Abstract
Objectives
An official guideline must be prepared for legalizing the doctor-patient telemedicine system based on the evaluations of the ongoing telemedicine demonstration project performed by the Korean government. In this study, critical items of the Korean telemedicine guideline are suggested based on the guidelines of developed countries.
Methods
To investigate the telemedicine guidelines of developed countries, a keyword of 'telemedicine guidelines' was used for Google search to find out US, Australian, and Japanese guidelines. The common items included in two or more of the followings were screened: US Core Operational Guidelines for Telehealth Services Involving Provider-Patient Interactions, the Australian New South Wales (NSW) Agency for Clinical Innovation Guidelines for the use of Telehealth for Clinical and Non Clinical Settings in NSW, and the Japanese Guidelines for the practice of home telemedicine.
Results
A total of 22 common items of the following four domains, which could be used for the Korean guideline were screened: the common features in overall considerations (6 items), the common features in clinical considerations (6 items), the common features in technical considerations (5 items), and the common features in privacy considerations (5 items). These 22 items were suggested as the critical items of the Korean telemedicine guideline.
Conclusions
The screened 22 items of the telemedicine guideline must be further organized for details. Additional studies and professional opinions on the telemedicine cases and on the guidelines of developed countries are required to establish the Korean guideline in the near future.
Keywords: Telemedicine, Health Care Reform, Telecommunications, Remote Consultation, Guideline
I. Introduction
The telemedicine service has been introduced to the large-territory countries such as US and Australia to improve the medical accessibility in the remote areas of islands or mountains, and to save medical service costs.
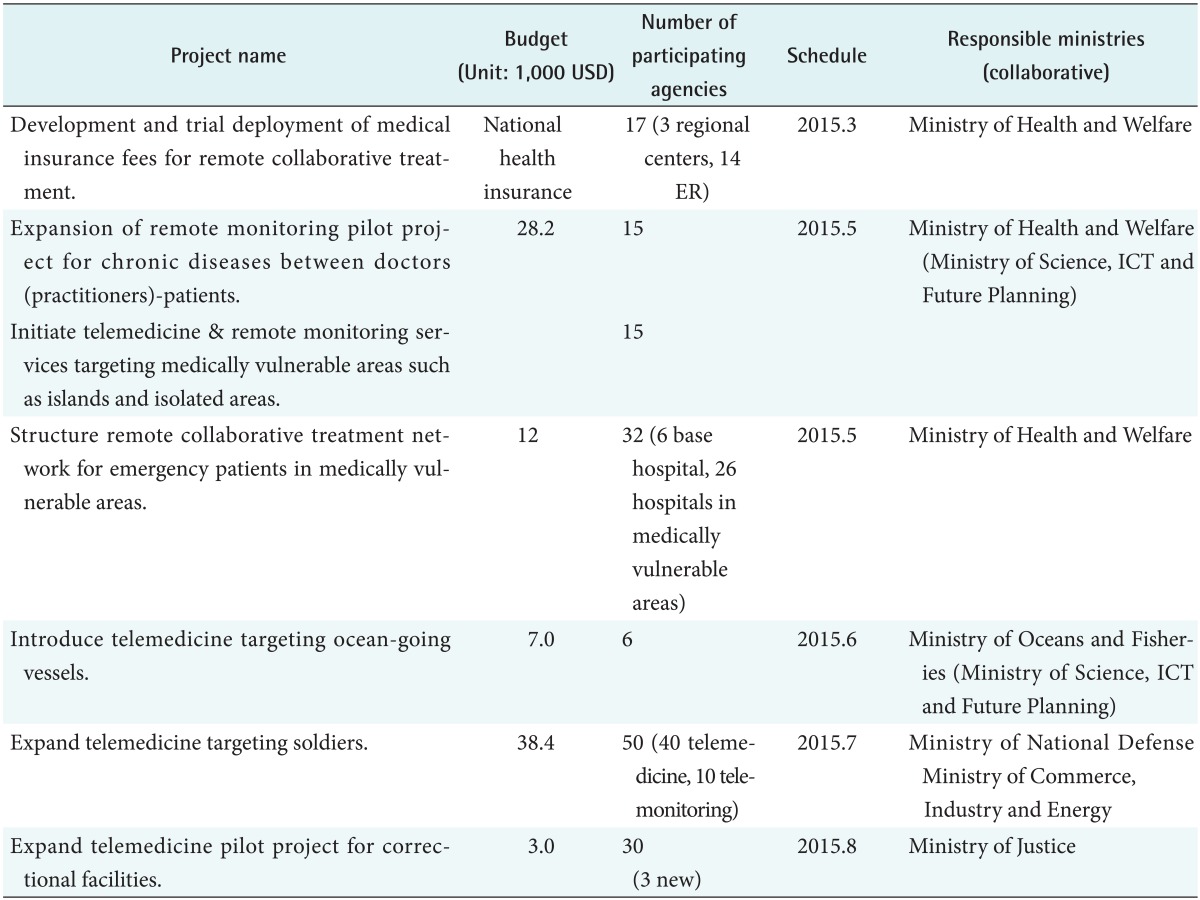
In South Korea, telemedicine was introduced for the first time in 1991. The stage-one telemedicine demonstration project was initiated using the Public Switched Telephone Network (PSTN) of Korea Telecomm among the three groups of medical institutions: between Woori Yeonchon Health Care Center in Gyungi-do and Seoul National University Hospital, between Hwachon Health Care Center in Gangwon-do and Hallym University Chuncheon Sacred Heart Hospital, and between Uljin Health Care Center in Gyeongbuk and Kyungpook National University Hospital [1]. Since then, various telemedicine demonstration projects have been performed, but only improvised guidelines have been used for individual projects without official telemedicine guidelines. The representative projects of the ministries according to the inter-ministry u-health industrialization promotion plan are shown in Table 1.
Table 1. Current pilot project list in 2015 in Korea.
1 USD=1,200 KRW
As the healthcare environment has changed according to technological developments and medical service demands, doctor-patient telemedicine has been discussed. The Korean government proposed a draft/partial amendment of the doctor-patient telemedicine law to the National Assembly in October 2013 in an effort to improve the convenience of and the accessibility to national medical services [2].
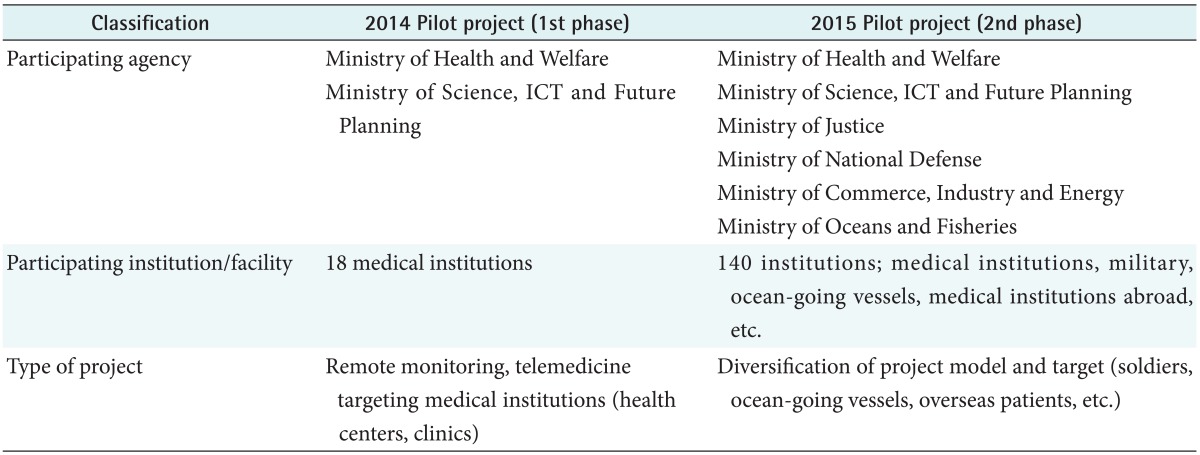
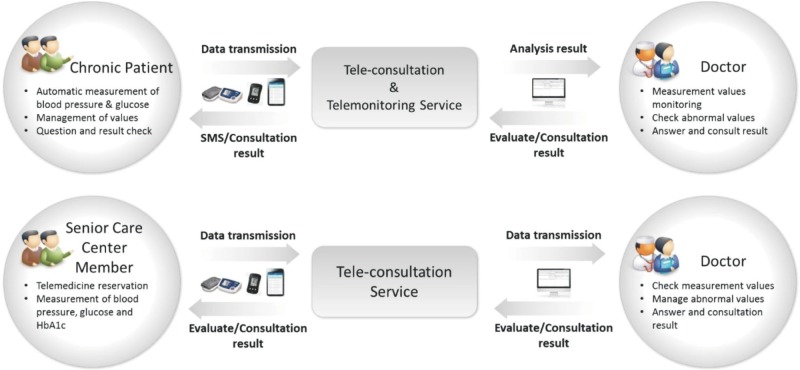
The government performed the stage-one demonstration project for six months between September 2014 and March 2015 to verify the stability and effectiveness of telemedicine [3]. According to the results of the stage-one demonstration project, which were reported by the Ministry of Health and Welfare, 92% of the surveyees answered intermediate or higher satisfaction with a 77% satisfaction level. In the stage-one demonstration project, 18 medical institutes (5 community health centers and 13 private general practitioners) and 845 returning hypertension and diabetes patients participated. 84.3% of the patients answered the telemedicine service was good for managing their chronic diseases. Furthermore, their compliance to drug administration improved [4]. The stage-two, 6-month demonstration project is under process between April 2015 and September 2015 with increased number of ministries, medical institutes, and facilities. The Table 2 shows expanded scopes of the stage-two telemedicine pilot project from those of the stage-one. Details of the pilot projects are shown in Figure 1 [5].
Table 2. Summary of pilot projects of telemedicine in 2014-2015 in Korea.
Figure 1. Telemedicine pilot service model.
In the stage-two project, the clinical safety, effectiveness, and usefulness (including the satisfaction level) of telemedicine are evaluated (Ministry of Health and Welfare). Even though expanded demonstration project is under process, studies on the guidelines for telemedicine services have not been clearly established. "The development and verification of the telemedicine service guideline (facilities, human resources, and equipment)" prepared by the Korea Health Industry Development Institute in 2011 was based on overseas guidelines and Korean u-health demonstration project guidelines, and it suggests standards on the facilities, human resources, and equipment required for telemedicine services. The guideline covers doctor-doctor and doctor-nurse services for patients, and cooperative services among medical professionals of the community health centers or hospitals in the medically underserved areas [6]. Minimal standards on telemedicine service facilities, medical professionals, governmental officials, and equipment are suggested in the guideline. However, it has not been established as an official telemedicine guideline but its outline has been posted at the library of u-health knowledge portal website (www.khidi.or.kr/uhips) [7].
If doctor-patient telemedicine services are legalized based mainly on the evaluations on the telemedicine demonstration projects without through studies and political considerations on the telemedicine guideline, no smooth progress and stable settlement of telemedicine services may be possible. Due to the necessity of establishing the basic items of the Korean telemedicine guideline, the common items of the telemedicine guidelines of developed countries were investigated in this study.
II. Methods
In this study, the guidelines of the US, Australia, and Japan were compared to each other. The country selection criteria are as follows: First, the countries that had a nation-level official guideline were selected. The official guideline applied to telemedicine services was verified already, and is practically used in the clinical setting. Second, the countries that officially and legally provide nationwide telemedicine services were selected. Third, the countries whose health care systems were different from each other were selected. The US medical industry is working based on the market principle and free competition among both profit and non-profit service providers to respect the free will of consumers. In comparison, Australia has established a government-led health care system that provides free medical services. The US has promoted telemedicine services to enhance efficiency, while Australia has promoted them to reinforce the medical resources in the medically underserved areas. The Japanese system was used as a reference when South Korea established its medical insurance system, so the South Korean and Japanese medical insurance systems are similar to each other [8,9]. Accordingly, Japan was selected to investigate its telemedicine service system. These three countries were selected to compare their characteristics of policy making and health care system. The guidelines of the US, Australia, and Japan were compared through the coincidence methods for generalization.
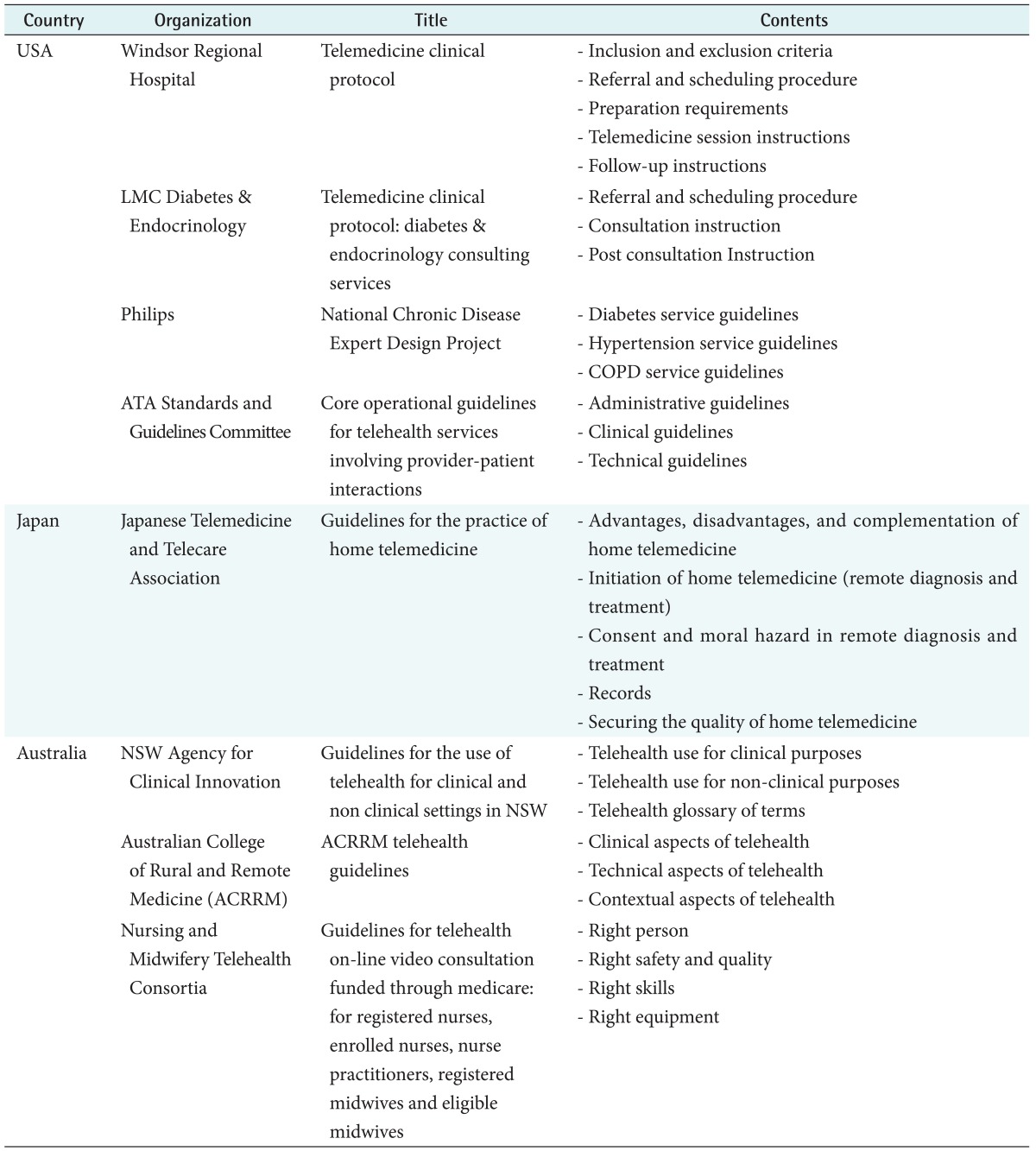
Through Google search, the guidelines posted by government agencies or telemedicine agencies were searched. The searched US guidelines included: Telemedicine Clinical Protocol [10] published by Windsor Regional Hospital, Telemedicine Clinical Protocol: Diabetes & Endocrinology Consulting Services [11] published by Local Medical Center (LMC) Diabetes & Endocrinology, National Chronic Disease Expert Design Project [12] published by Philips, and Core Operational Guidelines for Telehealth Services Involving Provider-Patient Interactions [13] published by Standards and Guidelines Committee. The searched Australian guidelines included: ACRRM Telehealth Guidelines [14] published by Australian College of Rural and Remote Medicine (ACRRM), Guidelines for Telehealth On-line Video Consultation Funded Through Medicare: For Registered Nurses, Enrolled Nurses, Nurse Practitioners, Registered Midwives and Eligible Midwives [15] published by Nursing and Midwifery Telehealth Consortia, and Guidelines for the use of Telehealth for Clinical and Non Clinical Settings in NSW (New South Wales) [16] published by NSW Agency for Clinical Innovation. The searched Japanese guidelines included: Guidelines for the practice of home telemedicine [17] published by Japanese Telemedicine and Telecare Association (JTTA).
For macroscopic comparison, the guidelines that include general items may be suitable rather than those include specific areas of telemedicine such as teledermatology or teleemergency. The scope and details of the guidelines were compared, and the guidelines that described telemedicine in the most macroscopic perspective were selected from each country for comparison. The main subjects of the considered guidelines of each country are listed in Table 3.
Table 3. The main subjects of telemedicine guidelines of each country.
III. Results
1. Guideline Selection Process
Considering the scope and details of the guidelines of each country, Core Operational Guidelines for Telehealth Services Involving Provider-Patient Interactions published by American Telemedicine Association was considered most comprehensive among the US guidelines; Clinical Innovation Guidelines for the use of Telehealth for Clinical and Non Clinical Settings in NSW published by NSW Agency for Clinical Innovation, among Australian guidelines; and Guidelines for the practice of home telemedicine published by JTTA, among Japanese guidelines.
The US guideline that was compared with those of Australia and Japan was Core Operational Guidelines for Telehealth Services Involving Provider-Patient Interactions published by American Telemedicine Association in 2014. In the guideline, fundamental requirements for providing patients, practitioners and other healthcare providers with medical and other healthcare services using telecommunication technologies and any other electronic communications were described. This guideline is applied to individual practitioners, group and specialty practices, hospitals and health care systems, and other providers of health related services in the cases of telehealth interactions between patients and service providers for the purposes of health care delivery. In the administrative guideline, operation policies and procedures that must be obeyed by organizations and health professions while providing telehealth services were defined. In the clinical guidelines, items that must be clinically considered and followed while providing telehealth services were defined. In the technical guidelines, technical items that must be considered and followed while providing telehealth services were defined. In the communication modes & applications, devices & equipment, connectivity for real-time interactive encounters, and privacy were separately defined. This guideline is the version that the items of: enhances guidance on educating patients about telehealth treatment; adds several new items related to verification of patient/provider identity and service delivery location; provides guidance related to mobile devices and services delivered to patients in nonfacility settings; and expands guidelines on privacy and security requirements were updated from the Core Standards for Telemedicine Operations published in February 2008. This version was the most macroscopic US guideline.
The Australian guideline that was selected for comparing with the US and Japanese guidelines was Clinical Innovation Guidelines for the use of Telehealth for Clinical and Non Clinical Settings in NSW. This was the most comprehensive Australian guideline.
This guideline was published by NSW Agency for Clinical Innovation in 2015. This guideline was developed to provide the sites of clinician to patient and clinician to clinician telehealth consultations with a common communications framework for treatment activities (ED, acute care, triaging, case conferences and follow up). This guideline was developed for NSW health staff, Non-government organizations, Medicare Locals (Primary Health Networks), General Practitioners (GPs), Specialists, Aboriginal Medical Services, Residential Aged Care Facility Services and affiliated Colleges and Organizations, and the guideline for telehealth use for clinical purposes and non-clinical purposes were separately described. The telehealth use for clinical purposes is composed of implementation considerations, financial considerations, and technical considerations. The telehealth use for non-clinical purpose chapter is composed of the items of remote education, analysis of case studies, peer review and networking, health promotion, mentoring and supervision the doesn't involve a patient, research and evaluation, metropolitan and rural profession support. In this study, only the telehealth use for clinical purposes part was included.
The Japanese guideline that was selected for comparing with US and Australian guidelines was Guidelines for the practice of home telemedicine published by Japanese Telemedicine and Telecare Association in 2011. This guideline was developed to reduce confusions, and to help healthcare providers understanding advantages and limitations of home telemedicine. In this guideline, home telemedicine terminologies, strengths and weakness of home telemedicine, and considerations of home telemedicine are described.
The guideline was developed to minimize the anxiety of less experienced medical professionals, the variation of the medical equipment and the communication environment, and the variations in the primary diseases and complications home telemedicine targets. Information on home care strategy is also included. In this study, selected guideline chapters on the initiation of home telemedicine (remote diagnosis and treatment), consent and moral hazard in remote diagnosis and treatment, records, securing the quality of home telemedicine and responsibilities were compared.
The US, Australia, and Japanese guidelines were compared to screen common items that could be applied to the development of Korean telemedicine guideline.
2. Common Guidelines of Each Country
As a result of reviewing and comparing the guidelines of each country, although a slight difference exists according to the legal issues, size and definition of telemedicine and so on. In overall, an argument on the necessity of regulation was similar. Therefore in this section, telemedicine guidelines of the US, Japan and Australia will be reviewed and the guidelines commonly defined by each country will be examined.
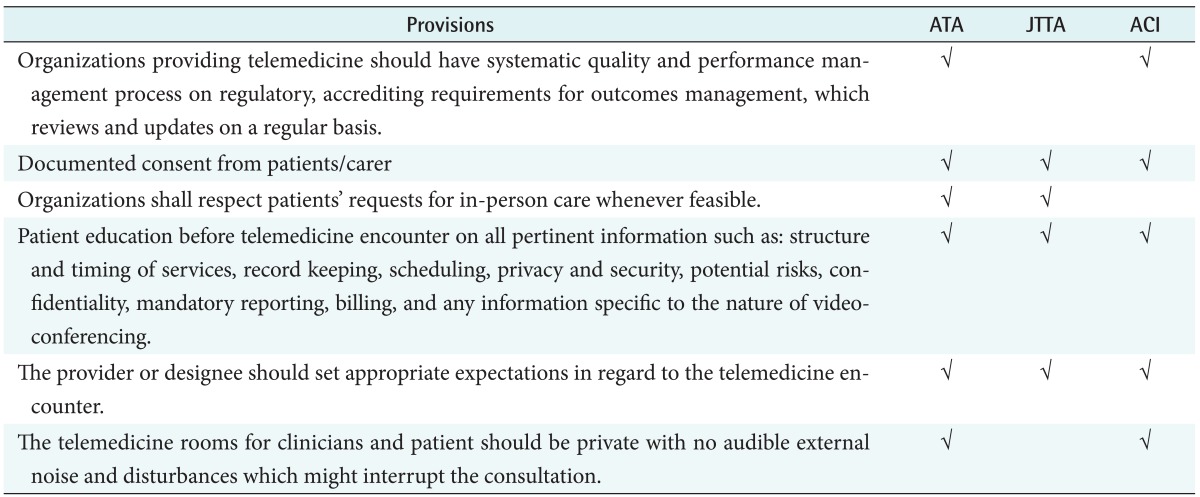
1) Overall considerations
The items that should be generally considered for telemedicine were commonly found in the guidelines as shown in Table 4. In all the guidelines of three countries, patient or carer consent was required for patient documentation. Prior to the start of the telemedicine encounter, the provider shall inform and educate the patient in real-time of all pertinent information such as discussion of the structure and timing of services, record keeping, scheduling. In addition, the provider or designee should set appropriate expectations in regard to the telemedicine encounter.
Table 4. Common provisions in overall considerations.
ATA: American Telemedicine Association, JTTA: Japanese Telemedicine and Telecare Association, ACI: Agency for Clinical Innovation.
In the US and Australian guidelines, restrictions on the telemedicine space are described unlike the Japanese guideline. The Japanese guideline did not include evaluation on telemedicine services, which was emphasized in the US and Australian guidelines.
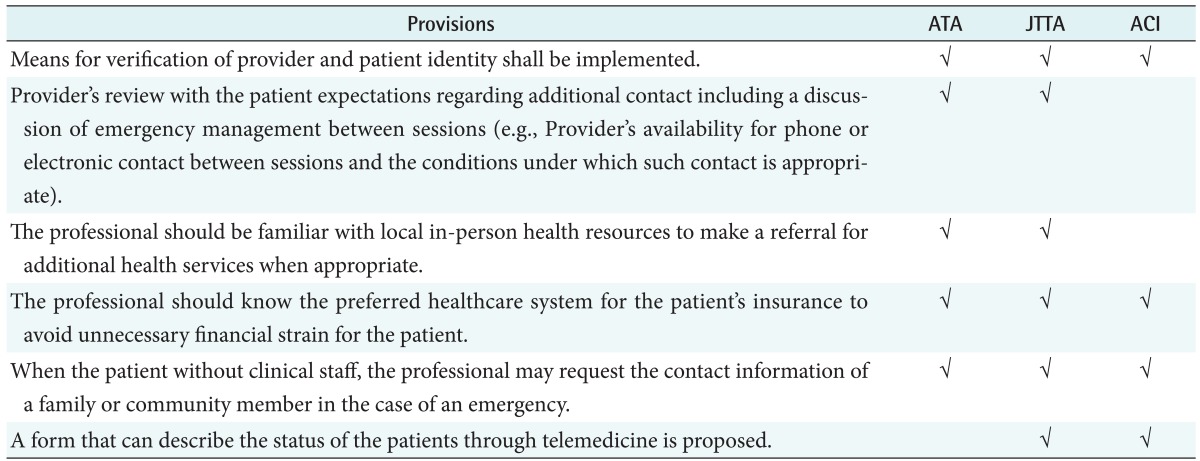
2) Clinical considerations by medical professionals
The common items of clinical consideration by medical professionals in telemedicine were: verification of provider and patient identity, professionals' consideration on preferred healthcare system for the patient's insurance to avoid unnecessary financial strain, and on the acquisition of contact information in the case of emergency when clinical staff is not on the patient site. In Australia ACI, no information was included unlike the guidelines of US and Japan as shown in Table 5.
Table 5. Common provisions in clinical considerations.
ATA: American Telemedicine Association, JTTA: Japanese Telemedicine and Telecare Association, ACI: Agency for Clinical Innovation.
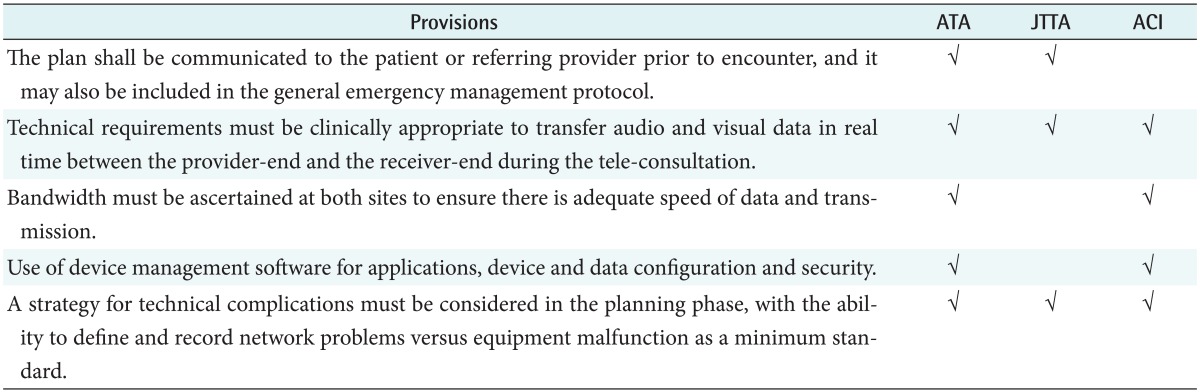
3) Technical considerations
In the US and Australian guidelines, issues on equipment and connection were described, but they were not described in detail in the Japanese guideline as shown in Table 6. However, an appropriate use of technical equipment was commonly described, and the guidelines agreed to prepare backup plans for any possible problem occurrence.
Table 6. The common provisions in technical considerations.
ATA: American Telemedicine Association, JTTA: Japanese Telemedicine and Telecare Association, ACI: Agency for Clinical Innovation.
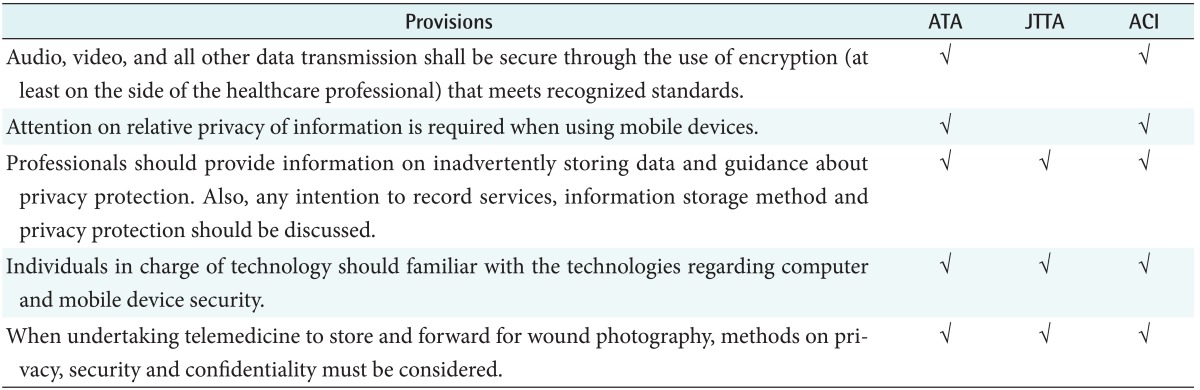
4) Privacy
Regarding privacy, all the three guidelines described issues of security system, maintenance of telemedicine service system by technicians, security management, and patient data recording, transmission, and storage as shown in Table 7. They commonly described the necessity of privacy protection, data storage, and patient consent. In addition, professional security check for equipment and the system was commonly emphasized. In the US and Australian guidelines, security of mobile devices was also described.
Table 7. The common provisions in privacy considerations.
ATA: American Telemedicine Association, JTTA: Japanese Telemedicine and Telecare Association, ACI: Agency for Clinical Innovation.
In this study, the telemedicine guidelines of the US, Japan, and Australia were compared to extract common items. As a result, many items were confirmed to have been commonly described in the guidelines of three countries or at least of two countries. Therefore, all the screened items are required to be modified to Korean circumstances to prepare the Korean guideline.
IV. Discussion
Upon completion of the Korean telemedicine demonstration projects that have been performed for the last two decades, telemedicine services may be eventually included in the category of medical services in the near future. Therefore, it is required to do groundwork for establishing guidelines for telemedicine services.
The telemedicine guidelines of US, Australia, and Japan were searched and 22 common items in four domains were screened: the common features in overall considerations (6 items), the common features in clinical considerations (6 items), the common features in technical considerations (5 items), and the common features in privacy considerations (5 items).
These items are suggested in this study as the critical items of the Korean telemedicine guideline.
In the US, video conference or teleconference was developed in 1950s, and was introduced to contemporary telemedicine services [18]. Thanks to its long history, they have more detailed guidelines on detailed items compared with other countries. In addition to the Core Operational Guidelines for telehealth services involving provider-patient Interactions, US has various guidelines such as Practice Guidelines for Live, On-Demand Primary and Urgent Care, and Clinical Guidelines for Telepathology [19].
Moreover, many data on telemedicine outcome are shared in the US websites of literature review, and various studies have been under process [20,21].
In the US and Australian guidelines, items on the evaluation on patients and medical professionals are established, and evaluation examples are provided.
Korean guidelines must be established, and studies on telemedicine outcomes must be actively conducted through systemic sharing of the results of the demonstration projects and literature review. In the long-term, evaluations on the target, type, quality, and usefulness of telemedicine must be performed based on data analyses.
The telemedicine guideline should be established for each category of the service upon clearly classifying the objects as medical professionals-patient or medical professionals-medical professionals. Service protocols that are adequate for Korean circumstances are required to provide medical professionals with a workflow and methods of coping with unexpected situations.
Related to the security aspect in telemedicine, Identity Theft Resource Center in the US reported health related data theft and leakage cases increased by 300% from 2005 to 2013. Therefore, medical information protection and security issue should be handled heavily in the telemedicine guideline establishment [22].
The limitations of this study may include that considerations were focused on general conditions, medical professionals, technical issues, and privacy, and that various databases were not investigated. Additionally, the telemedicine guidelines of only three countries were searched and their common items were screened, so this approach may not be appropriate for universal benchmarking.
In the future studies, not only the guidelines of these three countries but also those of UK, Canada, India, Norway, and Malaysia must be searched and analyzed. The common items of the guidelines of these countries that actively provide telemedicine services must be screened, and the guideline details must be discussed by professionals to establish the Korean telemedicine guideline in the near future. Even after the guideline is established, regular updates are required based on new rationale.
Acknowledgments
This study was supported by the Senior-friendly Product R&D program funded by the Ministry of Health & Welfare through the Korea Health Industry Development Institute (HI14C1435).
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Park YH. Super-high-speed information communications usage plan. J Korean Inst Commun Sci. 1994;11:34–37. [Google Scholar]
- 2.Park JS. New convergence industry development for health industry and policy support: telemedicine policy trend and economical evaluation structure development. Cheongju: Korea Health Industry Development Institute; 2014. [Google Scholar]
- 3.Ministry of Health & Welfare. Pilot program for telemedical service kicks off at the end of September [Internet] Sejong, Korea: Ministry of Health & Welfare; 2014. [cited at 2015 Aug 1]. Available from: http://english.mw.go.kr/front_eng/sg/ssg0111vw.jsp?PAR_MENU_ID=1001&MENU_ID=100111&page=1&CONT_SEQ=305455&SEARCHKEY=TITLE&SEARCHVALUE=tele. [Google Scholar]
- 4.Ministry of Health & Welfare. Telemedicine overall satisfaction 77% (usually more than 91.8%) appeared as high as [Internet] Sejong, Korea: Ministry of Health & Welfare; 2015. [cited at 2015 Aug 1]. Available from: http://www.mw.go.kr/front_new/al/sal0301vw.jsp?PAR_MENU_ID=04&MENU_ID=0403&page=32&CONT_SEQ=322478. [Google Scholar]
- 5.Biotech Policy Research Center. Recent domestic trend about telemedicine [Internet] Daejeon, Korea: Biotech Policy Research Center; 2015. [cited at 2015 Aug 1]. Available from: http://www.bioin.or.kr/InnoDS/data/upload/issue/1433988718282.pdf. [Google Scholar]
- 6.Korea Health Industry Development Institute. Development and verification of guideline to telemedicine service. Cheongju, Korea: Korea Health Industry Development Institute; 2011. [Google Scholar]
- 7.Korea Health Industry Development Institute. IT health [Internet] Cheongju: Korea Health Industry Development Institute; c2015. [cited at 2015 Oct 8]. Available from: http://www.khidi.or.kr/board/view?pageNum=2&rowCnt=10&no1=5&linkId=140027&menuId=MENU00511&maxIndex=00001400409998&minIndex=00001400239998&schType=0&schText=&boardStyle=&categoryId=&continent=&country= [Google Scholar]
- 8.Nam SY, Kwon OJ, Kim YJ, Nishiyama T, Okamoto E, Kudo T, et al. The study on Japanese health care insurance and medical service reimbursement system. Seoul: Korean Medical Association; 2010. [Google Scholar]
- 9.Park JS. u-Health policy status and future direction. Sci Technol Policy. 2012;188:20–29. [Google Scholar]
- 10.Windsor Regional Hospital. Telemedicine clinical protocol [Internet] Ontario, Canada: Windsor Regional Hospital; 2013. [cited at 2015 Jun 30]. Available from: http://www.wrh.on.ca/Site_Published/wrh_internet/Document.aspx?Body.Id=62471. [Google Scholar]
- 11.LMC Diabetes and Endocrinology. Telemedicine clinical protocol: diabetes & endocrinology consulting services. Ontario, Canada: LMC Diabetes and Endocrinology; 2009. [cited at 2015 Jun 30]. Available from: https://www.lmc.ca/2011/wp-content/uploads/LMC-OTN-ProtocolvApril2013.pdf. [Google Scholar]
- 12.Philips National Chronic Disease Expert Design Project [Internet] Northampton (MA): Fazzi Associates Inc.; 2009. [cited at 2015 Aug 1]. Available from: http://www.fazzi.com/tl_files/documents/research-and-resources/Philips_Chronic_Disease_Report.pdf. [Google Scholar]
- 13.American Telemedicine Association. Core operational guidelines for telehealth services involving providerpatient interaction [Internet] Washington (DC): American Telemedicine Association; 2014. [cited at 2015 Jun 30]. Available from: http://www.americantelemed.org/docs/default-source/standards/core-operationalguidelines-for-telehealth-services.pdf?sfvrsn=6. [Google Scholar]
- 14.Australian College of Rural & Remote Medicine. ACRRM Telehealth guidelines [Internet] Brisbane: Australian College of Rural & Remote Medicine; 2012. [cited at 2015 Jun 30]. Available from: http://www.ehealth.acrrm.org.au/system/files/private/ACRRM%20Telehealth%20Guidelines_v1.0_20120827.pdf. [Google Scholar]
- 15.Australian Nursing Federation. Guidelines for telehealth on-line video consultation funded through medicare [Internet] Melbourne: Australian Nursing Federation; 2013. [cited at 2015 Jun 30]. Available from: https://crana.org.au/files/pdfs/Telehealth_Guidelines.pdf. [Google Scholar]
- 16.Agency for Clinical Innovation. Guidelines for the use of telehealth for clinical and non clinical settings in NSW [Internet] Chatswood, Australia: Agency for Clinical Innovation; 2015. [cited at 2015 Jun 30]. Available from: http://www.aci.health.nsw.gov.au/__data/assets/pdf_file/0010/258706/ACI-telehealth-guidelines.pdf. [Google Scholar]
- 17.Japanese Telemedicine and Telecare Association. Guidelines for the practice of home telemedicine (2011 edition) [Internet] Takasaki: Japanese Telemedicine and Telecare Association; 2011. [cited at 2015 Jun 30]. Available from: http://jtta.umin.jp/eng/pdf/guidelines/guidelines01.pdf. [Google Scholar]
- 18.Jung YY. Telemedical law. Paju: Korean Studies Information; 2008. pp. 43–47. [Google Scholar]
- 19.American Telemedicine Association. Telemedicine practice guidelines [Internet] Washington (DC): American Telemedicine Association; 2012. [cited at 2015 Oct 8]. Available from: http://www.americantelemed.org/resources/telemedicine-practice-guidelines/telemedicinepractice-guidelines. [Google Scholar]
- 20.American Telemedicine Association. Telemedicine case studies [Internet] Washington (DC): American Telemedicine Association; 2012. [cited at 2015 Oct 8]. Available from: http://www.americantelemed.org/abouttelemedicine/telemedicine-case-studies. [Google Scholar]
- 21.American Telemedicine Association. Research outcomes: telemedicine's impact on healthcare cost and quality [Internet] Washington (DC): American Telemedicine Association; 2015. [cited at 2015 Oct 8]. Available from: http://www.americantelemed.org/docs/default-source/policy/examples-of-research-outcomes--telemedicine's-impact-on-healthcare-cost-and-quality.pdf. [Google Scholar]
- 22.The Identity Theft Resource Center. ITRC 2013 Breach List Tops 600 in 2013 [Internet] San Diego (CA): The Identity Theft Resource Center; 2015. [cited at 2015 Nov 5]. Available from: http://www.idtheftcenter.org/ITRCSurveys-Studies/2013-data-breaches.html. [Google Scholar]