Abstract
Impairments in attention and concentration are distinctive features of melancholic depression, and may diminish the ability to shift focus away from internal dysphoric states. Disrupted brain networks may underlie the inability to effectively disengage from interoceptive signals in this disorder. This study investigates changes in effective connectivity between cortical systems supporting attention, interoception, and perception in those with melancholic depression when shifting attention from rest to viewing dynamic film stimuli. We hypothesised that those with melancholia would show impaired attentional shifting from rest to emotional film viewing, captured in neuronal states that differed little across conditions. Functional magnetic resonance imaging (fMRI) data were acquired from 48 participants (16 melancholic depressed, 16 non-melancholic depressed, and 16 healthy controls) at rest and whilst viewing emotionally salient movies. Using independent component analysis, we identified 8 cortical modes (default mode, executive control, left/right frontoparietal attention, left/right insula, visual and auditory) and studied their dynamics using dynamic causal modelling. Engagement with dynamic emotional material diminished in melancholia and was associated with network-wide increases in effective connectivity. Melancholia was also characterised by an increase in effective connectivity amongst cortical regions involved in attention and interoception when shifting from rest to negative film viewing, with the converse pattern in control participants. The observed involvement of attention- and insula-based cortical systems highlights a potential neurobiological mechanism for disrupted attentional resource allocation, particularly in switching between interoceptive and exteroceptive signals, in melancholia.
Keywords: Attention, Dynamic causal modelling, Film viewing, fMRI, Interoception, Melancholic depression
Highlights
-
•
Neurobiology of impaired attention, a key feature of melancholia, is unknown.
-
•
Effective connectivity increased in melancholia when shifting attention.
-
•
Disrupted connectivity was amongst regions supporting attention and interoception.
-
•
May reflect disrupted neuronal adaptation during processing of dynamic emotion.
-
•
Synchronisation of BOLD and neuronal states during film viewing validates approach.
1. Introduction
Melancholia, a canonical depressive subtype, is associated with persistent, internal dysphoria (Sims, 1995). This interoceptive preoccupation is commonly associated with impaired concentration and mental “fogginess” that undermine adaptive engagement with the external environment (Tancer et al., 1990, Taylor and Fink, 2006). Other overrepresented melancholic features such as anhedonia and attentional dysfunction have been proposed to contribute to this interoceptive focus (Pizzagalli et al., 2005). For example, disturbances in attention may impair reorienting away from distressing internal emotional states towards exogenous stimuli. This aligns with notions of ineffective attentional resource allocation in depression (Thomas et al., 1999), likely involving disturbances in complex brain networks known to support attentional control (Cole et al., 2014). The distinct attentional impairments (Austin et al., 2001) and ‘endogenous quality’ of affect (Parker et al., 1994) (e.g., nonreactivity) in melancholia motivate research into these complex brain networks.
Whilst inattention is a commonly observed sign of melancholia (Parker and Hadzi-Pavlovic, 1996), knowledge of underlying neurobiological markers is minimal (Elliott et al., 2002, Soriano-Mas et al., 2011), partly reflecting challenges in identifying brain correlates of depression more broadly. It is yet to be determined whether increases or decreases in connectivity strength between cortical regions best characterise ‘depression’ (Davey et al., 2012, Greicius et al., 2007, Veer et al., 2010). Inconsistencies across such neurobiological studies likely relate to diagnostic heterogeneity (e.g., major depression may effectively comprise differing depressive subsets with differing causes) (Parker, 2000). We recently identified disconnectivity between cortical systems involved in attention, executive control and interoception in melancholia in resting state fMRI data (Hyett et al., 2015), suggesting that this disorder may be selectively underpinned by disrupted neuronal integration.
Although task-based functional magnetic resonance imaging (fMRI) studies have provided insight into neural mechanisms of basic cognitive processes (Cabeza and Nyberg, 2000), behavioural tasks may have limited ecological validity (Chaytor and Schmitter-Edgecombe, 2003) and are often difficult to implement in those with depression given disturbances in attention and response biases (Elliott et al., 1996). Advances in imaging protocol design and analysis (i.e., use of naturalistic film viewing stimuli in a scanner environment) offer an ecologically informative platform for investigating the neurobiology of cognition and emotion in depression. Films minimise task demands, can manipulate emotion in a manner closely reflecting everyday emotional dynamics (Gross and Levenson, 1995), and reliably elicit consistent neuronal responses across cortical regions (Hasson et al., 2004, Jääskeläinen et al., 2008).
Such advances parallel theories positioning the brain as a complex network (Sporns et al., 2004). Large-scale brain networks have been identified by analysis of resting-state fMRI data (Damoiseaux et al., 2006), and correspond to those activated by cognitive and emotional processes such as attention and interoception (Laird et al., 2011, Seeley et al., 2007, Smith et al., 2009), both of which are disrupted in depression (Drevets, 2001). Network models of brain function emphasise the importance of interactions within and between brain regions (Fox et al., 2005), offering a framework to understand pathophysiological processes across complex disease phenotypes (Fornito et al., 2015, Menon, 2011). Further, analyses of neuronal dynamics at rest (Breakspear et al., 2004, Deco and Jirsa, 2012, Haimovici et al., 2013) and during active perception (Friston et al., 2012) have documented the spontaneous formation and dissolution of large-scale networks, hence advancing the notion of the brain as a ‘self-organising’ system, that is delicately poised to dynamically switch between different states (Rubinov et al., 2011). However, the dynamic properties of complex brain networks, in both health and disease, are frequently overlooked (Deco et al., 2011). The current study leverages these advances to examine whether brain networks related to attention and interoception are disrupted in melancholia when reorienting attention from rest to the free viewing of emotional film stimuli.
We examined the neural properties of the redirection of attention from resting state to exogenous, emotional film stimuli, in melancholic depressed, non-melancholic depressed and healthy control groups. We hypothesised that those with melancholia would show impaired attentional shifting from rest to emotional film viewing, reflected in neuronal states that differed little across conditions (remaining in an “at rest” state regardless of task demands). Independent component analysis (ICA) was applied across resting-state and film viewing fMRI conditions to identify brain modes corresponding to attentional, salience and sensory processing. We then applied stochastic dynamic causal modelling (sDCM) (Friston et al., 2003, Li et al., 2011) to estimate hidden neuronal states and causal influences amongst these. Network-based statistics (NBS) (Zalesky et al., 2010) were used to identify different patterns of interactions between depressed and control groups across resting-state and film viewing fMRI conditions.
2. Material and methods
2.1. Participants
This observational study was approved by the University of New South Wales Human Research Ethics Committee (approval 08077). Written informed consent was obtained and no monetary incentive was provided. Participants comprised 32 unipolar depressed patients, consecutively assessed and recruited through a specialist depression clinic at the Black Dog Institute in Sydney between August 30, 2010, and June 27, 2012. Sixteen matched healthy non-depressed control participants were also recruited. The age range for all participants was 18 to 75 years. Clinical participants met DSM-IV criteria for a current major depressive episode, but did not meet criteria for current and/or lifetime hypomania, mania, or psychosis on the Mini-International Neuropsychiatric Interview (MINI) (Sheehan et al., 1998). Clinical participants were assigned to a melancholic (n = 16) or non-melancholic (n = 16) class by clinic psychiatrists, weighting previously detailed criteria (Hyett et al., 2015), as detailed in the Supplementary material. Exclusion criteria for all participants included current and/or past drug or alcohol dependence, neurological disorder, invasive neurosurgery, traumatic brain injury, electroconvulsive therapy in the preceding six months, and/or an estimated IQ of below 80 on the Wechsler Test of Adult Reading (Holdnack, 2001). Additional participant details are provided in the Supplement.
2.2. Imaging protocol
All participants underwent four temporally consecutive fMRI scans. First, a 6 1/4 min resting-state fMRI scan during which participants were instructed to lie awake with their eyes closed, then three separate scans involving presentation of 6-minute films of positive, negative and neutral valence, with their order counter-balanced between participants.
2.3. Naturalistic stimuli — film clips
For the positive condition, participants viewed an excerpt from a stand-up comedy routine. For the negative condition, participants watched a scene from the movie “The Power of One”, depicting the degrading and inhumane treatment of a prisoner during the apartheid era. For the neutral condition, participants watched dynamic footage of landscapes and flowing water. The films were viewed through an MRI-compatible monitor, with matching audio inputs provided via an insert earphone system (Sensimetrics T14). Prior to each film, participants were shown text stating, “Video will begin soon. Please relax and watch.”
To validate the emotional salience of the dynamic film stimuli, an independent cohort of 18 non-depressed participants was recruited from the community to provide continuous ratings of emotion whilst viewing the positive and negative films. Overall, ratings were highly consistent between participants and with the intended valence of the film clips (reliability data provided in the Supplement).
2.4. Image acquisition and preprocessing
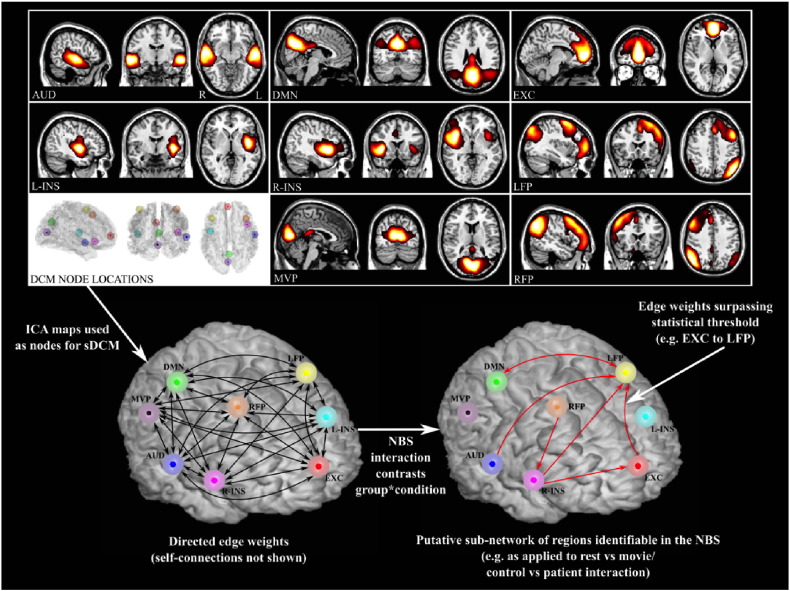
Functional images were acquired on a Philips 3-Tesla scanner equipped with a 12-channel head coil. Each image was realigned, normalised (unwarped) and smoothed using statistical parametric mapping software (SPM8) (Friston et al., 1995). Spatial ICA was applied across all subjects and (concatenated) sessions in the fMRI Software Library (FSL). The ICA decomposition generated 70 components, or “modes”, of neuronal, physiological and artefactual origin (Beckmann and Smith, 2004). We identified eight canonical neuronal modes representing emotional, cognitive and perceptual systems of clear relevance to attention and interoception during movie viewing, namely: auditory (AUD); default mode network (DMN); executive control (EXC); left insula (L-INS); right insula (R-INS); left frontoparietal attention (LFP); medial visual pole (MVP); and right frontoparietal attention (RFP; Fig. 1) (Damoiseaux et al., 2006). All components, with the exception of L-INS and R-INS, were matched with previously identified cognitive and sensory networks (Smith et al., 2009), using spatial cross-correlation. The insula modes were identified by first determining the coordinates of bilateral anterior insula cortices using PickAtlas, and using these coordinates to identify anatomically concordant ICA components. Additional acquisition and analytic information is provided in the Supplement.
Fig. 1.
Analysis pipeline illustrating the use of ICA spatial maps to inform sDCMs. Directed edge weights derived from the sDCMs (both resting state and film viewing fMRI) were used in the NBS to test for condition by group effects.
2.5. Dynamic causal modelling
Time series of the eight spatial ICA maps were used as inputs for subject- and condition-specific sDCMs. DCM is a computational technique combining dynamic models of neuronal signals (“states”) with detailed forward models of hemodynamic responses (Friston et al., 2003). The neuronal models consist of a network of interacting cortical populations, driven from a stable, steady state by fluctuations – external sensory inputs or internal state noise – and coupled to each other through directed causal interactions. Estimates of the (hidden) neuronal dynamics and the influence of one neuronal population over another are obtained by inverting these models using (variational) Bayesian techniques. This model inversion returns posterior estimates of the neuronal dynamics as well as a matrix detailing connectivity between regions or modes. Here, we allowed DCM to estimate the responses of primary sensory (visual and auditory) cortex to the film stimuli as well as the constellation of attentional, interoceptive and executive regions that regulate and modulate these primary responses. We allowed all possible pair-wise effective connections to be present.
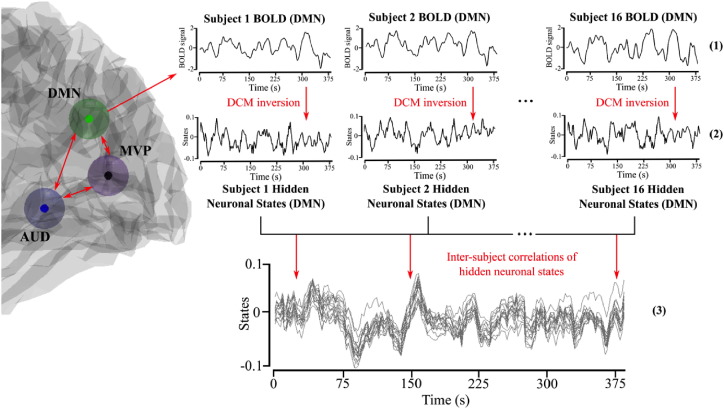
2.6. Inter-subject correlations (ISC)
Consistent neuronal responses to film stimuli between participants are a hallmark finding in the use of these dynamic stimuli during functional imaging experiments (Hasson et al., 2004). Previous research has imputed these responses from direct observation of the blood-oxygen-level-dependent (BOLD) signal. To validate the application of sDCM to film viewing, we examined inter-subject correlations (ISC) of the inferred neuronal states following model inversion, by extracting these from each ICA mode in each participant and then calculating the average pair-wise correlations between all participants (giving ISCs; Fig. 2). The statistical significance of these neuronal ISCs was tested using a permutation approach (for further details, see the Supplement). Family-wise control for multiple comparisons (across all possible pairs) was achieved using false discovery rate (FDR) correction (α = 0.0055). To validate the novel approach of inverting DCMs from film viewing data, we compared the ISCs of the inferred neuronal states to those of the observed BOLD signals, within and between each of the three participant groups.
Fig. 2.
Analysis pipeline for calculating inter-subject correlations of hidden neuronal states. Illustrated schematically for DMN mode. From top: BOLD time series of each subject; DCM inversion of BOLD to give neuronal states for each subject; inter-subject correlations calculated on hidden neuronal states.
2.7. Network-based statistic (NBS)
We used NBS (Zalesky et al., 2010) to study condition and group effects on the strength of interactions in the connectivity matrices obtained from inversion of the sDCMs. NBS is a permutation-based method that institutes control over family-wise error given the mass univariate testing required when there are multiple edges in a network. It identifies topologically connected networks that differ across conditions and/or groups. NBS follows the traditional principles of cluster-based thresholding of statistical parametric maps by first setting a preliminary height threshold (pair-wise connections) and then imposing a family-wise error (FWE)-adjusted cluster threshold (for networks of connections). We applied a height threshold of p < 0.01 (uncorrected), followed by cluster-wise thresholding of p < 0.05 (FWE corrected).
3. Results
3.1. Inter-subject correlations of hidden neuronal states
ISCs were calculated on the inferred neuronal states of the sDCMs for each group and for each ICA mode across positive and negative film viewing conditions (Fig. 2). These analyses revealed significant ISCs for the neuronal states of most modes across negative and positive film viewing conditions in both melancholic and control groups (Table 1). For example, ISCs in primary auditory (AUD) and visual (MVP) cortices were strong and significant across conditions and groups. ISCs were particularly robust for the AUD mode in the control group (r = 0.18) during negative film viewing. Both insula modes (L-INS and R-INS) showed significant ISCs in all groups for the negative film but were markedly weaker in all groups for the positive film. Hence, there were considerable effects of valence on ISCs.
Table 1.
Inter-subject correlations of negative and positive film viewing conditions.
| Negative film viewing |
Positive film viewing |
|||||
|---|---|---|---|---|---|---|
| Melancholic |
Non-melancholic |
Control |
Melancholic |
Non-melancholic |
Control |
|
| Mean (sig.) | Mean (sig.) | Mean (sig.) | Mean (sig.) | Mean (sig.) | Mean (sig.) | |
| AUD | 0.0610 (< 0.001) | 0.1325 (< 0.001) | 0.1833 (< 0.001) | 0.0437 (< 0.001) | 0.0755 (< 0.001) | 0.0778 (< 0.001) |
| DMN | 0.0422 (< 0.001) | 0.0156 (0.041) | 0.0318 (< 0.001) | 0.0347 (< 0.001) | 0.0547 (< 0.001) | 0.0438 (< 0.001) |
| EXC | 0.0417 (< 0.001) | 0.0187 (0.013) | 0.0400 (< 0.001) | 0.0210 (0.004) | 0.0434 (< 0.001) | 0.0359 (< 0.001) |
| L-INS | 0.0545 (< 0.001) | 0.0347 (< 0.001) | 0.0569 (< 0.001) | 0.0000 (0.467) | 0.0082 (0.140) | 0.0117 (0.069) |
| R-INS | 0.0456 (< 0.001) | 0.0339 (< 0.001) | 0.0351 (< 0.001) | 0.0131 (0.046) | 0.0234 (0.002) | 0.0233 (< 0.001) |
| LFP | 0.0678 (< 0.001) | 0.0236 (0.006) | 0.0310 (< 0.001) | 0.0225 (0.002) | 0.0173 (0.026) | 0.0093 (0.139) |
| MVP | 0.0235 (0.005) | 0.0107 (0.098) | 0.0379 (< 0.001) | 0.0373 (< 0.001) | 0.0437 (< 0.001) | 0.0357 (< 0.001) |
| RFP | 0.0267 (0.007) | 0.0091 (0.161) | 0.0455 (< 0.001) | 0.0395 (< 0.001) | 0.0529 (< 0.001) | 0.0733 (< 0.001) |
Bold denotes inter-subject correlation that was significant after adjusting for multiple comparisons.
In the melancholic group, ISCs for the RFP mode during negative film viewing were weak and not statistically significant, as were the insula modes during positive film viewing. There was no discernable correlation for the L-INS during positive film viewing in the melancholic group. ISCs for the RFP did not exceed corrected significance in the melancholic group during negative film viewing. In general, the non-melancholic group had fewer significant ISCs — namely, for negative film viewing: AUD, L-INS and R-INS, and during positive film viewing for all modes except the L-INS and LFP.
To further validate our approach, we performed a comparison of the ISCs of the inferred neuronal states to those of the BOLD signals obtained directly from the ICA decomposition. Those ICA modes whose neuronal states showed significant ISCs also showed consistent BOLD responses. Conversely, specificity was also consistent such that for those modes with weak and non-significant ISCs of the neuronal states, the accompanying BOLD signals also lacked inter-subject correlation. These additional analyses are provided in the Supplement.
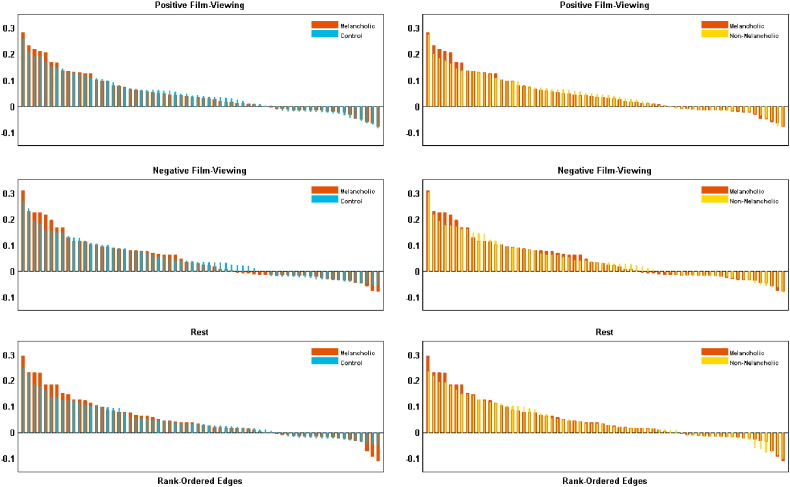
3.2. Connectivity amongst brain modes
In light of views of the brain as a self-organising system (Friston et al., 2012) – modulating its response to exogenous and endogenous perturbations – we examined the degree of network ‘homeostasis’ in our data through analysis of system-wide effective connectivity strength. We quantified the distributions of connection weights between all eight brain modes, yielding 64 unique edges, across conditions (Fig. 3). Those with melancholia had stronger connection weights in both directions (i.e., positive and negative) over more edges under both film viewing conditions compared to healthy controls (left column Fig. 3). The extent of this effect was less pronounced at rest. Effective connection weights were also increased in non-melancholic participants. Distributions of the edge weights for the neutral film viewing condition are provided in the Supplementary material.
Fig. 3.
Group comparisons of rank-ordered distributions of all 64 edge weights across positive and negative film viewing and resting state. Left column shows melancholic versus healthy controls: right column shows melancholia versus non-melancholic MDD.
We formally tested whether these edge weight distributions differed between groups using permutation testing (see the Supplement). There was a statistically significant difference between the melancholic and control group connectivity weight distribution across the network of edges during positive film viewing (difference = 0.0223; p < 0.001). An effect was also present for the negative film viewing condition between melancholic and control groups (difference = 0.0198; p = 0.0170), and a trend-level difference for the resting state condition (difference = 0.0140, p = 0.038) between these groups. Whilst the edges were slightly stronger in the melancholic than non-melancholic group (difference across all conditions = 0.7493), none reached significance.
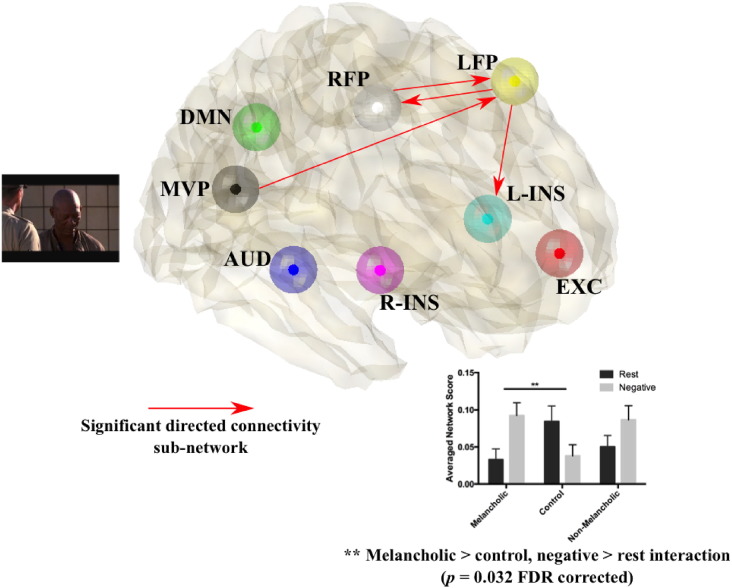
3.3. DCM analysis of naturalistic film viewing
We assessed for group differences in connected sub-networks of directed connections amongst the 8 modes (Fig. 1) using NBS to control for family-wise error across multiple edges (using a false discovery rate of p < 0.05). We focused on group by condition (rest versus film viewing) interaction effects. As illustrated in Fig. 4, a sub-network of edges, including directed edges LFP → L-INS, MVP → LFP, and a bidirectional connection LFP ↔ RFP, differed between melancholic and control groups between rest and negative film viewing conditions (p < 0.032, FDR corrected). Specifically, connectivity strengths for this sub-network of edges were substantially higher in the melancholic group during negative film viewing compared to rest. In contrast, the control group showed the reverse, with higher connectivity strength at rest compared to negative film viewing. Sub-network connectivity strengths of the non-melancholic group trended towards that of the melancholic group (bar graph Fig. 4). Univariate analyses of sub-network connectivity strengths suggested an interaction effect between non-melancholic and control groups across resting state and negative film viewing conditions (F = 5.541, p = 0.022), although this does not survive multiple comparisons correction across all edges. This confirms the specificity of the differences observed between the melancholic and control groups across resting state and negative film viewing conditions. Interaction effects between neutral film viewing and resting state conditions across the groups are provided in the Supplementary material.
Fig. 4.
Sub-network of edges distinguishing melancholic and control groups across resting state and negative film viewing conditions identified using the NBS.
3.4. The impact of medication on sub-network scores
We examined potential medication effects on sub-network scores using logistic regression. Patients were divided into two subsets: those in receipt of a selective serotonin reuptake inhibitor (SSRI) medication versus those not on any such medication; and those on any other non-SSRI medication (e.g., antipsychotics, mood stabilisers, all broad action antidepressants) versus those not on such medications. We controlled for clinical group and examined whether the interaction of resting state and negative film viewing sub-network edge weights predicted the presence or absence of the medication partitions. No significant effects were observed. Formal statistics are provided in the Supplement.
4. Discussion
Cognitive impairments are classical features of melancholic depression, highlighted by difficulties in shifting attention (Austin et al., 1999). We hypothesised that the inability to disengage from dysphoric internal states in response to emotionally salient exogenous stimuli in melancholia would be associated with dysfunction in brain networks supporting attention and interoception. Although our analyses conform to this hypothesis, our sub-network of brain regions underpinning attentional control and interoception increased in effective connectivity strength during negative film viewing, hence in the opposite direction conjectured, challenging the notion that cortical systems in such patients would dwell in a state of “rest” during emotional film viewing. Further, global connectivity strength was significantly increased when ‘attending’ to emotionally salient films in those with melancholia, possibly indicating a subtle, but more general, system-wide regulatory failure of neuronal interactions across cognitive, emotional and sensory cortical systems. More specifically, our findings revealed distinct disequilibria of neuronal states during sensory integration of emotional material in melancholia. Considering the brain as a self-organising system, such a breakdown in the optimal balance between sensory inputs and dynamic network organisation may be key to understanding the neurobiology of this disorder.
Effective connectivity decreases in cortical systems with learning (Büchel et al., 1999) and may be of relevance in light of the current findings. Increases in effective connectivity strength in melancholic compared to control participants during negative film viewing may reflect inefficient neuronal adaptation following the onset of the (initially unexpected) emotionally-valenced film (Siegle et al., 2002). Specifically, cortical systems may fail to adapt to dynamic exteroceptive emotional stimuli in this disorder. Wernicke (1906) used the term vital feelings to describe various somatic symptoms that occur in severe affective illnesses (e.g., “a feeling of misery, like a black cloud pressing against my head”) (Sims, 1995). Attentional focus on unpleasant ‘coenesthesic’ states in melancholia may contribute to a failure of cortical systems to adapt, and attend, to exteroceptive demands. Further, it may be possible that incoming emotional material is not effectively filtered and integrated due to competition for attentional resources, which then gives rise to disrupted network dynamics. When attention needs to be shifted to exteroceptive emotional content, attentional demands may be increased due to continued focus on interoceptive signals, consistent with observations that those with depression perform more poorly on tasks requiring effortful attentional processing (Thomas et al., 1999, Roy-Byrne et al., 1986). This again parallels theories of self-organisation in the brain; in essence, the brain may be unable to optimise a dynamic cognitive balance between neuronal representations of both the sensorium and distressing interoceptive states (Friston et al., 2012, Seth, 2013).
Emerging evidence suggests that consistent patterns of brain activity arise across individuals when viewing well-directed dynamic film stimuli (Hasson et al., 2004). Both lower and higher cortical regions, including occipital, temporal, parietal and frontal cortices, exhibit this pattern (Jääskeläinen et al., 2008, Bartels et al., 2008). Intriguingly, it appears that significant ISCs only arise in regions such as the insula when subjects view emotionally salient material (Nummenmaa et al., 2012). However, prior fMRI research has focused on ICSs of hemodynamics (e.g., the BOLD signal) in healthy individuals. Our ISC analyses were derived from inferred (hidden) neuronal states, converging with a prior study of consistent slow-wave fluctuations in invasive direct electrocortical recordings (Honey et al., 2012). The high level of consistency amongst these inferred states, in parallel with consistent BOLD responses (see the Supplement), provides construct validation of our experimental approach. The generally smaller ISCs that occur in both clinical groups compared to the healthy controls may either reflect weaker neuronal responses in the corresponding regions or activity that is not driven by the film and hence unique to each individual. In regard to the latter, the inconsistency of the RFP mode during negative film viewing in melancholia may reflect divergent attentional processing across individuals.
The non-melancholic group appeared to sit in between that of melancholic and control groups, albeit non-significantly, suggesting that this condition may be associated with an intermediate pattern of neurobiological dysfunction (with melancholia acting as the phenotypic condition). On their own, these neurobiological findings are hence agnostic about a categorical versus spectrum model of depressive disorders: A more highly powered study might well identify differences between melancholia and non-melancholic MDD, as previously identified in such patients at rest (Hyett et al., 2015). Given self-reported depression severity was comparable between the two clinical groups, it is unlikely that the lack of differences between melancholic and non-melancholic depression are purely a function of illness severity. In line with emerging perspectives that seek to unify objective markers of illness with clinical phenomena (Insel, 2014), we suggest the findings provide evidence of a neurobiological endophenotype of melancholia – but not of depression per se – given the specificity of the observed differences when compared with control subjects. In particular, melancholic, but not non-melancholic, depression was associated with disruptions to neurobiological processes underlying attention and interoception when attempting to engage with emotional film content. In light of emerging neuroscientific models, the present findings thus broadly implicate melancholia as a disorder of distorted perceptual and attentional inference, reflected predominantly in an inability to shift attention away from dysphoric internal states.
We note several study limitations. First, inferring cognitive states from neuroimaging data is prone to the fallacy of “reverse inference” (Poldrack, 2006). Whilst film viewing is arguably considerably more constrained than the widely used resting state paradigms, there are no behaviours that can be used to gauge “task completion”. Here, we have linked attentional and insula mode dysfunction to cognitive processes. However, as our cognitive manipulations are only implicitly inferred in the movie valence, limitations must be placed on the extent to which these relate to the formal constructs of “interoception”, “attentional control” or “ineffective learning”. Concurrent monitoring of physiological parameters, including eye movements and skin conductance, would provide a more refined index of the level of engagement with the naturalistic stimuli. Second, whilst the sample size was sufficient for identifying significant group effects (Friston, 2012), larger samples would assist in identifying relationships between neurobiological and other key illness attributes (e.g., behavioural markers, clinical trajectory). Third, as withholding medications in our clinical groups (whom were moderately to severely depressed at the time of scanning) was not feasible, addressing the impact of medications in future larger studies will also be important given the role that SSRIs (Wagner et al., 2010) and antipsychotics (Miller et al., 1997) have on cerebral blood flow.
5. Conclusions
As psychiatry aligns itself to computational neuroscience (Huys et al., 2011, Montague et al., 2012), it necessitates departure from more traditional views of brain function (i.e., representing cognition and emotion in increasingly reductionistic terms) to a model that considers observable behaviour (e.g., mood, affect) as emerging from interacting brain systems. The current findings align with previous work suggesting that psychiatric disorders may best be positioned as arising from disturbances to brain network organisation (Fornito et al., 2015). By highlighting a perturbed network of brain regions underpinning attention and interoception, we here advance the neurobiology of melancholia through such a lens. A number of practical implications should be noted. Our findings may help explain why those with melancholia have difficulty engaging interpersonally (observed as non-interactiveness and/or non-reactivity), and potentially assist in clarifying mechanisms of treatment resistance (i.e., to psychotherapy) and response to rational pharmacotherapy (Parker et al., 2013). Furthermore, the specificity of brain network disruptions in melancholia indicates that these disruptions have potential to serve as diagnostic biomarkers (Insel et al., 2010).
Abbreviations
- AUD
auditory
- BOLD
blood-oxygen-level-dependent
- DCM
dynamic causal modelling
- DMN
default mode network
- DSM-IV
Diagnostic and Statistical Manual for Mental Disorders (4th Ed.)
- EXC
executive control
- FDR
false discovery rate
- fMRI
functional magnetic resonance imaging
- FWE
family-wise error
- ICA
independent component analysis
- ISC
inter-subject correlation
- LFP
left frontoparietal
- L-INS
left insula
- MINI
Mini-International Neuropsychiatric Interview
- MVP
medial visual pole
- NBS
Network Based Statistic
- RFP
right frontoparietal
- R-INS
right insula
- sDCM
stochastic dynamic causal modelling
- SPM8
statistical parametric mapping
- SSRI
selective serotonin reuptake inhibitor
Author contributions
MPH, TY, GBP and MJB contributed to the design of the imaging protocol. MPH and MJB analysed the data and wrote the manuscript. AZ, CCG, VN and TY contributed to the analysis, and all authors provided interpretation of the data and assisted with the writing of the manuscript. GBP held primary responsibility for diagnostic decisions for clinical participants in the study, was lead chief investigator on the funded grant, and provided clinical and editorial input to the manuscript.
Disclosures and acknowledgements
The authors report no financial conflicts of interest in relation to this manuscript. Supported by grants from the National Health and Medical Research Council of Australia (Program Grants 510135 and 1037196: MPH, GBP, MJB; Career Development Fellowship GNT1047648: AZ); and a Health Research Fellowship from the QLD Office of Health and Medical Research (MJB, MPH).
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2015.10.011.
Appendix A. Supplementary data
Supplementary material.
References
- Sims A.C.P. 2nd ed. WB Saunders; Philadelphia, PA: 1995. Symptoms in the Mind: An Introduction to Descriptive Psychopathology. [Google Scholar]
- Tancer M.E., Brown T.M., Evans D.L. Impaired effortful cognition in depression. Psychiatry Res. 1990;31(2):161–168. doi: 10.1016/0165-1781(90)90118-o. [DOI] [PubMed] [Google Scholar]
- Taylor M.A., Fink M. Cambridge University Press; New York, NY: 2006. Melancholia: The Diagnosis, Pathophysiology and Treatment of Depressive Illness. [Google Scholar]
- Pizzagalli D.A., Jahn A.L., O'Shea J.P. Toward an objective characterization of an anhedonic phenotype: a signal-detection approach. Biol. Psychiatry. 2005;57(4):319–327. doi: 10.1016/j.biopsych.2004.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P., Goudemand M., Rousseaux M. Attentional resources in major depression. Eur. Arch. Psychiatry Clin. Neurosci. 1999;249(2):79–85. doi: 10.1007/s004060050070. [DOI] [PubMed] [Google Scholar]
- Cole M.W., Repovs G., Anticevic A. The frontoparietal control system: a central role in mental health. Neuroscientist. 2014;20(6):652–664. doi: 10.1177/1073858414525995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin M.P., Mitchell P., Goodwin G.M. Cognitive deficits in depression: possible implications for functional neuropathology. Br. J. Psychiatry. 2001;178(3):200–206. doi: 10.1192/bjp.178.3.200. [DOI] [PubMed] [Google Scholar]
- Parker G., Hadzi-Pavlovic D., Wilhelm K. Defining melancholia: properties of a refined sign-based measure. Br. J. Psychiatry. 1994;164(3):316–326. doi: 10.1192/bjp.164.3.316. [DOI] [PubMed] [Google Scholar]
- Parker G., Hadzi-Pavlovic D. Cambridge University Press; New York, NY: 1996. Melancholia: A Disorder of Movement and Mood. [Google Scholar]
- Elliott R., Rubinsztein J.S., Sahakian B.J., Dolan R.J. The neural basis of mood-congruent processing biases in depression. Arch. Gen. Psychiatry. 2002;59(7):597–604. doi: 10.1001/archpsyc.59.7.597. [DOI] [PubMed] [Google Scholar]
- Soriano-Mas C., Hernández-Ribas R., Pujol J. Cross-sectional and longitudinal assessment of structural brain alterations in melancholic depression. Biol. Psychiatry. 2011;69(4):318–325. doi: 10.1016/j.biopsych.2010.07.029. [DOI] [PubMed] [Google Scholar]
- Davey C.G., Harrison B.J., Yucel M., Allen N.B. Regionally specific alterations in functional connectivity of the anterior cingulate cortex in major depressive disorder. Psychol. Med. 2012;42(10):2071–2081. doi: 10.1017/S0033291712000323. [DOI] [PubMed] [Google Scholar]
- Greicius M.D., Flores B.H., Menon V. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol. Psychiatry. 2007;62(5):429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veer I.M., Beckmann C.F., van Tol M.J. Whole brain resting-state analysis reveals decreased functional connectivity in major depression. Front. Syst. Neurosci. 2010;4 doi: 10.3389/fnsys.2010.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker G. Classifying depression: should paradigms lost be regained? Am. J. Psychiatry. 2000;157(8):1195–1203. doi: 10.1176/appi.ajp.157.8.1195. [DOI] [PubMed] [Google Scholar]
- Hyett M.P., Breakspear M.J., Friston K.J., Guo C.C., Parker G.B. Disrupted effective connectivity of cortical systems supporting attention and interoception in melancholia. JAMA Psychiatry. 2015;72(4):350–358. doi: 10.1001/jamapsychiatry.2014.2490. [DOI] [PubMed] [Google Scholar]
- Cabeza R., Nyberg L. Imaging cognition II: an empirical review of 275 PET and fMRI studies. J. Cogn. Neurosci. 2000;12(1):1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Chaytor N., Schmitter-Edgecombe M. The ecological validity of neuropsychological tests: a review of the literature on everyday cognitive skills. Neuropsychol. Rev. 2003;13(4):181–197. doi: 10.1023/b:nerv.0000009483.91468.fb. [DOI] [PubMed] [Google Scholar]
- Elliott R., Sahakian B.J., McKay A.P., Herrod J.J., Robbins T.W., Paykel E.S. Neuropsychological impairments in unipolar depression: the influence of perceived failure on subsequent performance. Psychol. Med. 1996;26(5):975–989. doi: 10.1017/s0033291700035303. [DOI] [PubMed] [Google Scholar]
- Gross J.J., Levenson R.W. Emotion elicitation using films. Cogn. Emot. 1995;9(1):87–108. [Google Scholar]
- Hasson U., Nir Y., Levy I., Fuhrmann G., Malach R. Intersubject synchronization of cortical activity during natural vision. Science. 2004;303(5664):1634–1640. doi: 10.1126/science.1089506. [DOI] [PubMed] [Google Scholar]
- Jääskeläinen I.P., Koskentalo K., Balk M.H. Inter-subject synchronization of prefrontal cortex hemodynamic activity during natural viewing. Open Neuroimaging J. 2008;2:14–19. doi: 10.2174/1874440000802010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O., Chialvo D.R., Kaiser M., Hilgetag C.C. Organization, development and function of complex brain networks. Trends Cogn. Sci. 2004;8(9):418–425. doi: 10.1016/j.tics.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Damoiseaux J.S., Rombouts S.A., Barkhof F. Consistent resting-state networks across healthy subjects. Proc. Natl. Acad. Sci. U. S. A. 2006;103(37):13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird A.R., Fox P.M., Eickhoff S.B. Behavioral interpretations of intrinsic connectivity networks. J. Cogn. Neurosci. 2011;23(12):4022–4037. doi: 10.1162/jocn_a_00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Fox P.T., Miller K.L. Correspondence of the brain's functional architecture during activation and rest. Proc. Natl. Acad. Sci. U. S. A. 2009;106(31):13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets W.C. Neuroimaging and neuropathological studies of depression: implications for the cognitive–emotional features of mood disorders. Curr. Opin. Neurobiol. 2001;11(2):240–249. doi: 10.1016/s0959-4388(00)00203-8. [DOI] [PubMed] [Google Scholar]
- Fox M.D., Snyder A.Z., Vincent J.L., Corbetta M., Van Essen D.C., Raichle M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. U. S. A. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A., Zalesky A., Breakspear M. The connectomics of brain disorders. Nat. Rev. Neurosci. 2015;16(3):159–172. doi: 10.1038/nrn3901. [DOI] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn. Sci. 2011;15(10):483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Breakspear M., Williams L.M., Stam C.J. A novel method for the topographic analysis of neural activity reveals formation and dissolution of ‘dynamic cell assemblies’. J. Comput. Neurosci. 2004;16(1):49–68. doi: 10.1023/b:jcns.0000004841.66897.7d. [DOI] [PubMed] [Google Scholar]
- Deco G., Jirsa V.K. Ongoing cortical activity at rest: criticality, multistability, and ghost attractors. J. Neurosci. 2012;32:3366–3375. doi: 10.1523/JNEUROSCI.2523-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haimovici A., Tagliazucchi E., Balenzuela P., Chialvo D.R. Brain organization into resting state networks emerges at criticality on a model of the human connectome. Phys. Rev. Lett. 2013;110(17):178101. doi: 10.1103/PhysRevLett.110.178101. [DOI] [PubMed] [Google Scholar]
- Friston K., Breakspear M., Deco G. Perception and self-organized instability. Front. Comput. Neurosci. 2012;6:44. doi: 10.3389/fncom.2012.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M., Sporns O., Thivierge J.P., Breakspear M. Neurobiologically realistic determinants of self-organized criticality in networks of spiking neurons. PLoS Comput. Biol. 2011;7(6) doi: 10.1371/journal.pcbi.1002038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deco G., Jirsa V.K., McIntosh A.R. Emerging concepts for the dynamical organization of resting-state activity in the brain. Nat. Rev. Neurosci. 2011;12(1):43–56. doi: 10.1038/nrn2961. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Harrison L., Penny W. Dynamic causal modelling. Neuroimage. 2003;19(4):1273–1302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- Li B., Daunizeau J., Stephan K.E., Penny W., Hu D., Friston K. Generalised filtering and stochastic DCM for fMRI. Neuroimage. 2011;58(2):442–457. doi: 10.1016/j.neuroimage.2011.01.085. [DOI] [PubMed] [Google Scholar]
- Zalesky A., Fornito A., Bullmore E.T. Network-based statistic: identifying differences in brain networks. Neuroimage. 2010;53(4):1197–1207. doi: 10.1016/j.neuroimage.2010.06.041. [DOI] [PubMed] [Google Scholar]
- Sheehan D.V., Lecrubier Y., Sheehan K.H. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry. 1998;59(Suppl. 20):22–33. [PubMed] [Google Scholar]
- Holdnack H.A. 2001. Wechsler Test of Adult Reading (WTAR) [Google Scholar]
- Friston K.J., Holmes A.P., Worsley K.J., Poline J.-P., Frith C.D., Frackowiak R.S.J. Statistical parametric maps in functional imaging: a general linear approach. Hum. Brain Mapp. 1995;2(4):189–210. [Google Scholar]
- Beckmann C.F., Smith S.M. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans. Med. Imaging. 2004;23(2):137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- Austin M.P., Mitchell P., Wilhelm K. Cognitive function in depression: a distinct pattern of frontal impairment in melancholia? Psychol. Med. 1999;29(1):73–85. doi: 10.1017/s0033291798007788. [DOI] [PubMed] [Google Scholar]
- Büchel C., Coull J.T., Friston K.J. The predictive value of changes in effective connectivity for human learning. Science. 1999;283(5407):1538–1541. doi: 10.1126/science.283.5407.1538. [DOI] [PubMed] [Google Scholar]
- Siegle G.J., Steinhauer S.R., Thase M.E., Stenger V.A., Carter C.S. Can't shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biol. Psychiatry. 2002;51(9):693–707. doi: 10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- Wernicke C. Thieme; Berlin: 1906. Grundrisse der Psychiatrie. [Google Scholar]
- Roy-Byrne P.P., Weingartner H., Bierer L.M., Thompson K., Post R.M. Effortful and automatic cognitive processes in depression. Arch. Gen. Psychiatry. 1986;43(3):265–267. doi: 10.1001/archpsyc.1986.01800030083008. [DOI] [PubMed] [Google Scholar]
- Seth A.K. Interoceptive inference, emotion, and the embodied self. Trends Cogn. Sci. 2013;17(11):565–573. doi: 10.1016/j.tics.2013.09.007. [DOI] [PubMed] [Google Scholar]
- Bartels A., Zeki S., Logothetis N.K. Natural vision reveals regional specialization to local motion and to contrast-invariant, global flow in the human brain. Cereb. Cortex. 2008;18(3):705–717. doi: 10.1093/cercor/bhm107. [DOI] [PubMed] [Google Scholar]
- Nummenmaa L., Glerean E., Viinikainen M., Jääskeläinen I.P., Hari R., Sams M. Emotions promote social interaction by synchronizing brain activity across individuals. Proc. Natl. Acad. Sci. U. S. A. 2012;109(24):9599–9604. doi: 10.1073/pnas.1206095109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey C.J., Thesen T., Donner T.H. Slow cortical dynamics and the accumulation of information over long timescales. Neuron. 2012;76(2):423–434. doi: 10.1016/j.neuron.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T.R. The NIMH Research Domain Criteria (RDoC) project: precision medicine for psychiatry. Am. J. Psychiatry. 2014;171(4):395–397. doi: 10.1176/appi.ajp.2014.14020138. [DOI] [PubMed] [Google Scholar]
- Poldrack R.A. Can cognitive processes be inferred from neuroimaging data? Trends Cogn. Sci. 2006;10(2):59–63. doi: 10.1016/j.tics.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Friston K. Ten ironic rules for non-statistical reviewers. Neuroimage. 2012;61(4):1300–1310. doi: 10.1016/j.neuroimage.2012.04.018. [DOI] [PubMed] [Google Scholar]
- Wagner G., Koch K., Schachtzabel C. Differential effects of serotonergic and noradrenergic antidepressants on brain activity during a cognitive control task and neurofunctional prediction of treatment outcome in patients with depression. J. Psychiatry Neurosci. 2010;35(4):247–257. doi: 10.1503/jpn.090081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D.D., Andreasen N.C., O'Leary D.S. Effect of antipsychotics on regional cerebral blood flow measured with positron emission tomography. Neuropsychopharmacology. 1997;17(4):230–240. doi: 10.1016/S0893-133X(97)00042-0. [DOI] [PubMed] [Google Scholar]
- Huys Q.J., Moutoussis M., Williams J. Are computational models of any use to psychiatry? Neural Netw. 2011;24(6):544–551. doi: 10.1016/j.neunet.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Montague P.R., Dolan R.J., Friston K.J., Dayan P. Computational psychiatry. Trends Cogn. Sci. 2012;16(1):72–80. doi: 10.1016/j.tics.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker G., Blanch B., Paterson A. The superiority of antidepressant medication to cognitive behavior therapy in melancholic depressed patients: a 12-week single-blind randomized study. Acta Psychiatr. Scand. 2013;128(4):271–281. doi: 10.1111/acps.12049. [DOI] [PubMed] [Google Scholar]
- Insel T., Cuthbert B., Garvey M. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am. J. Psychiatry. 2010;167(7):748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.