In this randomized trial, raltegravir had more of a favorable lipid profile than ritonavir-boosted atazanavir or ritonavir-boosted darunavir–based regimens. Metabolic syndrome rates increased to the same degree for all 3 regimens. There was no relationship between ritonavir exposure and lipid levels.
Keywords: HIV/AIDS, cART, lipids, metabolic syndrome
Abstract
Background. Metabolic effects following combination antiretroviral therapy (cART) vary by regimen type. Changes in metabolic effects were assessed following cART in the AIDS Clinical Trials Group (ACTG) A5257 study, and correlated with plasma ritonavir trough concentrations (C24).
Methods. Treatment-naive adult subjects were randomized to ritonavir-boosted atazanavir or darunavir, or raltegravir-based cART. Changes in lipids and other metabolic outcomes over time were estimated. Differences between arms were estimated with 97.5% confidence intervals and compared using pairwise Student t tests. Associations between ritonavir C24 and lipid changes at week 48 were evaluated via linear regression.
Results. Analyses included 1797 subjects with baseline fasting data. Baseline lipid profiles and metabolic syndrome rates (approximately 21%) were similar across arms. Comparable increases occurred in total cholesterol, triglycerides, and low-density lipoprotein cholesterol with the boosted protease inhibitors (PIs); each PI had greater increases relative to raltegravir (all P ≤ .001 at week 96). Metabolic syndrome incident rates by week 96 (approximately 22%) were not different across arms. Ritonavir C24 was not different by arm (P = .89) (median, 69 ng/mL and 74 ng/mL in the atazanavir and darunavir arms, respectively) and were not associated with changes in lipid measures (all P > .1).
Conclusions. Raltegravir produced the most favorable lipid profile. Metabolic syndrome rates were high at baseline and increased to the same degree in all arms. Ritonavir C24 was not different in the PI arms and had no relationship with the modest but comparable increases in lipids observed with either atazanavir or darunavir. The long-term clinical significance of the lipid changes noted with the PIs relative to raltegravir deserves further evaluation.
Clinical Trials Registration. NCT 00811954.
Contemporary antiretroviral therapy (ART) regimens have improved virologic efficacy compared with earlier therapies. However, less is known about how these regimens compare to each other with respect to metabolic outcomes. Given the need for lifelong therapy, data on metabolic outcomes from randomized clinical trials can provide useful information to guide the selection of first-line therapy. Ritonavir-boosted protease inhibitor (PI)–containing therapy has been associated with the development of dyslipidemia and insulin resistance that may predispose to cardiovascular and cerebrovascular injuries [1–3]. On the contrary, raltegravir, an integrase strand inhibitor, has been shown to have milder lipid and metabolic effects relative to PI-based or nonnucleoside reverse transcriptase inhibitor–based therapy, producing minimal changes in plasma lipid levels, insulin resistance, or alteration of body habitus [4–6].
Furthermore, the extent of lipid and other metabolic changes in ritonavir-boosted PI regimens may vary by regimen type and by systemic ritonavir exposure [7]. Ritonavir is both a substrate and an inhibitor of the cytochrome P450 (CYP) 3A isoenzyme and, to a lesser extent, CYP2D6 [8]. All PIs vary in their affinity and impact on CYP3A4 isoenzyme [9]. As higher ritonavir exposure has been correlated with greater elevation in plasma lipids and the occurrence of other metabolic side effects [10, 11], the differential effect of coadministered PI on ritonavir plasma concentrations could impact the lipid profile of a given ritonavir-boosted PI regimen.
The AIDS Clinical Trials Group (ACTG) Study A5257 evaluated the virologic efficacy and tolerability of tenofovir disoproxil fumarate (TDF)/emtricitabine in combination with ritonavir-boosted atazanavir, raltegravir, or ritonavir-boosted darunavir in treatment-naive human immunodeficiency virus (HIV)–infected adults in the United States and Puerto Rico [12]. In the current report, we examined the lipid and other metabolic effects of these 3 antiretroviral regimens, with the aim of evaluating differences between the 2 PI arms, and between each of the PI arms and the raltegravir arm. Additionally, in the 2 PI arms of the study, the effects of atazanavir and darunavir on plasma trough ritonavir concentrations (C24) and the association between plasma ritonavir C24 and fasting lipid measures were evaluated.
METHODS
Study Population and Design
ACTG A5257 was a phase 3, randomized, open-label trial in which participants were randomly assigned 1:1:1 to receive 1 of 3 regimens—(1) 300 mg of atazanavir (Reyataz, Bristol-Myers Squibb) plus 100 mg of ritonavir (Norvir, Abbott Laboratories) once daily (ritonavir-boosted atazanavir); (2) 400 mg of raltegravir (Isentress, Merck Inc) twice daily; or (3) 800 mg of darunavir (Prezista, Janssen Therapeutics) plus 100 mg of ritonavir once daily (ritonavir-boosted darunavir)—each with the fixed-dose combination of 300 mg of TDF plus 200 mg of emtricitabine (Truvada, Gilead Sciences). This study was approved by the biomedical investigational research committee at each site, and all participants gave written informed consent before study enrollment (ClinicalTrials.gov identifier NCT00811954).
The study population included ART-naive, HIV-1–infected adults with plasma HIV-1 RNA level >1000 copies/mL. Study evaluations were completed before entry; at entry; at weeks 4, 8, 16, 24, and 32; and every 16 weeks thereafter. Participants were followed, regardless of meeting an endpoint, for 96 weeks after enrollment of the last participant. The primary objective of ACTG A5257 was to determine regimen equivalence with regard to virologic efficacy and tolerability over 96 weeks. Study A5257 had two important metabolic objectives. The first was to compare the impact of the study regimens on a variety of metabolic measures including fasting plasma lipid and glucose levels, prevalence of metabolic syndrome, and anthropometric measures. The study also aimed to detect an association between plasma ritonavir C24 at steady state and 48-week changes in fasting plasma lipid levels in the two ritonavir-boosted PI arms.
Fasting plasma lipid measurements evaluated included total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), triglycerides (TG), calculated non–HDL-C (calculated as TC – HDL-C), and calculated low-density lipoprotein cholesterol (LDL-C) (calculated as TC – [HDL-C + (TG/5)], if TG ≤400 mg/dL). Per protocol, these parameters were assessed at weeks 0, 24, 48, 96, and 144. Fasting was defined as nothing to eat or drink for at least 8 hours except for water or decaffeinated black coffee, and required prescription medications. Fasting lipid profile levels were performed at any Clinical Laboratory Improvement Amendments–compliant laboratory. Steady-state plasma ritonavir concentrations were evaluated by ritonavir C24 assessed once at least two weeks after initiation of ritonavir-boosted atazanavir or ritonavir-boosted darunavir and obtained within 22–26 hours after the last self-reported dose of ritonavir. Sampling for ritonavir C24 continued until a target sample size of 258 participants with evaluable steady-state ritonavir C24 measures was reached. Concentrations were measured by liquid chromatography–tandem mass spectrometry (LC-MS/MS) as previously described [13] at the University of California, San Francisco, on batched plasma samples. Incident metabolic syndrome was assessed based on the National Cholesterol Education Program (NCEP) Adult Treatment Panel III criteria [14, 15].
Statistical Analysis
Unless otherwise specified, all analyses included all eligible participants with fasting lipid measures available at study entry but otherwise followed the intention-to-treat principle; as-treated analysis including participants who completed the study on randomized treatment and sensitivity analyses excluding participants on lipid-lowering medications (primarily aimed at reducing TC, TG, and LDL) and antihypertensive agents were also performed. Missing data were considered missing completely at random. Mean changes in fasting plasma lipid measures over time were estimated with 95% confidence intervals (CIs). Pairwise treatment differences at weeks 24, 48, 96, and 144 were estimated with 97.5% CIs and compared between the treatment arms using pairwise Student t tests. The cumulative probability of developing metabolic syndrome over time was estimated by Kaplan–Meier method. Participants not meeting criteria for metabolic syndrome prior to end of follow-up were censored at the time of their last visit requiring metabolic evaluation; participants meeting criteria for metabolic syndrome at study entry were excluded from this analysis. All treatment group comparisons were assessed at a 2.5% level of statistical significance to provide modest control of the type I error in the setting of multiple comparisons.
Ritonavir C24 was analyzed on the natural logarithm scales and compared by treatment arms using Wilcoxon rank-sum tests. Associations between ritonavir C24 and change from baseline in levels of fasting TG, non–HDL-C, and calculated LDL-C at week 48 and week 96 were analyzed using linear regression; treatment-dependent associations were evaluated via 2-degrees of freedom tests for different intercept and slope. For analyses of these pharmacokinetic objectives, lipid values obtained following discontinuation of ritonavir-boosted PI or initiation of lipid-lowering agents were excluded, with values imputed with the last observations obtained prior to these events. The target sample size of 258 participants randomized to each of the ritonavir-boosted PI arms provided 90% power to detect an association between ritonavir C24 and 48-week change in fasting TG, equating to a 32 mg/dL lower change in fasting TG over 48 weeks per 12.6 ng/mL lower ritonavir C24, and allowed for a 20% loss due to missing data (providing effective sample size of 103 participants per ritonavir-boosted PI arm).
RESULTS
A total of 1809 evaluable participants were enrolled from 57 sites into A5257 between 22 May 2009 and 9 June 2011. Of these, 1797 with confirmed baseline fasting samples and clinical measures were included in the current analyses. Baseline demographics, metabolic and lipid measures, and clinical characteristics of the study population were well balanced between treatment arms (Table 1). The study population comprised 24% of women, 34% of non-Hispanic white, 42% of non-Hispanic black, and 21% of Hispanic. Full demographic details have been previously presented [9].
Table 1.
Baseline Characteristics and Metabolic Parameters Among Fasted Subjects
| Characteristic | Total (N = 1797) | Treatment |
||
|---|---|---|---|---|
| ATV/r (n = 602) | RAL (n = 600) | DRV/r (n = 595) | ||
| Female sex | 432 (24) | 143 (24) | 146 (24) | 143 (24) |
| Age, y, mean (SD) | 37.37 (11.02) | 37.60 (10.84) | 37.00 (10.81) | 37.52 (11.40) |
| Race/ethnicity | ||||
| White non-Hispanic | 612 (34) | 212 (35) | 212 (35) | 188 (32) |
| Black non-Hispanic | 753 (42) | 250 (42) | 253 (42) | 250 (42) |
| Hispanic (regardless of race) | 385 (21) | 124 (21) | 115 (19) | 146 (25) |
| Other | 43 (2) | 15 (2) | 18 (3) | 10 (2) |
| Weight, kg, mean (SD) | 78.64 (18.36) | 78.97 (18.92) | 78.84 (18.49) | 78.11 (17.66) |
| BMI, kg/m2, mean (SD) | 26.14 (5.93) | 26.22 (6.10) | 26.26 (5.85) | 25.94 (5.82) |
| Baseline HIV-1 RNA, log10 copies/mL, mean (SD) | 4.63 (0.72) | 4.63 (0.73) | 4.65 (0.71) | 4.60 (0.71) |
| Baseline CD4+ count, cells/µL, mean (SD) | 308.45 (192.27) | 309.16 (188.96) | 306.07 (198.59) | 310.15 (189.38) |
| Fasting TC, mg/dL | ||||
| Median (Q1, Q3) | 154 (133, 178) | 154 (134, 176) | 155 (134, 181) | 154 (133, 179) |
| <200 mg/dL | 1,589 (88) | 537 (89) | 523 (87) | 529 (89) |
| Fasting HDL-C, mg/dL | ||||
| Median (Q1, Q3) | 38 (31, 46) | 37 (30, 45) | 38 (31, 46) | 38 (31, 47) |
| >40 mg/dL | 780 (43) | 249 (41) | 272 (45) | 259 (44) |
| Fasting TG, mg/dL | ||||
| Median (Q1, Q3) | 103 (73, 148) | 105 (74, 150) | 103 (73, 146) | 99 (73, 148) |
| <150 mg/dL | 1,352 (75) | 449 (75) | 456 (76) | 447 (75) |
| Fasting non-HDL-C, mg/dL | ||||
| Median (Q1, Q3) | 114 (95, 138) | 115 (96, 137) | 115 (96, 140) | 112 (93, 138) |
| <160 mg/dL | 1,606 (89) | 542 (90) | 533 (89) | 531 (89) |
| Fasting calculated LDL-C, mg/dLa | ||||
| Median (Q1, Q3) | 92 (74, 112) | 93 (75, 111) | 93 (74, 115) | 89 (73, 111) |
| <130 mg/dL | 1,606 (89) | 542 (90) | 528 (88) | 536 (90) |
| Fasting glucose, mg/dL | ||||
| Median (Q1, Q3) | 84 (78, 92) | 85 (79, 93) | 85 (78, 92) | 83 (77, 91) |
| <100 mg/dL | 1602 (89) | 529 (88) | 539 (90) | 534 (90) |
| Presence of metabolic syndrome | 381 (21) | 141 (23) | 121 (20) | 119 (20) |
| Smoking status | ||||
| History of smoking | 1,044 (58) | 358 (59) | 349 (58) | 337 (57) |
| Smoking history >10 y | 678 (38) | 249 (41) | 225 (38) | 204 (34) |
| Current smoker | 706 (39) | 251 (42) | 241 (40) | 214 (36) |
| On lipid-lowering treatment | 106 (6) | 33 (5) | 35 (6) | 38 (6) |
| On antihypertensive agents | 273 (15) | 95 (16) | 89 (15) | 89 (15) |
| Hypoglycemic agents | 64 (4) | 24 (4) | 21 (3) | 19 (3) |
Data are presented as No. (%) unless otherwise specified.
Abbreviations: ATV/r, ritonavir-boosted atazanavir; BMI, body mass index; DRV/r, ritonavir-boosted darunavir; HDL-C, high-density liproprotein cholesterol; HIV-1, human immunodeficiency virus type 1; LDL, low-density liproprotein cholesterol; RAL, raltegravir; SD, standard deviation; TC, total cholesterol; TG, triglycerides.
a Calculated as [fasting calculated LDL-C (mg/dL) = fasting TC – fasting HDL-C – (fasting TG/5)], only for subjects with fasting TG ≤400 mg/dL; subjects with fasting TG >400 mg/dL were excluded.
Eighty-eight percent of participants had the NCEP target TC <200 mg/dL, 75% had TG <150 mg/dL, 89% had non–HDL-C <160 mg/dL, and 89% (of 1170 with TG ≤400 mg/dL) had calculated LDL <130 mg/dL, whereas only 43% had HDL-C ≥40 mg/dL. Median (Q1, Q3) fasting blood glucose level was 84 (78, 92) mg/dL; 89% had fasting blood glucose levels <100 mg/dL. Twenty-one percent of participants had evidence of metabolic syndrome at study entry. Fifty-eight percent of participants reported a history of smoking, 38% reported a smoking history >10 years, and 39% were current smokers. At study entry, 106 (6%) were taking lipid-lowering agents, 273 (15%) were taking antihypertensive agents, and 64 (4%) were on hypoglycemic therapy at study entry (Table 1).
Changes from baseline and absolute values over time in lipid measures and other metabolic outcomes following therapy are summarized in Figure 1, Supplementary Figure 1, and Table 2, respectively. In pairwise comparisons, there were no differences in the mean change from baseline to all study weeks (24, 48, and 96) in any of the lipid measures between the ritonavir-boosted atazanavir and the ritonavir-boosted darunavir arms (all P > .05). However, each of the ritonavir-boosted PI arms had greater increases relative to the raltegravir arm in TC, TG, non–HDL-C, and LDL-C (all P ≤ .001). HDL-C increased modestly in all 3 arms (an average increase of 6 mg/dL over 96 weeks), with no significant differences in mean change from baseline to all study weeks evaluated between treatment arms (all P > .06) (Figure 1A–C, Supplementary Figure 1A). From baseline to week 96, the percentage of participants who had taken lipid-lowering agents increased from 5% to 11% in the ritonavir-boosted atazanavir arm, 6%–9% in the raltegravir arm, and 6%–14% in the ritonavir-boosted darunavir arm. Sensitivity analyses of changes in lipid measures over time by treatment arms that included as-treated analyses with participants who completed the study on their original randomized treatment, or limited to participants not taking lipid-lowering agents, yielded findings that were consistent with the intention-to-treat analyses.
Figure 1.
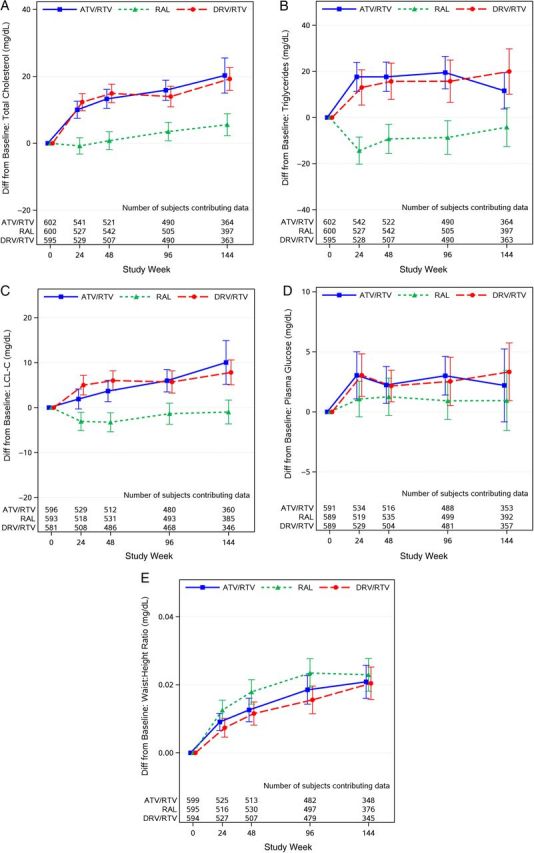
Mean changes from baseline in metabolic outcomes and anthropometric measures, over time, with 95% confidence interval. Abbreviations: ATV/RTV, ritonavir-boosted atazanavir; Diff, difference; DRV/RTV, ritonavir-boosted darunavir; LDL-C, low-density lipoprotein cholesterol; RAL, raltegravir.
Table 2.
Summary of the Absolute Levels: Treatment Group Comparison of Lipids Over Time
| Metabolic Parameters | Study Week | ATV/r | RAL | DRV/r |
|---|---|---|---|---|
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | ||
| Fasting total cholesterol, mg/dL | 0 | 156.7 (154.0–159.4) | 158.3 (155.4–161.2) | 157.0 (154.0–160.0) |
| 24 | 166.3 (163.1–169.4) | 157.9 (154.9–160.9) | 169.2 (166.1–172.3) | |
| 48 | 169.8 (166.4–173.2) | 159.5 (156.5–162.4) | 172.3 (168.9–175.6) | |
| 96 | 172.3 (169.0–175.6) | 163.4 (160.3–166.4) | 172.4 (169.0–175.8) | |
| Fasting HDL-C, mg/dL | 0 | 38.8 (37.8–39.8) | 39.5 (38.3–40.6) | 40.4 (39.2–41.5) |
| 24 | 43.4 (42.2–44.6) | 43.9 (42.7–45.0) | 44.4 (43.1–45.7) | |
| 48 | 45.1 (43.8–46.5) | 44.5 (43.3–45.7) | 45.9 (44.6–47.1) | |
| 96 | 45.2 (43.9–46.5) | 45.4 (44.2–46.7) | 45.6 (44.2–47.0) | |
| Fasting triglycerides, mg/dL | 0 | 123.8 (117.2–130.4) | 123.4 (116.9–129.9) | 124.3 (117.1–131.5) |
| 24 | 140.3 (133.0–147.6) | 109.3 (103.4–115.2) | 137.3 (129.7–144.9) | |
| 48 | 139.7 (132.1–147.4) | 115.3 (108.8–121.9) | 139.5 (131.3–147.6) | |
| 96 | 140.9 (133.0–148.8) | 116.3 (109.6–122.9) | 141.1 (131.1–151.1) | |
| Fasting non–HDL-C, mg/dL | 0 | 117.9 (115.4–120.4) | 118.8 (116.2–121.5) | 116.6 (113.9–119.3) |
| 24 | 122.9 (119.9–126.0) | 114.0 (111.1–116.9) | 124.8 (121.8–127.9) | |
| 48 | 124.6 (121.4–127.9) | 115.0 (112.1–117.8) | 126.5 (123.2–129.7) | |
| 96 | 127.1 (123.9–130.3) | 118.0 (114.9–121.0) | 126.9 (123.6–130.1) | |
| Fasting calculated LDL, mg/dL | 0 | 93.7 (91.4–96.0) | 94.9 (92.4–97.5) | 93.0 (90.5–95.4) |
| 24 | 95.4 (92.8–98.1) | 92.2 (89.7–94.7) | 98.0 (95.3–100.6) | |
| 48 | 97.4 (94.5–100.2) | 92.0 (89.6–94.3) | 99.1 (96.3–101.9) | |
| 96 | 99.4 (96.5–102.3) | 95.1 (92.5–97.7) | 99.9 (97.1–102.7) | |
| Fasting plasma glucose, mg/dL | 0 | 88.0 (86.2–89.8) | 87.4 (85.6–89.2) | 85.6 (84.0–87.1) |
| 24 | 90.3 (88.1–92.5) | 89.2 (87.4–91.0) | 88.9 (87.0–90.9) | |
| 48 | 89.5 (87.9–91.2) | 89.0 (86.8–91.3) | 87.0 (85.6–88.5) | |
| 96 | 90.2 (88.3–92.2) | 88.6 (87.0–90.3) | 88.9 (86.9–90.9) | |
| Waist:height ratio, cm:cm | 0 | 0.53 (.52–.53) | 0.53 (.52–.53) | 0.52 (.51–.53) |
| 24 | 0.54 (.53–.54) | 0.54 (.53–.54) | 0.53 (.52–.53) | |
| 48 | 0.54 (.53–.55) | 0.54 (.54–.55) | 0.53 (.52–.54) | |
| 96 | 0.55 (.54–.55) | 0.55 (.54–.56) | 0.53 (.52–.54) |
Abbreviations: ATV/r, ritonavir-boosted atazanavir; CI, confidence interval; DRV/r, ritonavir-boosted darunavir; HDL-C, high-density lipoprotein cholesterol; LDL, low-density liproprotein cholesterol; RAL, raltegravir.
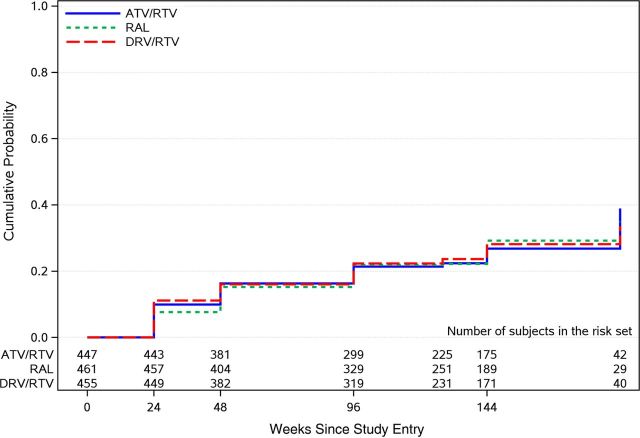
Fasting plasma glucose, waist circumference, and waist-to-height ratio increased following ART initiation in all 3 treatment arms (Figure 1D and 1E; Supplementary Figure 1B). In pairwise comparisons, larger increases in waist circumference were observed with the raltegravir arm compared with the ritonavir-boosted darunavir arm at weeks 48 and 96 (all P ≤ .023) but not compared with the ritonavir-boosted atazanavir arm (P ≥ .07); no other treatment group differences were apparent. The cumulative probability of incident of metabolic syndrome by week 96 was 21% (95% CI, 18%–26%) for the ritonavir-boosted atazanavir arm, 22% (95% CI, 18%–26%) for the raltegravir arm, and 22% (95% CI, 19%–27%) for the ritonavir-boosted darunavir arm, with no apparent difference between the treatment arms (all P ≥ .7; Figure 2).
Figure 2.
Cumulative probability of metabolic syndrome, by treatment group. A total of 1363 subjects were included in this analysis; 381 subjects who had metabolic syndrome at baseline and 53 subjects who were censored at baseline were excluded. Abbreviations: ATV/RTV, ritonavir-boosted atazanavir; DRV/RTV, ritonavir-boosted darunavir; RAL, raltegravir.
Of the 230 participants who had plasma obtained for evaluation of drug concentrations, 109 in the ritonavir-boosted atazanavir arm and 121 in the ritonavir-boosted darunavir arm had evaluable steady-state ritonavir C24. Median (Q1, Q3) ritonavir C24 was 69 (40–105) ng/mL in the ritonavir-boosted atazanavir arm, and 74 (38–110) ng/mL in the ritonavir-boosted darunavir arm, with no apparent difference between the arms (P = .89). Associations between ritonavir C24 and changes in fasting plasma lipid measures were not apparent (P ≥ .4) at either week 48 or week 96. While treatment group specific estimates of associations between ritonavir C24 and lipid change were in opposite directions (negative in the atazanavir group, positive in the darunavir group; Supplementary Figure 2), none of these associations were statistically significant (P > .09), and no evidence of PI-specific associations was apparent (P ≥ .09) (Table 3).
Table 3.
Linear Regression Estimates Evaluating the Association Between Plasma Ritonavir Trough Concentrations and Changes in Lipid Parameters Over 48 and 96 Weeks
| Parameter | LDL |
TG |
Non-HDL |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate (95% CI) | P Value | Estimate (95% CI) | P Value | Estimate (95% CI) | P Value | ||||
| Associations with change to week 48, mg/dL | |||||||||
| Intercept | 3.78 | (−0.02 to 7.57) | … | 15.85 | (5.17–26.52) | … | 8.21 | (4.10–12.31) | … |
| RTV C24 (per 1 log [ng/mL])a | 0.51 | (−1.58 to 2.61) | .63 | 1.71 | (−4.25 to 7.68) | .57 | 0.99 | (−1.30 to 3.28) | .40 |
| Test for PI-specific association (2 df) | P = .09 | P = .23 | P = .10 | ||||||
| Associations with change to week 96, mg/dL | |||||||||
| Intercept | 4.23 | (0.30–8.16) | … | 17.45 | (6.18–28.73) | … | 8.87 | (4.54–13.20) | … |
| RTV C24 (per 1 log [ng/mL])a | −0.15 | (−2.28 to 1.99) | .89 | −0.14 | (−6.36 to 6.08) | .97 | −0.25 | (−2.63 to 2.14) | .84 |
| Test for PI-specific association (2 df) | P = .53 | P = .35 | P = .22 | ||||||
Estimates (mg/dL) are from simple linear regression analysis of RTV C24 on change from baseline to the given week for each lipid parameter. RTV C24 values (on the natural log scale) were centered on the group mean for modeling, so the intercept estimate represents the average lipid change from baseline for an average (geometric mean) RTV C24 of approximately 55 ng/mL. The test for a PI-specific association is a 2-df F test for nonzero treatment group main (intercept) and interaction (RTV C24 × treatment) coefficients.
Abbreviations: C24, plasma trough concentration; CI, confidence interval; df, degrees of freedom; HDL, high-density lipoprotein; LDL, low-density lipoprotein; PI, protease inhibitor; RTV, ritonavir; TG, triglycerides.
a Estimate represents the estimated difference in the lipid change from baseline to given week for each 1-log (natural) difference in RTV C24; note that 1 log is approximately the difference between the first and third quartiles of observed RTV C24 in the A5257 study.
DISCUSSION
In this prospective, randomized, open-label study evaluating raltegravir and the two ritonavir-boosted PI regimens as initial treatment of HIV infection, we identified differences in lipid changes and anthropometric changes between treatment regimens. As expected based on prior small studies, we found increases in fasting lipid measures including TC, TG, non–HDL-C, and LDL-C following therapy with the two ritonavir-boosted PI arms but not with the raltegravir arm. These lipid changes were similar at every study evaluation point between the two ritonavir-boosted PI arms, confirming previously reported equivalence in the lipid effects of these two widely used PI regimens in clinical practice [16]. Of note, the mean HDL-C level was low in the majority of participants at entry, as expected for ART-naive subjects [17]. However, small increases were observed for this lipid measure in all 3 arms with no apparent differences between arms, also consistent with prior data suggesting that initiation of ART modestly improves HDL-C levels [17].
Metabolic syndrome is a term that describes a cluster of clinical, anthropometric, and laboratory measures that confer heightened risk of cardiovascular disease and type 2 diabetes [18]. There is a relatively high prevalence of metabolic disorders among HIV-infected individuals [19], which has been attributed to lipid abnormalities, particularly high plasma TG and low HDL-C commonly seen in untreated HIV disease [20], and to antiretroviral-induced metabolic and body shape changes [21, 22]. At baseline, the fasting lipid profile was below NCEP lipid thresholds for individuals with one or more cardiovascular risk factors for a large proportion of participants, fasting blood glucose levels were <100 mg/dL in 89%, and the use of hypoglycemic agents was modest. The presence of pretherapy metabolic syndrome was high (21%), and similar to that previously reported for HIV-infected individuals [23, 24]. In contrast to the general population, among whom metabolic syndrome is primarily driven by hypertension, hyperglycemia, and abdominal obesity [25], baseline metabolic syndrome in this study was driven largely by a combination of low plasma HDL-C levels and hypertriglyceridemia—two lipid parameters directly affected by HIV infection [17].
During the study, the cumulative probability of developing new metabolic syndrome was high (approximately 22% at 96 weeks). However, contrary to expectation due to the known effects of ritonavir-boosted PI regimens on lipids, no differences between the study arms were apparent. The greater increase in waist circumference observed with the raltegravir-based therapy counterbalanced the contribution of higher TG levels in the PI arms, accounting for the comparable cumulative probability of metabolic syndrome in all three treatment arms. This notwithstanding, the high rate of metabolic syndrome at baseline and following ART in this population should raise some concern, as individuals with metabolic syndrome are susceptible to a variety of conditions including cardiovascular diseases, type 2 diabetes, certain malignancies, polycystic ovarian syndrome, and reactive airway disease [26–28].
The lipid changes experienced with ritonavir-boosted PI therapy are speculated to be driven to a larger extent by the ritonavir component of the regimens. In the TMC114-C159 trial [29], ritonavir-boosted atazanavir increased the plasma exposure of ritonavir, whereas ritonavir-boosted darunavir had an opposite effect. That healthy volunteer study with only 28 days’ duration could not evaluate the metabolic consequences of this differential ritonavir systemic exposure. The present study provided an opportunity to rigorously evaluate this question. Steady-state ritonavir trough concentrations in our study population were comparable to previous reports for the tablet formulation [30], and were not different between the ritonavir-boosted atazanavir and darunavir regimens. Associations between ritonavir exposure and lipid changes over 48 and 96 weeks were also not apparent (both overall and within specific PI regimens). Several reasons may account for the differences between our findings and the TMC114-C159 trial, including the larger size of our study population, which may have limited the confounding effect of intrasubject variability in drug concentrations; the use of HIV-infected rather than healthy volunteers; and the longer study follow-up time points at which lipid sampling was performed in the current study. It should also be noted that it is possible that ritonavir exposure may affect other metabolic outcomes other than plasma lipids, although the scant published literature to date does not support an association between ritonavir concentration and changes in anthropometric measurements [31].
The diversity of the study population with relatively high proportions of women and participants of ethnic minority backgrounds, which closely mirrors the demography of the HIV/AIDS epidemic in many parts of the United States, makes these findings of the current analyses generalizable to clinical practice. Furthermore, the pharmacokinetic/pharmacodynamic analysis included a robust sample of >200 ritonavir C24 samples collected within a specific time interval. This robust sample size is large enough to militate against the confounding effect of intrasubject variability in plasma antiretroviral concentrations that have limited the interpretation of previous smaller studies. In addition, this study captured data from baseline to week 144, allowing a longer assessment of changes in metabolic measures relative to other studies. Self-reported adherence to ART and nonobservance of ritonavir administration prior to pharmacokinetic sampling limits nearly all studies of this type. Fasting plasma lipid fractions were limited only to TC, TG, non–HDL-C, LDL-C, and HDL-C. Typically, dual-energy X-ray absorptiometry (DEXA) data are used to assess changes in body fat composition with therapy. However, DEXA scans were not obtained in the general study participants, but were obtained on a subset of participants and will be reported separately.
In this large, randomized prospective trial, raltegravir produced the most favorable lipid profile compared with 2 ritonavir-boosted PIs. Metabolic syndrome rates at baseline were high, and the cumulative probability of developing new metabolic syndrome post-ART was similar across all arms. Ritonavir C24 was not different between the 2 ritonavir-boosted PIs arms, and had no relationship with the modest but similar increases in plasma lipids observed with either the ritonavir-boosted atazanavir–based or ritonavir-boosted darunavir–based regimen. The long-term clinical significance of the lipid changes noted with the two ritonavir-boosted PI regimens relative to the raltegravir-based regimen deserves further evaluation, particularly when initiating ART in patients with high cardiovascular risk profiles.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases (NIAID) or the National Institutes of Health.
Financial support. The project described was supported by the NIAID (award number UM1AI068636); the National Institute of Mental Health; and the National Institute of Dental and Craniofacial Research. The protocol received support from the AIDS Clinical Trial Group (ACTG); the Site Data Management Center (grant number UM1AI68634); the ACTG specialty laboratories listed in the manuscript; and the 37 clinical research sites.
Potential conflicts of interest. All authors: No potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
APPENDIX
We acknowledge the following personnel and AIDS Clinical Trials Unit grants:
Michelle Saemann, RN and Jennifer Baer, RN: Cincinnati CRS (Site 2401) Grant AI069439; Dr Susan Koletar, Mark Hite RN: Ohio State University CRS (Site 2301) Grant UM1AI069494; Linda Meixner, RN, and Edward Seefried, RN: UCSD Antiviral Research Center CRS (Site 701) Grant AI69432; Vicki Bailey, RN, and Rebecca Basham, CCRP: Vanderbilt Therapeutics CRS (Site 3652) Grant 2UM1AI069439-08, CTSA Grant UL1 TR000445; David Currin, RN, and Miriam Chicurel-Bayard RN: Chapel Hill CRS (Site 3201) Grant UM1 AI069423-08, CTSA Grant 1UL1TR001111, CFAR Grant P30 AI50410; Teresa Spitz and Judy Frain: Washington University Therapeutics CRS (Site 2101) Grant UM1AI069439; Elizabeth Lindsey, RN, and Tamara James: Alabama Therapeutics CRS (Site 5801) Grant 2UM1AI069452-08; Beverly Putnam and Cathi Basler: University of Colorado Hospital CRS (Site 6101) Grant 2UM1AI069432, CTSA Grant UL1 TR001082; Michael P. Dube, MD, and Bartolo Santos, RN: University of Southern California CRS (Site 1201) Grant AI069432; Eric Daar and Sadia Shaik : Harbor UCLA CRS (Site 603) Grant AI069424, CTSA Grant UL1TR000124; Pablo Tebas MD, and Aleshia Thomas, RN, BSN: Penn Therapeutics CRS (Site 6201) Grant UM1-AI069534-08, CFAR Grant 5-P30-AI-045008-15; Roger Bedimo, MD, and Michelle Mba, MPH: Trinity Health and Wellness Center (Site 31443) Grant U01 AI069471; David Cohn, MD, and Fran Moran, RN: Denver Public Health CRS (Site 31470) Grant UM1 AI069503; Jorge L. Santana Bagur, MD, and Ileana Boneta Dueño, RN: Puerto Rico AIDS Clinical Trials Unit CRS (Site 5401) Grant 2UM1AI069415-09; Babafemi Taiwo, MBBS, Baiba Berzins, MPH: Northwestern University CRS (Site 2701) Grant 5U01 AI069471; Dr Emery Chang and Maria Palmer: UCLA CARE Center CRS (Site 601) Grant A1069424; Mary Adams, RN, and Christine Hurley, RN : University of Rochester ACTG CRS/AIDS CARE CRS/Trillium Health (Site 1101/Site 1108) Grant 2UM1 AI069511-08, CTSA Grant UL1 TR024160; Timothy Lane and Cornelius Van Dam: Greensboro CRS (Site 3203) Grant A1069423-08; Karen Tashima, MD, and Helen Patterson, LPN : The Miriam Hospital (TMH) CRS (Site 2951) Grant 2UM1A1069412-08; Carlos del Rio, MD, and Ericka Patrick, RN: The Ponce de Leon Ctr. CRS (Site 5802) Grant 2UM1 AI069418-08, CFAR Grant P30 AI050409, CTSA Grant UL1 TR000454; Norman Markowitz and Indira Brar: Henry Ford Hospital CRS (Site 31472) Grant UM 1 A1069503; Roberto C. Arduino, MD, and Maria Laura Martinez: Houston AIDS Research Team CRS (Site 31473) Grant 2 UM1 AI069503-08, 2 UM1 AI068636-08; Rose Kim, MD, and Yolanda Smith, BA: Cooper University Hosp. CRS (Site 31476) Grant UM1 AI069503; Hector Bolivar, MD, Margaret A. Fischl, MD: University of Miami AIDS Clinical Research Unit (ACRU) CRS (Site 901) Grant AI069477; Edward Telzak, MD, and Richard Cindrich, MD: Bronx-Lebanon Hospital Ctr. CRS (Site 31469) Grant UM1 AI069503; Paul Sax, MD and Cheryl Keenan, RN, BC: Brigham and Women's Hospital Therapeutics CRS (Site 107) Grant UM1AI069472; CFAR grant P30 AI060354, CTSA UL1 TR000170; Kim Whitely, RN, and Traci Davis, RN: MetroHealth CRS (Site 2503) Grant AI 69501; CTSA Grant UL1TR000439; Dr Rodger D. MacArthur and Marti Farrough, RN, BSN: Wayne State University CRS (Site 31478) Grant 2UM1AI069503-08; Judith A. Aberg, MD, and Michelle S. Cespedes, MPH, MD: New York University HIV/AIDS CRS (Site 401) Grant UM1 AI069532; Shelia Dunaway, MD, and Sheryl Storey, PA-C: University of Washington AIDS CRS (Site 1401) Grant UM AI069481; Joel Gallant, MD, and Ilene Wiggins, RN : Johns Hopkins University CRS (Site 201) Grant 2UM1 AI069465, CTSA Grant UL1TR001079; Beverly Sha, MD, and Veronica Navarro, RN: Rush University CRS (Site 2702) Grant AI-069471; Vicky Watson, RN, and Daniel Nixon, DO, PhD : Virginia Commonwealth University Medical Ctr. CRS (Site 31475) CPCRA CTU award UM1 AI069503, CTSA UL1TR000058; Annie Luetkemeyer, MD, and Jay Dwyer, RN: UCSF HIV/AIDS CRS (Site 801) Grant UM1 AI069496, UCSF-CTSA Grant UL1 TR000004; Kristen Allen, RN and Patricia Walton, RN: Case CRS (Site 2501) Grant AI069501; Dr Princy Kumar and Dr Joseph Timpone: Georgetown University CRS (Site 1008) Grant 1U01AI069494; Mehri McKellar, MD, and Jacquelin Granholm, RN: Duke University Medical Ctr. Adult CRS (Site 1601) Grant 5UM1 AI069484-07; Michael T Yin, MD, MS, and Madeline Torres, RN: Columbia Physicians and Surgeons CRS (Site 30329) Grant 2UM1-AI069470-08, CTSA 5UL1 RR024156; Sandra Valle, PA-C, and Debbie Slamowitz, RN: Stanford CRS (Site 501) Grant AI069556; Charles E. Davis Jr, MD, and William A. Blattner, MD: IHV Baltimore Treatment CRS (Site 4651) Grant U01AI069447; BMC ACTG CRS (Site 104); Benjamin Linus, MD: UM1 AI069472; Beth Israel Deaconess Medical Ctr., ACTG CRS (Site 103); Mary Albrecht, MD: UM1 AI069472; CFAR grant P30 AI060354; Christina Megill, PA-C, and Valery Hughes, NP: Cornell Chelsea CRS (Site 7804) Grant UM1AI069419, CTSA Grant UL1TR000457; Teri Flynn, MSN, ANP, and Amy Sbrolla, BSN, RN : Massachusetts General Hospital CRS (Site 101) Grant 2UM1AI069412-08; CFAR grant P30 AI060354; Sharon Riddler, MD, and Lisa Klevens, BSN: University of Pittsburgh CRS (Site 1001) Grant UM1 AI069494.
Contributor Information
Collaborators: for the AIDS Clinical Trials Group (ACTG) A5257 Team, Michelle Saemann, Jennifer Baer, Susan Koletar, Linda Meixner, Edward Seefried, Vicki Bailey, Rebecca Basham, David Currin, Miriam Chicurel-Bayard, Teresa Spitz, Judy Frain, Elizabeth Lindsey, Tamara James, Beverly Putnam, Cathi Basler, Michael P. Dube, Bartolo Santos, Eric Daar, Sadia Shaik, Pablo Tebas, Aleshia Thomas, Roger Bedimo, Michelle Mba, David Cohn, Fran Moran, Jorge L. Santana Bagur, Ileana Boneta Dueño, Babafemi Taiwo, Baiba Berzins, Emery Chang, Maria Palmer, Mary Adams, Christine Hurley, Timothy Lane, Cornelius Van Dam, Karen Tashima, Helen Patterson, Carlos del Rio, Ericka Patrick, Norman Markowitz, Indira Brar, Roberto C. Arduino, Maria Laura Martinez, Rose Kim, Yolanda Smith, Hector Bolivar, Margaret A. Fischl, Edward Telzak, Richard Cindrich, Paul Sax, Cheryl Keenan, Kim Whitely, Traci Davis, Rodger D. MacArthur, Marti Farrough, Judith A. Aberg, Michelle S. Cespedes, Shelia Dunaway, Sheryl Storey, Joel Gallant, Ilene Wiggins, Beverly Sha, Veronica Navarro, Vicky Watson, Daniel Nixon, Annie Luetkemeyer, Jay Dwyer, Kristen Allen, Patricia Walton, Princy Kumar, Joseph Timpone, Mehri McKellar, Jacquelin Granholm, Michael T. Yin, Madeline Torres, Sandra Valle, Debbie Slamowitz, Charles E. Davis, William A. Blattner, Benjamin Linus, Mary Albrecht, Christina Megill, Valery Hughes, Teri Flynn, Amy Sbrolla, Sharon Riddler, and Lisa Klevens
References
- 1.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services, 2014:521–7. Available at: http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf Accessed 2 May 2014. [Google Scholar]
- 2.World Health Organization. WHO consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Geneva, Switzerland: WHO, 2013:1–272 Available at: http://www.who.int/hiv/pub/guidelines/arv2013/en/ Accessed 2 May 2014. [PubMed] [Google Scholar]
- 3.Periard D, Telenti A, Sudre P, et al. Atherogenic dyslipidemia in HIV-infected individuals treated with protease inhibitors. The Swiss HIV Cohort Study. Circulation 1999; 100:700–5. [DOI] [PubMed] [Google Scholar]
- 4.Lennox JL, Dejesus E, Berger DS, et al. Raltegravir versus efavirenz regimens in treatment-naive HIV-1-infected patients: 96-week efficacy, durability, subgroup, safety, and metabolic analyses. J Acquir Immune Defic Syndr 2010; 55:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eron JJ, Young B, Cooper DA, et al. Switch to a raltegravir-based regimen versus continuation of a lopinavir-ritonavir-based regimen in stable HIV-infected patients with suppressed viraemia (SWITCHMRK 1 and 2): two multicentre, double-blind, randomised controlled trials. Lancet 2010; 375:396–407. [DOI] [PubMed] [Google Scholar]
- 6.Martinez E, Larrousse M, Llibre JM, et al. Substitution of raltegravir for ritonavir-boosted protease inhibitors in HIV-infected patients: the SPIRAL study. AIDS 2010; 24:1697–707. [DOI] [PubMed] [Google Scholar]
- 7.Boffito M. Differences between PI effects on plasma RTV levels: analysis of cross-over pK trials and clinical trials. In: 8th International Workshop on Clinical Pharmacology of HIV Therapy, Budapest, Hungary, 16–18 April 2007. [Google Scholar]
- 8.Kumar GN, Rodrigues AD, Buko AM, Denissen JF. Cytochrome P450-mediated metabolism of the HIV-1 protease inhibitor ritonavir (ABT-538) in human liver microsomes. J Pharmacol Exp Ther 1996; 277:423–31. [PubMed] [Google Scholar]
- 9.Lillibridge JH, Liang BH, Kerr BM, et al. Characterization of the selectivity and mechanism of human cytochrome P450 inhibition by the human immunodeficiency virus-protease inhibitor nelfinavir mesylate. Drug Metab Dispos 1998; 26:609–16. [PubMed] [Google Scholar]
- 10.de Gonzalez RD, Blanco F, Garcia-Benayas T, Jimenez-Nacher I, Gonzalez-Lahoz J, Soriano V. Correlation between lopinavir plasma levels and lipid abnormalities in patients taking lopinavir/ritonavir. AIDS Patient Care STDS 2003; 17:443–5. [DOI] [PubMed] [Google Scholar]
- 11.Gatti G, Di Biagio A, Casazza R, et al. The relationship between ritonavir plasma levels and side-effects: implications for therapeutic drug monitoring. AIDS 1999; 13:2083–9. [DOI] [PubMed] [Google Scholar]
- 12.Lennox JL, Landovitz RJ, Ribaudo HJ, et al. Efficacy and tolerability of 3 nonnucleoside reverse transcriptase inhibitor-sparing antiretroviral regimens for treatment-naive volunteers infected with HIV-1: a randomized, controlled equivalence trial. Ann Intern Med 2014; 161:461–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chi J, Jayewardene AL, Stone JA, Motoya T, Aweeka FT. Simultaneous determination of five HIV protease inhibitors nelfinavir, indinavir, ritonavir, saquinavir and amprenavir in human plasma by LC/MS/MS. J Pharm Biomed Anal 2002; 30:675–84. [DOI] [PubMed] [Google Scholar]
- 14.Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 2001; 285:2486–97. [DOI] [PubMed] [Google Scholar]
- 15.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009; 120:1640–5. [DOI] [PubMed] [Google Scholar]
- 16.Aberg JA, Tebas P, Overton ET, et al. Metabolic effects of darunavir/ritonavir versus atazanavir/ritonavir in treatment-naive, HIV type 1-infected subjects over 48 weeks. AIDS Res Hum Retroviruses 2012; 28:1184–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riddler SA, Smit E, Cole SR, et al. Impact of HIV infection and HAART on serum lipids in men. JAMA 2003; 289:2978–82. [DOI] [PubMed] [Google Scholar]
- 18.Kassi E, Pervanidou P, Kaltsas G, Chrousos G. Metabolic syndrome: definitions and controversies. BMC Med 2011; 9:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Volberding PA, Deeks SG. Antiretroviral therapy and management of HIV infection. Lancet 2010; 376:49–62. [DOI] [PubMed] [Google Scholar]
- 20.Grunfeld C, Pang M, Doerrler W, Shigenaga JK, Jensen P, Feingold KR. Lipids, lipoproteins, triglyceride clearance, and cytokines in human immunodeficiency virus infection and the acquired immunodeficiency syndrome. J Clin Endocrinol Metab 1992; 74:1045–52. [DOI] [PubMed] [Google Scholar]
- 21.Wohl DA, Brown TT. Management of morphologic changes associated with antiretroviral use in HIV-infected patients. J Acquir Immune Defic Syndr 2008; 49(suppl 2):S93–100. [DOI] [PubMed] [Google Scholar]
- 22.Krishnan S, Schouten JT, Atkinson B, et al. Metabolic syndrome before and after initiation of antiretroviral therapy in treatment-naive HIV-1infected individuals. J Acquir Immune Defic Syndr 2012; 61:381–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Worm SW, Lundgren JD. The metabolic syndrome in HIV. Best Pract Res Clin Endocrinol Metab 2011; 25:479–86. [DOI] [PubMed] [Google Scholar]
- 24.Worm SW, Friis-Moller N, Bruyand M, et al. High prevalence of the metabolic syndrome in HIV-infected patients: impact of different definitions of the metabolic syndrome. AIDS 2010; 24:427–35. [DOI] [PubMed] [Google Scholar]
- 25.Ervin RB. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States, 2003–2006. Natl Health Stat Report 2009:1–7. [PubMed]
- 26.Westley RL, May FE. A twenty-first century cancer epidemic caused by obesity: the involvement of insulin, diabetes, and insulin-like growth factors. Int J Endocrinol 2013; doi:10.1155/2013/632461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teede H, Deeks A, Moran L. Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med 2010; 8:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brumpton BM, Camargo CA, Jr, Romundstad PR, Langhammer A, Chen Y, Mai XM. Metabolic syndrome and incidence of asthma in adults: the HUNT study. Eur Respir J 2013; 42:1495–502. [DOI] [PubMed] [Google Scholar]
- 29.Tomaka F, Lefebvre E, Sekar V. Similar changes in metabolic parameters of darunavir (TMC114) and atazanavir, each coadministered with low-dose ritonavir in healthy volunteers (TMC114-C159). In: American Conference for the Treatment of HIV (ACTHIV), Dallas, TX, 31 May–3 June 2007. [Google Scholar]
- 30.Heat-stable Norvir tablet provides similar drug levels to capsule. AIDS Patient Care STDS. 2008; 22:761–2. [PubMed] [Google Scholar]
- 31.Autar RS, Boyd MA, Wit FW, et al. Relationships between drug exposure, changes in metabolic parameters and body fat in HIV-infected patients switched to a nucleoside sparing regimen. Antivir Ther 2007; 12:1265–71. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.