Abstract
Plant regeneration through rapid in vitro clonal propagation of nodal explants of Morus alba L. variety S-1 was established along with genetic stability analysis of regenerates. Axillary shoot bud proliferation was achieved on Murashige and Skoog (MS) medium in various culture regimes. Highest number of shoots (5.62 ± 0.01), with average length 4.19 ± 0.01 cm, was initially achieved with medium containing 0.5 mg/l N6-benzyladenine (BA) and 3% sucrose. Repeated subculturing of newly formed nodal parts after each harvest up to sixth passage, yielded highest number of shoots (about 32.27) per explants was obtained after fourth passage. Rooting of shoots occurred on 1/2 MS medium supplemented with 1.0 mg/1 Indole-3-butyric acid (IBA). About 90% (89.16) of the plantlets transferred to the mixture of sand:soil:organic manure (2:2:1) in small plastic pots acclimatized successfully. Genetic stability of the discussed protocol was confirmed by two DNA-based fingerprinting techniques i.e. RAPD (random amplified polymorphic DNA) and ISSR (inter-simple sequence repeat). This protocol can be used for commercial propagation and for future genetic improvement studies.
Keywords: In vitro, Micropropagation, Nodal explants, Morus alba L. variety S-1, DNA-based markers
1. Introduction
Mulberry is a typical woody plant, and it grows worldwide under different climatic conditions such as tropical, subtropical and temperate. In most mulberry-growing countries, especially in China and India, production is focused on enhancing the foliage. The leaves, being the sole food source of the silkworm (Bombyx mori L.), the cocoon of which is used in silk production, have high ecological and economic importance (Butkhup et al., 2013). Low rooting potential of cuttings from this cultivar is a serious bottleneck to their large-scale propagation. Mulberry plants are out breeder and as a result their progeny show genetic variability, which makes them unsuitable for commercial purposes. Methods of conventional vegetative propagation like production of plants through grafting is not economically viable because it involves lot of skilled manpower, expensive nursery facilities and a long wait of 4–5 years to obtain plants ready for harvest (Bhau and Wakhlu, 2001). Propagation of plants through cuttings is also not viable for this cultivar due to their extremely low rooting ability. In this backdrop, plant tissue culture techniques could offer a viable alternative and reliable procedure for mass propagation of this cultivar.
Plant tissue culture (micropropagation) is recognized as one of the key areas of biotechnology because of its potential use to regenerate elites, while conserving valuable plant genetic resources. However, the scaling up of any micropropagation methodology carries the risk of inducing genetic variability, namely somaclonal variation among sub-clones of one parental line (Larkin and Scowcroft, 1981). In vitro cultivation in particular poses a problem in recovering true-to-type regenerants due to chromosomal rearrangement, gene amplification, gene mutation and retrotransposon activation (Saker et al., 2000). Therefore study of somaclonal variation within regenerates is very much relevant to its commercial utilization and exploitation. Strategies available for detecting genetic variation may include phenotypic identification, cytological studies, or molecular analysis. Cytological analysis was one of the conventional and primary methods to detect somaclonal variation in many species. Recently, molecular markers have come up as the most desirable tool for establishing genetic similarity or dissimilarity of in vitro propagated plants.
Scaling up of any micropropagation protocol is severely hindered due to incidence of somaclonal variations, so a stringent quality check in terms of genetic similarity of progeny becomes mandatory. Therefore, it is important to first establish the suitability of a particular micropropagation protocol developed for a particular clone with respect to the production of genetically identical and stable plants before it is released for commercial purposes.
Plant regeneration has been achieved in mulberry in vitro using meristem/shoot tip cultures, axillary buds, internodal segments, hypocotyl regions, cotyledons, and leaves, but the most widely used method of plant regeneration in mulberry is micropropagation through axillary bud culture (Vijayan et al., 2014, in a review). These studies have revealed that in vitro micropropagation in mulberry is dependent on the growth regulator combinations, explant type and most of the protocols are genotype specific and may not be applicable to all valuable genotypes (Bhojwani, 1992). Perusal of literature also demonstrates that scant attention has been paid to the establishment of suitable regeneration and micropropagation protocol in Indian elite varieties which is a prerequisite for large scale true-to-type biomass production and exploitation for commercial purposes (Kavyashree, 2007, Chattopadhyay et al., 2011). With this background, the objective of this work was to develop a reliable plantlet regeneration protocol of Morus alba L. var. S-1 using nodal explants for large-scale production of plants and for long-term germplasm storage in vitro for conservation. An attempt was also made to validate the genetic stability of the regenerants using molecular approaches.
2. Material and methods
2.1. Plant material and disinfection
The nodal explants (2–2.5 cm) were collected from 1 year old plants growing in the experimental field garden of the Department of Botany, University of Kalyani, Kalyani, India, which is located at 22°57′ N latitude, 88°22′ E longitude with an average altitude of 9.75 m above mean sea level. Explants were washed thoroughly under running tap water and then treated with 5% (v/v) aqueous Teepol (Qualigen, Mumbai, India) for 15 min, followed by rinsing five times in sterile distilled water. The explants were then surface disinfested with 70% alcohol for 1 min followed by immersion in 0.1% (w/v) aqueous mercuric chloride (HgCl2) solution for 5–6 min and finally rinsed with sterilized distilled water (4–5 times) in a flow chamber. The surface sterilized explants were aseptically trimmed at cut ends to reduce the size to about 1–1.2 cm prior to placement on culture media.
2.2. Culture media and conditions
Individual disinfected nodal segments (1–1.2 cm) were cultured on MS (Murashige and Skoog, 1962) basal medium containing 3% (w/v) sucrose (Himedia, Mumbai, India) for culture initiation and served as explant sources for subsequent experiments. The pH of the medium (supplemented with respective membrane filtered sterile growth regulators) was adjusted to 5.7 with 1 N NaOH or 1 N HCl before gelling with 0.8% (w/v) agar (Hi-media, Mumbai, India). In all experiments, the chemicals used were of analytical grade. The explants initially were implanted vertically on the culture medium in test tubes (150 × 25 nm) and plugged tightly with non-absorbent cotton. All the cultures were incubated under cool fluorescent light (16 h photo period 55 μmol m− 2 s− 1, Philips, India at 25 ± 2 °C) at 60–70% relative humidity (RH).
2.3. Shoot induction and multiplication
For multiple shoot induction, the nodal explants were cultured on MS medium supplemented with various concentrations of (0.3–2.0 mg/l) N6-benzyladenine (BA)/Kinetin (KN)/2-isoPentenyladenine (2-iP), either individually or in combination with α-naphthalene acetic acid (NAA) (0.3–2.0 mg/l), Indole-3-butyric acid (IBA) (0.3–2.0 mg/l). The regenerated shoots were excised, cut into single axillary buds and further multiplied for five to six subcultures using fresh medium of the same composition. Induction of multiple shoots occurred after about 4 weeks. The frequency with which the explants produced shoots, the number of shoots per explant and average shoot length were recorded after 4 weeks of culture. The length of individual shoots per explant was measured and averaged.
2.4. Induction of rooting of micropropagated shoots
For root induction, regenerated shoots (3–4 cm length) with three to four fully expanded leaves from in vitro grown explants were excised and transferred to full or half strength basal MS medium supplemented with different concentrations of auxins, namely, NAA and IBA (0.5–2.0 mg/l). Data with respect to percentage of rooting, the mean number of roots per shoot and average root length were recorded after four weeks of transfer onto the rooting medium.
2.5. Acclimatization and transfer of plantlets to soil
The rooted plantlets with 4–5 fully expanded leaves were removed from the culture vessels, washed with sterilized distilled water and acclimatized initially to the ex vitro controlled environment (primary hardening). The plantlets were first planted in perforated plastic cups (6 cm diameter) containing four different types of sterilized potting mixture viz. sand:soil:organic manure (1:1:1), sand:soil:organic manure (2:1:1), sand:soil:organic manure (2:2:1) and garden soil: organic manure (1:1). Then they were incubated in a closed growth chamber at 20–28 °C and a relative humidity of 70–90%. The light was provided with cool fluorescent tube light (16 h photoperiod; 55 μmol m−2 s−1, Philips, India). The over-head watering of the plants were done using a hand sprayer as and when required. After 2 weeks, the plantlets were transferred to a separate polythene growth chamber providing photon flux density of 200 μmol m−2 s−1 and relative humidity of 70–100%, and maintained at 20–28 °C. Three comparable experiments were performed for primary hardening each with 40 plantlets and the percentage survival was scored after 4 weeks.
The primarily hardened plants were further established under greenhouse conditions in 15 cm earthen pots. This process is mentioned here as secondary hardening. The plants were removed gently from the plastic cups without hampering the root ball and potted in a mixture of soil, coarse sand and cattle manure (2:2:1). The potted plants were kept within greenhouse under 50% shade provided by Agro shade net (B&V Agro Irrigation Co., India). A 10-h photoperiod with light intensity of 200 μmo l m−2 s−1, RH 70% and temperature of 28 ± 2° were maintained. The watering and fertilizer application of individual plants was done with drip irrigation (Netafilm, Israel). The hardening experiments were conducted during the winter (November–February) season. Seventy five randomly selected plants from each treatment combination of potting mixture were subjected to secondary hardening. Each secondary hardening experiment was repeated thrice with 25 replicates each and the percentage survival was recorded after 30 and 60 days of transferring into earthen pots.
2.6. Molecular analysis
The genetic stability of the donor plant as well as 20 randomly selected in vitro raised hardened plants was assessed by PCR-based RAPD and ISSR analysis. Genomic DNA was extracted from young/juvenile leaves of the donor plant and in vitro-raised field grown plants according to Cytl trimethyl ammonium bromide (CTAB) procedure (Murray and Thompson, 1980) with minor modifications. Quality and quantity of DNA was checked on 0.8% agarose gel and also from values obtained by 260/280 nm UV absorbance ratio.
2.7. Primer selection and reproducibility
Initially, 23 (10-mer) arbitrary decamer RAPD primers and 13 ISSR primers (Genni, Bangalore, India) were randomly selected for screening for the PCR standardization. The reproducibility of the PCR amplification was assessed using selected primers with different DNA samples isolated independently from the control cultures and amplified at different times. The detailed description of the RAPD and ISSR primers used in the present study were given in Table 6, Table 7 respectively.
Table 6.
List of primers, their sequences and size of the amplified fragments generated by randomly amplified polymorphic DNA (RAPD) markers for DNA fingerprinting of micropropagated plants of Morus alba L. variety S-1.
| No. | Primer code | Primer sequence (5′–3′) | No. of scorable loci per primer | Total no. of bands amplified | Size range (bp) |
|---|---|---|---|---|---|
| 1. | OPS-01 | GGTCCCTGAC | 4 | 84.00 | 800–1480 |
| 2. | OPS-02 | CAGGCCCTTC | 2 | 42.00 | 450–800 |
| 3. | OPS-03 | TGCCGAGCTG | 3 | 63.00 | 400–1500 |
| 4. | OPS-04 | AGTCAGCCAC | 2 | 42.00 | 600–1200 |
| 5. | OPS-05 | AATCGGGCTG | 3 | 63.00 | 500–1500 |
| 6. | OPS-06 | AGGGGTCTTG | 3 | 63.00 | 400–1500 |
| 7. | OPS-07 | GTGACGTAGG | 2 | 42.00 | 550–1000 |
| 8. | OPS-08 | CAATCGCCGT | 3 | 63.00 | 800–1500 |
| 9. | OPS-09 | AGCCGTGGAA | 4 | 84.00 | 450–1350 |
| 10. | OPS-10 | AGAGCCGTCA | 3 | 63.00 | 700–1500 |
| 11. | OPS-11 | GGGTCTCGGT | 2 | 42.00 | 500–1000 |
| Total | 31 | 651.00 | |||
Table 7.
List of primers, their sequences and size of the amplified fragments generated by Inter-simple sequence repeats (ISSR) markers for DNA fingerprinting of micropropagated plants of Morus alba L. variety S-1.
| No. | Primer code | Primer sequence (5′–3′) | Annealing temperature (°C) | No. of scorable loci per primer | Total no. of bands amplified | Size range (bp) |
|---|---|---|---|---|---|---|
| 1. | ISSR-01 | (AG)8T | 50.0 | 4 | 84.00 | 300–1500 |
| 2. | ISSR-02 | (AG)8C | 55.0 | 3 | 63.00 | 600–1500 |
| 3. | ISSR-03 | (AG)8G | 50.0 | 5 | 105.00 | 500–1500 |
| 4. | ISSR-04 | (GA)8T | 45.0 | 3 | 63.00 | 600–2000 |
| 5. | ISSR-05 | (GT)8C | 50.0 | 3 | 63.00 | 600–1300 |
| 6. | ISSR-06 | (TC)8G | 53.0 | 2 | 42.00 | 500–1000 |
| 7. | ISSR-07 | (AC)8T | 50.0 | 2 | 42.00 | 500–700 |
| Total | 22 | 462.00 | ||||
2.8. RAPD analysis
PCR amplifications of genomic DNA with RAPD primers (Genni, Bangalore, India) were used with minor modification of Williams et al. (1990). PCR reaction mixture was 25 μl containing 1 unit of PCR buffer (Genni, Bangalore, India), 200 μM dNTPs, 1 unit (U) Taq DNA polymerase, 50 ng template DNA, 1.0 μM of each primer (Genni, Bangalore, India) and 2.0 mM MgCl2. The amplification reaction consisted of an initial denaturation step at 94 °C for 4 min, followed by 40 cycles of 15 s denaturation at 94 °C, 15 s annealing at 40 °C, 1.15 min extension at 72 °C with a final extension of 72 °C for 7 min using thermal cycles (Perkin Elmer gene Amp 2400 PCR system,). The PCR products were resolved by electrophoresis on a 1.5% agarose gel (Himedia, Mumbai, India) in 1.0% tris-acetate EDTA buffer, stained with ethidium bromide (0.5 μg/ml) and visualized under UV light. The number of bands was recorded using a Gel Doc System (Bio-Rad, Hercules, Calif). The size of the amplification products was estimated using a 100–3000 bp DNA ladder (Genni, Bangalore, India).
2.9. ISSR analysis
ISSR analysis was carried out as described by Zietkiewicz et al. (1994). PCR amplifications were performed in a volume of 25 μl containing 1 unit of PCR buffer, 200 μM dNTP mix, 1 unit Taq DNA polymerase, 1.0 μM of each primers (Bangalore Genei Pvt. Ltd., India), 25 ng of template DNA, with various concentrations of MgCl2 depending on the primer (data not shown). The amplification reaction consisted of an initial denaturation at 94 °C for 4 min followed by 35 cycles of 1 min at 94 °C, 1 min annealing at a temperature 3 °C lower than melting point for each primer and 1.15 min extension at 72 °C with a final extension of 72 °C for 7 min using a thermal cycle. The PCR products were separated on a 2% (m/v) agarose gel as mentioned elsewhere.
2.10. Experimental design and statistical analysis
All tissue culture experiments were set up in completely randomized block design. Each experiment was repeated three times with 12–15 replicates. Data were analyzed statistically using one-way analysis of variance (ANOVA) and the significant differences between means were assessed by Duncan's multiple range test p ≤ 0.05 (Duncan, 1955) on the statistical package of SPSS (Version 10). For molecular studies, only consistently reproducible and well resolved bands, ranging from 100 to 3000 bp in size (for RAPD and ISSR markers), were manually scored and the scoring of bands was recorded in the form of their presence (“1”) or absence (“0”) in the gel.
3. Results and discussion
3.1. Effect of cytokinins on shoot regeneration from explants
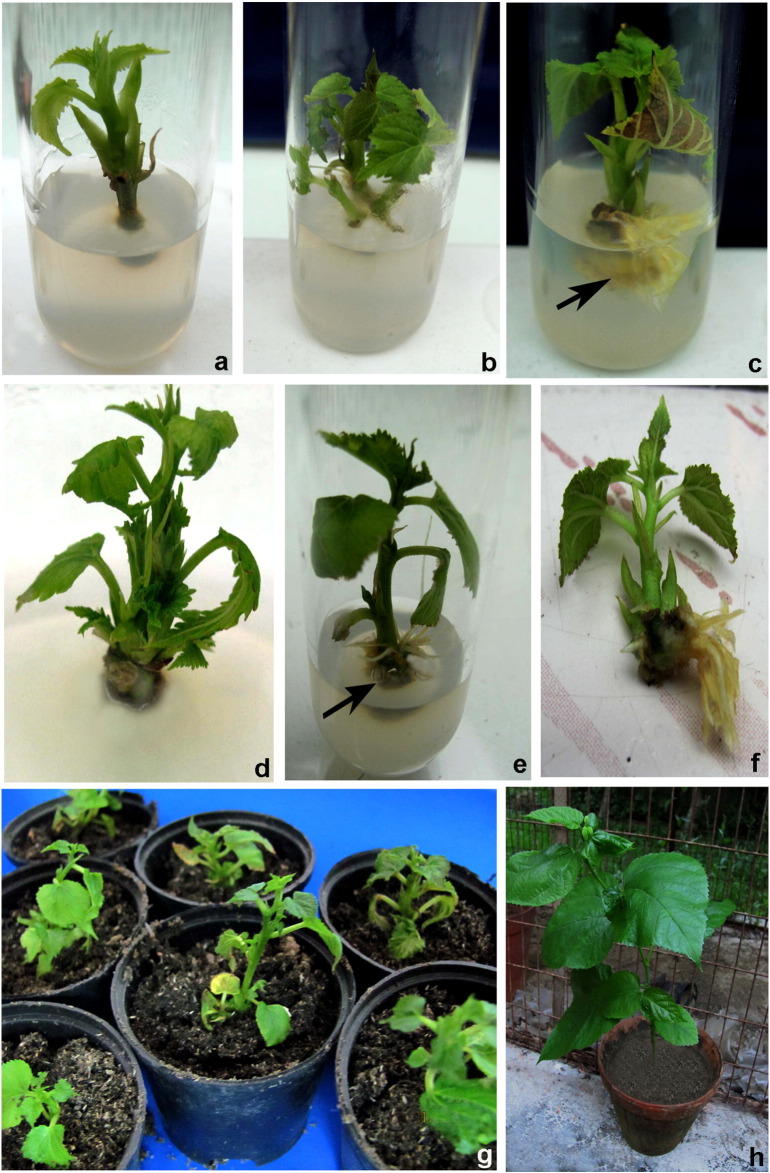
Different kinds of cytokinins (BA/KN/2-iP) were tested for their morphogenetic potential in the regeneration from nodal explant of 1 year old M. alba L. variety S-1 are shown in Table 1. Culture of nodal explants on MS medium without any cytokinin failed to produce shoots even after 4 weeks of inoculation, but the explants cultured on MS basal medium supplemented with cytokinins at different concentrations showed variation in the regeneration percentage and number of shoots formed. Nodal explants cultured on 1/2 strength MS basal medium showed no visible signs of tissue differentiation (not shown with table). Working with other plants similar results were obtained earlier and have been explained as due to greater demand of nitrogen and potassium for tissue differentiation (Guru et al., 1999) that is lacking in 1/2 MS medium. Auxillary buds started to break after 10–12 days of inoculation (Fig. 1a). Data on different growth parameters from different treatments were recorded after 4 weeks of culture initiation following one transfer to the new medium. Among the three cytokinins tested, the best response (92.10%) was obtained in the presence of 0.5 mg/l BA (Fig. 1b) and was found to be significantly higher than shoots induced per nodal explant in other concentrations of cytokinins (KN/2-iP). The concentration and type of cytokinins used significantly affected the percentage of regenerated shoots, shoot number, and shoot length. It was found that nodes cultured on MS medium with different concentrations of KN and 2-iP showed lower induction of axillary shoot bud proliferation. The maximum number of multiple shoots was obtained (5.62 ± 0.01) in the medium containing 0.5 mg/l of BA. The shoots developed in this medium also attained maximum height of 4.19 ± 0.01 cm after 4 weeks. In the BA concentrations higher than 0.5 mg/l, the number of shoots and percent response was reduced (Table 1). Reduction in the number of shoots generated from each node at BA concentration higher than the optimal level has been reported earlier for several plant species (Pattnaik and Chand, 1997, Anis et al., 2010) where higher concentration of BA resulted in complete suppression of bud break. Although this condition may not to be valid for all tree or woody species, at least it can be suggested that high level of BA is not appropriate for culture of a number of tropical species such as Pterocarpus marsupium (Chand and Singh, 2004), Holarrhena antidysenterica (Kumar et al., 2005). Of the three cytokinins (BA, KN and 2-iP) tested, BA has been found to be most effective in inducing multiple shoot formation. The stimulating effect of BA on multiple shoot formation has been reported earlier for several plants including Morus species (Pattnaik and Chand, 1997, Bhau and Wakhlu, 2003, Balakrishnan et al., 2009, Chattopadhyay et al., 2011). Zhang et al. (2010) while studying the metabolism of BA-treated mature pine plant suggested that BA causes reinvigoration of mature/old tissues and causes bud induction, a prerequisite for cloning of mature trees. George (1993) reported that BA can overcome optical dominance which causes breakdown of dormancy of lateral buds and promote shoot formation.
Table 1.
Effect of different concentrations of cytokinins namely N6-benzyladenine/Kinetin/2-isoPentenyladenine on shoot regeneration from nodal explants of Morus alba L. variety S-1 in Murashige and Skoog medium after 4 weeks of culture.
| Plant growth regulators (mg/l) | Response of explants (%) | Average no. of shoots per explant⁎ | Average shoot length⁎ (cm) |
|---|---|---|---|
| MS basal medium | 0.00 ± 0.0 | 0.00 ± 0.00l | 0.00 ± 0.00k |
| MS + BA | |||
| 0.3 | 75.4 ± 1.3 | 3.43 ± 0.03c | 3.34 ± 0.03cd |
| 0.5 | 92.1 ± 0.5 | 5.62 ± 0.01a | 4.19 ± 0.01a |
| 1.0 | 83.2 ± 1.1 | 4.16 ± 0.03b | 3.73 ± 0.03b |
| 1.5 | 71.3 ± 1.4 | 3.11 ± 0.03d | 2.31 ± 0.04de |
| 2.0 | 70.5 ± 2.1 | 2.12 ± 0.05f | 2.23 ± 0.01fg |
| MS + Kn | |||
| 0.3 | 56.8 ± 0.5 | 1.21 ± 0.02j | 1.86 ± 0.01i |
| 0.5 | 67.5 ± 1.1 | 2.11 ± 0.03f | 2.12 ± 0.02h |
| 1.0 | 75.2 ± 2.3 | 2.81 ± 0.01e | 2.26 ± 0.01ef |
| 1.5 | 79.2 ± 2.1 | 3.09 ± 0.05d | 3.52 ± 0.01c |
| 2.0 | 72.3 ± 1.3 | 1.90 ± 0.03h | 2.21 ± 0.03fg |
| MS + 2-iP | |||
| 0.3 | 54.3 ± 2.3 | 1.03 ± 0.03k | 1.77 ± 0.01j |
| 0.5 | 61.4 ± 2.1 | 1.19 ± 0.04j | 1.87 ± 0.02i |
| 1.0 | 73.3 ± 1.4 | 2.05 ± 0.02g | 2.24 ± 0.01fg |
| 1.5 | 74.1 ± 3.8 | 1.94 ± 0.11gh | 3.36 ± 0.05cd |
| 2.0 | 71.5 ± 2.4 | 1.42 ± 0.03i | 2.20 ± 0.03g |
N6-benzyladenine (BA), Kinetin (KN), 2-isoPentenyladenine (2-iP); Do, same as above.
Each value represents the mean ± SE; each experiment consisted of 12–15 replicates and repeated three times. Each mean value followed by the same letter does not differ significantly according to Duncan's Multiple Range Test (p ≤ 0.05).
Fig. 1.
In vitro clonal propagation of Morus alba L. a. Shoot proliferation from nodal explant on MS medium supplemented with 0.5 mg/1 BA after 10–12 days of culture. b. Shoot multiplication on MS medium supplemented with 0.5 mg/l BA after 4 weeks of culture. c. Higher concentration of NAA induced callus at the base. d. High rate of shoot multiplication on MS medium supplemented with 0.5 mg/1 BA. e. Formation of roots from regenerated shoots cultured on 1/2 MS medium supplemented with 1.0 mg/1 IBA. f. Well developed root system and complete plantlet. g. 1-month old primary hardened plants in plastic pot. h. 3-month old secondary hardened plants in earthen pots.
3.2. Effect of cytokinin and auxin on shoot regeneration
Auxin and cytokinin play fairly important roles in many aspects of plant growth and development. The interaction between auxin and cytokinin is particularly important to control a few developmental processes, such as the formation and maintenance of meristems that are essential to establish the whole plant body. Therefore, in the present study interaction of the optimal concentration of BA (0.5 mg/l) and concentrations (0.3–2.0 mg/l) of auxins (NAA or IBA) was evaluated (Table 2). BA in combination with NAA markedly enhanced the percent regeneration, number of shoots and shoot length whereas IBA did not improve the parameters evaluated. Nodal explants cultured on MS medium supplemented with 0.5 mg/l BA and 0.3 mg/l NAA exhibited 84.6 ± 2.91% shoot regeneration. This combination gave the highest number 4.86 ± 0.03 of shoots per explants along with the maximum length 3.87 ± 0.01 cm of shoot. Upon increasing the concentration of NAA up to 2.0 mg/l, a gradual decrease in regeneration frequency, number of shoots per explants and shoot length. Increase in concentration of NAA also caused induction of callus at the base and consequently the explant failed to produce further shoot regeneration (Fig. 1c). Among the various concentration of IBA with optimal concentration of BA used, the highest shoot regeneration frequency 77.6% and number of shoots per explants 4.18 ± 0.01 along with the maximum shoot length 3.36 ± 0.01 cm were recorded on MS medium after 4 weeks of inoculation (Table 2). The results showed that, in M. alba L. variety S-1 combination of hormones did not produce synchronized effect towards large scale multiple shoot formation. These differential responses, exhibited by different type and concentrations of the hormones used indicate possible existence of genotype specific optimum responses within large number of taxa (Tewary et al., 1996). It is now established that, in cultured tissues, the requirement for exogenous hormone depends on the endogenous level in plant tissue which varies with organ, plant genotype, and the phases of growth (Suresh and Ajay, 2004). Our results are suggestive of the need of independent standardization and examination of micropropagation protocols for different plant species.
Table 2.
Effect of different concentrations of auxins namely α-naphthalene acetic acid/Indole-3-butyric acid in combination with optimal concentration of N6-benzyladenine on shoot regeneration form nodal explants of Morus alba L. variety S-1 in Murashige and Skoog medium after 4 weeks of culture.
| Growth regulators (mg/l) | Shoot proliferation (%) | Average no. of shoots per explant⁎a | Average shoot length⁎ (cm) |
|---|---|---|---|
| MS + BA + NAA | |||
| 0.5 + 0.3 0.5 + 0.5 0.5 + 1.0 0.5 + 1.5 0.5 + 2.0 |
84.6 ± 2.91 | 4.86 ± 0.03a | 3.87 ± 0.01a |
| 76.7 ± 3.10 | 4.16 ± 0.02b | 3.55 ± 0.02b | |
| 68.7 ± 1.12 | 3.67 ± 0.04c | 3.35 ± 0.01c | |
| 65.3 ± 2.89 | 2.20 ± 0.04d | 2.95 ± 0.03d | |
| 61.2 ± 2.23 | 1.67 ± 0.01e | 2.97 ± 0.01d | |
| MS + BA + IBA | |||
| 0.5 + 0.3 0.5 + 0.5 0.5 + 1.0 0.5 + 1.5 0.5 + 2.0 |
77.6 ± 3.89 | 4.18 ± 0.01b | 3.36 ± 0.01c |
| 72.6 ± 1.66 | 3.65 ± 0.02c | 3.32 ± 0.01c | |
| 62.2 ± 2.22 | 2.18 ± 0.04d | 2.96 ± 0.02d | |
| 59.4 ± 1.54 | 1.65 ± 0.02e | 2.86 ± 0.02e | |
| 57.1 ± 3.09 | 1.27 ± 0.02f | 1.85 ± 0.03f | |
N6-benzyladenine (BA), α-naphthalene acetic acid (NAA), Indole-3-butyric acid (IBA), Do, same as above.
Each value represents the mean ± SE; each experiment consisted of 12–15 replicates and repeated three times. Each mean value followed by the same letter does not differ significantly according to Duncan's Multiple Range Test (p ≤ 0.05).
3.3. Effect of subculturing on shoot regeneration
To be successful in large scale commercial multiplication, cost effective technology is required. Subculturing significantly increased multiple shoot induction and direct organogenesis. Effect of subculture passages on shoot multiplication rate of the test plant by transferring the regenerated shoots from fresh shoot induction medium (MS supplemented with 0.5 mg/l BA) after every 4 week interval (Table 3) was evaluated. The shoot generation ability was investigated up to the sixth subculture passages. The highest number of shoots (32.27 ± 0.01) with shoot length (6.38 ± 0.01 cm) was observed during the fourth passages (Fig. 1d) which become stabilized in the fifth passage and reduced thereafter (Table 3). The increase in shoot number may be due to the suppression of apical dominance during subculture that induced basal dominant meristematic cells to form new shoots (Tripathi and Kumari, 2010). Another possible reason of increase shoot number may be due to horizontal position of nodal explants. This approach has also been reported in the micropropagation of a number of woody trees (Tripathi and Kumari, 2010, Phulwaria et al., 2011). The effects of subculture on the multiplication rate are known to differ from one species to another. In Terminalia bellirica, increase in shoot induction and multiplication has been reported up to the fourth subculture passage beyond which a decline in multiplication rate was observed (Phulwaria and Rai, 2012) whereas in Aegle marmelos (L.) Corr. frequency of shoot proliferation and growth of shoot continued through five subculture passages without any sign of decline (Ajithkumar and Seeni, 1998). In many plant species, micropropagation requires two media i.e. a propagation medium and a shoot elongation medium, which makes the micropropagation procedures cumbersome and uneconomical (Bapat et al., 1987). In this work, shoot multiplication and subsequent elongation were achieved on the same medium.
Table 3.
Influence of repeated subculture of nodes in Murashige and Skoog medium supplemented with 0.5 mg/l N6-benzyladenine on shoot proliferation of Morus alba L. variety S-1.
| Subculture passages | No. of shoots/explant⁎ | Shoot length⁎ (cm) |
|---|---|---|
| First | 5.62 ± 0.01d | 4.19 ± 0.01c |
| Second | 11.39 ± 0.02c | 4.17 ± 0.02c |
| Third | 17.89 ± 0.02b | 5.16 ± 0.01b |
| Fourth | 32.27 ± 0.01a | 6.38 ± 0.01a |
| Fifth | 32.23 ± 0.02a | 6.34 ± 0.01a |
Medium: 0.8% agar-solidified full-strength MS medium + 3% sucrose + 0.5 mg/l BA. Data recorded after 4 weeks of each subculture.
Each value represents the mean ± SE of three repeated experiments. Each mean value followed by the same letter does not differ significantly according to Duncan's Multiple Range Test (p ≤ 0.05).
3.4. Effect of auxin on rooting of shoots
Successful rooting of microshoots is a prerequisite to facilitate their establishment in soil. Auxins stimulate the occurrence of adventitious roots in the majority of plant species. Therefore, in the present study effects of auxins (IBA and NAA) on rooting of regenerated shoots were examined (Table 4). Rooting occurred sporadically when shoots from fourth subculture passage were transferred to hormone free different strength MS basal media. Half strength MS medium was found to be superior as compared to full strength medium. Strength of MS medium appeared to be an important factor in influencing the rooting efficiency. Sometimes, the endogenous level of auxins present in the tissue is sufficient to induce roots in hormone free MS medium. However, at times no rooting occurred in hormone free full strength MS medium. Between two auxins tried, IBA had a more pronounced effect on in vitro rooting than NAA. Among all treatment tested, half-strength MS medium supplemented with 1.0 mg/l IBA was most effective for rooting of shoots (Fig. 1e; Table 4). In this medium a maximum frequency of root formation (89.33%), number of roots (6.61) per explant, and root length (2.67 cm) were obtained. A similar observation were also reported by Balakrishnan et al. (2009) and Sajeevan et al. (2011) in M. alba L. Root development was however; slow at higher concentration of NAA or IBA. Optimum rooting response using IBA has been reported in several tree species including Ziziphus spina-christi (Sudhersan and Hussain, 2003) and Ziziphus jujuba (Hossain et al., 2003). Although induction of root is usually affected by several factors like medium strength, concentration and type of auxins, auxin treatment duration, and supplementation of additive (Pan and Staden, 1998), Taiz and Zeiger (2002) opined that roots require a smaller concentration of auxin to grow but root growth is strongly inhibited at higher concentration. The exact mechanism of auxin induced inhibition of root growth warrants investigation.
Table 4.
Effect of MS strength and different auxin namely Indole-3-butyric acid/Indole-3-acetic acid/α-naphthalene acetic acid concentrations on root induction from in vitro raised shoots of Morus alba L. variety S-1 after 4 weeks of culture.
| Media | Plant growth regulators (mg/l) |
||||
|---|---|---|---|---|---|
| IBA | NAA | % rooting | Average no. of roots per shoot⁎ | Average root length⁎ (cm) | |
| MS | − | − | 00.00 ± 0.00 | 0.00 ± 0.00j | 0.00 ± 0.00g |
| 1/2 MS | − | − | 43.88 ± 3.09 | 2.15 ± 0.02h | 1.28 ± 0.03f |
| 1/3 MS | – | − | 40.03 ± 2.13 | 1.81 ± 0.03i | 1.25 ± 0.05f |
| 1/2 MS | 0.5 | − | 79.44 ± 2.41 | 4.36 ± 0.07d | 2.09 ± 0.01c |
| 1/2 MS | 1.0 | − | 89.33 ± 2.52 | 6.61 ± 0.02a | 2.67 ± 0.01a |
| 1/2 MS | 1.5 | − | 72.33 ± 1.66 | 4.16 ± 0.09c | 2.14 ± 0.02bc |
| 1/2 MS | 2.0 | − | 68.77 ± 1.11 | 4.08 ± 0.04e | 1.79 ± 0.02d |
| 1/2 MS | − | 0.5 | 72.66 ± 2.54 | 4.18 ± 0.02de | 1.84 ± 0.02d |
| 1/2 MS | − | 1.0 | 74.42 ± 2.40 | 5.47 ± 0.03b | 2.17 ± 0.01b |
| 1/2 MS | − | 1.5 | 52.77 ± 2.73 | 2.48 ± 0.04f | 1.54 ± 0.02e |
| 1/2 MS | − | 2.0 | 47.66 ± 2.33 | 2.33 ± 0.04fg | 1.30 ± 0.01f |
Indole-3-butyric acid (IBA), α-naphthalene acetic acid (NAA), Do, same as above.
Each value represents the mean ± SE; each experiment consisted of 12–15 replicates and repeated three times. Each mean value followed by the same letter does not differ significantly according to Duncan's Multiple Range Test (p ≤ 0.05).
3.5. Acclimatization and field establishment of regenerated shoots with roots
The successful acclimatization of micropropagated plants and their subsequent transfer to the field is a crucial step of commercial exploitation of in vitro technology. In this study complete plantlet regeneration was achieved within 18 weeks of culture (Fig. 1f). Rooted plantlets were hardened in pots containing different sterilized potting mixtures (Table 5) in plastic cups for primary hardening in growth chamber condition (Fig. 1g). Mean survival percentages of the potted plants after one month of primary hardening were significantly higher in sand:soil:organic manure (2:2:1) mixture showing 89.16% survival (p < 0.05). After 30 days, randomly selected successfully hardened plants (75 plants) from each potting mixture combination were transferred to earthen pots containing soil:sand:cattle manure (2:2:1) under greenhouse conditions for secondary hardening. About 85.30% of the plants subjected to secondary hardening survived after 60 days and could be grown in pots kept in a greenhouse or in open shady conditions (Fig. 1h).
Table 5.
Acclimatization of regenerated plantlets of Morus alba L. variety S-1.
| Potting mixtures | Primary hardening |
Secondary hardening |
|||
|---|---|---|---|---|---|
| Total number of plants transferreda | Survival % after 30 daysc | Number of plants transferredb | Survival % after 30 days | Survival % after 60 daysd | |
| Sand:soil:organic manure (1:1:1) | 120 | 77.50 ± 1.44 (93)bc | 75 | 81.33 ± 2.66bc | 72.01 ± 2.26bc |
| Sand:soil:organic manure (2:1:1) | 120 | 80.83 ± 1.66(97)b | 75 | 86.66 ± 1.33ab | 76.91 ± 0.36b |
| Sand:soil:organic manure (2:2:1) | 120 | 89.16 ± 0.83(107)a | 75 | 90.66 ± 1.33a | 85.30 ± 1.36a |
| Garden soil:organic manure (1:1) | 120 | 73.33 ± 3.63(88)c | 75 | 77.33 ± 1.33c | 70.70 ± 1.55c |
Each experiment (different potting mixtures) was repeated thrice with 40 replicates each.
Seventy-five samples were randomly selected from each treatment for secondary hardening. Three repeated experiments were performed using 25 replicates each.
Means followed by the same letter are not significantly different at 5% level after DMRT test (p < 0.05). Values in parenthesis represent total number of plants survived after primary hardening.
Values (mean ± S.E.) represent mean survival percentage 60 days after secondary hardening. Means followed by same letter are not significantly different at 5% level after DMRT test (p < 0.05). Values in parenthesis represent total number of plants which survived after 60 days of secondary hardening.
3.6. Molecular analysis
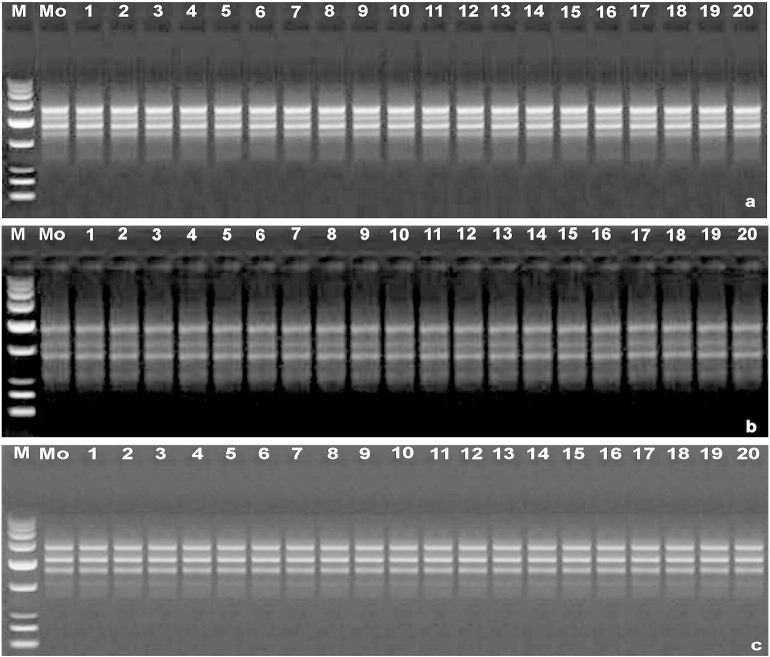
Utility of molecular analysis of in vitro regenerated plants has been well documented by many workers (Bhatia et al., 2011, Saha et al., 2010, Saha et al., 2012, Saha et al., 2014a, Saha et al., 2014b). Genetic fidelity of micropropagated plants has immense practical utility and commercial implications. In this background, we assessed fingerprinting profiles of the culture regenerants and of the donor plants done by RAPD and ISSR markers to confirm whether the plantlets were genetically stable or not. A total of 23 random RAPD primers were tested for initial screening, among them only 11 primers gave clear and reproducible loci. The number of scorable loci for each RAPD primer varied from 2 (OPS-02, OPS-04, OPS-07 and OPS-11) to 4 (OPS-01 and OPS-09) (Table 6). The 11 RAPD primers produced 31 distinct and scorable loci, with an average of 2.81 loci per primer. Each primer generated a unique set of amplification products ranging in size from 400 bp (OPS-03 and OPS-06) to 1500 bp (OPS-03, OPS-05, OPS-06, OPS-08 and OPS-10). The maximum number of 15 loci was present within the size range of 500 to 1000 bp, followed by 4 loci of less than 500 bp in size and 12 loci between 1000 to 1500 bp. A total of 651 bands (number of samples analyzed × number of scorable loci with all 11 primers) were generated during RAPD analysis and all bands were found to be monomorphic. Primers OPS-01 and OPS-09 amplified the highest number of bands (84), while only 42 bands were obtained by primers OPS-02, OPS-04, OPS-07 and OPS-11. No polymorphism was detected during the RAPD analysis of in vitro raised plants with the mother plant (Fig. 2a–c). It was proven that the uniformity of the regenerated plants was maintained, indicating high genetic stability among the clones. Our results corroborate the reports of genetic stability of cryopreserved dormant buds of Morus germplasm (Choudhary et al., 2013). For instance, the absence of somaclonal variation in plant material micropropagated by nodal segments was reported for the tree species such as Pithecellobium dulce (Roxb.) Benth (Goyal et al., 2012) and woody species like Guadua angustifolia Kunth (Nadha et al., 2011).
Fig. 2.
Polymerase chain reaction (PCR) amplification products obtained with a random amplified polymorphic DNA (RAPD). a. Primer (OPS-01). b. Primer (OPS-05). c. Primer (OPS-08). Lane M—molecular marker (100 bp–3 kbp), Lane Mo—represents mother plant and lanes 1–20 represent in vitro-raised hardened plants.
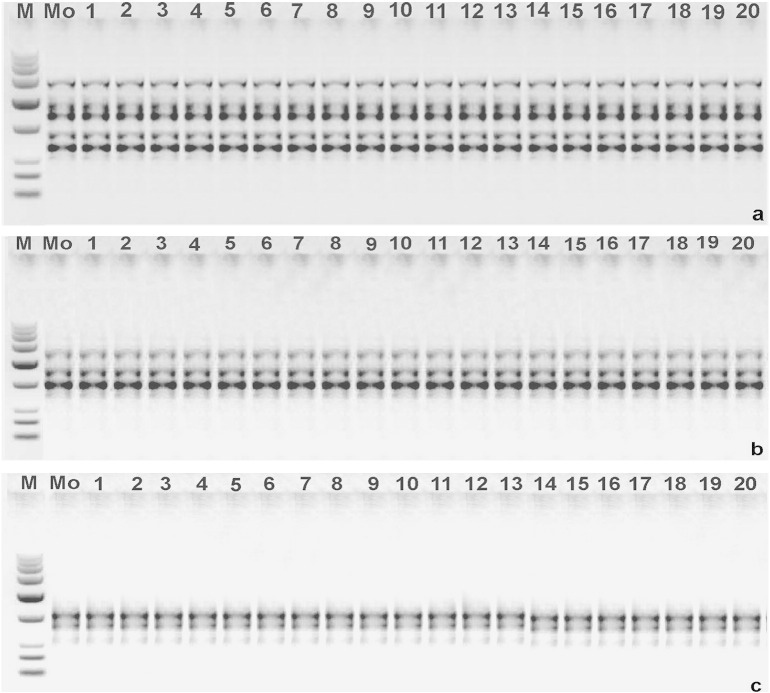
Out of 13 ISSR primers used in the initial screening, only 7 primers produced clear and reproducible loci. The optimum annealing temperature for ISSR markers varied from 45.0 to 55.00 °C (Table 7). The 7 ISSR primers produced 22 distinct and scorable loci in the size range of 300 bp (ISSR-01) to 2000 bp (ISSR-04). The number of scorable loci for each primer varied from 2 (ISSR-06 and ISSR-07) to 5 (ISSR-03), with an average of 3.14 loci per primer. A maximum number of 19 loci were confined within the ladder size of 500 to1500 bp, which was followed by 2 loci of less than 500 bp in size and 1 locus between 1500 and 2000 bp. A total of 462 bands (number of samples analyzed × number of scorable loci with all 7 primers) were generated during ISSR analysis and all bands were found to be monomorphic. Primer ISSR-03, amplified the highest number of bands (105), while only 42 bands were obtained by primers ISSR-06 and ISSR-07. Furthermore, no difference was observed in banding patterns of any of the sample population for a particular primer with their respective mother plants, indicating absence of variation among the in vitro raised plants (Fig.3a–c). Similar results have been reported by Choudhary et al. (2013) in Morus spp., Senapati et al. (2013) in Celastrus paniculatus.
Fig. 3.
Polymerase chain reaction (PCR) amplification products obtained with an inter simple sequence repeat (ISSR) a. Primer (ISSR-03). b. Primer (ISSR-05). c. Primer (ISSR-07). Lane M—molecular marker (100 bp–3 kbp), Lane Mo—represents mother plant and lanes 1–20 represent in vitro-raised hardened plants.
The overall banding profile obtained from the total of 1113 bands (number of plants analyzed × total number of scorable loci from RAPD and ISSR primers) was generated from the mother plant and 20 in vitro raised clones. All banding profiles from micropropagated plants were monomorphic and similar to those of the mother plants. The possible reason may be multiple shoot bud differentiation without intervening callus phase is least vulnerable to genetic changes. A similarity matrix based on Jaccard's coefficient revealed that the pair-wise value between the mother plant and the plantlets derived from different explants was 1, indicating 100% similarity. A UPGMA (Unweighted Pair Group Method with Arithmetic Mean) dendrogram was generated from the Jaccard's similarity values using NTsys-pc software. A phenetic dendrogram based on UPGMA analysis further confirmed the true-to-type nature of clones. Similar results were obtained by Senapati et al. (2013) during the clonal fidelity analysis of in vitro propagated C. paniculatus by RAPD and ISSR markers. In this study, two PCR-based techniques, i.e., RAPD and ISSR were chosen because of their simplicity and cost-effectiveness. The clonal fidelity assessment by the use of two types of DNA markers by earlier workers showed similar results with respect to long-term micropropagated shoot cultures of almond (Martins et al., 2004) and Gerbera jamesonii (Bhatia et al., 2011). It has been assumed that the use of two markers, which amplify different regions of the genome, allows for better chances for the identification of genetic variations in the clones. The ISSRs are widely distributed throughout the genomic DNA and make amplification of genomic DNA possible in much larger numbers of fragments per primer. Besides, the advantage of higher reproducibility and relatively low cost, this higher amplification efficiency of ISSRs makes the genetic inferences much clearer with some clues for genetic variation at inter- and even intra-specific levels (Powell and Machray, 1996).
The presence or absence of variations during in vitro propagation depends upon the source of explants and the method of regeneration (Goto et al., 1998). The sub and supra-optimal levels of plant growth substance, especially synthetic ones, have also been associated with somaclonal variation (Martins et al., 2004). Even at optimal levels, long term multiplication may often lead to somaclonal or epigenetic variations in micropropagated plants, thus, questioning the very fidelity of their clonal nature. In our study, plantlets were obtained from the sprouting of dormant buds situated in the axils of the bracts. These findings support the fact that a meristem-based micropropagation system is much more stable genetically than those in which regeneration occurs via the callus phase (Bhatia et al., 2011). Plants regenerated from adventitious buds around axillary buds or from other well developed meristematic tissue showed the lowest tendency for genetic variation (Joshi and Dhawan, 2007). Even plants derived from organized meristems are not always genetically true to the type in many crops (Devarumath et al., 2002). Hence, it becomes imperative to regularly check the genetic purity of the micropropagated plants in order to produce clonally uniform progeny while using different techniques of micropropagation. Our results further suggest that the molecular marker approach is a useful tool in the evaluation of the genetic stability of in vitro propagated plants. But a detailed study of the other markers and relative suitability of each one of them is warranted.
4. Conclusion
To the best of our knowledge, this is the first report of genetically sustainable micropropagation protocol of M. alba L. variety S-1 from nodal explants. Our experimentation establishes that multiple shoot bud induction and regeneration in M. alba L. variety S-1 is regulated by appropriate cytokinin and auxin concentration and combination of hormones does not influence towards large scale multiple shoot formation. The technique of ex vitro rooting coupled with simultaneous hardening shortened the micropropagation protocol, made the protocol more economical, and increased the survival rate in the field, which can be explored for continuous supply of plant material for conservation. Owing to the importance of genetic stability, our protocol appears to be highly stable and supports our claim that micropropagated plantlets are true to type to the donor plant.
Acknowledgments
This research is supported by a Grant from the University Grants Commission, New Delhi, Govt. of India. The authors are grateful to Dr. Prasenjit Paul, Agricultural Statistics Department, IARI, New Delhi for his kind co-operation in statistical analysis. The first author (S. Saha) and fourth author (P.D. Ghosh) wishes to acknowledge the support of the University Grants Commission, New Delhi for the award of Post Doctoral Fellowship and Emeritus fellowship respectively. Authors also gratefully acknowledge the DST-PURSE and Department of Botany, University of Kalyani for Central Instrumental facilities.
References
- Ajithkumar D., Seeni S. Rapid clonal multiplication through in vitro axillary shoot proliferation of Aegle marmelos (L.) Corr., a medicinal tree. Plant Cell Rep. 1998;17:422–426. doi: 10.1007/s002990050418. [DOI] [PubMed] [Google Scholar]
- Anis M., Varshney A., Siddique I. In vitro clonal propagation of Balanites aegyptiaca (L.) Del. Agrofor. Syst. 2010;78:151–158. [Google Scholar]
- Balakrishnan V., Latha M.R., Ravindran K.C., Robinson J.P. Clonal propagation of Morus alba L. through nodal and axillary bud explants. Bot. Res. Int. 2009;2:42–49. [Google Scholar]
- Bapat V.A., Mhatre M., Rao P.S. Propagation of Morus indica L. (mulberry) by encapsulated shoot buds. Plant Cell Rep. 1987;6:393–395. doi: 10.1007/BF00269570. [DOI] [PubMed] [Google Scholar]
- Bhatia R., Singh K.P., Sharma T.R., Jhang T. Evaluation of the genetic fidelity of in vitro propagated gerbera (Gerbera jamesonii Bolus) using DNA based markers. Plant Cell Tissue Organ Cult. 2011;104:131–135. [Google Scholar]
- Bhau B.S., Wakhlu A.K. Effect of genotype, explant type and growth regulators on organogenesis in Morus alba. Plant Cell Tissue Organ Cult. 2001;66:25–29. [Google Scholar]
- Bhau B.S., Wakhlu A.K. Rapid micropropagation of five cultivars of mulberry. Biol. Plant. 2003;46:349–355. [Google Scholar]
- Bhojwani S.S. Brainstorming Meeting on Genetics and Biotechnology of Silk Worm and Mulberry, 1–11. India; CSRTI, Mysore: 1992. Plant tissue culture and its relevance to mulberry breeding. [Google Scholar]
- Butkhup L., Samappito W., Samappito S. Phenolic composition and antioxidant activity of white mulberry (Morus alba L.) fruits. Int. J. Food Sci. Tech. 2013;48:934–940. [Google Scholar]
- Chand S., Singh A.K. In vitro shoot regeneration from cotyledon node explants of a multipurpose leguminous tree Pterocarpus marsupium Roxb. In Vitro Cell Dev. Biol. Plant. 2004;40:464–466. [Google Scholar]
- Chattopadhyay S., Doss S.G., Halder S., Ali A.K., Bajpai A.K. Comparative micropropagation efficiency of diploid and triploid mulberry (Morus alba cv. S1) from axillary bud explants. Afr. J. Biotechnol. 2011;10:18153–18159. [Google Scholar]
- Choudhary R., Chaudhury R., Malik S.K., Kumar S., Pal D. Genetic stability of mulberry germplasm after cryopreservation by two-step freezing technique. Afr. J. Biotechnol. 2013;12:5983–5993. [Google Scholar]
- Devarumath R.M., Nandy S., Rani V., Marimuthu S., Muraleedharan N. RAPD, ISSR and RFLP finger printing as useful markers to evaluate genetic integrity of micropropagated plants of three diploid and triploid elite tea clones representing Camellia sinensis (China type) and C. assamica ssp. assamica (Assam-India type) Plant Cell Rep. 2002;21:166–173. [Google Scholar]
- Duncan D.B. Multiple range and multiple F test. Biometrics. 1955;11:1–42. [Google Scholar]
- George E.F. The Technology Exegetics Ltd, Edington. 1993. Plant propagation by tissue culture. Part-I. [Google Scholar]
- Goto S., Thakur R.C., Ishii K. Determination of genetic stability in long-term micropropagated shoots of Pinus thunbergii Parl. using RAPD markers. Plant Cell Rep. 1998;18:193–197. doi: 10.1007/s002990050555. [DOI] [PubMed] [Google Scholar]
- Goyal P., Kachhwaha S., Kothari S.L. Micropropagation of Pithecellobium dulce (Roxb.) Benth—a multipurpose leguminous tree and assessment of genetic fidelity of micropropagated plants using molecular markers. Physiol. Mol. Biol. Plants. 2012;18:169–176. doi: 10.1007/s12298-012-0112-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guru S.K., Chandra R., Khetrapal S., Raj A., Palisetty R. Protein pattern in differentiating explants of chickpea (Cicer arietinum L. Ind. J. Plant Physiol. 1999;4:147–151. [Google Scholar]
- Hossain S.N., Munshi M.K., Islam M.R., Hakim L., Hossain M. In vitro propagation of plum (Zyziphus jujube Lam.) Plant Tissue Cult. 2003;13:81–84. [Google Scholar]
- Joshi P., Dhawan V. Assessment of genetic fidelity of micropropagated Swertia chirayita plantlets by ISSR marker assay. Biol. Plant. 2007;51:22–26. [Google Scholar]
- Kavyashree R. A repeatable protocol for in vitro micropropagation of mulberry variety S54. Indian J. Biotechnol. 2007;6:385–388. [Google Scholar]
- Kumar R., Sharma K., Agrawal V. In vitro clonal propagation of Holarrhena antidysenterica (L.) Wall. through nodal explants from mature trees. In Vitro Cell Dev. Biol. Plant. 2005;41:137–144. [Google Scholar]
- Larkin P.J., Scowcroft W.R. Somaclonal variation—a novel source of variability from cell cultures for plant improvement. Theor. Appl. Genet. 1981;60:197–214. doi: 10.1007/BF02342540. [DOI] [PubMed] [Google Scholar]
- Martins M., Sarmento D., Oliveira M.M. Genetic stability of micropropagated almond plantlets, as assessed by RAPD and ISSR markers. Plant Cell Rep. 2004;23:492–496. doi: 10.1007/s00299-004-0870-3. [DOI] [PubMed] [Google Scholar]
- Murashige T., Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962;15:473–497. [Google Scholar]
- Murray M.G., Thompson E.F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980;8:4321–4325. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadha H.K., Kumar R., Sharma R.K., Anand M., Sood A. Evaluation of clonal fidelity of in vitro raised plants of Guadua angustifolia Kunth using DNA-based markers. J. Med. Plants Res. 2011;5:5636–5641. [Google Scholar]
- Pan M.J., Staden J.V. The use of charcoal in in vitro culture—a review. Plant Growth Regul. 1998;26:155–163. [Google Scholar]
- Pattnaik S.K., Chand P.K. Rapid clonal propagation of three mulberries, Morus cathayana Hemsl., M.Ihou Koiz. and M. serrata Roxb., through in vitro culture of apical shoot buds and nodal explants from mature trees. Plant Cell Rep. 1997;16:503–508. doi: 10.1007/BF01092774. [DOI] [PubMed] [Google Scholar]
- Phulwaria M., Ram K., Gahlot P., Shekhawat N.S. Micropropagation of Salvadora persica — a tree of arid horticulture and forestry. New For. 2011;42:317–327. [Google Scholar]
- Phulwaria M., Rai M.K., Harish, Gupta A.K., Ram K., Shekhawat N.S. An improved micropropagation of Terminalia bellirica from nodal explants of mature tree. Acta Physiol. Plant. 2012;34:299–305. [Google Scholar]
- Powell W., Machray G.C., Provan J. Polymorphism revealed by simple sequence repeats. Trends Plant Sci. 1996;1:215–222. [Google Scholar]
- Saha S., Dey T., Ghosh P.D. Micropropagation of Ocimum kilimandscharicum Guerke (Labiatae) Acta Biol. Cracov. Ser. Bot. 2010;52:50–58. [Google Scholar]
- Saha S., Kader A., Sengupta C., Ghosh P.D. In vitro propagation of Ocimum gratissimum L. (Lamiaceae) and its evaluation of genetic fidelity using RAPD markers. Am. J. Plant Sci. 2012;3:64–74. [Google Scholar]
- Saha S., Roy S., Sengupta C., Ghosh P.D. Micropropagation and analysis of genetic stability in regenerated plantlets of Ocimum canum Sims. Indian J. Plant Physiol. 2014;19:174–183. [Google Scholar]
- Saha S., Sengupta C., Ghosh P.D. Encapsulation, short-term storage, conservation and molecular analysis to assess genetic stability in alginate-encapsulated microshoots of Ocimum kilimandscharicum Guerke. Plant Cell Tissue Organ Cult. 2014 [Google Scholar]
- Sajeevan R.S., Singh S.J., Nataraja K.N., Shivanna M.B. An efficient in vitro protocol for multiple shoot induction in mulberry, Morus alba L. variety V1. Int. Res. J. Plant Sci. 2011;2:254–261. [Google Scholar]
- Saker M.M., Bekheet S.A., Taha H.S., Fahmy A.S., Moursy H.A. Detection of somaclonal variations in tissue culture-derived date palm plants using isoenzyme analysis and RAPD fingerprints. Biol. Plant. 2000;43:347–351. [Google Scholar]
- Senapati S.K., Aparajita S., Rout G.R. Micropropagation and assessment of genetic stability in Celastrus paniculatus: an endangered medicinal plant. Biologia. 2013;68:627–632. [Google Scholar]
- Sudhersan C., Hussain J. In vitro clonal propagation of a multipurpose tree, Ziziphus spina-christi (L.) Derf. Turk. J. Bot. 2003;27:167–171. [Google Scholar]
- Suresh C., Ajay K.S. In vitro shoot regeneration from cotyledonary node explants of a multipurpose leguminous tree Pterocarpus marsupium Roxb. In Vitro Cell Dev. Biol. Plant. 2004;40:167–170. [Google Scholar]
- Taiz L., Zeiger E. 3rd ed. Publishers, Sunderland; Sinauer Associates Inc.: 2002. Plant Physiology. [Google Scholar]
- Tewary P.K., Raghunath M.K., Venkateshwarulu M., Sarkar A. Genotypic difference in response to in vitro shoot development of mulberry (Morus spp.). Indian J Sericult. 1996;35:104–106. [Google Scholar]
- Tripathi M., Kumari N. Micropropagation of a tropical fruit tree Spondias mangifera Willd. through direct organogenesis. Acta Physiol. Plant. 2010;32:1011–1015. [Google Scholar]
- Vijayan K., Jayarama Raju P., Tikader A., Saratchnadra B. Biotechnology of mulberry (Morus L.)—a review. Emir. J. Food Agric. 2014;26:472–496. [Google Scholar]
- Williams J.G.K., Kubelik A.R., Livak K.J., Rafalski J.A., Tingey S.V. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res. 1990;18:6531–6535. doi: 10.1093/nar/18.22.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Horgan K.J., Reynolds P.H., Jameson P.E. 6-Benzyladenine metabolism during reinvigoration of mature Pinus radiate buds in vitro. Tree Physiol. 2010;30:514–526. doi: 10.1093/treephys/tpp130. [DOI] [PubMed] [Google Scholar]
- Zietkiewicz E., Rafalski A., Labuda D. Genome fingerprinting by simple sequence repeat (SSR)-anchored polymerase chain reaction amplification. Genomics. 1994;20:176–183. doi: 10.1006/geno.1994.1151. [DOI] [PubMed] [Google Scholar]