Abstract
Background
It is still difficult to prevent partial or full-thickness flap necrosis. In this study, the effects of a cream containing menthol and methyl salicylate on the viability of randompattern skin flaps were studied.
Methods
Forty female Sprague-Dawley rats were divided into two equal groups. Caudally based dorsal random-pattern skin flaps were elevated, including the panniculus carnosus. In the study group, 1.5 mL of a cream containing menthol and methyl salicylate was applied to the skin of the flap, and saline solution (0.9%) was used in the control group. Upon completion of the experiment, flap necrosis was analyzed with imaging software and radionuclide scintigraphy. Histopathological measurements were made of the percentage of viable flaps, the number of vessels, and the width of the panniculus carnosus muscle.
Results
According to the photographic analysis, the mean viable flap surface area in the study group was larger than that in the control group (P=0.004). According to the scintigrams, no change in radioactivity uptake was seen in the study group (P>0.05). However, a significant decrease was observed in the control group (P=0.006). No statistically significant differences were observed between the groups in terms of the percentage of viable flaps, the number of vessels, or the width of the panniculus carnosus muscle (P>0.05).
Conclusions
Based on these results, it is certain that the cream did not reduce the viability of the flaps. Due to its vasodilatory effect, it can be used as a component of the dressing in reconstructive operations where skin perfusion is compromised.
Keywords: Surgical flaps, Menthol, Methyl salicylate, Vasodilator agents, Perfusion
INTRODUCTION
The introduction of flaps to plastic surgery had a major impact on wound closure and the reconstruction of normal and functional anatomical features. Local skin flaps provide an excellent match in terms of color, texture, and thickness. However, it is not always possible to predict the viability of flaps, especially in random-pattern skin flaps.
Partial or total flap loss may result in a new tissue defect at the flap donor area, increasing the total defect area requiring reconstruction. Such complications may increase the length of the hospital stay, the cost of the treatment, and most importantly, may damage the patient's confidence and trust in his/her doctor. In the literature, numerous surgical and pharmacological methods of increasing flap viability have been described. Pharmacological agents such as sympatholytics, vasodilators, calcium channel blockers, hemorheologics, prostaglandin inhibitors, anticoagulants, glucocorticoids, and free radical scavengers have been applied topically [1,2,3] and administered enterally or parenterally [4,5]. The use of some drugs with proven experimental efficacy has been restricted by side effects, high cost, and/or poor availability.
Menthol is a natural and readily available agent used for a vast range of purposes, including hygiene products, pharmaceuticals, and as a flavor [6,7]. Menthol has antipruritic, antiseptic, analgesic, anesthetic, and cooling effects when used topically. In addition, it has antibacterial, antiviral, and antifungal effects [7].
Calcium (Ca2+) channels play a major role in the regulation of vascular tone. The transient receptor potential (TRP) channel superfamily has non-voltage-gated cation-permeable channels. The vanilloid-related (TRPV) and melastatin-related (TRPM) TRP receptors are two major families that are responsible for sensing acidity, osmolarity, mechanical forces, and temperature in non-vascular tissues [8]. TRPM8, known as the menthol or cold receptor, has been found in arterial smooth muscle. Limited and contradictory data exist regarding the effect of menthol on vessels, with disagreement about whether it results in vasodilatation [8,9] or vasoconstriction [10,11,12,13].
Non-steroid anti-inflammatory drugs (NSAIDs) are the active ingredients of topical analgesics that are widely used to relieve muscle and joint pains. NSAIDs inhibit the cyclooxygenase enzyme that transforms arachidonic acid into various prostaglandins. A decrease in the amount of prostaglandins, which activate nociceptors, alleviates the pain. Methyl salicylate (MeSa) is hydrolyzed to salicylate, which has anti-inflammatory and analgesic effects [14]. In addition, it irreversibly inhibits platelet aggregation [15] and increases local blood flow and the temperature of the tissue.
We hypothesized that a combination of menthol and MeSa could affect the viability of skin flaps due to the anti-inflammatory properties of these compounds and their effects on blood vessels. No similar previous studies were found in a thorough review of the English-language, French-language, and Turkish-language literature. In this experimental study, the effects of a topical cream containing 10% menthol and 15% MeSa (Kamfolin, Münir Şahin, Istanbul, Turkey) on the viability of dorsal random-pattern skin flaps in rats were investigated.
METHODS
This research was conducted with the permission of the local ethical committee and in accordance with international health and medical research guidelines for animal welfare. The rats were housed in separate cages, placed in an environmentally controlled room, and fed with standard rat chow and tap water ad libitum. Three days before surgery, 200 mg/kg of paracetamol (Parol, Atabay, Istanbul, Turkey) was added to their water for analgesia. All surgical procedures and imaging studies were performed under general anesthesia, which was carried out with an intraperitoneal injection of 75-100 mg/kg of ketamine hydrochloride (Ketalar, Eczacıbaşı, Istanbul, Turkey) and 10 mg/kg of 2% xylazine (Rompun, Bayer, Leverkusen, Germany). The dorsal hair was removed with an electric shaver and all surgical procedures were carried out under sterile conditions by a single surgeon (UCD).
Experimental protocol
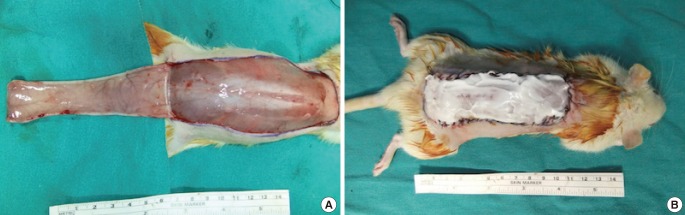
Forty adult (three months old) female Sprague-Dawley rats weighing 179-267 g were divided into two groups of 20 each. Modified version of the McFarlane was performed. A caudally based skin flap (10 cm × 3 cm) was marked on the shaved dorsum of the rat, starting from the line that connects the iliac spines. The flap was elevated beneath the panniculus carnosus muscle (PCM) in both groups, and the deep circumflex iliac artery and perforator vessels were cauterized in order to ensure a random pattern of blood circulation. The flaps were sutured back to their donor site after the scintigraphic measurements were performed (Fig. 1). A sterile silicone sheet (0.5 mm) was placed on the donor site in order to isolate the flap from the underlying bed.
Fig. 1. A modified McFarlane flap.
(A) Elevation of a caudally based dorsal skin flap, (B) skin closure and application of 1.5 mL of cream on a rat in the study group.
Study group (n=20)
1.5 mL (0.05 mL/cm2) of a cream containing 10% menthol and 15% MeSa was applied via massage on the skin of the flap immediately after the skin closure and once a day for six days. Before each application, debris from the previous application was removed with sterile isotonic saline solution (0.9%).
Control group (n=20)
Sterile isotonic saline solution (0.9%) was applied via massage on the skin of the flap immediately after the skin closure and once a day for six days, in order to control for the vasodilatory effect of the massage [16].
Weight analysis of the rats
All the rats were weighed before and after the experimental protocol with a digital scale in order to evaluate their physiological response to the surgery and to the cream.
Clinical and photographic analysis
All flaps were evaluated daily. On the seventh postoperative day, the rats were anesthetized and standardized photographs of the flaps were taken with a high-resolution digital camera (Fujifilm FinePix F80EXR, Fujifilm Holdings Corp., Beijing, China) before suture removal. These images were then uploaded to a computer, and the surface areas of the entire flap and the necrotic part of the flap were calculated in mm2 using Adobe Photoshop CS5 software (Adobe Systems Inc., San Jose, CA, USA) (Fig. 2). The surface area of the viable part of the flap was calculated by subtracting the surface area of the necrotic part of the flap from the surface area of the entire flap. Comparisons were made within and between the two groups.
Fig. 2. Measurement of the necrotic area of the flap surface.
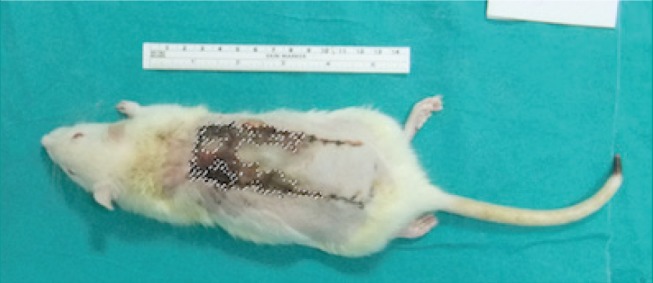
The dashed line represents the measured surface area.
Radionuclide scintigraphic analysis
On the first day of the experiment, scintigrams of the flaps were taken before skin closure; on the seventh day, scintigrams were obtained and the rats were then sacrificed. The rats were injected with 1 mCi (37 MBq) of technetium-99m pertechnetate (Tc99m-PO4) in 0.1 mL of isotonic saline solution through the tail vein. The rats were placed prone under a single-head gamma camera equipped with a pinhole collimator (Siemens E.Cam, Siemens Medical Solutions, Hoffman Estates, IL, USA). Images of both groups were taken five minutes after injection and were visualized for five minutes in a 256×256-pixel matrix. One lead plate was placed under the flap and two other plates covered the rest of the rat in order to obtain only the image of the flap. The lead plates were covered with waterproof paper; separate sets of waterproof paper were used in each rat in order to protect the plates from radioactive contamination.
Tc99m-PO4-soaked cotton in a needle cap was placed at the distal end of the flap in order to visualize the distal border of the flap on the scintigram. After five seconds, it was removed and the actual scintigram was obtained.
An experienced nuclear medicine specialist who was blinded to the groups interpreted the scintigrams. On the computer, a rectangle was drawn that enclosed the borders of the flap, encompassing the total region of interest (ROI). The total ROI was divided into three equal ROIs: the proximal ROI (from the pedicle) was referred to as ROI A, the middle third was referred to as ROI B, and the distal third was referred to as ROI C (Fig. 3). The borders of the hyperemic parts of the flap were drawn manually, using the free hand method, and the flap area was calculated (Fig. 4). Those values were referred to as the manually marked count of the viable flap area (MCFA). All results were compared within each group and between the two groups.
Fig. 3. Measurements of the regions of interest (ROIs).
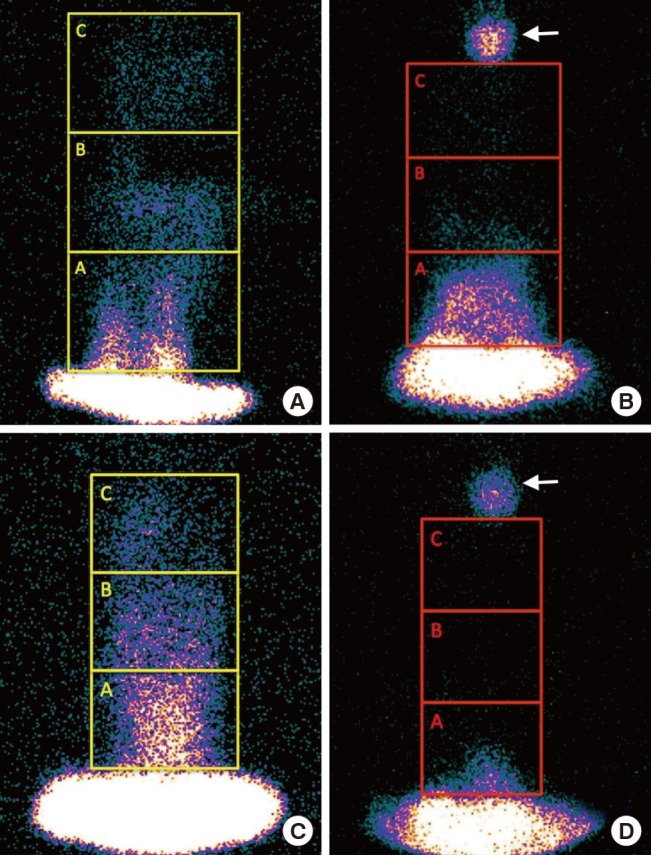
Measurements of the ROIs on flaps in the (A, B) study and (C, D) control groups. The flaps were divided into three ROIs, referred to as ROI A, ROI B, and ROI C. Scintigrams (A, C) before and (B, D) after the experiment. The white arrow shows the radionuclide-soaked material that was used to identify the end of the flap.
Fig. 4. Measurement of the manually marked count of the viable flap area.
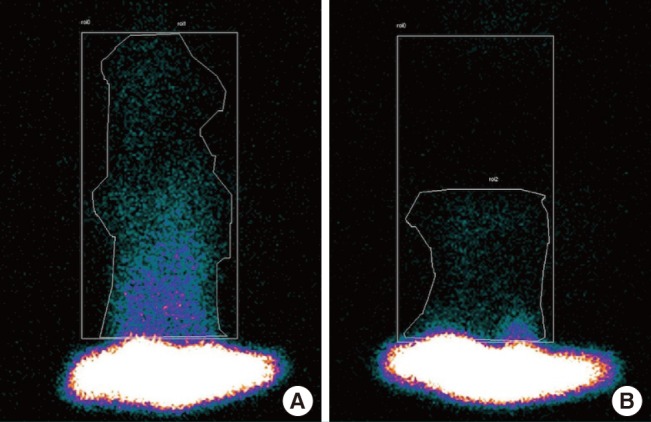
The measurement of the manually marked count of the viable flap area is shown by an asymmetric line that encloses the hyperemic area of the flap. The rectangle represents the entire flap area. A scintigram was taken (A) before and (B) after the experiment.
The photographs and the scintigrams were merged digitally with Adobe Photoshop CS5 software to better visualize the viable part of the flap (Figs. 5, 6).
Fig. 5. Combination of a scintigram and a photograph.
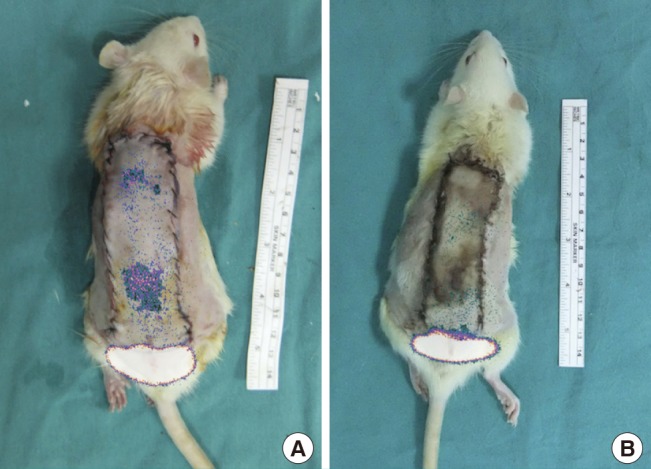
An image obtained after digitally combining the photographs and the scintigrams. In the study group, (A) scintigrams taken before the experiment showed little perfusion in the flap, while (B) scintigrams taken after the experiment showed almost no perfusion in the flap.
Fig. 6. Combination of a scintigram and a photograph.
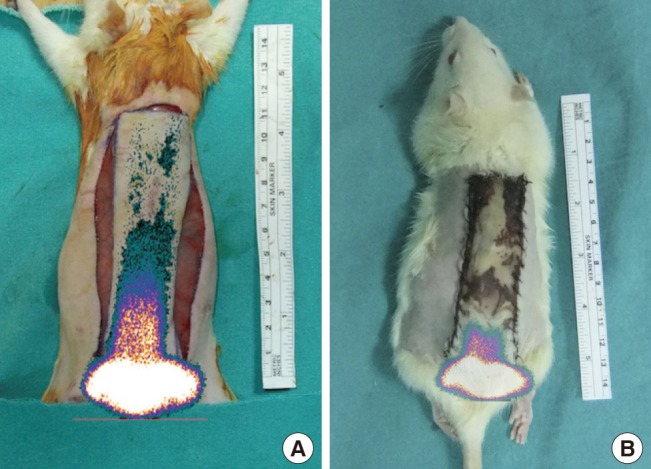
In the control group, (A) half of the flap was perfused before the experiment, and (B) perfusion was only found in the pedicle region after the experiment.
Histopathologic assessment
On the seventh day, the rats were sacrificed with high-dose ketamine after scintigraphic imaging. The flaps were divided from their base between the two iliac spines. The entire flap was fixed in 10% formaldehyde solution. After routine hospital-specified histopathological procedures, the paraffin-embedded specimens were sliced longitudinally into 5-µm thick segments along the long axis of the flap. All specimens were stained with hematoxylin and eosin and examined under light microscopy (Shanghai Optical Instrument Factory, Shanghai, China) by the same pathologist who was kept blind to the groups.
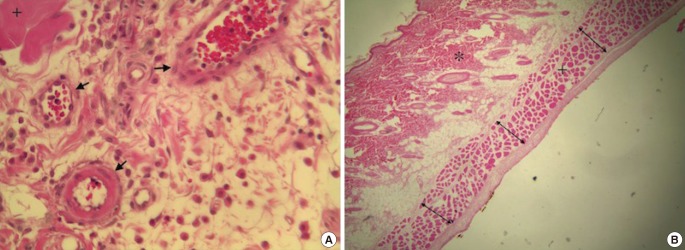
The percentages of the viable and necrotic parts of each flap were measured. The erythrocyte-containing capillary vessels were then counted in the pedicle region, before the necrotic region, and at the transition zone between those regions. These counts were performed at five different areas under high magnification (400×) in each region in order to calculate the density of capillary vessels per 1 mm2. The vessels were counted both at the level of the papillary dermis and inferior to the PCM, and the results were compared between the two groups (Fig. 7).
Fig. 7. Histopathology.
(A) Measurement of capillary vessel density (H&E, ×400). (B) Measurement of the thickness of the panniculus carnosus muscle (PCM) at three different points in the necrotic region. An asterisk shows the dermal layer, the plus sign indicates the PCM, black arrows show the vessels, and double arrows indicate the thickness of the PCM (H&E, ×40).
Microscopic images (40×) of the flaps were photographed. The thickness of PCM muscle was measured at the pedicle region, the necrotic region, and at the transition zone between these regions with the help of Adobe Photoshop CS5. Muscle thickness was measured in three different places in each region, and the arithmetic average of these measurements was used as the muscle thickness value for each region (Fig. 7).
Statistical analysis
Since the scintigraphy results were not normally distributed, a base-10 logarithmic transformation was applied. The transformed values were used for statistical analysis.
The Mann-Whitney U test was used to compare the necrotic, viable, and total surface areas of the flaps, as calculated by photographic analysis. The ROIs and the MCFA were measured using radionuclide scintigraphy, and the percentage of necrotic areas, capillary vessel density, and the thickness of the PCM were analyzed by histopathology and compared between the study group and the control group. The Wilcoxon signed-rank test was used to compare the weight of the rats, the ROIs, and the MCFA within the same group. The Kruskal-Wallis test was used to compare the thickness of the PCM in different regions of the same flap.
Statistical analysis was performed using SPSS ver. 15.0 (SPSS Inc., Chicago, IL, USA). Data were expressed as the mean±standard deviation, and the cut-off point for statistical significance was 0.05.
RESULTS
Two rats in the control group and one rat in the study group died of unknown causes before the study was completed. Neither wounds nor systemic infections were observed in the surviving rats.
Weight analysis of the rats
Before the experimental protocol, the median weight of the study group was 227 g (range, 204-249 g), and that of the control group was 211 g (range, 193-233.3 g). On the seventh postoperative day, the median weight of the study group was 205 g (range, 183-227 g), and that of the control group was 195.5 g (range, 182-213.8 g). No statistically significant difference was observed between the two groups before (P=0.13) and after (P=0.28) the experimental protocol. These figures show that the rats were distributed randomly. The weight of the rats decreased to a statistically significant extent by the end of the experiment in both the study (P<0.001) and control groups (P=0.001).
Clinical and photographic analysis
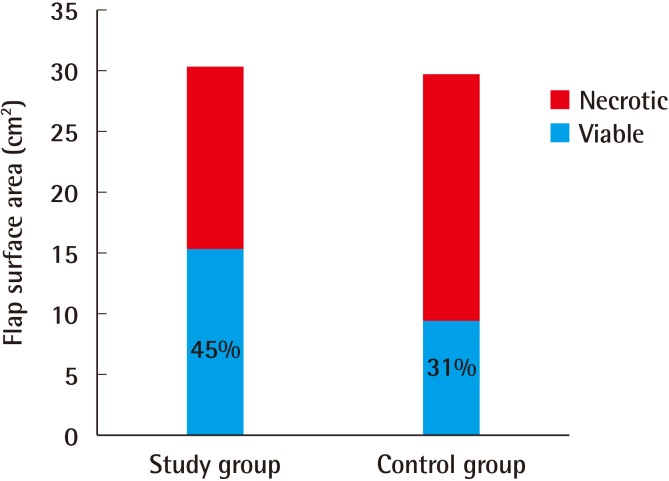
No statistically significant difference between the groups was found in the total surface areas of the flaps (P=0.6). This finding indicates that similarly sized flaps were elevated in both groups. The viable flap area of the study group (median, 15.8 cm2) was larger than that of the control group (median, 8.5 cm2), which was a statistically significant difference (P=0.004) (Table 1, Fig. 8).
Table 1. Distribution of the mean flap surface areas in the study and the control group.
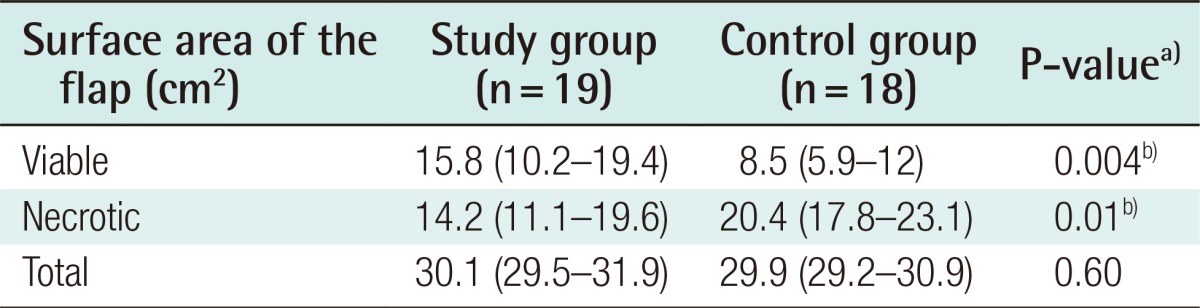
A comparison of the control and study groups according to the median viable, necrotic, and total flap areas. Values are presented as median (interquartile range).
a)Mann-Whitney U test; b)Statistically significant.
Fig. 8. Mean flap surface areas.
Radionuclide scintigraphic analysis
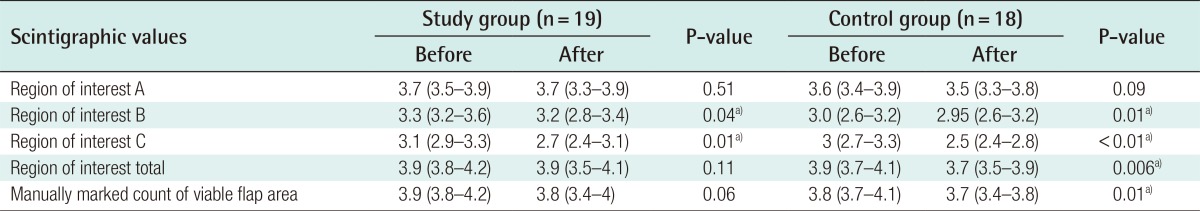
No statistical difference was observed in the values of ROI A before and after the experimental protocol in either the study group (P=0.51) or the control group (P=0.09). A statistically significant decrease was observed in the values of ROI B and ROI C, both in the study group (P=0.04 and P=0.01, respectively) and in the control group (P=0.01 and P=0.00, respectively).
A statistically significant decrease was found in the total ROI of the control group after the experiment (P=0.006). No statistically significant difference was observed in the values of the total ROI of the study group (P=0.11). Despite the reduction in both ROI B and ROI C in the study group, the total ROI did not change significantly (Table 2).
Table 2. In-group comparison between pre- and post-experimental scintigraphic values.
Values are presented as median (interquartile range). P-value is calculated with Wilcoxon signed-rank test.
a)Statistically significant.
No statistical differences were observed in all ROI values between the study and the control group before and after the experimental protocol. This finding shows that the pattern of blood perfusion was similar among the flaps.
In the study group, the MCFA did not change after the experimental protocol (P=0.32). However, it decreased in the control group (P=0.01) (Table 2).
No statistically significant difference was found in the pre-experimental scintigraphic values of the study group and the control group (MCFA, P=0.17). No statistically significant difference was found in the post-experimental scintigraphic values of the two groups (MCFA, P=0.11).
Histopathologic assessment
It was observed under the microscope that 36.18%±20.79% of the flaps in the study group and 28.22%±10.31% of the flaps in the control group survived. The difference between the two groups with respect to the percentage of the viable flaps was not statistically significant (P=0.18).
Capillary vessel density (the number of vessels in 1 mm2) did not significantly differ between the study group and the control group in the pedicle region (17 [range, 11-20] vs. 18 [range, 12.3-22], P=0.94), the transitional region (21 [range, 15-25] vs. 19.5 [range, 11-27], P=0.78), and in the pre-necrotic region (17 [range, 5-21] vs. 18 [range, 12-24.5], P=0.22). Likewise, no significant difference was found between the dermal papillary layer and the layer inferior to the PCM.
The thickness of the PCM (µm) did not show a significant difference between the study and the control groups in the pedicle region (212 [range, 163.1-235.2] vs. 210.2 [range, 192.4-248.9], P=0.60), the middle region (205.9 [range, 181.9-244.2] vs. 181.1 [range, 156.8-236.9], P=0.13), or in the necrotic region (264.8 [range, 194.4-329.5] vs. 306.1 [range, 213.6-378.9], P=0.83).
DISCUSSION
Flaps are the most important weapon in the arsenal of plastic surgeons. Even though their use is as old as the history of humankind, some aspects of flap viability remain unknown. Sir Harold Gillies said that "plastic surgery is a constant battle between blood supply and beauty," and in this battle, the mission of plastic surgeons is to elevate a flap without partial or total flap loss. Especially in random-pattern flaps, blood perfusion is the major issue that limits the size of the flap. Surgical delay is an efficient method, but requires multiple operations that not all patients can tolerate or accept. In contrast, pharmacologic agents do not require additional surgery, but are often of limited utility due to their high costs and restricted availability. Among these agents, topical drugs are the most advantageous because they can reach a therapeutic dose without causing any systemic side effects. In addition, patients can easily use these drugs immediately after surgery, even if they are experiencing nausea, and they can apply them by themselves at home without medical supervision. Topical agents require a skin permeation enhancer, such as terpenes, in order to be absorbed through the cutaneous layers. Due to its lipophilic structure, menthol can easily pass the stratum corneum and its chiral center enhances the absorption of other molecules [17]. It has already been shown that menthol facilitates and accelerates the absorption of MeSa [18].
A sterile silicone sheet was used to prevent the skin flap from surviving as a graft [19,20]. We observed loose capsule formation and a small seroma around the sheet at the end of the experiment. In the literature, the necrotic part of random-pattern dorsal skin flaps has been reported to vary widely, from 22% to 50% [21]. In our study, the percentage of necrotic areas in the flaps in the control group was found to be 69% in the photographic analysis and 72% in the histopathological analysis. We interpreted this high percentage of necrosis as the result of the silicone sheet, which prevented the flaps from undergoing plasmatic imbibition and revascularization from the underlying bed. We concluded that the cream did not cause any pain or irritation, because no significant differences were observed between the two groups in terms of post-experimental weight and in the consumption of water and food.
Johnson et al. [8] showed that rat blood vessels contain TRPM8 channels, which cause vasodilatation or vasoconstriction depending on the existing vascular tone. TRPM8 channels are also present in human blood vessels and regulate vasomotor tone. It has already been reported that Ca2+ channel blockers increase flap survival [15,22] and if menthol uses the same pathway, it can be hypothesized to increase flap viability [23].
Namer et al. [9] tested the effect of 40% menthol solution on healthy humans. They used laser Doppler to show that menthol increased the superficial blood flow in the area of the forearm where menthol was applied. Olive et al. [10] compared the effects of a cream containing 3.5% menthol and ice on the brachial artery of 12 healthy humans using ultrasound. Contrary to Namer et al., they found that menthol decreased blood flow. Topp et al. [24] also performed a similar study and found that both 3.5% menthol gel and 0.5 kg ice decreased the blood flow of the radial artery without changing the diameter of the artery or the pulse of the subjects. In another study, they investigated the effect of 3.5% menthol gel and a 10% menthol wipe on the thigh after three isokinetic maximum voluntary muscular contractions of the quadriceps and hamstrings. They found that the application of the gel decreased the blood flow in the popliteal artery, which had been increased after isokinetic exercises [11]. It was hypothesized that topical menthol causes vasoconstriction by inhibiting the nitric oxide system [13], and that its local cooling property acts through a mechanism similar to that of cold temperature sensations, which are perceived through α2c adrenergic receptors [12].
In addition to adrenergic vasoconstriction, platelet aggregation in the microvascular system plays a major role in ischemic necrosis of skin flaps. After trauma or surgery, thromboxane A2 (TXA2) released from the platelets causes vasoconstriction and platelet aggregation. In normal conditions, prostacyclin released from the vascular endothelium blocks the effect of TXA2. However, after vascular damage, the amount of TXA2 rises and that of prostacyclin decreases, and the perfusion of the tissue is therefore decreased. Salicylates, TXA2 inhibitors, and selective TXA2 antagonists increase the viability of random-pattern skin flaps [15,25]. MeSa, like all other salicylates, is a strong inhibitor of prostaglandin synthase and platelet aggregation. We hypothesized that MeSa would potentially increase flap survival through this mechanism.
As described above, conflicting data exist regarding the effect of menthol on vascular tone and blood flow. However, the studies showing a vasoconstrictive effect of menthol only investigated the blood flow of the major arteries and did not evaluate cutaneous blood flow. In our experiment, we indirectly observed the blood circulation of the skin while investigating flap survival. Photographic analysis showed that the cream increased flap viability, although the number of the vessels did not change after the experiment. The finding that the total ROI and MCFA of the study group did not change, while the total ROI of the control group decreased after the experiment, may be attributed to the vasodilatory effect of the cream. This effect could possibly be used in flap surgeries or replantations in order to increase the perfusion of the flap. However, it was not found to increase the overall viability of the flaps.
A limitation of our study was the absence of a control group using a control cream instead of saline. Moreover, laser Doppler or angiography could have provided more reliable results regarding the vasodilatory effect of the cream than scintigraphy, which provides functional imaging of living cells. Immunological stains could have shown neoangiogenesis more clearly.
Based on these results, it is certain that the cream containing 10% menthol and 15% methyl salicylate did not reduce the viability of the flaps. Its vasodilatory effect means that it can be incorporated as part of the dressing in reconstructive operations where skin perfusion is compromised. Nevertheless, further experiments are necessary to determine the optimal concentrations of menthol and MeSa (alone or in combination) for improving flap viability.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Rinker B, Fink BF, Barry NG, et al. The effect of calcium channel blockers on smoking-induced skin flap necrosis. Plast Reconstr Surg. 2010;125:866–871. doi: 10.1097/PRS.0b013e3181ccdc60. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Yang D, Wang P, et al. Microencapsulated myoblasts transduced by the vascular endothelial growth factor (VEGF) gene for the ischemic skin flap. Aesthetic Plast Surg. 2011;35:326–332. doi: 10.1007/s00266-010-9610-y. [DOI] [PubMed] [Google Scholar]
- 3.Miyawaki T, Jackson IT, Bier UC, et al. The effect of capsaicin ointment on skin for the survival of a cutaneous flap. Eur J Plast Surg. 2001;24:28–30. [Google Scholar]
- 4.Pal S, Khazanchi RK, Moudgil K. An experimental study on the effect of nifedipine on ischaemic skin flap survival in rats. Br J Plast Surg. 1991;44:299–301. doi: 10.1016/0007-1226(91)90076-v. [DOI] [PubMed] [Google Scholar]
- 5.Stadelmann WK, Hess DB, Robson MC, et al. Aprotinin in ischemia-reperfusion injury: flap survival and neutrophil response in a rat skin flap model. Microsurgery. 1998;18:354–361. doi: 10.1002/(sici)1098-2752(1998)18:6<354::aid-micr2>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 6.Patel T, Ishiuji Y, Yosipovitch G. Menthol: a refreshing look at this ancient compound. J Am Acad Dermatol. 2007;57:873–878. doi: 10.1016/j.jaad.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Kamatou GP, Vermaak I, Viljoen AM, et al. Menthol: a simple monoterpene with remarkable biological properties. Phytochemistry. 2013;96:15–25. doi: 10.1016/j.phytochem.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Johnson CD, Melanaphy D, Purse A, et al. Transient receptor potential melastatin 8 channel involvement in the regulation of vascular tone. Am J Physiol Heart Circ Physiol. 2009;296:H1868–H1877. doi: 10.1152/ajpheart.01112.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Namer B, Seifert F, Handwerker HO, et al. TRPA1 and TRPM8 activation in humans: effects of cinnamaldehyde and menthol. Neuroreport. 2005;16:955–959. doi: 10.1097/00001756-200506210-00015. [DOI] [PubMed] [Google Scholar]
- 10.Olive JL, Hollis B, Mattson E, et al. Vascular conductance is reduced after menthol or cold application. Clin J Sport Med. 2010;20:372–376. doi: 10.1227/NEU.0b013e3181e57bca. [DOI] [PubMed] [Google Scholar]
- 11.Topp R, Winchester LJ, Schilero J, et al. Effect of topical menthol on ipsilateral and contralateral superficial blood flow following a bout of maximum voluntary muscle contraction. Int J Sports Phys Ther. 2011;6:83–91. [PMC free article] [PubMed] [Google Scholar]
- 12.Chotani MA, Flavahan S, Mitra S, et al. Silent alpha(2C)-adrenergic receptors enable cold-induced vasoconstriction in cutaneous arteries. Am J Physiol Heart Circ Physiol. 2000;278:H1075–H1083. doi: 10.1152/ajpheart.2000.278.4.H1075. [DOI] [PubMed] [Google Scholar]
- 13.Green DJ, O'Driscoll G, Blanksby BA, et al. Control of skeletal muscle blood flow during dynamic exercise: contribution of endothelium-derived nitric oxide. Sports Med. 1996;21:119–146. doi: 10.2165/00007256-199621020-00004. [DOI] [PubMed] [Google Scholar]
- 14.Ohta T, Imagawa T, Ito S. Involvement of transient receptor potential vanilloid subtype 1 in analgesic action of methylsalicylate. Mol Pharmacol. 2009;75:307–317. doi: 10.1124/mol.108.051292. [DOI] [PubMed] [Google Scholar]
- 15.Davis RE, Wachholz JH, Jassir D, et al. Comparison of topical anti-ischemic agents in the salvage of failing random-pattern skin flaps in rats. Arch Facial Plast Surg. 1999;1:27–32. doi: 10.1001/archfaci.1.1.27. [DOI] [PubMed] [Google Scholar]
- 16.Hong CZ, Shellock FG. Effects of a topically applied counterirritant (Eucalyptamint) on cutaneous blood flow and on skin and muscle temperatures: a placebo-controlled study. Am J Phys Med Rehabil. 1991;70:29–33. doi: 10.1097/00002060-199102000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Kunta JR, Goskonda VR, Brotherton HO, et al. Effect of menthol and related terpenes on the percutaneous absorption of propranolol across excised hairless mouse skin. J Pharm Sci. 1997;86:1369–1373. doi: 10.1021/js970161+. [DOI] [PubMed] [Google Scholar]
- 18.Martin D, Valdez J, Boren J, et al. Dermal absorption of camphor, menthol, and methyl salicylate in humans. J Clin Pharmacol. 2004;44:1151–1157. doi: 10.1177/0091270004268409. [DOI] [PubMed] [Google Scholar]
- 19.Hammond DC, Brooksher RD, Mann RJ, et al. The dorsal skin-flap model in the rat: factors influencing survival. Plast Reconstr Surg. 1993;91:316–321. doi: 10.1097/00006534-199302000-00017. [DOI] [PubMed] [Google Scholar]
- 20.Jones M, Zhang F, Blain B, et al. Influence of recipient-bed isolation on survival rates of skin-flap transfer in rats. J Reconstr Microsurg. 2001;17:653–658. doi: 10.1055/s-2001-18821. [DOI] [PubMed] [Google Scholar]
- 21.Cheon YW, Tark KC, Kim YW. Better survival of random pattern skin flaps through the use of epigallocatechin gallate. Dermatol Surg. 2012;38:1835–1842. doi: 10.1111/j.1524-4725.2012.02566.x. [DOI] [PubMed] [Google Scholar]
- 22.Komorowska-Timek E, Chen SG, Zhang F, et al. Prolonged perivascular use of verapamil or lidocaine decreases skin flap necrosis. Ann Plast Surg. 1999;43:283–288. doi: 10.1097/00000637-199909000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Cheang WS, Lam MY, Wong WT, et al. Menthol relaxes rat aortae, mesenteric and coronary arteries by inhibiting calcium influx. Eur J Pharmacol. 2013;702:79–84. doi: 10.1016/j.ejphar.2013.01.028. [DOI] [PubMed] [Google Scholar]
- 24.Topp R, Ledford ER, Jacks DE. Topical menthol, ice, peripheral blood flow, and perceived discomfort. J Athl Train. 2013;48:220–225. doi: 10.4085/1062-6050-48.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghavami A, Nutt MP, Hardy SP. Heat shock protein and high-dose aspirin: effects on random skin flap survival in a rat model. Ann Plast Surg. 2002;48:60–67. doi: 10.1097/00000637-200201000-00009. [DOI] [PubMed] [Google Scholar]