Abstract
Pediatric traumatic brain injury often results in significant long-term deficits in mastery of reading ability. This study aimed to identify white matter pathways that, when damaged, predicted reading deficits in children. Based on the dual-route model of word reading, we predicted that integrity of the inferior fronto-occipital fasciculus would be related to performance in sight word identification while integrity of the superior longitudinal fasciculus would be related to performance in phonemic decoding. Reading fluency and comprehension were hypothesized to relate to the superior longitudinal fasciculus, inferior fronto-occipital fasciculus, and cingulum bundle. The connectivity of white matter pathways was used to predict reading deficits in children aged 6 to 16 years with traumatic brain injury (n = 29) and those with orthopedic injury (n = 27) using tract-based spatial statistics. Results showed that children with traumatic brain injury and reduced microstructural integrity of the superior longitudinal fasciculus demonstrated reduced word-reading ability on sight word and phonemic decoding tasks. Additionally, children with traumatic brain injury and microstructural changes involving the cingulum bundle demonstrated reduced reading fluency. Results support the association of a dorsal pathway via the superior longitudinal fasciculus with both sight word reading and phonemic decoding. No association was identified between the inferior fronto-occipital fasciculus and sight word reading or phonemic decoding. Reading fluency was associated with the integrity of the cingulum bundle. These findings support dissociable pathways predicting word reading and fluency using Diffusion Tensor Imaging and provide additional information for developing models of acquired reading deficits by specifying areas of brain damage which may predict reading deficits following recovery from the acute phase of TBI.
Keywords: Reading, Diffusion Tensor Imaging, Traumatic brain injury, Pediatric
Highlights
-
•
We apply models of white matter and reading ability to pediatric brain trauma.
-
•
We report dissociable effects for integrity of specific white matter pathways and specific reading skills following injury.
-
•
We report a relationship between the cingulum bundle and reading ability.
-
•
The implications of these findings are discussed in terms of brain-based reading models as they relate to brain injury.
1. Introduction
Skilled reading ability relies on the integration of information between widespread cortical regions with notable left-hemisphere lateralization of most functions within this network (McCandliss and Noble, 2003, Vandermosten et al., 2012a). The development of reading is a complex process involving the intersecting forces of brain maturation and education. Consequently, neurological trauma during the developmental period where reading skills are acquired can negatively affect both skills that have been mastered and skills that are still to be learned (Taylor and Alden, 1997). In neurodevelopmental disorders, such as dyslexia, atypical maturation of the left frontal and posterior Perisylvian cortical regions, and the white matter bundles connecting those regions, has been linked to deficits in reading skills (Misra et al., 2004, Pugh et al., 2001, Rimrodt et al., 2010, Vandermosten et al., 2012a, Vandermosten et al., 2012b). Unlike children with neurodevelopmental reading disabilities, the majority of children with a traumatic brain injury (TBI) have a history of typically developing white matter pathways and reading ability prior to injury. Despite this typical history, children often demonstrate long-lasting deficits in word decoding, reading fluency, and comprehension following TBI (Anderson et al., 2009, Barnes et al., 1999). Therefore, TBI provides a model for examining the relation between changes in white matter and acquired reading deficits in the developing brain. Analysis of pathway integrity and reading ability following TBI may reveal information about separable neurological networks underlying specific reading abilities, such as sight word identification, phonemic decoding, reading fluency, and comprehension of passages.
1.1. Models of reading
In relation to reading words, the hypothesis that words are read through dual processes was initially proposed by Marshall and Newcombe (1973). These cognitive models propose one route for grapheme–phoneme decoding of words and another route where words are identified by sight based on orthography. Coltheart et al. (2001) developed the Dual Route Cascaded Model which proposes that simultaneous attempts at word reading are undertaken by quasi-independent dual routes. This model includes a lexical route, sometimes referred to as the direct route, by which words are identified by their orthographic form and directly associated with semantic meaning. This is the route through which skilled readers identify and read common words that have been frequently encountered in the past. The second is an indirect route through which words are first broken down and decoded using a grapheme-to-phoneme conversion system to read novel words through phonemic decoding. In the skilled reader, these two large, reading networks interact to increase speed of reading text, creating a more efficient reading system (Coltheart et al., 2001). This cognitive model has received some support from studies of developmental dyslexia and of word reading in typically developing children. In both populations, there are dissociable differences in the use of direct access versus phonological decoding strategies for decoding regular and irregular words as well as nonwords (Castles and Coltheart, 1993, Schmalz et al., 2013), However, the heterogeneity of word decoding strategies in both disordered and typically developing samples of different ages (Pritchard et al., 2012, Zoccolotti and Friedmann, 2010) may limit the extension of the model directly to acquired reading deficits in children and adolescents (Castles, 2006).
Several neurological models of reading skills have proposed dorsal, ventral, and anterior neurological substrates believed to contribute to different aspects of word reading, fluency, and comprehension. Based on findings from several functional imaging studies, Pugh and colleagues (Das et al., 2011, Pugh et al., 2001) proposed a dual circuit model of word reading for alphabetic languages with opaque orthography, such as English, in which phonemic decoding was associated with a dorsal route involving temporo-parietal and anterior brain regions, including the angular gyrus and superior temporal gyrus. A ventral route was also proposed that includes the inferior occipito-temporal area and is more associated with skilled sight word reading. An anterior termination and processing center was also proposed that is involved specifically in the articulation and oral reading aspects of reading ability. This substrate involves the inferior frontal gyrus and is the termination point of both pathways, presumably through the arcuate portion of the superior longitudinal fasciculus and the inferior fronto-occipital fasciculus in the pars orbitalis and triangularis. Taken together, Pugh's neuroanatomical model and Coltheart's cognitive reading model suggest that sight words are most efficiently processed by the direct or ventral route, while words requiring phonemic decoding or phonemically decoded pseudowords would rely on the indirect or dorsal route. The Dual Route Cascaded Model suggests that these pathways compete for word recognition, resulting in a “horse race” for quickly identifying a word (Frost, 1998).
1.2. Functional Magnetic Resonance Imaging (fMRI) studies of reading
A meta-analysis of functional imaging studies involving word reading revealed some evidence of individualized activation to differentiate the indirect route from the direct route; however, there was not clear support for areas solely associated with the direct route (Jobard et al., 2003). This may either indicate that a unique pathway for the direct route does not exist, or is activated regardless of the word task presented. The latter is consistent with a dual route model as described above. Consequently, functional imaging studies using a traditional baseline versus activity model may not provide specific enough information about the relation between direct sight reading and the primary direct route of that information through the neural reading network.
Alternatively, approaches to studying reading pathways using dynamic causal modeling allow for further parsing of pathways into unique components. Using dynamic causal modeling, Mechelli and colleagues have identified unique, effective connectivity between the posterior fusiform and dorsal premotor area for pseudowords and between the anterior fusiform and pars triangularis for exception words (2005). This pattern is consistent with a dual pathway model for word reading and with the Dual Route Cascaded Model.
Unlike individual word reading ability, which has been studied extensively in functional imaging studies, reading fluency and comprehension have received only limited fMRI investigation. In particular, the study of reading fluency is inherently difficult with functional imaging procedures, where it is difficult to individualize the speed of stimulus presentation and measure changes in activity while providing the participant with the means to pace their own reading. However, Mechelli and colleagues identified that activation in areas of the occipital lobe, fusiform gyrus, temporal-occipital junction, and precentral gyrus were rate-sensitive to the presentation of single words or pseudowords (Mechelli et al., 2000).
With regards to reading comprehension, functional imaging of skilled adult readers has revealed involvement of the occipitotemporal junction, middle and superior temporal gyri, and the inferior frontal gyrus in sentence comprehension (Constable et al., 2004). In addition, in this study increased sentence complexity was associated with increased activation in the inferior frontal gyrus and superior temporal gyrus. Functional imaging of reading comprehension disorders has also revealed that areas of frontal activation near the inferior frontal gyrus are also related to poor reading comprehension (Cutting et al., 2013). Functional studies of typical readers have also revealed involvement of cortical areas associated with proposed ventral, dorsal, and anterior networks during reading comprehension (Moss et al., 2011). Furthermore, in this study, areas of increased activation during strategic reading comprehension also included brain regions associated with executive functioning and cognitive control, including the anterior cingulate gyrus. As such, the functional imaging research in reading fluency and comprehension seem to indicate that these skills rely on broadly defined ventral, dorsal, and anterior cortical areas for skilled and fluid reading, consistent with existing neurological pathway models (Pugh et al., 2001).
1.3. Diffusion tensor imaging of reading pathways
As the reading networks of the brain are widespread, the efficiency of the reading system may hinge on the efficiency by which information can travel between cortical areas implicated in reading (Jobard et al., 2003, Lebel et al., 2013, Philipose et al., 2007, Swett et al., 2013). Damage that results in reduced integrity of white matter along these networks will likely reduce reading efficiency. Therefore, the white matter bundles connecting cortical areas related to reading may serve as biomarkers of performance and further, may allow for the identification of specific white matter tracts associated with the dorsal and ventral informational streams associated with specific functions (Vandermosten et al., 2012b).
The integrity of large white matter bundles in the brain can be estimated through the use of Diffusion Tensor Imaging (DTI) methods. This approach involves measurement of the diffusion of water in tissues to estimate density of structures and directionality of white matter pathways (Basser, 1997). The common diffusion metrics derived from this method, particularly fractional anisotropy (FA) and mean diffusivity (MD) provide information regarding myelination and axonal integrity (Mori and Zhang, 2006, Song et al., 2003).
DTI has been used to examine the white matter substrates associated with reading performance. Studies of children with dyslexia and typically developing children have identified reduced FA in a variety of pathways and regions implicated in reading performance. In addition to left Perisylvian regions (Klingberg et al., 2000, Rimrodt et al., 2010), microstructural differences related to reading performance have been reported in commissural pathways, the superior longitudinal fasciculus, inferior longitudinal fasciculus, and the inferior frontal occipital fasciculus (Beaulieu et al., 2005, Cutting et al., 2013, Dougherty et al., 2007, Frye et al., 2011: Lebel et al., 2013) as well as in connectivity of other regions including thalamocortical (Fan et al., 2014) and cerebellar (Stoodley and Stein, 2013) pathways.
DTI methods have been employed in identifying white matter pathways associated with word reading in typically developing and dyslexic populations. Despite variability between studies, review of findings in both typical and impaired readers has revealed that the integrity of the superior longitudinal fasciculus and the inferior fronto-occipital fasciculus have frequently been implicated in word reading ability (Frye et al., 2011, Vandermosten et al., 2012a). The superior longitudinal fasciculus has been implicated in multiple region-of-interest reading studies for individual words (Carter et al., 2009, Hoeft et al., 2011, Zhang et al., 2014). Voxel-based analysis of the inferior fronto-occipital fasciculus, inferior longitudinal fasciculus and the superior longitudinal fasciculus have revealed correlations with word reading in both typical and dyslexic readers (Horowitz-Kraus et al., 2014, Odegard et al., 2009, Steinbrink et al., 2008). Similarly, tractography studies suggest involvement of the inferior fronto-occipital fasciculus or inferior longitudinal fasciculus as well as the superior longitudinal fasciculus in word reading ability (Vandermosten et al., 2012b, Yeatman et al., 2012).
The physical integrity of these two white matter bundles may be related to the direct and indirect routes for word reading. Within a dyslexic population, Vandermosten et al. (2012b) identified separable associations between the pathways associated with the ventral, direct route (inferior fronto-occipital fasciculus) and the dorsal, indirect route (superior longitudinal fasciculus). This appears to be the first DTI study to doubly dissociate these two pathways and the theoretically separable functions of sight word reading and phonemic decoding. However, as the relation between DTI metrics and reading abilities can vary between populations of interest (Frye et al., 2011), it is unknown if this relation holds in the acquired deficits associated with TBI. With regard to reading fluency, voxel-based DTI analysis with healthy adolescent and adult readers has correlated reading fluency with FA in the frontal lobes, suggesting a larger frontal lobe involvement in reading fluency than in individual word reading (Lebel et al., 2013). Consistent with studies of speeded performance across a range of tasks (Turken et al., 2008), dysfluent readers demonstrated a pattern of lower FA in voxels of the superior longitudinal fasciculus and inferior longitudinal fasciculus (Lebel et al., 2013).
Reading comprehension has been similarly correlated with integrity of the superior longitudinal fasciculus (Carter et al., 2009). However, reading comprehension is a complexly determined skill that is related to several supporting abilities, including working memory and processing speed (Cain et al., 2004, Christopher et al., 2012, Wolf and Katzir-Cohen, 2001). Reading comprehension may, therefore, rely on a combination of intact white matter pathways. Previous TBSS research with older adolescents has revealed that mean FA of both the superior longitudinal fasciculus and inferior longitudinal fasciculus are associated with reading comprehension (Horowitz-Kraus et al., 2014). While not specifically investigated in previous reading studies, the cingulum bundle, which has been implicated in executive function skills, including working memory and processing speed, may support reading fluency and/or comprehension (Peters et al., 2014).
1.4. Traumatic brain injury (TBI)
TBI initiates a cascade of pathological cellular processes that may produce diffuse and/or multifocal insult. Axonal injury can occur as a consequence of the immediate, direct acceleration forces as well as by subsequent neurochemical and metabolic cascades and apoptotic processes (Povlishock, 2000, Reeves et al., 2005). Cumulatively, these injuries may result in shearing and reduced integrity of white matter pathways and interrupt maturation of white matter (Ewing-Cobbs et al., 2008). Due to its sensitivity to white matter change, DTI has become a valuable method for integrating indices of microstructural damage in specific pathways with cognitive outcomes associated with TBI (Johnson et al., 2011, Kinnunen et al., 2011, Levin et al., 2008, Niogi et al., 2008). In children with TBI, white matter damage has been associated with significant behavioral and cognitive deficits which are related to the location and extent of the white matter injury (Ewing-Cobbs et al., 2008, Johnson et al., 2011, McCauley et al., 2011, Wozniak et al., 2007).
Academic deficits are among the most common and disabling, particularly deficits in reading (Ewing-Cobbs et al., 1998, Jaffe et al., 1992, Kinsella et al., 1997). Reading problems acquired through TBI often persist for years post-injury, with impairment in reading ability being common even five years after insult (Catroppa et al., 2008). Recent meta-analysis of educational outcomes has also demonstrated that reading deficits persist with little evidence of recovery among those with severe injuries between the 5 months post-injury and 24 months, whereas other academic skills, such as math ability, show some recovery of during this period (Vu et al., 2011). White matter in the frontal lobes has been associated with performance on tasks of reading and executive functioning in the TBI population (Adamson et al., 2013). In addition, the integrity of the cingulum bundle has been implicated in speeded task performance and working memory in TBI, though not with performance in word-reading (Wilde et al., 2010). It is currently unknown how microstructural changes in white matter pathways may affect specific reading abilities. Due to the sparse literature, there are no clear white matter biomarkers that might help predict reading outcomes in TBI.
1.5. Present study
The purpose of the current study was to investigate whether white matter integrity as measured by Tract-Based Spatial Statistics at 3 months after TBI, predicts reading outcomes at 12 months post-injury. Pathways were selected a priori based on developmental imaging studies and on cognitive models of reading performance. Using pediatric TBI as a model for acquired reading deficits, three left-sided white matter pathways were selected as possible predictors of phonemic decoding and sight word reading, as well as reading fluency and comprehension.
The first aim of the study was to identify if patterns of reduced white matter integrity and acquired reading deficits were consistent with a dual pathway model of word reading ability. Using the theoretical model of dual pathways suggested by Vandermosten et al. (2012b), we hypothesized that the superior longitudinal fasciculus, with its dorsal path, would predict performance on phonemic decoding tasks, while the integrity of the inferior fronto-occipital fasciculus, a major ventral pathway associated with word reading, would predict performance on sight word tasks.
The second aim was to investigate if fluency and comprehension could be linked with specific white matter pathways. Based on the few structural neuroimaging studies, we hypothesized that reduced integrity of the superior longitudinal fasciculus, cingulum bundle, and inferior fronto-occipital fasciculus after TBI would predict fluency and comprehension of reading connected text.
2. Materials and methods
2.1. Participants
All participants were hospitalized at an urban Level One Trauma Center for either a TBI or orthopedic injury (OI). The pool of participants was drawn from a larger study pool of 119 children recruited for a prospective, longitudinal study of academic outcomes following TBI. Of these, three were removed for not meeting exclusion criteria after consent, 13 did not participate in an MRI session, 13 had a bad acquisition due to motion or artifact, three withdrew from the study, and 13 failed to participate in the 12 month neuropsychological testing session. The remaining 74 participants included 39 children with TBI and 35 with OI. Children with OI were included as a comparison group due to factors such as stress, pain, hospitalization, and loss of school time which are associated with severe injuries.
Inclusionary criteria for children with TBI were (a) hospitalization following complicated mild, moderate, or severe TBI resulting from acceleration-deceleration or blunt impact injuries caused by vehicular accidents, falls, or impact with a blunt object; (b) no history of previous TBI; (c) primarily English-speaking or bilingual; (d) residing within the 125 mile radius of the catchment area; and (e) no known premorbid neurologic, metabolic, major developmental, or psychiatric disorders that would interfere with assessment of the impact of TBI on outcomes. An orthopedic injury group was included as a comparison to factor for confounding variables such as pain, length of absence from school, and psychological trauma following injury. The OI group met inclusionary criteria (b) through (e). Additionally, they had to be hospitalized for their injuries and have an absence of facial injuries so as to minimize the possibility of co-occurring mild TBI. Participants in both groups were also screened with the 2 subtest Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999). Three participants were removed for scores on the WASI that fell greater than two standard deviations below the mean.
2.2. Procedure
Participants were recruited and consented following procedures approved by the University of Texas Health Science Center at Houston's Committee for the Protection of Human Subjects. Structural T1, T2, and DTI sequences were completed at approximately 3 months from injury (Table 2). Neuropsychological testing was completed around 12 months from injury. Participants were tested by a trained examiner. All images were reviewed by a board certified radiologist (LK) for evidence of neurological injury. Injury characteristics from this review for the TBI group are provided in Table 1. Due to the multifocal nature of TBI and frequent presence of extensive shear injury, individuals were included who met injury criteria regardless of lesion location. Shear injury was identified by the presence of prominent hypointense foci on susceptibility weighted pulse sequences representing the superparamagnetic blooming artifact of hemosiderin deposition secondary to petechial hemorrhage typically found at grey–white matter interfaces.
Table 2.
Group differences between orthopedic injury (OI) and traumatic brain injury (TBI) groups.
| Demographics | Orthopedic group | Traumatic brain injury group | Statistics |
|
|---|---|---|---|---|
| x2(n = 56) | p | |||
| N | 27 | 29 | ||
| Gender (% male) | 56 | 72 | 1.73 | 0.19 |
| Dominant Hand (% right) | 85 | 93 | 0.92 | 0.34 |
| t(54) | p | |||
| Age at Scan (mean [SD]) | 10.1 [2.71] | 11.8 [2.74] | 2.39 | 0.02 |
| Maternal Education (mean [SD])⁎ | 13.8 [3.65] | 12.8 [4.74] | 0.84 | 0.41 |
| WASI IQ (Mean [SD]) | 107.0 [13.63] | 103.0 [11.57] | 1.19 | 0.24 |
| Injury Severity Score (mean [SD]) | 6.33 [2.54] | 21.62 [10.90] | 7.34 | 0.00 |
| Days From Injury to Scan (mean [SD]) | 115.93 [102.67] | 103.93 [46.25] | 0.57 | 0.57 |
| Days From Injury to Test (mean [SD]) | 374.96 [39.092] | 383.52 [37.829] | − 0.83 | 0.41 |
| Cause of injury (N) | ||||
| Assault | 0 | 1 | ||
| Fall | 11 | 5 | ||
| Motor vehicle accident | 1 | 17 | ||
| Pedestrian struck | 8 | 4 | ||
| Sports or play | 7 | 2 | ||
| Glasgow outcome score-1 year after injury (N) | ||||
| 1 – good outcome | 26 | 19 | ||
| 2 – moderate disability | 1 | 10 | ||
| Lowest GCS score (N) | ||||
| 3 – 8 | 17 | |||
| 9 – 12 | 9 | |||
| 13 – 15 | 3 |
As classified by the Hollingshead Four Factor Scale (1975).
Table 1.
Injury characteristics from radiological review of the traumatic brain injury (TBI) group.
| Age in years | Imaging findings | Hemisphere | Regions implicated | Encephalomalacia | Shear injuries |
|---|---|---|---|---|---|
| 6 | Yes | Left | Temporal, parietal, occipital | No | Yes |
| 7 | Yes | Bilateral | Frontal | Yes | Yes |
| 7 | No | ||||
| 8 | No | ||||
| 8 | No | ||||
| 9 | No | ||||
| 9 | No | ||||
| 9 | No | ||||
| 10 | Yes | Bilateral | Extensive | No | Yes |
| 10 | Yes | Bilateral | Frontal | Yes | Yes |
| 10 | Yes | Left | Frontal, parietal | No | Yes |
| 10 | Yes | Left | Extensive | Yes | Yes |
| 11 | No | ||||
| 11 | No | ||||
| 11 | Yes | Bilateral | Extensive | No | Yes |
| 12 | No | ||||
| 12 | Yes | Left | Temporal | Yes | No |
| 12 | No | ||||
| 12 | Yes | Bilateral | Frontal, temporal | Yes | No |
| 13 | No | ||||
| 13 | Yes | Bilateral | Extensive | No | Yes |
| 13 | No | ||||
| 14 | No | ||||
| 14 | Yes | Bilateral | Extensive | No | Yes |
| 15 | No | ||||
| 15 | Yes | Bilateral | Frontal | Yes | No |
| 15 | Yes | Left | Frontal, temporal, parietal | Yes | No |
| 15 | Yes | Bilateral | Extensive | Yes | Yes |
| 16 | Yes | Right | Frontal, parietal | No | Yes |
2.2.1. Reading outcomes
Reading subtests from the Woodcock–Johnson Tests of Achievement – Third Edition (WJ-III) were administered (Woodcock et al., 2007). The WJ-III is a nationally standardized, broad measure of academic achievement. The Letter-Word subtest is an untimed measure of word reading accuracy. The Reading Fluency subtest is a timed measure that requires the child to read a sentence which is either clearly true or clearly false and circle yes or no to the sentence. Standardized scores were used which have a mean of 100 and standard deviation of 15.
The Test of Word Reading Efficiency (TOWRE) is a timed measure of rapid word identification (Sight Word subtest) and rapid phonemic decoding of non-words (Phonemic Decoding subtest; Torgesen et al., 1999). It is a measure of fluent, efficient word-level reading. Standardized scores were used which have a mean of 100 and standard deviation of 15.
The Gray Oral Reading Tests – 4th Edition (GORT-4) evaluated fluency and comprehension based on oral reading of passages of meaningful text (Wiederholt and Brian, 2001). Scores from this measure include an overall Fluency composite, calculated as a linear combination of performance on Speed and Accuracy scores, and a Comprehension score. Scaled scores were used which have a mean of 10 and standard deviation of 3.
2.2.2. Imaging parameters
All MRIs were performed on a Philips 3 T scanner using an eight-channel phase array head coil. Sagittal 3D T1 and T2-weighted acquisitions were obtained in addition to an axial DTI sequence. The DTI sequence consisted of a single-shot spin-echo diffusion sensitized echo-planar imaging sequence with the following parameters: 21 non-collinear equally distributed diffusion encoding directions (e.g., Icosa21; Hasan and Narayana, 2003); repetition time/echo time = 6100/84 ms; b = 0, and 1000 s/mm2; field-of-view = 240 × 240 mm2; matrix = 256 × 256; slice thickness = 3.0 mm; in-plane pixel dimensions (x,y) = 0.94,0.94; acceleration factor = 2; the sequence was repeated twice and averaged with a total scan time of approximately 7 min.
2.2.3. Processing
Tract-based spatial statistics (TBSS) is a relatively recent method for estimating and comparing white matter pathway integrity (Smith et al., 2006). This data-driven approach for identifying and standardizing white matter pathways allows for estimation of diffusion metrics within large white matter bundles and has been employed in the study of dyslexia to identify pathways associated with reading deficits (Odegard et al., 2009). TBSS has also been employed to identify areas of significant white matter disruption in TBI (Niogi et al., 2008, Palacios et al., 2011, Wilde et al., 2012). The findings of these studies indicate that there is widespread disruption of white matter following TBI and that TBSS analyses can identify pathways associated with loss of cognitive functions.
Voxelwise statistical analysis of the FA and MD data was carried out using TBSS, part of the FSL software package (Smith et al., 2004, Smith et al., 2006). First, FA images were created by fitting a tensor model to the raw diffusion data using FDT, generating primary, secondary, and tertiary eigenvalues from the three primary, secondary, and tertiary eigenvectors, respectively. FA and MD values were automatically derived for each voxel from this data. All participants' FA data were then aligned into a common space using the nonlinear registration tool FNIRT (Andersson et al., 2007). Next, the mean FA image was created and thinned to create a mean FA skeleton which represents the centers of all tracts common to the group. Each subject's aligned FA and MD data was then projected onto this skeleton and the resulting data fed into voxelwise cross-subject statistics.
TBSS relies on standardization of white matter skeletons to a study template. However, due to the significant neurological variability introduced by severe traumatic brain injury, registration is not always successful, particularly in injuries involving significant bleeds or mass effect. Failure to investigate the white matter skeleton registration may result in artificially depressed white matter integrity measures of the pathways under investigation. To ensure that included participant skeletons were properly aligned, each registration was manually reviewed by a researcher (CPJ). Individual review of the skeletonized white matter pathways of all participants resulted in 14 participants being removed due to poor alignment to the study template. This left a final pool of participants that included 27 OI and 29 TBI cases.
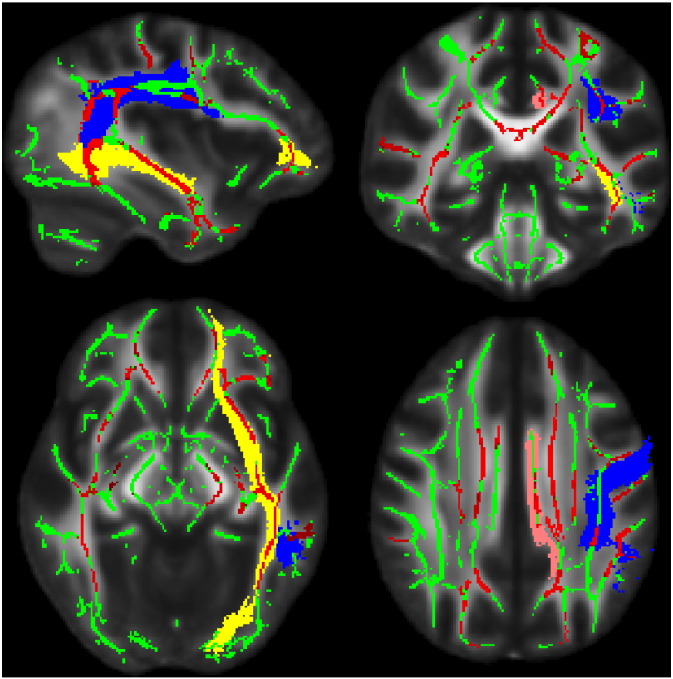
Probabilistic tracts derived from the Johns Hopkins University white-matter tractography atlas were overlaid onto each brain and masks were derived by thresholding the probability of white matter paths at FA values of 0.2 and then applied to the FA skeleton and summarized to generate FA and MD values for each of the tracts of interest in the left hemisphere (Hua et al., 2008; see Fig. 1). Left-hemisphere metrics were used for all participants regardless of dominant handedness. Despite the presence of left-hand dominant individuals in both groups, the decision to use left-hemispheric pathways was based on the tendency for language to be left-hemisphere lateralized regardless of handedness (Pujol et al., 1999) and in the interest of reducing Type I error rates. Using this approach, FA and MD values of the inferior fronto-occipital fasciculus, superior longitudinal fasciculus, and cingulum bundle values were all summarized and extracted from the white matter skeleton. These values were then exported to SAS 9.3 for further analysis.
Fig. 1.
TBSS Results. White matter skeleton appears in green. The cingulum bundle (pink), superior longitudinal fasciculus (blue), and inferior fronto-occipital fasciculus (yellow) regions from the Johns Hopkins University atlas are illustrated. Clusters where OI group FA is significantly greater than the TBI group (p < 0.05, multiple comparison correction) appear red. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
2.3. Statistical analyses
2.3.1. Group differences in reading and TBSS
Group comparisons of demographic information and reading performance were accomplished using chi-square and t-tests. Age-normed, standardized scores were used for all reading measures. Distributions of variables were assessed for significant outliers. One participant was removed as an outlier on reading fluency measures. FA values are reported as a ratio of relations between eigenvalues (ranging from 0 to 1) and MD values are reported in millimeters squared per second (mm2/s). To evaluate group differences in the spatial distribution of FA values along the TBSS white matter skeleton a threshold-free cluster enhancement t-test (corrected for multiple comparisons) was conducted using FSL's randomise statistical function.
Following the TBSS analysis for group differences, mean values for each DTI metric were extracted from each of the 3 left hemisphere tracts of interest (superior longitudinal fasciculus, inferior fronto-occipital fasciculus, and cingulum bundle) and exported to SAS for further statistical analyses. Group differences on DTI metrics were investigated using general linear models after covarying for the effects of age.
2.3.2. Relation of reading abilities to pathway integrity
To address the first aim of the study, three general linear models were developed to test whether the superior longitudinal fasciculus and inferior fronto-occipital fasciculus predicted reading outcomes at the word level as measured by the WJ-III Letter-Word subtest, TOWRE Sight Word subtest, and the TOWRE Phonemic Decoding subtest. Due to its relation with reading ability, maternal education was included as a covariate. Initial models included group membership, maternal education, both pathways, and the interaction of each pathway with group on each of the three word-reading measures. Non-significant interactions were removed and re-run to form the final models. All general linear models were generated using the SAS implementation of the GLM procedure, which uses the method of least squares to fit the models.
General linear models were also used to test whether any of the three pathways (superior longitudinal fasciculus, inferior fronto-occipital fasciculus, and the cingulum bundle) predicted reading fluency and reading comprehension as measured by the WJ-III Reading Fluency subtest, GORT-4 Reading Fluency composite, and the GORT-4 Comprehension score. Initial models included group membership, maternal education, the three pathways of interest, and the interactions of those pathways with group. Non-significant interactions were removed and re-run to form the final models.
2.3.3. Effect size
Measures of effect size, which are less influenced by sample size than are p values, were included to index the strength or magnitude of findings (Durlak, 2009, Lipsey, 1990). Cohen's d is commonly used for t-tests and was calculated to provide an estimate of the magnitude of group differences on reading measures. Partial eta squared was used to estimate effect sizes based on general linear models examining group differences in pathway TBSS metrics and in models relating pathway metrics to reading performance.
3. Results
The final sample of included participants did not differ from those excluded in terms of age at scan, t(114) = 0.55, p = 0.59, nor in terms of maternal education, t(114) = 0.28, p = 0.78. However, the TBI participants excluded from the study had significantly more severe injuries than those who remained based on Glasgow Coma Scale (GCS; Teasdale and Jennett, 1974) scores, t(59) = 3.35, p < 0.01. The TBI group contained 12 complicated mild or moderate injuries and 17 severe injuries following classification methods used by Levin et al. (2008).
Demographic information and injury-related variables for TBI and OI groups are provided in Table 2. While groups did not differ by sex or handedness, the TBI group was significantly older than the OI group. There were no differences in maternal education or WASI IQ. TBI severity was classified via the GCS. Injury severity for all participants was classified via the Injury Severity Scale (Baker et al., 1974). The TBI group had significantly higher Injury Severity Scores than the OI group (Baker et al., 1974). The TBI group also had more motor vehicle collisions than the OI group.
3.1. Group differences in reading and TBSS
The TBI group performed more poorly than the OI group on several reading measures (Table 3). The untimed word reading measure (WJ-III Letter-Word) and the GORT-4 reading comprehension score did not differ significantly between groups. Word reading fluency (TOWRE scores) was significantly lower and trends were noted for text reading fluency (WJ-III Reading Fluency; GORT-4 Reading Rate) to be lower in the TBI than OI group (p = .05). Except for comprehension, Cohen's d indicated medium group effect sizes for most reading measures, consistent with average scores in the TBI group falling about 13 to 24 percentile points lower than in the OI group.
Table 3.
Group differences on standardized reading measures.
| Reading measures M [SD] | Orthopedic group | Traumatic brain injury group | Statistics |
||
|---|---|---|---|---|---|
| t(54) | p | d | |||
| WJ-III Letter-Word | 102.9 [10.06] | 98.7 [11.32] | 1.45 | 0.08 | 0.39 |
| WJ-III Reading Fluency | 103.2 [14.12] | 96.8 [12.72] | 1.65 | 0.05 | 0.44 |
| TOWRE Sight Word | 102.2 [12.54] | 94.1 [12.24] | 2.44 | 0.01 | 0.65 |
| TOWRE Phonemic Decoding | 97.96 [13.91] | 91.55 [12.09] | 1.84 | 0.04 | 0.49 |
| GORT-4 Reading Rate | 10.4 [3.04] | 9.0 [3.08] | 1.72 | 0.05 | 0.46 |
| GORT-4 Reading Accuracy | 9.4 [2.53] | 8.2 [2.77] | 1.64 | 0.05 | 0.44 |
| GORT-4 Reading Comprehension | 10.4 [3.51] | 9.7 [2.65] | 0.82 | 0.21 | 0.22 |
Note: Significance values based on single-tailed t-tests. d = Cohen's d.
TBSS-based t-tests of group differences in FA along the white matter skeleton revealed significant differences throughout major white matter pathways of the brain. Consistent with previous reports in the published literature, these results from a whole-brain analysis reflect a pattern of diffuse white matter injury characteristic of TBI patients. The spatial distribution of significantly different voxels is highlighted for each of the 3 tracts of interest in the left hemisphere in Fig. 1.
Mean values for FA and MD were extracted from the superior longitudinal fasciculus, inferior fronto-occipital fasciculus, and cingulum bundle in the left hemisphere. GLM analyses run in SAS were used to test a priori hypotheses about the impact of TBI on pathway integrity. On all 3 pathways of interest, the TBI group had significantly lower FA than the OI group; in contrast, MD values did not differ between groups. Statistical tests and effect sizes for group differences are summarized in Table 4. Notably, the effect sizes for FA ranged from small (cingulum bundle & superior longitudinal fasciculus) to medium (inferior fronto-occipital fasciculus). Group accounted for from 7 to 18% of variability in pathway FA. Effect sizes for group differences in MD ranged from negligible (cingulum bundle) to small (inferior fronto-occipital fasciculus & superior longitudinal fasciculus).
Table 4.
Group differences in pathway microstructure after controlling for age.
| DTI Metric and Pathway M [SD] |
Orthopedic Group | Traumatic brain injury group | Statistics |
||
|---|---|---|---|---|---|
| F(1,53) | p | ηp2 | |||
| FA Measures | |||||
| Cingulum Bundle | 0.528 [0.042] | 0.515 [0.037] | 4.06 | 0.05 | 0.07 |
| Inferior Fronto-Occipital Fasciculus | 0.511 [0.023] | 0.492 [0.027] | 11.61 | < 0.001 | 0.18 |
| Superior Longitudinal Fasciculus | 0.454 [0.020] | 0.445 [0.028] | 4.34 | 0.04 | 0.08 |
| MD Measures x 103 | |||||
| Cingulum Bundle | 0.773 [0.047] | 0.767 [0.029] | 0.48 | 0.49 | 0.01 |
| Inferior Fronto-Occipital Fasciculus | 0.814 [0.049] | 0.821 [0.036] | 2.35 | 0.13 | 0.04 |
| Superior Longitudinal Fasciculus | 0.777 [0.057] | 0.780 [0.037] | 2.01 | 0.16 | 0.04 |
3.2. Relation of reading abilities to pathway integrity
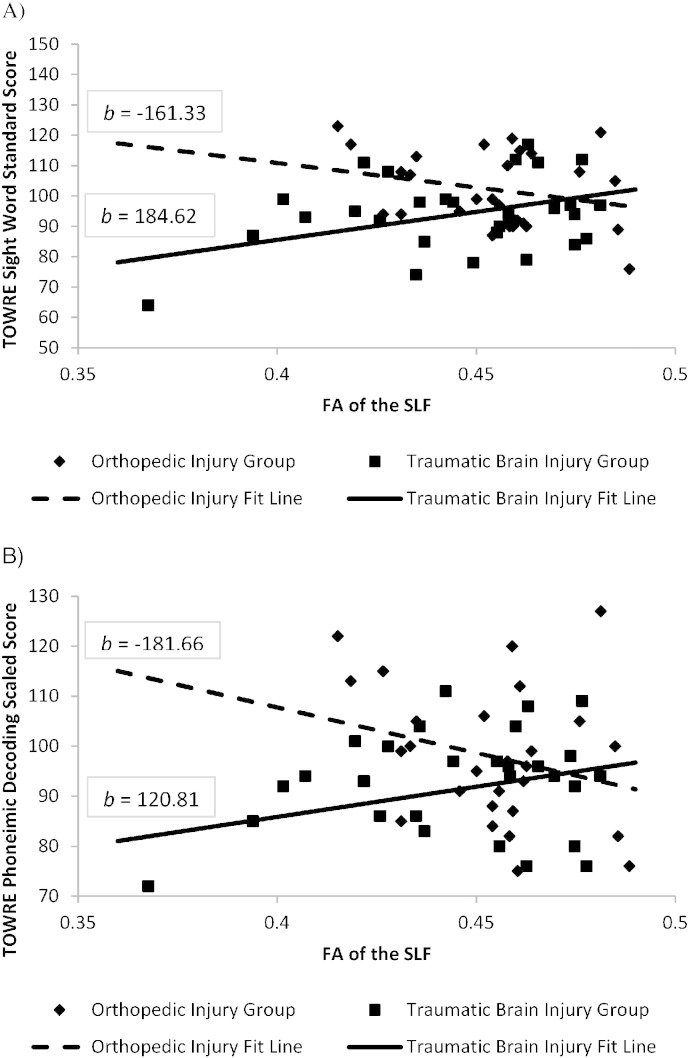
Prediction of single word decoding. General linear models examined group membership, FA values extracted from pathways hypothesized to support word decoding, and their interaction in relation to the standardized word reading measures after covarying for maternal education. Table 5 contains the statistical results and effect sizes for each analysis and the interaction effects are illustrated in Fig. 2. The inferior fronto-occipital fasciculus FA did not predict performance on any of the three measures in either group and the effect sizes were negligible. Although the main effect of the superior longitudinal fasciculus was not significant, the interaction between group and FA significantly predicted untimed word reading performance on the as well as timed sight word-reading and timed phonemic decoding on the TOWRE.
Table 5.
General Linear Models of White Matter Pathways Predicting Word Reading.
| WJ-III Letter-Word |
TOWRE Sight Word |
TOWRE Phonemic Decoding |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Predictor | F(1, 50) | P | ηp2 | F(1, 50) | p | ηp2 | F(1, 50) | p | ηp2 |
| Maternal Education | 5.34 | 0.03 | 0.09 | 4.37 | 0.04 | 0.07 | 2.88 | 0.10 | 0.05 |
| Group | 4.28 | 0.04 | 0.07 | 6.64 | 0.01 | 0.10 | 4.31 | 0.04 | 0.07 |
| Inferior Fronto-Occipital | 0.02 | 0.89 | 0.00 | 0.38 | 0.54 | 0.01 | 0.28 | 0.60 | 0.00 |
| Superior Longitudinal | 0.01 | 0.93 | 0.00 | 0.02 | 0.89 | 0.00 | 0.11 | 0.75 | 0.00 |
| Group x Superior Longitudinal | 4.07 | 0.05 | 0.07 | 6.03 | 0.02 | 0.09 | 3.93 | 0.05 | 0.07 |
Note: These three models were not corrected for multiple comparisons.
Fig. 2.
Interaction Effect of Group and Superior Longitudinal Fasciculus Integrity TOWRE sight word and Phonemic Decoding tasks.
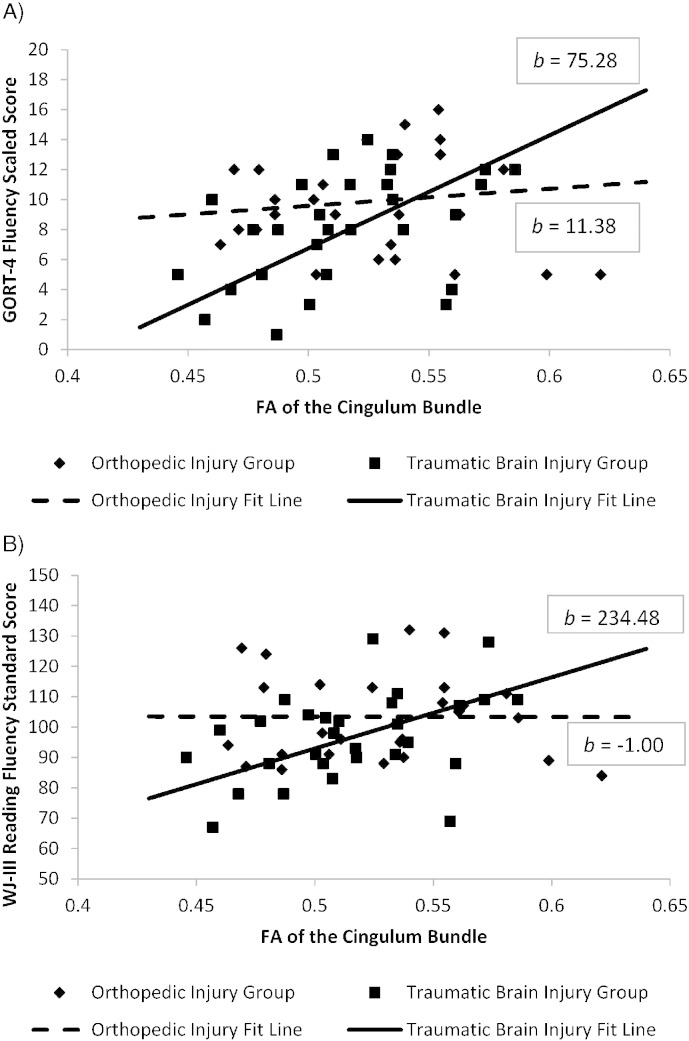
Prediction of fluency and comprehension. Neither the inferior fronto-occipital nor superior longitudinal fasciculi predicted fluency for reading sentences or connected text. There was an interaction effect between group and FA of the cingulum bundle in predicting both silent and oral reading fluency performance. This interaction indicates that lower FA of the cingulum bundle in the TBI group predicted lower reading fluency. The results of the general linear models for fluency and comprehension are presented in Table 6 and the interaction effects are illustrated in Fig. 3. No pathways predicted comprehension performance in either group.
Table 6.
General Linear Models of White Matter Pathways Predicting Fluency and Comprehension.
| WJ-III Reading Fluency |
GORT4 Fluency |
GORT-4 Comprehension |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Predictor | F(1, 49) | P | ηp2 | F(1, 49) | p | ηp2 | F(1, 50) | p | ηp2 |
| Maternal education | 3.85 | 0.06 | 0.06 | 4.78 | 0.03 | 0.07 | 6.24 | 0.02 | 0.10 |
| Group | 6.03 | 0.02 | 0.10 | 8.12 | 0.01 | 0.12 | 0.55 | 0.46 | 0.01 |
| Cingulum | 3.03 | 0.09 | 0.05 | 7.65 | 0.01 | 0.11 | 1.04 | 0.31 | 0.02 |
| Inferior fronto-occipital | 0.63 | 0.43 | 0.01 | 1.45 | 0.23 | 0.02 | 2.59 | 0.11 | 0.04 |
| Superior longitudinal | 0.00 | 0.99 | 0.00 | 0.77 | 0.38 | 0.01 | 0.63 | 0.43 | 0.01 |
| Group x cingulum | 5.58 | 0.02 | 0.09 | 7.53 | 0.01 | 0.11 | – | – | – |
Note: these three models were not corrected for multiple comparisons.
Fig. 3.
Interaction Effect of Group and Cingulum Bundle Integrity on GORT-4 and WJ-III Reading Fluency tasks.
4. Discussion
Consistent with dual route models of reading and theoretical models of associated pathways (Coltheart et al., 2001, Pugh et al., 2001), the integrity of the superior longitudinal fasciculus was associated with word reading outcomes in the TBI group. Additionally, the integrity of the cingulum bundle, a pathway not typically associated with reading ability, was associated with reading fluency outcomes on multiple tasks of text reading. As far as we know, this is the first study to demonstrate specific white matter pathway integrity in TBI as a predictor of reading outcomes. Notably, most effects were found in the forms of interactions where normal variations in white matter integrity in the orthopedic group were not related to reading ability, but post-traumatic loss of integrity was associated with lower reading ability.
4.1. White matter pathways associated with word reading
The first aim of this study was to test if reading ability following TBI could be predicted from patterns of white matter damage assuming a dual pathway model of reading (Coltheart et al., 2001, Jobard et al., 2003, Pugh et al., 2001). Under this model, it was expected that microstructural changes to the superior longitudinal fasciculus, a pathway associated with the dorsal, indirect route of phonemic decoding, would result in poorer phonemic decoding performance, while sparing sight word reading ability. Conversely, it was expected that microstructural changes to the inferior fronto-occipital fasciculus, a pathway associated with the ventral, direct route of sight word reading, would result in poorer sight word reading, particularly for common words with atypical grapheme–phoneme characteristics (Vandermosten et al., 2012b). In partial contrast to the proposed model, the current study found no relation between the inferior fronto-occipital fasciculus and word reading outcomes, but a relation between the FA of the superior longitudinal fasciculus and both direct and indirect route word-reading abilities.
Both the sight word and phonemic decoding tasks required oral responses, which may rely on connectivity of the superior longitudinal fasciculus to pre-motor areas of the frontal lobe, most notably the inferior frontal gyrus, which may explain the association between the superior longitudinal fasciculus and all word reading tasks. A second possibility is that disruptions in word reading following TBI are primarily related to efficiency of word reading, rather than accuracy of word reading. Support for this conclusion can be drawn from the group differences in reading ability which were more pronounced on timed word-reading measures. Therefore, following TBI, reduced microstructural integrity of the superior longitudinal fasciculus may primarily affect reading speed, rather than overall accuracy. However, it is also possible that models of normal reading and neurodevelopmental reading deficits are not adequate to illustrate the biological bases of word reading deficits in TBI. Specifically, it is unclear if the often diffuse damage characteristics of TBI can disrupt the established skill of grapheme–phoneme conversion, a reading system which never fully develops in a neurodevelopmental reading disorder (dyslexia) and is largely destroyed by focal injuries that result in acquired forms of dyslexia.
4.2. White matter pathways associated with reading fluency and comprehension
The second aim of the study was to determine if there were specific pathways associated with reading fluency and comprehension, areas of reading ability that tend to be impaired in TBI, particularly in children injured between the 5 to 15 year old age range of the study (Barnes et al., 1999). Under investigation were pathways previously implicated in word reading, the superior longitudinal fasciculus and the inferior fronto-occipital fasciculus (Vandermosten et al., 2012a), and also the cingulum bundle, which has been implicated in executive function tasks requiring self-monitoring and self-pacing (Peters et al., 2014). In children with TBI, only the integrity of the cingulum bundle predicted fluent reading of connected text. Both oral and silent reading fluency tasks were sensitive to reduced integrity of the cingulum bundle. Both tasks also required efficient reading of text, requiring a speed-accuracy tradeoff to be performed by the participant to pace his or her own reading and ensure accurate reading of the sentences and passages presented. This more complex task of monitoring performance while reading seems to rely heavily on the integrity of the cingulum bundle, which has been implicated in inhibitory control, working memory, and self-monitoring in TBI (Wilde et al., 2010) and with executive functioning in typically developing individuals across the lifespan (Peters et al., 2014). As far as we know, this is the first study to specifically implicate the cingulum bundle as a pathway related to reading fluency.
The relation of the cingulum bundle to reading fluency may also serve as a neuroanatomical biomarker separating classes of reading disorders. These findings support a neurological mechanism by which reading fluency might be impaired despite intact word reading accuracy. This may provide valuable information regarding the parsing of reading disabilities between those children with the word-level deficits of dyslexia and the speeded performance deficits seen in reading fluency disorders. As there are few neuroimaging studies investigating the neuroanatomical substrates of specific fluency impairment, the cingulum bundle may provide a starting point for investigating separable pathways that may be related to fluency (Fletcher et al., 2006, Lebel et al., 2013).
Additionally, reading fluency in the context of the current study relates to the speed and accuracy of reading text, which differs markedly from individual word reading, even when timed. This difference between content to be read has been related to separable cognitive processes in reading research (Klauda and Guthrie, 2008). In particular, while reading fluency for words seems to be primarily driven by phonological processing and rapid automatized naming abilities, reading of syntactically related text is also associated with cognitive processes of inferencing and background knowledge (Klauda and Guthrie, 2008). The independence of the cingulum bundle's relationship to fluency for text versus the speeded word reading task in this study may reflect the frontal lobe mediated function of inferencing, which has been demonstrated in fMRI of causal sentence reading (Kuperberg et al., 2006).
Reading comprehension was not predicted by any of the pathways of interest. Additionally, reading comprehension did not differ between groups within this sample. This may be a consequence of the limitations associated with a recognition-based multiple choice format for reading comprehension (Keenan et al., 2008). To better assess the relation of reading comprehension and TBI through white matter pathways, multiple tests of comprehension may be required, as the common tests of comprehension tend to correlate poorly (Cutting and Scarborough, 2012). The current findings may also be a consequence of the reading comprehension construct. Reading comprehension is a complexly determined ability that is related to other abilities, including working memory, inferencing, processing speed, and language comprehension, which are supported by widespread neural networks that may be differentially supported by various pathways (Christopher et al., 2012, Fletcher et al., 2006). As a consequence, more work may be required to characterize the brain networks supporting reading comprehension and its underlying cognitive construct. Some recent evidence suggests that right hemisphere pathways may be involved in reading comprehension (Horowitz-Kraus et al., 2014)
4.3. Limitations and future directions
Several study limitations may temper the conclusions derived from the results. First, the sample size is small which reduces power to detect group differences. Small sample sizes are problematic in TBI where heterogeneity is seen in both neurological and cognitive outcomes. Second, the TBI group was significantly older than the OI group, which may minimize our ability to characterize relations between group and pathway integrity as predictors of reading performance. Third, TBSS analysis, for all its strengths, has limitations for application to populations with significant gross lesion load, such as severe TBI. As a consequence, scans of patients with the most severe injuries had to be eliminated from this analysis for failure to register to the study template. Therefore, the characteristics of this less severe sample may not be representative of TBI as a population. Finally, recent studies using DTI have indicated that segmentation of the superior longitudinal fasciculus may enhance identification of tissue that may be differentially related to outcomes (Park and Friston, 2013). Conversely, the current TBSS method estimates the entirety of the white matter skeleton from anterior-to-posterior regions. Tractography methods will likely be required for adequate segmentation of pathways into neuroanatomically meaningful components.
TBI is associated with diffuse and heterogeneous injury across pathways. This heterogeneity may obscure relations of reading performance with pathways commonly associated with different aspects of reading in other populations. In addition, our finding that microstructure of the cingulum bundle predicted reading fluency may be related to the widespread axonal injury and concomitant slowed processing speed that are common consequences of TBI rather than to a specific relationship with reading fluency. Future studies may wish to explore the relation of cognitive processes, such as working memory and processing speed, which have been associated with the cingulum bundle, to reading fluency through the integrity of the cingulum bundle. Extension of the relation between the cingulum bundle and reading fluency in other populations may also elucidate whether this association is unique to the TBI population or a characteristic of reading fluency deficits in general. The impact of the age difference across groups on the findings is unclear as mean differences in FA between the groups occur in the opposite direction that age differences would imply; even though the TBI group was older, their FA values were lower than those of the OI group. As such, a replication of this study with a larger sample and longitudinal reading assessments would allow for closer study of the effect of age as it interacts with pathway integrity and components of reading. Longitudinal models could help shed light on mechanics underlying reading skill recovery or, alternatively, failure to develop new skills at age appropriate rates.
Funding
This work was funded by the National Institutes of Health R01 NS046308 awarded to LEC. The content is solely the responsibility of the authors and does not necessarily represent the official views of the granting institute. The authors report no financial or other conflict of interest.
Acknowledgments
We are grateful for the assistance of Emily Maxwell, Ph.D. in the preparation of the imaging data.
References
- Adamson C., Yuan W., Babcock L., Leach J.L., Seal M.L., Holland S.K., Wade S.L. Diffusion tensor imaging detects white matter abnormalities and associated cognitive deficits in chronic adolescent TBI. Brain Inj. 2013;27:454–463. doi: 10.3109/02699052.2012.750756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson V. Childhood brain insult: can age at insult help us predict outcome? Brain. 2009;132:45–56. doi: 10.1093/brain/awn293. [DOI] [PubMed] [Google Scholar]
- Andersson J.L., Jenkinson M., Smith S. FMRIB technical report TR07JA2. FMRIB Analysis Group of the University of Oxford; 2007. Non-linear Registration, aka Spatial Normalisation. [Google Scholar]
- Baker S.P., o'Neill B., Haddon W., Jr., Long W.B. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J. Trauma. 1974;14:187–196. [PubMed] [Google Scholar]
- Barnes M.A., Dennis M., Wilkinson M. Reading after closed head injury in childhood: effects on accuracy, fluency, and comprehension. Dev. Neuropsychol. 1999;15:1–24. [Google Scholar]
- Basser P.J. New histological and physiological stains derived from diffusion‐tensor MR images. Ann. N. Y. Acad. Sci. 1997;820:123–138. doi: 10.1111/j.1749-6632.1997.tb46192.x. [DOI] [PubMed] [Google Scholar]
- Beaulieu C., Plewes C., Paulson L.A., Roy D., Snook L., Concha L., Phillips L. Imaging brain connectivity in children with diverse reading ability. NeuroImage. 2005;25:1266–1271. doi: 10.1016/j.neuroimage.2004.12.053. [DOI] [PubMed] [Google Scholar]
- Cain K., Oakhill J., Bryant P. Children's reading comprehension ability: Concurrent prediction by working memory, verbal ability, and component skills. J. Educ. Psychol. 2004;96:31. [Google Scholar]
- Carter J.C. A dual DTI approach to analyzing white matter in children with dyslexia. Psychiatry Res. Neuroimaging. 2009;172:215–219. doi: 10.1016/j.pscychresns.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castles A. The dual route model and the developmental dyslexias. Lond. Rev. Educ. 2006;4:49–61. [Google Scholar]
- Castles A., Coltheart M. Varieties of developmental dyslexia. Cognition. 1993;47:149–180. doi: 10.1016/0010-0277(93)90003-e. [DOI] [PubMed] [Google Scholar]
- Catroppa C., Anderson V.A., Morse S.A., Haritou F., Rosenfeld J.V. Outcome and predictors of functional recovery 5 years following pediatric traumatic brain injury (TBI) J. Pediatr. Psychol. 2008;33:707–718. doi: 10.1093/jpepsy/jsn006. [DOI] [PubMed] [Google Scholar]
- Christopher M.E. Predicting word reading and comprehension with executive function and speed measures across development: a latent variable analysis. J. Exp. Psychol. Gen. 2012;141:470. doi: 10.1037/a0027375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coltheart M., Rastle K., Perry C., Langdon R., Ziegler J. DRC: a dual route cascaded model of visual word recognition and reading aloud. Psychol. Rev. 2001;108:204. doi: 10.1037/0033-295x.108.1.204. [DOI] [PubMed] [Google Scholar]
- Constable R.T., Pugh K.R., Berroya E., Mencl W.E., Westerveld M., Ni W., Shankweiler D. Sentence complexity and input modality effects in sentence comprehension: an fMRI study. NeuroImage. 2004;22:11–21. doi: 10.1016/j.neuroimage.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Cutting L.E., Scarborough H.S. Reaching an Understanding: Innovations in How We View Reading Assessment. 2012. Multiple bases for comprehension difficulties: the potential of cognitive and neurobiological profiling for validation of subtypes and development of assessments; p. 101. [Google Scholar]
- Cutting L.E. Not all reading disabilities are dyslexia: distinct neurobiology of specific comprehension deficits. Brain Connect. 2013;3:199–211. doi: 10.1089/brain.2012.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das T., Padakannaya P., Pugh K.R., Singh N.C. Neuroimaging reveals dual routes to reading in simultaneous proficient readers of two orthographies. NeuroImage. 2011;54:1476–1487. doi: 10.1016/j.neuroimage.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty R.F., Ben-Shachar M., Deutsch G.K., Hernandez A., Fox G.R., Wandell B.A. Temporal-callosal pathway diffusivity predicts phonological skills in children. Proc. Natl. Acad. Sci. 2007;104:8556–8561. doi: 10.1073/pnas.0608961104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durlak J.A. How to select, calculate, and interpret effect sizes. J. Pediatr. Psychol. 2009 doi: 10.1093/jpepsy/jsp004. (jsp004) [DOI] [PubMed] [Google Scholar]
- Ewing-Cobbs L., Fletcher J.M., Levin H.S., Iovino I., Miner M.E. Academic achievement and academic placement following traumatic brain injury in children and adolescents: a two-year longitudinal study. J. Clin. Exp. Neuropsychol. 1998;20:769–781. doi: 10.1076/jcen.20.6.769.1109. [DOI] [PubMed] [Google Scholar]
- Ewing-Cobbs L. Arrested development and disrupted callosal microstructure following pediatric traumatic brain injury: relation to neurobehavioral outcomes. NeuroImage. 2008;42:1305–1315. doi: 10.1016/j.neuroimage.2008.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Q., Davis N., Anderson A.W., Cutting L.E. Thalamo-cortical connectivity: what can diffusion tractography tell us about reading difficulties in children? Brain Connect. 2014;4:428–439. doi: 10.1089/brain.2013.0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher J.M., Lyon G.R., Fuchs L.S., Barnes M.A. Guilford Publications; 2006. Learning disabilities: from identification to intervention. [Google Scholar]
- Frost R. Toward a strong phonological theory of visual word recognition: true issues and false trails. Psychol. Bull. 1998;123:71. doi: 10.1037/0033-2909.123.1.71. [DOI] [PubMed] [Google Scholar]
- Frye R.E., Liederman J., Hasan K.M., Lincoln A., Malmberg B., McLean J., Papanicolaou A. Diffusion tensor quantification of the relations between microstructural and macrostructural indices of white matter and reading. Hum. Brain Mapp. 2011;32:1220–1235. doi: 10.1002/hbm.21103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan K.M., Narayana P.A. Computation of the fractional anisotropy and mean diffusivity maps without tensor decoding and diagonalization: theoretical analysis and validation. Magn. Reson. Med. 2003;50(3):589–598. doi: 10.1002/mrm.10552. [DOI] [PubMed] [Google Scholar]
- Hoeft F. Neural systems predicting long-term outcome in dyslexia. Proc. Natl. Acad. Sci. 2011;108:361–366. doi: 10.1073/pnas.1008950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead A.B. 1975. Four Factor Index of Social Status. [Google Scholar]
- Horowitz-Kraus T., Wang Y., Plante E., Holland S.K. Involvement of the right hemisphere in reading comprehension: a DTI study. Brain Res. 2014;1582:34–44. doi: 10.1016/j.brainres.2014.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua K. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. NeuroImage. 2008;39:336–347. doi: 10.1016/j.neuroimage.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe K.M., Fay G.C., Polissar N.L., Martin K.M., Shurtleff H., Rivara J.B., Winn H.R. Severity of pediatric traumatic brain injury and early neurobehavioral outcome: a cohort study. Arch. Phys. Med. Rehabil. 1992;73:540–547. [PubMed] [Google Scholar]
- Jobard G., Crivello F., Tzourio-Mazoyer N. Evaluation of the dual route theory of reading: a metanalysis of 35 neuroimaging studies. NeuroImage. 2003;20:693–712. doi: 10.1016/S1053-8119(03)00343-4. [DOI] [PubMed] [Google Scholar]
- Johnson C.P., Juranek J., Kramer L.A., Prasad M.R., Swank P.R., Ewing-Cobbs L. Predicting behavioral deficits in pediatric traumatic brain injury through uncinate fasciculus integrity. J. Int. Neuropsychol. Soc. 2011;17:663–673. doi: 10.1017/S1355617711000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan J.M., Betjemann R.S., Olson R.K. Reading comprehension tests vary in the skills they assess: differential dependence on decoding and oral comprehension. Sci. Stud. Read. 2008;12:281–300. [Google Scholar]
- Kinnunen K.M. White matter damage and cognitive impairment after traumatic brain injury. Brain. 2011;134:449–463. doi: 10.1093/brain/awq347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsella G.J. Predictors and indicators of academic outcome in children 2 years following traumatic brain injury. J. Int. Neuropsychol. Soc. 1997;3:608–616. [PubMed] [Google Scholar]
- Klauda S.L., Guthrie J.T. Relationships of three components of reading fluency to reading comprehension. J. Educ. Psychol. 2008;100:310. [Google Scholar]
- Klingberg T., Hedehus M., Temple E., Salz T., Gabrieli J.D., Moseley M.E., Poldrack R.A. Microstructure of temporo-parietal white matter as a basis for reading ability: evidence from diffusion tensor magnetic resonance imaging. Neuron. 2000;25:493–500. doi: 10.1016/s0896-6273(00)80911-3. [DOI] [PubMed] [Google Scholar]
- Kuperberg G.R., Lakshmanan B.M., Caplan D.N., Holcomb P.J. Making sense of discourse: An fMRI study of causal inferencing across sentences. NeuroImage. 2006;33:343–361. doi: 10.1016/j.neuroimage.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Lebel C., Shaywitz B., Holahan J., Shaywitz S., Marchione K., Beaulieu C. Diffusion tensor imaging correlates of reading ability in dysfluent and non-impaired readers. Brain Lang. 2013;125:215–222. doi: 10.1016/j.bandl.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Levin H.S. Diffusion tensor imaging in relation to cognitive and functional outcome of traumatic brain injury in children. J. Head Trauma Rehabil. 2008;23:197. doi: 10.1097/01.HTR.0000327252.54128.7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsey M.W. vol. 19. Sage; 1990. (Design Sensitivity: Statistical Power for Experimental Research). [Google Scholar]
- Marshall J.C., Newcombe F. Patterns of paralexia: a psycholinguistic approach. J. Psycholinguist. Res. 1973;2:175–199. doi: 10.1007/BF01067101. [DOI] [PubMed] [Google Scholar]
- McCandliss B.D., Noble K.G. The development of reading impairment: a cognitive neuroscience model. Ment. Retard. Dev. Disabil. Res. Rev. 2003;9:196–205. doi: 10.1002/mrdd.10080. [DOI] [PubMed] [Google Scholar]
- McCauley S.R. Diffusion tensor imaging of incentive effects in prospective memory after pediatric traumatic brain injury. J. Neurotrauma. 2011;28:503–516. doi: 10.1089/neu.2010.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechelli A., Humphreys G.W., Mayall K., Olson A., Price C.J. Differential effects of word length and visual contrast in the fusiform and lingual gyri during. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2000;267:1909–1913. doi: 10.1098/rspb.2000.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechelli A. Dissociating reading processes on the basis of neuronal interactions. J. Cogn. Sci. 2005;17:1753–1765. doi: 10.1162/089892905774589190. [DOI] [PubMed] [Google Scholar]
- Misra M., Katzir T., Wolf M., Poldrack R.A. Neural systems for rapid automatized naming in skilled readers: unraveling the RAN–reading relationship. Sci. Stud. Read. 2004;8:241–256. [Google Scholar]
- Mori S., Zhang J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron. 2006;51:527–539. doi: 10.1016/j.neuron.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Moss J., Schunn C.D., Schneider W., McNamara D.S., VanLehn K. The neural correlates of strategic reading comprehension: cognitive control and discourse comprehension. NeuroImage. 2011;58:675–686. doi: 10.1016/j.neuroimage.2011.06.034. [DOI] [PubMed] [Google Scholar]
- Niogi S. Extent of microstructural white matter injury in postconcussive syndrome correlates with impaired cognitive reaction time: a 3 T diffusion tensor imaging study of mild traumatic brain injury. Am. J. Neuroradiol. 2008;29:967–973. doi: 10.3174/ajnr.A0970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegard T.N., Farris E.A., Ring J., McColl R., Black J. Brain connectivity in non-reading impaired children and children diagnosed with developmental dyslexia. Neuropsychologia. 2009;47:1972–1977. doi: 10.1016/j.neuropsychologia.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Palacios E.M. Diffusion tensor imaging differences relate to memory deficits in diffuse traumatic brain injury. BMC Neurol. 2011;11:24. doi: 10.1186/1471-2377-11-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H.-J., Friston K. Structural and functional brain networks: from connections to cognition. Science. 2013;342:1238411. doi: 10.1126/science.1238411. [DOI] [PubMed] [Google Scholar]
- Peters B.D. Age-related differences in white matter tract microstructure are associated with cognitive performance from childhood to adulthood. Biol. Psychiatry. 2014;75:248–256. doi: 10.1016/j.biopsych.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipose L.E. Neural regions essential for reading and spelling of words and pseudowords. Ann. Neurol. 2007;62:481–492. doi: 10.1002/ana.21182. [DOI] [PubMed] [Google Scholar]
- Povlishock J. Pathophysiology of neural injury: therapeutic opportunities and challenges. Clin. Neurosurg. 2000;46:113. [PubMed] [Google Scholar]
- Pritchard S.C., Coltheart M., Palethorpe S., Castles A. Nonword reading: comparing dual-route cascaded and connectionist dual-process models with human data. J. Exp. Psychol. Hum. Percept. Perform. 2012;38:1268. doi: 10.1037/a0026703. [DOI] [PubMed] [Google Scholar]
- Pugh K.R. Neurobiological studies of reading and reading disability. J. Commun. Disord. 2001;34:479–492. doi: 10.1016/s0021-9924(01)00060-0. [DOI] [PubMed] [Google Scholar]
- Pujol J., Deus J., Losilla J.M., Capdevila A. Cerebral lateralization of language in normal left-handed people studied by functional MRI. Neurology. 1999;52:1038-1038. doi: 10.1212/wnl.52.5.1038. [DOI] [PubMed] [Google Scholar]
- Reeves T.M., Phillips L.L., Povlishock J.T. Myelinated and unmyelinated axons of the corpus callosum differ in vulnerability and functional recovery following traumatic brain injury. Exp. Neurol. 2005;196:126–137. doi: 10.1016/j.expneurol.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Rimrodt S.L., Peterson D.J., Denckla M.B., Kaufmann W.E., Cutting L.E. White matter microstructural differences linked to left perisylvian language network in children with dyslexia. Cortex. 2010;46:739–749. doi: 10.1016/j.cortex.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmalz X., Marinus E., Castles A. Phonological decoding or direct access? Regularity effects in lexical decisions of Grade 3 and 4 children. Q. J. Exp. Psychol. 2013;66:338–346. doi: 10.1080/17470218.2012.711843. [DOI] [PubMed] [Google Scholar]
- Smith S.M. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith S.M. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Song S.-K., Sun S.-W., Ju W.-K., Lin S.-J., Cross A.H., Neufeld A.H. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. NeuroImage. 2003;20:1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Steinbrink C., Vogt K., Kastrup A., Müller H.-P., Juengling F., Kassubek J., Riecker A. The contribution of white and gray matter differences to developmental dyslexia: insights from DTI and VBM at 3.0 T. Neuropsychologia. 2008;46:3170–3178. doi: 10.1016/j.neuropsychologia.2008.07.015. [DOI] [PubMed] [Google Scholar]
- Stoodley C.J., Stein J.F. Cerebellar function in developmental dyslexia. Cerebellum. 2013;12:267–276. doi: 10.1007/s12311-012-0407-1. [DOI] [PubMed] [Google Scholar]
- Swett K., Miller A.C., Burns S., Hoeft F., Davis N., Petrill S.A., Cutting L.E. Comprehending expository texts: the dynamic neurobiological correlates of building a coherent text representation. Front. Hum. Neurosci. 2013;7 doi: 10.3389/fnhum.2013.00853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor H.G., Alden J. Age-related differences in outcomes following childhood brain insults: an introduction and overview. J. Int. Neuropsychol. Soc. 1997;3:555–567. [PubMed] [Google Scholar]
- Teasdale G., Jennett B. Assessment of coma and impaired consciousness: a practical scale. Lancet. 1974;304:81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- Torgesen J.K., Wagner R., Rashotte C. Pearson; 1999. Test of Word Reading Efficiency. [Google Scholar]
- Turken, Whitfield-Gabrieli S., Bammer R., Baldo J.V., Dronkers N.F., Gabrieli J.D. Cognitive processing speed and the structure of white matter pathways: convergent evidence from normal variation and lesion studies. NeuroImage. 2008;42:1032–1044. doi: 10.1016/j.neuroimage.2008.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandermosten M., Boets B., Poelmans H., Sunaert S., Wouters J., Ghesquière P. A tractography study in dyslexia: neuroanatomic correlates of orthographic, phonological and speech processing. Brain. 2012;135:935–948. doi: 10.1093/brain/awr363. [DOI] [PubMed] [Google Scholar]
- Vandermosten M., Boets B., Wouters J., Ghesquière P. A qualitative and quantitative review of diffusion tensor imaging studies in reading and dyslexia. Neurosci. Biobehav. Rev. 2012;36:1532–1552. doi: 10.1016/j.neubiorev.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Vu J.A., Babikian T., Asarnow R.F. Academic and language outcomes in children after traumatic brain injury: a meta-analysis. Except. Child. 2011;77:263–281. [Google Scholar]
- Wechsler D. The Psychological Corporation; Harcourt Brace & Company. New York, NY: 1999. Wechsler Abbreviated Scale of Intelligence. [Google Scholar]
- Wiederholt J.L.B., Brian R. Fourth edition. Pearson; 2001. Gray Oral Reading Tests. [Google Scholar]
- Wilde E.A. Diffusion tensor imaging of the cingulum bundle in children after traumatic brain injury. Dev. Neuropsychol. 2010;35:333–351. doi: 10.1080/87565641003696940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde E.A. Diffusion tensor imaging in moderate-to-severe pediatric traumatic brain injury: changes within an 18 month post-injury interval. Brain Imaging Behav. 2012;6:404–416. doi: 10.1007/s11682-012-9150-y. [DOI] [PubMed] [Google Scholar]
- Wolf M., Katzir-Cohen T. Reading fluency and its intervention. Sci. Stud. Read. 2001;5:211–239. [Google Scholar]
- Woodcock R.W.M., Kevin S., Mather Nancy. Riverside; 2007. Woodcock-Johnson III Normative Update Complete. [Google Scholar]
- Wozniak J.R. Neurocognitive and neuroimaging correlates of pediatric traumatic brain injury: a diffusion tensor imaging (DTI) study. Arch. Clin. Neuropsychol. 2007;22:555–568. doi: 10.1016/j.acn.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman J.D., Dougherty R.F., Ben-Shachar M., Wandell B.A. Development of white matter and reading skills. Proc. Natl. Acad. Sci. 2012;109:E3045–E3053. doi: 10.1073/pnas.1206792109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M. Language-general and-specific white matter microstructural bases for reading. NeuroImage. 2014;98:435–441. doi: 10.1016/j.neuroimage.2014.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoccolotti P., Friedmann N. From dyslexia to dyslexias, from dysgraphia to dysgraphias, from a cause to causes: a look at current research on developmental dyslexia and dysgraphia. Cortex. 2010;46:1211–1215. doi: 10.1016/j.cortex.2010.09.003. [DOI] [PubMed] [Google Scholar]