OR for sudden cardiac death of 3.8 (95 % CI 1.57–9.7). A second study in the same primary care database designed to look specifically at current domperidone use versus no use found an adjusted OR for sudden cardiac death of 1.99 (95 % CI 0.80–4.96), based on ten exposed cases of 1304 and 28 exposed controls of 13,480 [6]. A nested case-control study in the Canadian Saskatchewan databases, with 169 exposed (of 1608) cases with sudden cardiac death or serious ventricular arrhythmia and 481 exposed (of 6428) controls, found an adjusted OR for sudden cardiac death or serious ventricular arrhythmia of 1.59 (95 % CI 1.28–1.98) for current domperidone use compared with no use [7]. Neither study in The Netherlands included a control group of individuals exposed to other gastrointestinal medications with indications similar to domperidone. In our study, the fully adjusted comparison of current use of domperidone with current use of PPIs resulted in an OR of 1.26 (95 % CI 0.68–2.34), similar to the value reported in the Canadian study of 1.44 (95 % CI 1.12–1.86).
With the comparison group of non-use of domperidone or PPIs, stratified analyses in the Canadian study suggested a slightly higher risk for subjects older than 60 years of age (OR 1.64) compared with those younger than 60 years of age (OR 1.10), but there were few cases and controls aged 60 years or younger [7]. The current study also found a higher risk of sudden cardiac death in subjects older than 60 years of age with domperidone use alone than in younger subjects, but the estimate in younger subjects was statistically unstable. Despite the finding that over half of the subjects using domperidone in the entire study exposure cohort were aged 41 years or younger at cohort entry, there was only one case of sudden cardiac death and three controls aged 60 years and younger with current exposure to domperidone alone at the index date. Of the three aforementioned studies, only the study by van Noord et al. [6] included an analysis of domperidone dosage and sudden cardiac death risk; the study found an OR of sudden cardiac death of 11.4 (95 % CI 2.0–65.2) associated with daily doses >30 mg (four cases and three controls) and 1.02 (95 % CI 0.23–4.42) associated with a daily dose of 30 mg (4 cases and 15 controls), but small sample sizes yielded imprecise ORs. Our study also found the highest OR associated with daily doses >30 mg. Although we had twice as many cases (n = 8) exposed to more than 30 mg/day of domperidone, the number was still insufficient to evaluate smaller groupings of doses in this category.
Our results provide support for the 1 September 2014 European Medicines Agency restrictions on the use of domperidone-containing medicines in the EU, particularly reduction of the recommended adult dose to 10 mg up to three times daily, and the contraindication for use in patients with impaired cardiac conduction, underlying cardiac disease such as congestive heart failure, and coadministration with CYP3A4 inhibitor QTc-prolonging medications [20]. An unexpected finding in our study was that current exposure to oral metoclopramide alone was associated with a fourfold adjusted increase in the risk of sudden cardiac death, which resulted in an inverse association when current use of domperidone alone was compared with current use of metoclopramide alone.
Our study used a large primary care database linked to death certificate data, and contributed additional information to previously published population-based studies by providing (i) an in-depth analysis of the potential effects of dose and duration of current domperidone exposure on the risk of sudden cardiac death; (ii) evaluation of metoclopramide and PPIs as comparator medications; and (iii) a case-crossover analysis to evaluate the possible impact of residual confounding by personal characteristics on risk estimates. An innovation of the study was the exclusion of cases with evidence of palliative care in a short period of time before death, in addition to excluding, to the extent possible, expected deaths and deaths of non-cardiac origin. Some potential limitations of the study include a possible risk for indication and protopathic bias despite the design because serious ventricular arrhythmia can be accompanied by nausea, and antiemetic medications may be administered. Also, although we excluded patients with cancer, domperidone and metoclopramide can be used for palliative care in diseases other than cancer; UK guidelines indicate that side effects of analgesics used for palliative care are nausea and vomiting, which are common in opioid-exposed patients and can be prevented by access to an antiemetic, e.g. metoclopramide [16]. Additional terminal patients with sudden cardiac death might not have been identified by our algorithm if they were prescribed oral domperidone or metoclopramide for side effects of palliative care. We did not exclude controls with evidence of palliative care because the prevalence among controls (0.09 %) was negligibly small, and the exclusion of only cases with ‘known’ causes will yield nearly the same result as the valid approach of excluding all such subjects [21].
Exposure misclassification could result because data are based on electronic recorded prescriptions issued by GPs without information on dispensed prescriptions and actual medication use by the patient. This issue could be particularly problematic for a drug such as domperidone, for which the usual recommended dose was variable during the time of the study (i.e. 10–20 mg orally three to four times daily), and which is often prescribed for ‘as needed’ use. Over half of the patients received a single prescription (median number of prescriptions per patient was one), making it difficult to ascertain the real dose taken or the real duration of the treatment. We used several approaches to lessen this potential misclassification, including revising the numeric daily doses derived from dosing instructions, surveying physicians, and conducting sensitivity analyses with different assumptions for the mean duration of the standard prescription. The results were consistent and did not indicate a great degree of misclassification. Misclassification of the outcome is also possible, even with the linkage to ONS data for cause of death, particularly if no autopsy was performed and there was uncertainty about the cause of death. In addition, cardiac arrests that did not result in sudden death because of increased public access to automated external defibrillators, and specialized cardiac emergency services would not have been captured in this study. It is unlikely that outcome misclassification or likelihood of successful resuscitation would be differential with respect to the study exposures, and therefore would likely have a minimal impact on the study effect estimates.
The increase in the risk of sudden cardiac death among patients with metoclopramide exposure was unexpected. In a prior study, users of metoclopramide did not show an increase in risk of ventricular arrhythmia compared with users of PPIs [22], but spontaneous events of QT prolongation have been reported with metoclopramide exposure, mainly via the intravenous route and in critically ill subjects [23–28]. A recent surveillance report from the EU-ADR network (http://www.euadr-project.org) which included over 20 million individuals, found that metoclopramide and domperidone were among nine drugs that satisfied criteria as prime suspects for drug-related acute myocardial infarction, with effect estimates similar to the results for sudden cardiac death in our study [29]. Another publication reported on subjects presenting with long QT syndrome and torsade de pointes in a wide network of 51 collaborating hospitals in Berlin, Germany. Metoclopramide was the drug related in the causality assessment in four (one probable and three possible) of 35 confirmed cases that were considered drug related [30]. A case-crossover study conducted in Taiwan’s Longitudinal Health Insurance Database that was published while our manuscript was under review also found that the odds of ventricular arrhythmia were elevated for both domperidone and metoclopramide (during periods of current exposure compared with periods of no exposure) [31]. The adjusted OR for domperidone use compared with non-use was 1.56 (95 % CI 1.41–1.72), based on 1644 exposed case periods and 1148 exposed control periods among 24,356 cases of ventricular arrhythmia. Compared with metoclopramide exposure, the OR for domperidone was close to the null value (adjusted OR 1.06; 95 % CI 0.91–1.23) [31]. We had considered metoclopramide to be a better comparator group than the PPIs since the indications for metoclopramide and domperidone are more similar than those for the PPIs and domperidone; in fact, no patient in the case-control analysis was exposed to domperidone and metoclopramide concomitantly, and age and other characteristics of users of domperidone were closer to those of users of metoclopramide than those of users of PPIs. The increase in risk among patients with metoclopramide should be evaluated in additional studies, given that metoclopramide is the alternative treatment to domperidone for some indications.
Conclusions
We found an increased odds of sudden cardiac death associated with current use of domperidone compared with non-use of study medications after adjusting for multiple potentially confounding factors, and also accounting for time-invariant intrinsic factors. The results suggested that the increased odds were concentrated in the first 15 days of exposure, in patients aged 60 years and older and in those taking more than 30 mg/day. This study is consistent with prior epidemiologic studies on the risk of sudden cardiac death with the use of domperidone, and identifies patient subgroups at increased risk. An unexpected finding of this study was the higher risk of sudden cardiac death associated with current exposure to oral metoclopramide than to oral domperidone. This finding should be further evaluated.
Electronic supplementary material
Acknowledgments
The authors would like to thank Amy Ladner for expert project management, Allen Mangel and Susana Perez-Gutthann for review and clinical direction, and Adele Monroe for expert editorial support.
Contribution to authorship
Alejandro Arana, Catherine B. Johannes, Lisa J. McQuay, Cristina Varas-Lorenzo, Daniel Fife, and Kenneth J. Rothman conceived and planned the research; Alejandro Arana, Catherine B. Johannes, Lisa J. McQuay, and Cristina Varas-Lorenzo carried out the research; Lisa J. McQuay analysed the data; and Alejandro Arana, Catherine B. Johannes, Lisa J. McQuay, and Cristina Varas-Lorenzo drafted the manuscript. All authors contributed to data interpretation and to critical revision of the manuscript, and have seen and approved the final version. All authors had full access to all of the data (including the tables) in the analysis.
Compliance with Ethical Standards
Funding
The study was performed by RTI Health Solutions under a research contract funded by Johnson & Johnson, who market domperidone.
Potential conflict of interest
All authors have completed the conflicts of interest disclosure form and declare that they received financial support from Johnson & Johnson for the submitted work. Daniel Fife is a full-time employee of Johnson & Johnson, holds stock and stock options in the company, and holds pension rights from Johnson & Johnson. Alejandro Arana, Catherine B. Johannes, Lisa J. McQuay, Cristina Varas-Lorenzo, and Kenneth J. Rothman have no financial relationships with organizations that might have an interest in the submitted work in the previous 3 years. None of the authors have other relationships or activities that could appear to have influenced the submitted work.
Research involving human participants and/or animals
Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required. The study was determined exempt from full review by the Institutional Review Board of RTI International. The study protocol received approval for database research from the Independent Scientific Advisory Committee of the Medicines and Healthcare Products Regulatory Agency (United Kingdom).
References
- 1.Doggrell SA, Hancox JC. Cardiac safety concerns for domperidone, an antiemetic and prokinetic, and galactogogue medicine. Expert Opin Drug Saf. 2014;13(1):131–138. doi: 10.1517/14740338.2014.851193. [DOI] [PubMed] [Google Scholar]
- 2.Rossi M, Giorgi G. Domperidone and long QT syndrome. Curr Drug Saf. 2010;5(3):257–262. doi: 10.2174/157488610791698334. [DOI] [PubMed] [Google Scholar]
- 3.Barone JA. Domperidone: a peripherally acting dopamine2-receptor antagonist. Ann Pharmacother. 1999;33(4):429–440. doi: 10.1345/aph.18003. [DOI] [PubMed] [Google Scholar]
- 4.Drolet B, Rousseau G, Daleau P, Cardinal R, Turgeon J. Domperidone should not be considered a no-risk alternative to cisapride in the treatment of gastrointestinal motility disorders. Circulation. 2000;102(16):1883–1885. doi: 10.1161/01.CIR.102.16.1883. [DOI] [PubMed] [Google Scholar]
- 5.Straus SM, Sturkenboom MC, Bleumink GS, Dieleman JP, van der Lei J, de Graeff PA, et al. Non-cardiac QTc-prolonging drugs and the risk of sudden cardiac death. Eur Heart J. 2005;26(19):2007–2012. doi: 10.1093/eurheartj/ehi312. [DOI] [PubMed] [Google Scholar]
- 6.van Noord C, Dieleman JP, van Herpen G, Verhamme K, Sturkenboom MC. Domperidone and ventricular arrhythmia or sudden cardiac death: a population-based case-control study in the Netherlands. Drug Saf. 2010;33(11):1003–1014. doi: 10.2165/11536840-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 7.Johannes CB, Varas-Lorenzo C, McQuay LJ, Midkiff KD, Fife D. Risk of serious ventricular arrhythmia and sudden cardiac death in a cohort of users of domperidone: a nested case-control study. Pharmacoepidemiol Drug Saf. 2010;19(9):881–888. doi: 10.1002/pds.2016. [DOI] [PubMed] [Google Scholar]
- 8.Clinical Practice Research Datalink. 2014. Available at: http://www.cprd.com/intro.asp. Accessed 20 Oct 2014.
- 9.Campbell J, Dedman DJ, Eaton SC, Gallagher AM, Williams RJ. Is the CPRD GOLD population comparable to the U.K. population? Pharmacoepidemiol Drug Saf. 2013;22(Suppl 1):280. [Google Scholar]
- 10.Gelfand JM, Margolis DJ, Dattani H. The UK general practice research database. In: Strom BL, editor. Pharmacoepidemiology. 4th ed. Wiley; 2005. p. 337–46.
- 11.Herrett E, Thomas SL, Schoonen WM, Smeeth L, Hall AJ. Validation and validity of diagnoses in the general practice research database: a systematic review. Br J Clin Pharmacol. 2010;69(1):4–14. doi: 10.1111/j.1365-2125.2009.03537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallagher AM, van Staa TP, Murray-Thomas T, Schoof N, Clemens A, Ackermann D, et al. Population-based cohort study of warfarin-treated patients with atrial fibrillation: incidence of cardiovascular and bleeding outcomes. BMJ Open. 2014;4(1):e003839. doi: 10.1136/bmjopen-2013-003839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung CP, Murray KT, Stein CM, Hall K, Ray WA. A computer case definition for sudden cardiac death. Pharmacoepidemiol Drug Saf. 2010;19(6):563–572. doi: 10.1002/pds.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ray WA, Chung CP, Murray KT, Hall K, Stein CM. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med. 2009;360(3):225–235. doi: 10.1056/NEJMoa0806994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arana A, Johannes C. Post-authorisation safety study: risk of out-of-hospital sudden cardiac death in users of domperidone, users of proton pump inhibitors, and users of metoclopramide. Final protocol, version 1.1. 2012. Available at: http://www.encepp.eu/encepp/openAttachment/fullProtocolLatest/3861;jsessionid=lnZSZ_LMk9WgG4UatCesNibvPEwU0soaotce51ef3tfrg7cI8ohn!68387223. Accessed 14 Aug 2015. [DOI] [PMC free article] [PubMed]
- 16.North of England Cancer Network. Palliative and end of life care guidelines for cancer and non-cancer patients. 3rd ed. 2012. Available at: http://www.nesra.co.uk/files/acutepain/NECNPalliativeCareGuidelinesBooklet2012.pdf. Accessed 14 Aug 2015.
- 17.Shah AD, Martinez C. An algorithm to derive a numerical daily dose from unstructured text dosage instructions. Pharmacoepidemiol Drug Saf. 2006;15(3):161–166. doi: 10.1002/pds.1151. [DOI] [PubMed] [Google Scholar]
- 18.Brunelli SM, Gagne JJ, Huybrechts KF, Wang SV, Patrick AR, Rothman KJ, et al. Estimation using all available covariate information versus a fixed look-back window for dichotomous covariates. Pharmacoepidemiol Drug Saf. 2013;22(5):542–550. doi: 10.1002/pds.3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rothman KJ. Episheet—spreadsheets for the analysis of epidemiologic data. 2012. Available at: http://www.krothman.org/episheet.xls. Accessed 14 Aug 2015.
- 20.European Medicines Agency. Restrictions on the use of domperidone-containing medicines. 2014. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Domperidone_31/European_Commission_final_decision/WC500172573.pdf. Accessed 14 Aug 2015.
- 21.Rothman KJ, Ray W. Should cases with a ‘known’ cause of their disease be excluded from study? Pharmacoepidemiol Drug Saf. 2002;11(1):11–14. doi: 10.1002/pds.683. [DOI] [PubMed] [Google Scholar]
- 22.Hennessy S, Leonard CE, Newcomb C, Kimmel SE, Bilker WB. Cisapride and ventricular arrhythmia. Br J Clin Pharmacol. 2008;66(3):375–385. doi: 10.1111/j.1365-2125.2008.03249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baguley WA, Hay WT, Mackie KP, Cheney FW, Cullen BF. Cardiac dysrhythmias associated with the intravenous administration of ondansetron and metoclopramide. Anesth Analg. 1997;84(6):1380–1381. doi: 10.1097/00000539-199706000-00038. [DOI] [PubMed] [Google Scholar]
- 24.Bentsen G, Stubhaug A. Cardiac arrest after intravenous metoclopramide: a case of five repeated injections of metoclopramide causing five episodes of cardiac arrest. Acta Anaesthesiol Scand. 2002;46(7):908–910. doi: 10.1034/j.1399-6576.2002.460725.x. [DOI] [PubMed] [Google Scholar]
- 25.Chou CC, Wu D. Torsade de pointes induced by metoclopramide in an elderly woman with preexisting complete left bundle branch block. Chang Gung Med J. 2001;24(12):805–809. [PubMed] [Google Scholar]
- 26.Grenier Y, Drolet P. Asystolic cardiac arrest: an unusual reaction following IV metoclopramide. Can J Anaesth. 2003;50(4):333–335. doi: 10.1007/BF03021028. [DOI] [PubMed] [Google Scholar]
- 27.Midttun M, Øberg B. Total heart block after intravenous metoclopramide. Lancet. 1994;343(8890):182–183. doi: 10.1016/S0140-6736(94)90978-4. [DOI] [PubMed] [Google Scholar]
- 28.Siddique SM, Shariff N, Vesuwala N, Hafiz T. Metoclopramide as a possible cause of prolonged QT syndrome and torsade de pointes in a patient with heart failure and renal insufficiency. Ann Intern Med. 2009;150(7):502–504. doi: 10.7326/0003-4819-150-7-200904070-00016. [DOI] [PubMed] [Google Scholar]
- 29.Coloma PM, Schuemie MJ, Trifiro G, Furlong L, van Mulligen E, Bauer-Mehren A, et al. Drug-induced acute myocardial infarction: identifying ‘prime suspects’ from electronic healthcare records-based surveillance system. PLoS One. 2013;8(8):e72148. doi: 10.1371/journal.pone.0072148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarganas G, Garbe E, Klimpel A, Hering RC, Bronder E, Haverkamp W. Epidemiology of symptomatic drug-induced long QT syndrome and torsade de pointes in Germany. Europace. 2014;16(1):101–108. doi: 10.1093/europace/eut214. [DOI] [PubMed] [Google Scholar]
- 31.Chen HL, Hsiao FY. Domperidone, cytochrome P450 3A4 isoenzyme inhibitors and ventricular arrhythmia: a nationwide case-crossover study. Pharmacoepidemiol Drug Saf. 2015;24(8):841–848. doi: 10.1002/pds.3814. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.