Abstract
Premature infants suffer significant respiratory morbidity during infancy with long-term negative consequences on health, quality of life, and health care costs. Enhanced susceptibility to a variety of infections and inflammation play a large role in early and prolonged lung disease following premature birth, although the mechanisms of susceptibility and immune dysregulation are active areas of research. This article reviews aspects of host-pathogen interactions and immune responses that are altered by preterm birth and that impact chronic respiratory morbidity in these children.
Keywords: Prematurity, Neonatal immunology, Neonatal infection, Virus, Lymphocytes, Bronchopulmonary dysplasia, Chronic lung disease of prematurity, Preterm
Key points
-
•
In the first year of life, preterm infants are rehospitalized twofold to fivefold times more frequently than infants born at term, primarily for respiratory symptoms.
-
•
Mediators of inflammation tend to enhance lung maturation but impair alveolar septation and developmental vascular remodeling.
-
•
The developmental age of the immune system at birth, and at early-age infections, may significantly alter the acute response, and the sequelae, to inflammatory stimuli.
-
•
Prenatal and postnatal infection and immune responses contribute to the severity of chronic lung disease of prematurity.
Introduction
Each year, approximately 1 in 9 infants in the United States, more than 440,000 infants yearly, are born prematurely (<37 weeks gestation).1 These infants suffer from complications of exposure to a diverse environment at a time in development when the respiratory tract and immune system are intended to be protected and maintained in a relatively naïve intrauterine state. During infancy and early childhood, premature infants suffer significant inflammatory and infectious respiratory morbidities with extended negative consequences for health, quality of life, and health care costs. As compared with approximately 8% of full-term newborns, 17% of late-preterm (LPT, born at 34 0/7–36 6/7 weeks) and 30% to 40% of early preterm infants (EPT, born at <32 weeks) are rehospitalized within the first year of life, most commonly for viral respiratory infections.2, 3, 4 Respiratory infections that are less severe, not requiring hospitalization, are even more common, recurrent and, in total, costly in the very young.5 The incidence and severity of respiratory tract infections in infants younger than 1 year is attributed at least in part to immune immaturity, a problem magnified by preterm birth and influenced by genetic traits and environmental exposures. Differences in gastrointestinal tract colonization patterns and the development and balance of the intestinal microbiome have been shown to influence immunologic development in full-term infants, and have begun to be evaluated in the premature.6, 7, 8 Viral infections, either subclinical or severe, may also alter immunologic development both directly and by altering the bacterial microflora. Preterm infants are exposed to maternal and hospital-based flora, frequently with additional pressures of antibiotics, indwelling catheters, and tubes, that alter the establishment of diverse, health-promoting microbiota on the skin and respiratory mucosa, as well as in the gastrointestinal tract, and increase the risk of invasive disease with predominant organisms.
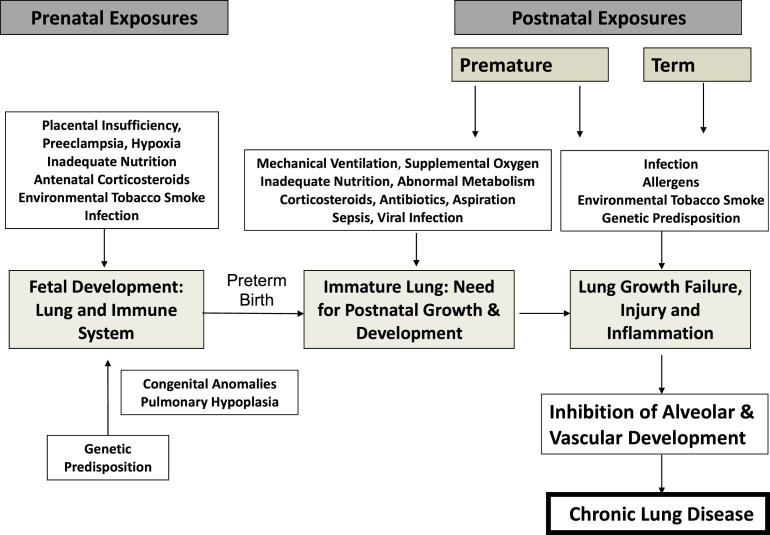
Recurrence of respiratory symptoms in the first year of life correlates inversely with gestational age at birth, directly with in utero exposure to inflammation (chorioamnionitis), and with non-white race. The pathogenesis of chronic lung disease of prematurity, bronchopulmonary dysplasia (BPD), has been recently reviewed and is closely correlated with in utero inflammation, oxygen toxicity, ventilator-induced trauma, and prealveolar lung development at birth (Fig. 1 ).9, 10, 11 Premature birth induces a slowing or arrest of lung development that underlies BPD and likely occurs in a spectrum of severity in all prematurely born infants. Perinatal therapeutic and environmental exposures, most notably oxygen exposure and environmental tobacco smoke, have been reproducibly related to chronic respiratory morbidity, independent of mechanical ventilation and the diagnosis of BPD. A recent study of very low birth weight (VLBW) infants without BPD demonstrated significant relationships between an integrated estimate of oxygen exposure in the first 3 to 14 days of life and symptomatic respiratory disease (SRD) over the first year of life.12 Lower gestational age, non-white race, greater oxygen exposure, and chorioamnionitis significantly increased the odds ratio of infants having SRD. A recent murine model demonstrated that early neonatal exposure to hyperoxia dramatically increases, in a dose-responsive manner, the severity of influenza infection when induced in adulthood, with markedly enhanced inflammation and fibrotic repair.9, 10 These observations, and an increasing understanding of the preterm infant immune system, as well as their exposures, colonization, and infections with microorganisms, suggest that interventions to modify the immunologic response may significantly improve respiratory and general outcome for these children. This article reviews prenatal and postnatal exposures that induce lung inflammation in preterm infants in the context of unique susceptibility factors that occur because of premature delivery.
Fig. 1.
Factors implicated in the pathogenesis of chronic lung disease of prematurity.
Inflammation as a mechanism for respiratory morbidity
Several lines of evidence suggest that the inflammatory response of the fetal or premature lung to injury or infection, if not causative of disease, exacerbates the severity of chronic lung disease in infants at risk.11, 13 Recent reviews highlight the current understanding of the role of inflammatory mediators and the immunobiology of BPD.14, 15, 16 Increased levels of proinflammatory mediators in amniotic fluid,17, 18 early tracheal effluents,19, 20, 21, 22, 23, 24 lung tissue,23 and serum25, 26 of at-risk premature infants support a role for both intrauterine and extrauterine inflammation in the development and severity of BPD. Airway and bronchoalveolar lavage samples demonstrate increased inflammatory cells and multiple proinflammatory mediators in ventilated, oxygen-exposed infants progressing toward BPD.19, 20, 21, 22, 23 Genome-wide expression profiling of BPD lungs, as compared with gestational age–matched controls, identified 159 differentially expressed genes.27 Pathway analysis identified cell cycle, immunodeficiency signaling, and B-cell development pathways associated with BPD. In addition, of the top 25 differentially expressed gene sets, 9 were related to chymase-expressing mast cells, the presence of which was confirmed by polymerase chain reaction (PCR) and immunohistochemistry. Consistent with active inflammation, the transcription factor, NF-κB, a prototypical regulator of inflammation and cell survival, was elevated in neutrophils and macrophages in preterm infant airways, correlating with the presence of Ureaplasma urealyticum and need for prolonged mechanical ventilation.28 Interestingly, NF-κB activation in fetal lung and fetal lung macrophages has been shown to inhibit airway morphogenesis and activity of fibroblast growth factor 10, a critical factor in lung development, linking inflammation to the growth arrest of the preterm lung.29, 30 Several animal models demonstrate that mediators of inflammation, including endotoxins, tumor necrosis factor α (TNF-α), and transforming growth factor α, enhance lung maturation but also impair alveolar septation and vascular remodeling, and thus contribute to the development of BPD even without frank tissue destruction.31, 32, 33
Proinflammatory stimuli come from multiple sources in the premature infant both prenatally and after birth. The most common causes are considered next.
Prenatal induction of inflammation and respiratory morbidity
Chorioamnionitis
Once thought to be sterile, modern molecular techniques independent of culture demonstrated that amniotic fluid and placental tissues frequently contain microbes.34, 35, 36, 37, 38, 39, 40 Maternal-fetal inflammation is clinically identified as chorioamnionitis by maternal fever with one or more of maternal/fetal tachycardia, maternal leukocytosis, uterine tenderness, and/or foul amniotic fluid. A recent study, using amniocentesis to sample amniotic fluid of 46 mothers with signs and symptoms of clinical chorioamnionitis, detected microorganisms by culture and/or PCR/mass spectrometry, frequently more than one microbe, in 61%.41 Fifteen percent had neither inflammation nor infection and 24% had amniotic fluid evidence of inflammation without detectable microorganisms, suggesting other noninfectious causes of clinical symptoms. Of those with clinical chorioamnionitis, 51% to 62% also have histologic evidence of placental inflammation.41, 42 Severity of acute histologic chorioamnionitis has been correlated with amniotic fluid matrix-metalloproteinase-8 and interleukin (IL)-6 levels supporting the presence of active inflammation.43, 44 It is not uncommon, however, to have evidence of acute histologic chorioamnionitis without detectable microorganisms, ranging from 30% to more than 50%. The cause of “sterile inflammation” of the fetal-placental tissues may be noninfectious disease or lack of sensitivity for microbial detection. Inflammatory placental lesions of a more chronic form, characterized by lymphocytes, plasma cells, and macrophages, sometimes eosinophils, also occur in association with preterm birth and recurrent placental failure. Most frequently, these lesions are of unknown etiology.45
Chorioamnionitis has been associated with chronic lung disease of prematurity in multiple small series46 and in focused studies of specific organisms, such as Ureaplasma.47 In experimental models, chorioamnionitis caused by intra-amniotic injections of endotoxin or Ureaplasma initially cause fetal lung inflammation followed by persistent low-grade inflammation and evidence of enhanced lung maturation.48, 49, 50 A more aggressive inflammatory response to oxygen or mechanical ventilation in newborns with a history of chorioamnionitis has been suggested in animal models51 and some clinical reports.52 The severity of the fetal inflammatory response to infection, as indicated by amniotic fluid IL-6, is inversely related to gestational age, suggesting that more premature infants are at greater risk of inflammatory injury.44
The most common organisms isolated from infected amniotic fluid and placentas are Ureaplasma parvum and U urealyticum. Likewise, it is relatively common to identify these organisms in the bodily fluids of preterm infants. Compelling evidence for an association between pulmonary Ureaplasma colonization and BPD in preterm infants has been recently reviewed.53, 54 Further details and discussion of clinical trials for treatment of Ureaplasma found in respiratory secretions of preterm infants are reviewed by Viscardi and Kallapur.55
However, the role of chorioamnionitis as a risk factor for BPD remains controversial and recently debated.56, 57 Several large studies question the relationship of in utero infection to chronic lung disease. As part of the Extremely Low Gestational Age Newborns (ELGAN) Study, exhaustive placental bacterial cultures were done from deliveries at 23 to 27 weeks of gestation.58 There was no correlation between placental culture results and the phenotypes of the infants assessed by oxygen need at day of life 14 or the development of BPD. The Canadian Neonatal Network also reported that 3094 infants born at less than 33 weeks’ gestation exposed to clinical chorioamnionitis had no increase in the incidence of BPD.59 Further, Lahra and colleagues60 reported, using a 13-year experience from Sydney, that a fetal inflammatory response was protective for BPD.
These and other similar studies demonstrate that clinical or culture-proven chorioamnionitis are not good predictors of BPD. Chorioamnionitis/infection has a major association with preterm premature rupture of membranes and preterm labor at early gestations.61, 62 Also, chorioamnionitis is associated with inflammation in lungs of preterm infants soon after birth63 and causes lung inflammation and altered immune modulation in animal models where the type and duration of fetal exposures can be controlled.64 Clinically, variation in detection and virulence of causative organisms, as well as in the duration of infection and the maternal-fetal inflammatory response, complicates the determination of effect on outcomes. The assessment of influence on preterm infant chronic lung disease is further confounded by the imprecise diagnosis of BPD.65
Other Prenatal Proinflammatory Exposures
As outlined in Fig. 1, there are a number of other maternal-fetal-placental abnormalities that alter lung growth and/or induce fetal inflammation. The association of maternal preeclampsia, placental insufficiency, and associated intrauterine growth restriction with BPD, however, remains controversial, with some studies suggesting increased and others decreased or no effect.66, 67, 68, 69, 70, 71, 72 Antenatal corticosteroids enhance fetal lung maturation and likely reduce inflammation but, although one study suggested that corticosteroids reduced BPD in those with histologic chorioamnionitis, overall they have had little effect on rates of BPD.73
Postnatal induction of inflammation and respiratory morbidity
Many exposures in the postnatal period promote inflammation.52
Oxygen and Mechanical Ventilation
Both oxygen and mechanical ventilation, together and independently, induce inflammation via direct cellular injury, induction of cytokines and chemokines, recruitment of neutrophils and macrophages, and oxidation of DNA, lipids, and proteins. Oxygen toxicity and barotrauma or volutrauma are important hazards of mechanical ventilation that are associated with the release of inflammatory cytokines and chemokines that cause pulmonary injury.74 Higher levels of cytokines correlate with more prolonged duration of ventilation.74 Supplemental oxygen also contributes to inflammation through biochemical pathways of oxidant stress.75, 76, 77
Bacterial Infection and Sepsis
Sepsis beyond the first days of life is frequent in extremely low birthweight (ELBW) infants at risk of BPD and often presents with respiratory instability.60 Both early and late microbial presence in neonatal lung fluid samples was significantly associated with the development of chronic lung disease, suggesting that both antenatal and postnatal infection play a role in the development of disease.24 Numerous studies associate postnatal sepsis, both early-onset and late-onset and typically with common infectious agents, such as coagulase-negative Staphylococcus and gram-negative bacteria, with BPD, suggesting that sepsis-induced inflammation compromises lung development and healing.52, 78, 79, 80, 81, 82 Administration of intravenous immunoglobulin, however, although associated with a small reduction in sepsis, was not shown by meta-analysis of randomized controlled trials to reduce the incidence of BPD.83
Viral Infections
Broad respiratory virus surveillance in the neonatal intensive care unit (NICU) is a relatively new approach augmented by more readily available culture-independent methods of detection. Previous NICU viral studies targeted patients with threshold symptoms. With this approach, small pandemics of viral infection, such as with adenovirus or respiratory syncytial virus (RSV) were detected, but the overall infection rate in NICUs appeared relatively low.84 As example, using a symptom-based testing strategy, viral infection was confirmed in 51 (1%) of 5396 infants admitted to the NICU; of these, 20 (39%) had an enterovirus/Parechovirus infection, 15 (29%) RSV, 5 (10%) rotavirus, 3 (6%) cytomegalovirus (CMV), 2 (4%) adenovirus, 2 (4%) parainfluenza virus, 2 (4%) herpes simplex virus, 1 (2%) rhinovirus, and 1 (2%) rubella virus.85
Recent data, including that from our collection of expedited autopsy human neonatal distal lung tissue, suggest a relatively high prevalence of lung viral infections in those who succumb to respiratory failure in the NICU; 21 of 63 samples tested were virus positive (Ref.86 and data not shown). Coronavirus, rhinovirus, parainfluenza, and CMV were detected by reverse-transcriptase PCR (RT-PCR). Interestingly, in this small postmortem sample, RSV, influenza A and B, parainfluenza type 1, and metapneumovirus were not detected.
Surveillance studies using PCR and genomic sequencing for detection have begun to report a closer to true incidence of nosocomial viral respiratory infections (NVRI) in neonates and children hospitalized in pediatric intensive care units and NICUs. In a NICU surveillance study, nasal brush samples were taken weekly from all neonates (age ≤28 days) and children (age >28 days) hospitalized through a winter viral season. Of a total of 120 patients enrolled (64 neonates and 56 children), 20 patients were virus positive by PCR (incidence 16.7%). Seven positive samples for human coronaviruses were detected (incidence 11%). Risk factors for NVRI in the neonates were duration of hospitalization, antibiotic treatment, and duration of parenteral nutrition (P<.01).87
A 1-year NICU surveillance study of infants born at less than 33 weeks’ gestation, using PCR detection of 17 viral subtypes, identified at least one positive respiratory virus during the hospitalization in 26 of 50 subjects, most asymptomatic. Testing positive was associated with longer length of stay and length of mechanical ventilation, as well as diagnosis of BPD. Similar ongoing studies should determine if viral infection is such a common occurrence in the NICU as to warrant more frequent surveillance and development of interventions to reduce exposure and illness.
Neonatal Cytomegalovirus
Human CMV, a Betaherpesvirinae virus, latent in leukocytes, is highly prevalent in the human population; approximately 50% of adults are CMV seropositive and 60% of mothers of preterm infants. Congenital, in utero, infection of the fetus occurs in 0.1% to 2.0% of all pregnancies and may arise through primary infection of the mother, reactivation during pregnancy of a latent infection or reinfection with a different strain of CMV. Postnatal, the virus is spread even more efficiently from mother to the newborn via breastmilk. Because it reactivates in 95% or more of CMV-seropositive women in the postpartum period and can be detected in breastmilk as early as 3.5 days after delivery, CMV is a relatively common viral infection of the newborn period.88 Transmission to full-term newborns is reported in approximately 40%, whereas in preterm infants it varies from 6% to 55%, potentially due to differing strains, use of fresh/frozen milk, and maternal factors affecting viral shedding.88 A surveillance study of 175 NICU neonates, testing serum CMV-titers and CMV-DNA, demonstrated an overall prevalence of CMV of 12.6%. Ten (5.71%) of the infants had congenital infection, whereas 12 cases (6.86%) had perinatal infection.89 Postnatal infection in the newborn can be detected by molecular diagnostics as early as 12 days of life. Infection remains clinically silent in most, but 9% to 12% of postnatally infected low birth weight preterm infants have been reported to demonstrate severe, sepsislike infection.90 Although infants at lower gestational age are at increased risk of developing symptoms with postnatal infection and are also at greatest risk of BPD, there remains relatively little evidence of cause and effect. Prosch and colleagues91 found approximately 29% of VLBW infants with BPD to be CMV positive, but 12% of those without BPD. This study and others have found postnatal infection symptoms in preterms to be transient and to have no effect on neonatal outcome including BPD or necrotizing enterocolitis.92, 93 A review of PubMed articles describing CMV pneumonitis, however, concludes that CMV infection can be protracted with diffuse interstitial pneumonitis associated with fibrosis and BPD.94 It would appear that more surveillance and outcome studies are needed to determine if a causative relationship exists and if anti-CMV therapy or methods to reduce transmission of CMV to the fetus and neonates could effectively reduce disease.
Interestingly, CMV has a notable influence on the human immune system inducing a substantial cytolytic CD8+ T-cell population.95 CMV infection in infants induces the differentiation of not only phenotypically mature cells, but also functionally active cells that produce interferon gamma (IFN-γ) on restimulation.96 Serum cytokine concentrations measured in CMV congenitally infected infants show evidence of a strong Th1 bias with a predominance of IFN-γ, IL-2, IL-12, and IL-8 production and diminished IL-4.97 Because the generation of IFN-γ secreting T cells and CD8+ effector cells is associated with successful recovery from viral infections in general and RSV in particular, such data suggest that CMV infection in infancy could be beneficial. There is, however, concern that CMV-induced immuno-ageing of lymphocytes may ultimately result in immunosuppression suggested by poor vaccine response in the elderly.98
Respiratory Syncytial Virus and Other Common Viruses
Recurrent wheezing in later childhood has been associated with infections with RSV, metapneumovirus (hMPV), parainfluenza (PIV), rhinovirus, and human coronavirus NL63.99, 100, 101, 102, 103 RSV infections have best demonstrated that effects of viral respiratory tract infections in infancy may be long-lived. In premature infants born at less than 32 weeks’ gestation, with and without BPD, those with a history of RSV lower respiratory tract infection (LRTI) were found to have more days of cough and wheeze at 1 year of age than those without RSV LRTI.104 Additionally, those with RSV LRTI and hMPV LRTI were found to have increased airway resistance at 1 year of age on pulmonary function testing.102 In some infants, airway function has been shown to deteriorate during the first years of life.105 When the group with BPD was followed up at school entry, those who had been hospitalized with RSV LRTI or another respiratory illness within the first 2 years of life had a greater cumulative number of outpatient visits and costs of care compared with former premature infants with BPD without a respiratory hospitalization.106 A subset of these children with pulmonary function testing at 8 to 10 years of age demonstrated significantly reduced lung function (lower forced expiratory volume in 0.75 s [FEV0.75], FEV0.75/forced vital capacity, and flows at 50% and 75% of vital capacity) in those with an RSV LRTI compared with children without. Whether viral LRTIs cause subsequent airway disease or are merely markers for preexisting abnormal lung function has not been definitively determined.101 The role of atopy, predisposition to asthma, and postinfection airway remodeling in relationship to LRTI and subsequent wheezing in childhood is also not clear.101, 107 A combination of viral factors and innate and adaptive immune responses, in the setting of a susceptible genetic background and a young or elderly host appear to drive clinical outcome.108, 109 An ongoing study of premature infants and viral LRTIs, including baseline pulmonary function testing, seeks to determine if the viral respiratory infection is causal of the increased long-term morbidity or merely a marker for children with more severe preexisting lung disease, as has been suggested for term infants (ClinicalTrials.gov NCT01789268). This question is important when evaluating selective vaccine strategies to prevent severe LRTI and chronic disease.
Susceptibility factors for enhanced inflammation
The immune system is a double-edged sword: too little response and microbes invade and injure, too robust a host response may result in bystander injury and disease. In addition to exposure to infectious agents, there appear to be certain intrinsic factors that result in enhanced inflammatory responses in some individuals as adult, child, or preterm infant, when compared with others. There is evidence to suggest that preterm infants may be affected by both relative immunosuppression and more robust immune responses than full-term infants. We conclude this article with a review of susceptibility factors identified or suggested to enhance inflammation in the prematurely born.
Genetics
Genetics that predispose to shortened pregnancy, especially if related to increased inflammation, naturally increase the risk of inflammatory lung disease of prematurity. For example, elevated mid-trimester vaginal IL-1β is associated with increased risk for spontaneous preterm birth. Homozygous carriers of IL1RN*1, a single nucleotide polymorphism (SNP) in the IL-1 receptor antagonist (IL-1ra) gene, a genotype associated with elevated IL-1β, are at increased risk for preterm birth and an example of genetic polymorphisms that affect the innate immune system and risks of prematurity.110 In women who had a preterm birth, the combination of clinical chorioamnionitis and IL-10 (-1082)*G allele was associated with an increased risk for delivery before 29 weeks’ gestation, suggesting a gene-environment interaction.111
In infants, twin studies suggest significant genetic susceptibility to BPD.112 Relative to inflammation, genotype analysis, after multiple comparisons correction, revealed 2 significant SNPs, rs3771150 (IL-18RAP) and rs3771171 (IL-18R1), in African American individuals with BPD (vs African American individuals without BPD; q <0.05). No associations with Caucasian BPD, African American or Caucasian respiratory distress syndrome (RDS), or prematurity in either African American or Caucasian individuals were identified with these SNPs.113 Functional polymorphisms in the promoter of NFKBIA that encodes IκBα, a negative regulator of NF-κB, is associated with differential susceptibility to severe bronchopulmonary dysplasia, as well as other common inflammatory diseases of infant lung.114 A number of additional studies, evaluating exome sequencing in extremes of disease, epigenomic regulation, transcriptome responses to exposures such as hyperoxia, and pathway analyses are ongoing to identify gene and gene regulatory susceptibility factors involved in pathogenesis of BPD.115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125
Alterations in Immune Responses Due to Developmental Window of Preterm Delivery
Recent developments in miniaturization of technologies, including assays based on polychromatic flow cytometry, multiplexed protein assays, and low-input transcriptional analyses, have begun to advance the field of neonatal immunology. Dowling and Levy126 provide a recent review of both in vivo and in vitro approaches to studying early-life immuno-development, as well as a summary of unique characteristics of the preterm and term innate and adaptive immune systems.
The innate immune responses of full-term infants, including the function and recruitment of granulocytes, natural killer (NK) cells, and antigen-presenting cells are characterized as immature and functionally suppressed.126 Innate immune responses in human preterm infants have been less well characterized.127, 128 Fetal cells, including NK cells, have enhanced sensitivity to the immuno-suppressive effects of transforming growth factor beta.129 Early-life antigen-presenting cells tend to produce more IL-6, IL-10, and IL-27, predominantly immunosuppressive cytokines. Intriguingly, a recent study has in addition suggested that CD71+ nucleated red blood cells (erythroid precursor cells) that are typically increased in fetal blood, especially in pregnancies complicated by placental insufficiency, appear to suppress phagocyte and antigen-presenting cell stimulus-induced TNF-α production suggesting an immunosuppressive function.127
Lymphocytes and the adaptive immune system provide a critical defense against intracellular, including viral, infections. Reduced CD4+ T cells result in impaired immune response to pathogens. CD8+ T cells and NK cells provide protection from viral infection but also contribute to immunopathology by contact-dependent effector functions (eg, perforin and FasL). IFN-γ and, particularly, TNF-α are thought to be primary perpetrators of T-cell–mediated lung injury, yet are also important for antimicrobial defense.130
The fetal and neonatal periods are unique immune developmental stages in which adaptive responses are highly plastic and dependent on gestational age.131, 132 Although relatively little literature refers to detailed phenotyping of lymphocytic maturation in the prematurely born infant, investigators have numerically evaluated classes of lymphocytes in the human fetus and young child. The total circulating white cell counts increase through the latter half of gestation until term delivery and then decrease slightly to adult levels. The percentage of lymphocytes decreases from approximately 80% at 18 to 36 weeks (median 26 weeks) to 40% at term delivery to 21% in the adult human, based on cord blood sampling at delivery or cordocentesis.133, 134 The proportion of CD3+ cells, however, increases with gestational age and in the presence of an infection. In normal pregnancies, circulating CD4+ T-cell numbers are inversely related to gestational age and the fetal percentage of CD8+ T cells was reduced, increasing before term (9.5%–15.7%) such that CD4+/CD8+ ratios also vary inversely with gestational age, higher in VLBW infants than full-term.135, 136 Maternal disease may alter fetal lymphocytes. Preeclampsia had a significant effect on T-cell distribution associating with fewer CD4+ cells and CD4+CD8+ double-positive cells, decreased CD4+/CD8+ ratios, reduced Th2 and regulatory T-cell subsets in cord blood, whereas maternal betamethasone therapy also associates with higher CD3+ cell proportion and a lower proportion of NK cells.137, 138
Evidence for Lymphocytic Abnormalities in Premature Infants with Lung Disease
Several lines of investigation suggest a role for dysregulation of CD4+ responses in BPD. In animal models, T cells accumulate in the lungs of preterm lambs exposed to lipopolysaccharide in utero139 and preterm baboons that develop BPD were found to have abundant CD4+ T cells in the lung parenchyma.140 Significant infiltrates of T cells were noted in distal lung of infants who died with BPD as compared with gestational age–matched infants without lung disease.141 In serial blood samples from premature infants with RDS born 1200 g and less than 30 weeks’ gestation, Ballabh and colleagues142 demonstrated a reduction in absolute lymphocyte count, as well as the percentage and the absolute number of CD4+ T cells, in those who progressed to BPD (P<.03), significant even on day 1 of life. More activated T cells in those who go on to develop BPD may reflect sequestration and activation of cells within the lung.142, 143 CD4+ T-cell percentage continued to decrease with postnatal age. Berrington and colleagues144 measured lymphocyte subclasses in premature infants just before first immunizations. At 7 to 8 weeks of age, prematurely born infants had lower absolute lymphocyte, T-cell, B-cell, and T-helper cell counts, and lower CD4+/CD8+ T-cell ratio, than term infants, as well as increased proportion of T-regulatory (Treg) (CD4+CD25+) cells and decreased CD45RA + naïve cells. By 6 months, the B-cell population had numerically normalized but T-cell abnormalities persisted.
Recent studies have challenged the concept that CD3+ T-cell responses are uniformly impaired in neonates, especially in preterm infants. Although most of the newborn CD4+ T cells are naïve, activation markers like CD25, CD69, and CD45RO+ are enhanced on CD4+ cells of prematurely born infants.145 Likewise, the proportion of cord blood CD8+ T cells that are CD45RO+, suggesting activation, is also higher at lower gestational age.146 Several reports now demonstrate a correlation between T-cell activation, as measured by CD45RO expression, and premature infants’ adverse outcomes, such as BPD, necrotizing enterocolitis, and periventricular leukomalacia.25, 142, 147 CD4+ and CD8+ T cells at lower gestational age are also shown to have enhanced cytokine production with in vitro stimulation, suggesting that enhanced CD45RO expression in preterms is accompanied by inducible effector functions that may contribute to the severity of lung disease.146, 148 A report that regulatory CD4+ T cells (CD4+CD25hiFoxP3+CD127Dim) were significantly reduced in cord blood of preterm infants who developed BPD further raises the potential for enhanced inflammation due to reduced inhibitory control.148 It has been suggested that the relatively activated CD4+ and CD8+ T-cell phenotype at early gestational ages is reminiscent of recovery from bone marrow ablation in adults and represents rapid homeostatic expansion in a lymphopenic host.147 Further, intra-amniotic administration of IL-1beta to rhesus monkeys at 80% gestation resulted in reduction in frequency of Treg cells in lymphoid organs, whereas Th17, IL-17A-producing, cells were increased, potentially linking in utero innate immune activity to inflammatory lymphocyte-mediated injury.149
Some insight into potential T-cell immunopathology in BPD may be gained from animal and adult models of inflammatory lung disease. In a baboon model of BPD, thymic involution, increased peripheral T cells carrying markers of maturation, robust nonspecific cytokine secretion, and increased autoreactive CD4+ T cells in the lung interstitium, were associated with an increase in bombesin-like peptides (BLP).140, 150 Treatment with a neutralizing antibody to BLP corrected the thymic and lung pathology seen in preterm baboons treated with 100% oxygen.140 BLP is also elevated in preterm human infants with BPD,151 suggesting a mechanism linking lymphocyte dysregulation and BPD.
Age at First Infection
The degree of lung and immune system maturation at the time of infection influences cytopathogenic responses to virus and perhaps bacteria but also appears to set a trajectory of immune response to subsequent challenge. Newborn mice infected with RSV have, compared with mice infected at a slightly later age, increased bronchoalveolar lavage fluid numbers of Th2 type CD4+IL-4+ cells and fewer CD4+IFN-γ+ cells when reinfected in adulthood.152 Likewise, mice infected with influenza A within 1 week of birth showed enhanced airway hyperreactivity, chronic pulmonary inflammation, and diffuse emphysematous-type lesions as adults. An adaptive immune insufficiency was most apparent in the neonatal CD8+ T cells. Newborn infection was associated with reduced and delayed IFN-γ responses as compared with infection in older animals. RSV-infected neonatal mice recruited CD8+ T cells defective in IFN-γ production in association with mild symptoms. Reinfection as adults, however, resulted in limited viral replication but enhanced inflammation and T-cell recruitment, including Th2 cells and eosinophils.153, 154 Depletion of CD8+ T cells (but not CD4) cells during the primary neonatal infection was protective against the adult challenge. Recall responses from neonatal-primed and adult-primed mice were associated with IFN-γ secretion, indicative of a Th1 response. However, IL-4 and IL-5 secretion were enhanced only in neonatal-primed mice. Rechallenge of these mice, primed as newborns, was also associated with increased concentrations of monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-1α (MIP-1α), and RANTES in the lung. It is suggested then that neonatal T cells, in particular IFN-γ–deficient CD8+ T cells, play a crucial role in regulation of immune responses after neonatal infection. In these neonatal animal models, adoptive transfer of naive CD8+ cells, from wild-type but not from IFN-γ–deficient donors, significantly lowered pulmonary viral titers and greatly improved pulmonary function as adults, supporting the importance of IFN-γ secreting CD8+ T cells in determining disease outcome.155
A strong argument has now been advanced that childhood wheezing and atopy are related to reduced cord blood IFN-γ. In a study of infants predisposed to asthma and atopy, less robust mitogen-induced or specific antigen-induced IFN-γ and IL-13 responses from cord blood cells were associated with more wheezing episodes in the first year of life in children infected with RSV and rhinovirus.156 In the Childhood Origins of Asthma (COAST) Project, cytokine-response profiles of cord blood and 1-year mononuclear cells stimulated in vitro identified that cord blood IFN-γ responses were inversely related to the frequency of viral respiratory infections and wheezing in infancy while enhanced IFN-γ responses at 1 year correlated positively with the frequency of preceding viral infections.157 Severity of asthma has been associated with excessive IFN-γ production, particularly by CD8+ T cells, potentially reflecting the cytotoxic effect of the cytokine. These data suggest that neonatal IFN-γ responses influence subsequent antiviral activity. Conversely, the frequency of viral infections in infancy can influence IFN-γ responses.
Neutralizing antibodies provide important antiviral protection in infants. Higher RSV neutralizing antibody titers in both premature and term infants are associated with protection from infection and LRTI, an effect also supported by the success of palivizumab in preventing severe RSV disease in premature infants.158 Transplacental transfer of maternal antibodies is inversely related to length of gestation such that the more preterm infants have relatively less humoral protection contributing to disease risk. Because viral loads of RSV, hMPV, PIV, and rhinovirus correlate with the severity of clinical disease,159, 160, 161, 162 it is suggested that infants with a greater ability to control viral replication on first infection, via the presence of neutralizing antibody and a more robust IFN-γ response, are successful in limiting excessive antigen presentation, generating protective immune responses associated with viral clearance, and avoiding immuno-pathogenesis. To date, no study has evaluated antibody and cellular immune phenotype together with viral load measurements in infants with respiratory infections. Additionally, the association between these factors and disease severity has not been explored in premature infants.
Overall, alterations in lymphocyte-related immunity occur and are dependent on gestational age, maternal influences, postnatal oxidant stress, and viral diseases. There are burgeoning data in this area in premature infants, although as yet minimal knowledge of specific mechanisms by which lymphocytes participate in respiratory outcomes in premature infants.
Altered Establishment of Colonizing Microbiota
A developing body of research suggests that both the acquisition and maintenance of bacterial populations in the gut soon after birth are important drivers of the development of both systemic and mucosal immunity.7 Recent advances in high-throughput sequencing technology have provided insight into the gut microbiome and are beginning to describe the diversity and dynamics of the microbial populations in both health and disease. Although the exact factors that control the interactions between the gut epithelial cells, the gut-associated lymphoid tissue, and the gut microbiome are not yet clear, all 3 components appear to play a significant role in the induction of immune tolerance to luminal bacterial antigens and the maintenance of homeostasis. Proposed mechanisms identified in animal models include the blocking of innate signaling via Toll-like receptor-4, the development or expansion of Fox P3+ Treg cells, and enhanced IL-10 production in the gut induced by commensal bacteria.163, 164 The presence of specific species of bacteria also may be crucial to the development of gut tolerance as suggested by the relatively decreased amounts of Bacteroides, Bifidobacterium, and Lactobacillus species in patients with inflammatory bowel disease.7 Additionally, the timing of acquisition of gut bacteria may be critical for the positive effects on health. Neonatal IL-10–deficient mice exposed to bacterial antigens had delayed development of colitis at 18 weeks of age compared with those not exposed. Decreased IFN-γ and IL-17 production in explanted intestinal tissue and spleen cells following stimulation with gut bacteria also suggests that exposure of the neonatal immune system to antigens of the microbiome is associated with both mucosal and systemic immune tolerance.8 These findings may be especially relevant to the preterm infant in view of recent data showing an inverse relationship between antibiotic therapy and parenteral nutrition with fecal diversity in the infants born at less than 29 weeks’ gestation.165 A recent study in elderly adults also suggests improved protection from influenza infection with oral provision of a Bifidobacterium longum species.166
Attention has recently turned to determining the microbiome of the respiratory tract in premature infants. The conventional theory that the lower airways are sterile has been challenged by identification of organisms in the deep lung of adults and now infants and children, initially, not surprisingly associated with diseases such as cystic fibrosis, chronic obstructive pulmonary disease, and asthma, but also now as a “normal microbiome” in healthy patients.167, 168, 169 In preterm infants, Lohmann and colleagues170 described nonsterile tracheal aspirates with a predominance of Acinetobacter in samples taken at intubation in the delivery room, and a persistent decrease in diversity of organisms over the first month of life in those who went on to develop BPD. Early sustained airway bacterial colonization in infants less than 1250 g at birth and intubated for at least 3 weeks was detected within 7 days of life, dominated by Staphylococcus and Ureaplasma.171 Ongoing studies promise further longitudinal intestinal and respiratory microbiome and viral infection data and correlations to respiratory outcomes in preterm and full-term infants (Clinicaltrials.gov: NCT01607216 and NCT01789268, funding U01HL101813 and HHSN272201200005C, respectively).
That the microbes, the bacteria, viruses, fungi, and others, that flourish on human skin and mucosa affect the metabolism, immune system, health, and disease of their host is becoming more clear. Just what those effects are in the premature infant and how they affect susceptibility to infections and alter respiratory outcomes is an important area of current research.
Footnotes
References
- 1.Martin J.A., Hamilton B.E., Osterman M.J. Births: final data for 2013. Natl Vital Stat Rep. 2015;64(1):1–65. [PubMed] [Google Scholar]
- 2.McLaurin K.K., Hall C.B., Jackson E.A. Persistence of morbidity and cost differences between late-preterm and term infants during the first year of life. Pediatrics. 2009;123(2):653–659. doi: 10.1542/peds.2008-1439. [DOI] [PubMed] [Google Scholar]
- 3.Gunville C.F., Sontag M.K., Stratton K.A. Scope and impact of early and late preterm infants admitted to the PICU with respiratory illness. J Pediatr. 2010;157(2):209–214.e1. doi: 10.1016/j.jpeds.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Underwood M.A., Danielsen B., Gilbert W.M. Cost, causes and rates of rehospitalization of preterm infants. J Perinatol. 2007;27(10):614–619. doi: 10.1038/sj.jp.7211801. [DOI] [PubMed] [Google Scholar]
- 5.Wade K.C., Lorch S.A., Bakewell-Sachs S. Pediatric care for preterm infants after NICU discharge: high number of office visits and prescription medications. J Perinatol. 2008;28(10):696–701. doi: 10.1038/jp.2008.74. [DOI] [PubMed] [Google Scholar]
- 6.Dimmitt R.A., Staley E.M., Chuang G. Role of postnatal acquisition of the intestinal microbiome in the early development of immune function. J Pediatr Gastroenterol Nutr. 2010;51(3):262–273. doi: 10.1097/MPG.0b013e3181e1a114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conroy M.E., Shi H.N., Walker W.A. The long-term health effects of neonatal microbial flora. Curr Opin Allergy Clin Immunol. 2009;9(3):197–201. doi: 10.1097/ACI.0b013e32832b3f1d. [DOI] [PubMed] [Google Scholar]
- 8.Sydora B.C., McFarlane S.M., Doyle J.S. Neonatal exposure to fecal antigens reduces intestinal inflammation. Inflamm Bowel Dis. 2011;17(4):899–906. doi: 10.1002/ibd.21453. [DOI] [PubMed] [Google Scholar]
- 9.O'Reilly M.A., Marr S.H., Yee M. Neonatal hyperoxia enhances the inflammatory response in adult mice infected with influenza A virus. Am J Respir Crit Care Med. 2008;177(10):1103–1110. doi: 10.1164/rccm.200712-1839OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maduekwe E.T., Buczynski B.W., Yee M. Cumulative neonatal oxygen exposure predicts response of adult mice infected with influenza A virus. Pediatr Pulmonol. 2014 doi: 10.1002/ppul.23063. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Speer C.P. New insights into the pathogenesis of pulmonary inflammation in preterm infants. Biol Neonate. 2001;79(3–4):205–209. doi: 10.1159/000047092. [DOI] [PubMed] [Google Scholar]
- 12.Stevens T.P., Dylag A., Panthagani I. Effect of cumulative oxygen exposure on respiratory symptoms during infancy among VLBW infants without bronchopulmonary dysplasia. Pediatr Pulmonol. 2010;45(4):371–379. doi: 10.1002/ppul.21199. [DOI] [PubMed] [Google Scholar]
- 13.Jobe A.H., Ikegami M. Mechanisms initiating lung injury in the preterm. Early Hum Dev. 1998;53(1):81–94. doi: 10.1016/s0378-3782(98)00045-0. [DOI] [PubMed] [Google Scholar]
- 14.Viscardi R.M. Perinatal inflammation and lung injury. Semin Fetal Neonatal Med. 2012;17(1):30–35. doi: 10.1016/j.siny.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhandari V. Postnatal inflammation in the pathogenesis of bronchopulmonary dysplasia. Birth Defects Res A Clin Mol Teratol. 2014;100(3):189–201. doi: 10.1002/bdra.23220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryan R.M., Ahmed Q., Lakshminrusimha S. Inflammatory mediators in the immunobiology of bronchopulmonary dysplasia. Clin Rev Allergy Immunol. 2008;34(2):174–190. doi: 10.1007/s12016-007-8031-4. [DOI] [PubMed] [Google Scholar]
- 17.Baud O., Emilie D., Pelletier E. Amniotic fluid concentrations of interleukin-1beta, interleukin-6 and TNF-alpha in chorioamnionitis before 32 weeks of gestation: histological associations and neonatal outcome. Br J Obstet Gynaecol. 1999;106(1):72–77. doi: 10.1111/j.1471-0528.1999.tb08088.x. [DOI] [PubMed] [Google Scholar]
- 18.Viscardi R.M., Muhumuza C.K., Rodriguez A. Inflammatory markers in intrauterine and fetal blood and cerebrospinal fluid compartments are associated with adverse pulmonary and neurologic outcomes in preterm infants. Pediatr Res. 2004;55(6):1009–1017. doi: 10.1203/01.pdr.0000127015.60185.8a. [DOI] [PubMed] [Google Scholar]
- 19.Kotecha S., Wilson L., Wangoo A. Increase in interleukin (IL)-1 beta and IL-6 in bronchoalveolar lavage fluid obtained from infants with chronic lung disease of prematurity. Pediatr Res. 1996;40(2):250–256. doi: 10.1203/00006450-199608000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Kotecha S., Mildner R.J., Prince L.R. The role of neutrophil apoptosis in the resolution of acute lung injury in newborn infants. Thorax. 2003;58(11):961–967. doi: 10.1136/thorax.58.11.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baier R.J., Majid A., Parupia H. CC chemokine concentrations increase in respiratory distress syndrome and correlate with development of bronchopulmonary dysplasia. Pediatr Pulmonol. 2004;37(2):137–148. doi: 10.1002/ppul.10417. [DOI] [PubMed] [Google Scholar]
- 22.Munshi U.K., Niu J.O., Siddiq M.M. Elevation of interleukin-8 and interleukin-6 precedes the influx of neutrophils in tracheal aspirates from preterm infants who develop bronchopulmonary dysplasia. Pediatr Pulmonol. 1997;24(5):331–336. doi: 10.1002/(sici)1099-0496(199711)24:5<331::aid-ppul5>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 23.Bose C.L., Dammann C.E., Laughon M.M. Bronchopulmonary dysplasia and inflammatory biomarkers in the premature neonate. Arch Dis Child Fetal Neonatal Ed. 2008;93(6):F455–F461. doi: 10.1136/adc.2007.121327. [DOI] [PubMed] [Google Scholar]
- 24.Beeton M.L., Maxwell N.C., Davies P.L. Role of pulmonary infection in the development of chronic lung disease of prematurity. Eur Respir J. 2011;37(6):1424–1430. doi: 10.1183/09031936.00037810. [DOI] [PubMed] [Google Scholar]
- 25.Ambalavanan N., Carlo W.A., D'Angio C.T., Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network Cytokines associated with bronchopulmonary dysplasia or death in extremely low birth weight infants. Pediatrics. 2009;123(4):1132–1141. doi: 10.1542/peds.2008-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bose C., Laughon M., Allred E.N., Elgan Study Investigators Blood protein concentrations in the first two postnatal weeks that predict bronchopulmonary dysplasia among infants born before the 28th week of gestation. Pediatr Res. 2011;69(4):347–353. doi: 10.1203/PDR.0b013e31820a58f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhattacharya S., Go D., Krenitsky D.L. Genome-wide transcriptional profiling reveals connective tissue mast cell accumulation in bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2012;186(4):349–358. doi: 10.1164/rccm.201203-0406OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheah F.C., Winterbourn C.C., Darlow B.A. Nuclear factor kappaB activation in pulmonary leukocytes from infants with hyaline membrane disease: associations with chorioamnionitis and Ureaplasma urealyticum colonization. Pediatr Res. 2005;57(5 Pt 1):616–623. doi: 10.1203/01.PDR.0000156209.37627.82. [DOI] [PubMed] [Google Scholar]
- 29.Blackwell T.S., Hipps A.N., Yamamoto Y. NF-kappaB signaling in fetal lung macrophages disrupts airway morphogenesis. J Immunol. 2011;187(5):2740–2747. doi: 10.4049/jimmunol.1101495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benjamin J.T., Carver B.J., Plosa E.J. NF-kappaB activation limits airway branching through inhibition of Sp1-mediated fibroblast growth factor-10 expression. J Immunol. 2010;185(8):4896–4903. doi: 10.4049/jimmunol.1001857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jobe A.H., Ikegami M. Antenatal infection/inflammation and postnatal lung maturation and injury. Respir Res. 2001;2(1):27–32. doi: 10.1186/rr35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le Cras T.D., Hardie W.D., Deutsch G.H. Transient induction of TGF-alpha disrupts lung morphogenesis, causing pulmonary disease in adulthood. Am J Physiol Lung Cell Mol Physiol. 2004;287(4):L718–L729. doi: 10.1152/ajplung.00084.2004. [DOI] [PubMed] [Google Scholar]
- 33.Kallapur S.G., Bachurski C.J., Le Cras T.D. Vascular changes after intra-amniotic endotoxin in preterm lamb lungs. Am J Physiol Lung Cell Mol Physiol. 2004;287(6):L1178–L1185. doi: 10.1152/ajplung.00049.2004. [DOI] [PubMed] [Google Scholar]
- 34.Combs C.A., Gravett M., Garite T.J. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am J Obstet Gynecol. 2014;210(2):125.e1–125.e15. doi: 10.1016/j.ajog.2013.11.032. [DOI] [PubMed] [Google Scholar]
- 35.Wang X., Buhimschi C.S., Temoin S. Comparative microbial analysis of paired amniotic fluid and cord blood from pregnancies complicated by preterm birth and early-onset neonatal sepsis. PLoS One. 2013;8(2):e56131. doi: 10.1371/journal.pone.0056131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ardissone A.N., de la Cruz D.M., Davis-Richardson A.G. Meconium microbiome analysis identifies bacteria correlated with premature birth. PLoS One. 2014;9(3):e90784. doi: 10.1371/journal.pone.0090784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wassenaar T.M., Panigrahi P. Is a foetus developing in a sterile environment? Lett Appl Microbiol. 2014;59(6):572–579. doi: 10.1111/lam.12334. [DOI] [PubMed] [Google Scholar]
- 38.Payne M.S., Bayatibojakhi S. Exploring preterm birth as a polymicrobial disease: an overview of the uterine microbiome. Front Immunol. 2014;5:595. doi: 10.3389/fimmu.2014.00595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Antony K.M., Ma J., Mitchell K.B. The preterm placental microbiome varies in association with excess maternal gestational weight gain. Am J Obstet Gynecol. 2015;212(5):653.e1–653.e16. doi: 10.1016/j.ajog.2014.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aagaard K., Ma J., Antony K.M. The placenta harbors a unique microbiome. Sci Transl Med. 2014;6(237):237ra265. doi: 10.1126/scitranslmed.3008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Romero R., Miranda J., Kusanovic J.P. Clinical chorioamnionitis at term I: microbiology of the amniotic cavity using cultivation and molecular techniques. J Perinat Med. 2015;43(1):19–36. doi: 10.1515/jpm-2014-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smulian J.C., Shen-Schwarz S., Vintzileos A.M. Clinical chorioamnionitis and histologic placental inflammation. Obstet Gynecol. 1999;94(6):1000–1005. doi: 10.1016/s0029-7844(99)00416-0. [DOI] [PubMed] [Google Scholar]
- 43.Kim S.M., Romero R., Park J.W. The relationship between the intensity of intra-amniotic inflammation and the presence and severity of acute histologic chorioamnionitis in preterm gestation. J Matern Fetal Neonatal Med. 2014:1–10. doi: 10.3109/14767058.2014.961009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Romero R., Miranda J., Chaemsaithong P. Sterile and microbial-associated intra-amniotic inflammation in preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med. 2014:1–16. doi: 10.3109/14767058.2014.958463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Katzman P.J. Chronic inflammatory lesions of the placenta. Semin Perinatol. 2015;39(1):20–26. doi: 10.1053/j.semperi.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 46.Watterberg K.L., Demers L.M., Scott S.M. Chorioamnionitis and early lung inflammation in infants in whom bronchopulmonary dysplasia develops. Pediatrics. 1996;97:210–215. [PubMed] [Google Scholar]
- 47.Viscardi R.M., Hasday J.D. Role of Ureaplasma species in neonatal chronic lung disease: epidemiologic and experimental evidence. Pediatr Res. 2009;65(5 Pt 2):84R–90R. doi: 10.1203/PDR.0b013e31819dc2f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kallapur S.G., Moss J.T.M., Newnham J.P. Recruited inflammatory cells mediate endotoxin-induced lung maturation in preterm fetal lambs. Am J Respir Crit Care Med. 2005;172:1315–1321. doi: 10.1164/rccm.200506-1007OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moss T.J.M., Knox C.L., Kallapur S.G. Experimental amniotic fluid infection in sheep; effects of Ureaplasma parvum. Am J Obstet Gynecol. 2008;198(1):122.e1–122.e8. doi: 10.1016/j.ajog.2007.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Willet K.E., Jobe A.H., Ikegami M. Antenatal endotoxin and glucocorticoid effects on lung morphometry in preterm lambs. Pediatr Res. 2000;48:782–788. doi: 10.1203/00006450-200012000-00014. [DOI] [PubMed] [Google Scholar]
- 51.Ikegami M., Jobe A. Postnatal lung inflammation increased by ventilation of preterm lambs exposed antenatally to E. coli endotoxin. Pediatr Res. 2002;52:356–362. doi: 10.1203/00006450-200209000-00008. [DOI] [PubMed] [Google Scholar]
- 52.Van Marter L.J., Dammann O., Allred E.N. Chorioamnionitis, mechanical ventilation, and postnatal sepsis as modulators of chronic lung disease in preterm infants. J Pediatr. 2002;140(2):171–176. doi: 10.1067/mpd.2002.121381. [DOI] [PubMed] [Google Scholar]
- 53.Lowe J., Watkins W.J., Edwards M.O. Association between pulmonary Ureaplasma colonization and bronchopulmonary dysplasia in preterm infants: updated systematic review and meta-analysis. Pediatr Infect Dis J. 2014;33(7):697–702. doi: 10.1097/INF.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 54.Kallapur S.G., Kramer B.W., Jobe A.H. Ureaplasma and BPD. Semin Perinatol. 2013;37(2):94–101. doi: 10.1053/j.semperi.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Viscardi R.M., Kallapur S.G. Role of Ureaplasma Respiratory Tract Colonization in Bronchopulmonary Dysplasia Pathogenesis-Current Concepts and Update. Clin Perinatol. 2015 doi: 10.1016/j.clp.2015.08.003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lacaze-Masmonteil T. That chorioamnionitis is a risk factor for bronchopulmonary dysplasia–the case against. Paediatr Respir Rev. 2014;15(1):53–55. doi: 10.1016/j.prrv.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 57.Thomas W., Speer C.P. Chorioamnionitis is essential in the evolution of bronchopulmonary dysplasia–the case in favour. Paediatr Respir Rev. 2014;15(1):49–52. doi: 10.1016/j.prrv.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 58.Laughon M., Allred E.N., Bose C. Patterns of respiratory disease during the first 2 postnatal weeks in extremely premature infants. Pediatrics. 2009;123(4):1124–1131. doi: 10.1542/peds.2008-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Soraisham A.S., Singhal N., McMillan D.D. A multicenter study on the clinical outcome of chorioamnionitis in preterm infants. Am J Obstet Gynecol. 2009;200(4):372.e1–372.e6. doi: 10.1016/j.ajog.2008.11.034. [DOI] [PubMed] [Google Scholar]
- 60.Lahra M.M., Beeby P.J., Jeffery H.E. Intrauterine inflammation, neonatal sepsis, and chronic lung disease: a 13-year hospital cohort study. Pediatrics. 2009;123(5):1314–1319. doi: 10.1542/peds.2008-0656. [DOI] [PubMed] [Google Scholar]
- 61.Goldenberg R.L., Hauth J.C., Andrews W.W. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342(20):1500–1507. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 62.Stimac M., Juretic E., Vukelic V. Effect of chorioamnionitis on mortality, early onset neonatal sepsis and bronchopulmonary dysplasia in preterm neonates with birth weight of <1,500 grams. Coll Antropol. 2014;38(1):167–171. [PubMed] [Google Scholar]
- 63.Watterberg K.L., Scott S.M., Naeye R.L. Chorioamnionitis, cortisol, and acute lung disease in very low birth weight infants. Pediatrics. 1997;99:E6. doi: 10.1542/peds.99.2.e6. [DOI] [PubMed] [Google Scholar]
- 64.Kramer B.W., Ikegami M., Moss T.J. Endotoxin-induced chorioamnionitis modulates innate immunity of monocytes in preterm sheep. Am J Respir Crit Care Med. 2005;171(1):73–77. doi: 10.1164/rccm.200406-745OC. [DOI] [PubMed] [Google Scholar]
- 65.Maitre N.L., Ballard R.A., Ellenberg J.H., Prematurity and Respiratory Outcomes Program Investigators Respiratory consequences of prematurity: evolution of a diagnosis and development of a comprehensive approach. J Perinatol. 2015;35(5):313–321. doi: 10.1038/jp.2015.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vinnars M.T., Nasiell J., Holmstrom G. Association between placental pathology and neonatal outcome in preeclampsia: a large cohort study. Hypertens Pregnancy. 2014;33(2):145–158. doi: 10.3109/10641955.2013.842584. [DOI] [PubMed] [Google Scholar]
- 67.Yen T.A., Yang H.I., Hsieh W.S., Taiwan Premature Infant Developmental Collaborative Study Group Preeclampsia and the risk of bronchopulmonary dysplasia in VLBW infants: a population based study. PLoS One. 2013;8(9):e75168. doi: 10.1371/journal.pone.0075168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lees C., Marlow N., Arabin B. Perinatal morbidity and mortality in early-onset fetal growth restriction: cohort outcomes of the trial of randomized umbilical and fetal flow in Europe (TRUFFLE) Ultrasound Obstet Gynecol. 2013;42(4):400–408. doi: 10.1002/uog.13190. [DOI] [PubMed] [Google Scholar]
- 69.Eriksson L., Haglund B., Odlind V. Prenatal inflammatory risk factors for development of bronchopulmonary dysplasia. Pediatr Pulmonol. 2014;49(7):665–672. doi: 10.1002/ppul.22881. [DOI] [PubMed] [Google Scholar]
- 70.Ozkan H., Cetinkaya M., Koksal N. Increased incidence of bronchopulmonary dysplasia in preterm infants exposed to preeclampsia. J Matern Fetal Neonatal Med. 2012;25(12):2681–2685. doi: 10.3109/14767058.2012.708371. [DOI] [PubMed] [Google Scholar]
- 71.O'Shea J.E., Davis P.G., Doyle L.W., Victorian Infant Collaborative Study Group Maternal preeclampsia and risk of bronchopulmonary dysplasia in preterm infants. Pediatr Res. 2012;71(2):210–214. doi: 10.1038/pr.2011.27. [DOI] [PubMed] [Google Scholar]
- 72.Mestan K.K., Check J., Minturn L. Placental pathologic changes of maternal vascular underperfusion in bronchopulmonary dysplasia and pulmonary hypertension. Placenta. 2014;35(8):570–574. doi: 10.1016/j.placenta.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ahn H.M., Park E.A., Cho S.J. The association of histological chorioamnionitis and antenatal steroids on neonatal outcome in preterm infants born at less than thirty-four weeks' gestation. Neonatology. 2012;102(4):259–264. doi: 10.1159/000339577. [DOI] [PubMed] [Google Scholar]
- 74.Jonsson B., Tullus K., Brauner A. Early increase of TNF alpha and IL-6 in tracheobronchial aspirate fluid indicator of subsequent chronic lung disease in preterm infants. Arch Dis Child Fetal Neonatal Ed. 1997;77(3):F198–F201. doi: 10.1136/fn.77.3.f198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lorch S.A., Banks B.A., Christie J. Plasma 3-nitrotyrosine and outcome in neonates with severe bronchopulmonary dysplasia after inhaled nitric oxide. Free Radic Biol Med. 2003;34(9):1146–1152. doi: 10.1016/s0891-5849(03)00063-7. [DOI] [PubMed] [Google Scholar]
- 76.Varsila E., Pesonen E., Andersson S. Early protein oxidation in the neonatal lung is related to development of chronic lung disease. Acta Paediatr. 1995;84(11):1296–1299. doi: 10.1111/j.1651-2227.1995.tb13552.x. [DOI] [PubMed] [Google Scholar]
- 77.Schlenzig J.S., Bervoets K., von Loewenich V. Urinary malondialdehyde concentration in preterm neonates: is there a relationship to disease entities of neonatal intensive care? Acta Paediatr. 1993;82(2):202–205. doi: 10.1111/j.1651-2227.1993.tb12639.x. [DOI] [PubMed] [Google Scholar]
- 78.Shah J., Jefferies A.L., Yoon E.W., Canadian Neonatal Network Risk factors and outcomes of late-onset bacterial sepsis in preterm neonates born at <32 weeks' gestation. Am J Perinatol. 2015;32(7):675–682. doi: 10.1055/s-0034-1393936. [DOI] [PubMed] [Google Scholar]
- 79.Landry J.S., Menzies D. Occurrence and severity of bronchopulmonary dysplasia and respiratory distress syndrome after a preterm birth. Paediatr Child Health. 2011;16(7):399–403. doi: 10.1093/pch/16.7.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Klinger G., Levy I., Sirota L., Israel Neonatal Network Outcome of early-onset sepsis in a national cohort of very low birth weight infants. Pediatrics. 2010;125(4):e736–e740. doi: 10.1542/peds.2009-2017. [DOI] [PubMed] [Google Scholar]
- 81.Lardon-Fernandez M., Uberos J., Molina-Oya M. Epidemiological factors involved in the development of bronchopulmonary dysplasia in very low birth-weight preterm infants. Minerva Pediatr. 2015 doi: 10.23736/S0026-4946.16.04215-8. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 82.Ivarsson M., Schollin J., Bjorkqvist M. Staphylococcus epidermidis and Staphylococcus aureus trigger different interleukin-8 and intercellular adhesion molecule-1 in lung cells: implications for inflammatory complications following neonatal sepsis. Acta Paediatr. 2013;102(10):1010–1016. doi: 10.1111/apa.12350. [DOI] [PubMed] [Google Scholar]
- 83.Ohlsson A., Lacy J.B. Intravenous immunoglobulin for preventing infection in preterm and/or low-birth-weight infants. Cochrane Database Syst Rev. 2004;(1) doi: 10.1002/14651858.CD000361.pub2. CD000361. [DOI] [PubMed] [Google Scholar]
- 84.Faden H., Wynn R.J., Campagna L. Outbreak of adenovirus type 30 in a neonatal intensive care unit. J Pediatr. 2005;146(4):523–527. doi: 10.1016/j.jpeds.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 85.Verboon-Maciolek M.A., Krediet T.G., Gerards L.J. Clinical and epidemiologic characteristics of viral infections in a neonatal intensive care unit during a 12-year period. Pediatr Infect Dis J. 2005;24(10):901–904. doi: 10.1097/01.inf.0000180471.03702.7f. [DOI] [PubMed] [Google Scholar]
- 86.Maniscalco W.M., Watkins R.H., Pryhuber G.S. Angiogenic factors and alveolar vasculature: development and alterations by injury in very premature baboons. Am J Physiol Lung Cell Mol Physiol. 2002;282(4):L811–L823. doi: 10.1152/ajplung.00325.2001. [DOI] [PubMed] [Google Scholar]
- 87.Gagneur A., Sizun J., Vallet S. Coronavirus-related nosocomial viral respiratory infections in a neonatal and paediatric intensive care unit: a prospective study. J Hosp Infect. 2002;51(1):59–64. doi: 10.1053/jhin.2002.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Meier J., Lienicke U., Tschirch E. Human cytomegalovirus reactivation during lactation and mother-to-child transmission in preterm infants. J Clin Microbiol. 2005;43(3):1318–1324. doi: 10.1128/JCM.43.3.1318-1324.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Morgan M.A., el-Ghany el-S.M., Khalifa N.A. Prevalence of cytomegalovirus (CMV) infection among neonatal intensive care unit (NICU) and healthcare workers. Egypt J Immunol. 2003;10(2):1–8. [PubMed] [Google Scholar]
- 90.Maschmann J., Hamprecht K., Dietz K. Cytomegalovirus infection of extremely low-birth weight infants via breast milk. Clin Infect Dis. 2001;33(12):1998–2003. doi: 10.1086/324345. [DOI] [PubMed] [Google Scholar]
- 91.Prosch S., Lienicke U., Priemer C. Human adenovirus and human cytomegalovirus infections in preterm newborns: no association with bronchopulmonary dysplasia. Pediatr Res. 2002;52(2):219–224. doi: 10.1203/00006450-200208000-00014. [DOI] [PubMed] [Google Scholar]
- 92.Neuberger P., Hamprecht K., Vochem M. Case-control study of symptoms and neonatal outcome of human milk-transmitted cytomegalovirus infection in premature infants. J Pediatr. 2006;148(3):326–331. doi: 10.1016/j.jpeds.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 93.Capretti M.G., Lanari M., Lazzarotto T. Very low birth weight infants born to cytomegalovirus-seropositive mothers fed with their mother's milk: a prospective study. J Pediatr. 2009;154(6):842–848. doi: 10.1016/j.jpeds.2008.12.046. [DOI] [PubMed] [Google Scholar]
- 94.Coclite E., Di Natale C., Nigro G. Congenital and perinatal cytomegalovirus lung infection. J Matern Fetal Neonatal Med. 2013;26(17):1671–1675. doi: 10.3109/14767058.2013.794207. [DOI] [PubMed] [Google Scholar]
- 95.Kuijpers T.W., Vossen M.T., Gent M.R. Frequencies of circulating cytolytic, CD45RA+CD27-, CD8+ T lymphocytes depend on infection with CMV. J Immunol. 2003;170(8):4342–4348. doi: 10.4049/jimmunol.170.8.4342. [DOI] [PubMed] [Google Scholar]
- 96.Miles D.J., van der Sande M., Jeffries D. Cytomegalovirus infection in Gambian infants leads to profound CD8 T-cell differentiation. J Virol. 2007;81(11):5766–5776. doi: 10.1128/JVI.00052-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hassan J., Dooley S., Hall W. Immunological response to cytomegalovirus in congenitally infected neonates. Clin Exp Immunol. 2007;147(3):465–471. doi: 10.1111/j.1365-2249.2007.03302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Saurwein-Teissl M., Lung T.L., Marx F. Lack of antibody production following immunization in old age: association with CD8(+)CD28(-) T cell clonal expansions and an imbalance in the production of Th1 and Th2 cytokines. J Immunol. 2002;168(11):5893–5899. doi: 10.4049/jimmunol.168.11.5893. [DOI] [PubMed] [Google Scholar]
- 99.Lemanske R.F., Jr., Dick E.C., Swenson C.A. Rhinovirus upper respiratory infection increases airway hyperreactivity and late asthmatic reactions. J Clin Invest. 1989;83(1):1–10. doi: 10.1172/JCI113843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Simoes E.A., Carbonell-Estrany X., Rieger C.H. The effect of respiratory syncytial virus on subsequent recurrent wheezing in atopic and nonatopic children. J Allergy Clin Immunol. 2010;126(2):256–262. doi: 10.1016/j.jaci.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Stein R.T., Martinez F.D. Respiratory syncytial virus and asthma: still no final answer. Thorax. 2010;65(12):1033–1034. doi: 10.1136/thx.2009.133967. [DOI] [PubMed] [Google Scholar]
- 102.Broughton S., Thomas M.R., Marston L. Very prematurely born infants wheezing at follow-up: lung function and risk factors. Arch Dis Child. 2007;92(9):776–780. doi: 10.1136/adc.2006.112623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lee K.K., Hegele R.G., Manfreda J. Relationship of early childhood viral exposures to respiratory symptoms, onset of possible asthma and atopy in high risk children: the Canadian Asthma Primary Prevention Study. Pediatr Pulmonol. 2007;42(3):290–297. doi: 10.1002/ppul.20578. [DOI] [PubMed] [Google Scholar]
- 104.Broughton S., Roberts A., Fox G. Prospective study of healthcare utilisation and respiratory morbidity due to RSV infection in prematurely born infants. Thorax. 2005;60(12):1039–1044. doi: 10.1136/thx.2004.037853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jacob S.V., Coates A.L., Lands L.C. Long-term pulmonary sequelae of severe bronchopulmonary dysplasia. J Pediatr. 1998;133(2):193–200. doi: 10.1016/s0022-3476(98)70220-3. [DOI] [PubMed] [Google Scholar]
- 106.Greenough A., Alexander J., Boit P. School age outcome of hospitalisation with respiratory syncytial virus infection of prematurely born infants. Thorax. 2009;64(6):490–495. doi: 10.1136/thx.2008.095547. [DOI] [PubMed] [Google Scholar]
- 107.Sigurs N., Aljassim F., Kjellman B. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax. 2010;65(12):1045–1052. doi: 10.1136/thx.2009.121582. [DOI] [PubMed] [Google Scholar]
- 108.Collins P.L., Graham B.S. Viral and host factors in human respiratory syncytial virus pathogenesis. J Virol. 2008;82(5):2040–2055. doi: 10.1128/JVI.01625-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Graham B.S. Biological challenges and technological opportunities for respiratory syncytial virus vaccine development. Immunol Rev. 2011;239(1):149–166. doi: 10.1111/j.1600-065X.2010.00972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Genc M.R., Onderdonk A. Endogenous bacterial flora in pregnant women and the influence of maternal genetic variation. BJOG. 2011;118(2):154–163. doi: 10.1111/j.1471-0528.2010.02772.x. [DOI] [PubMed] [Google Scholar]
- 111.Kerk J., Dordelmann M., Bartels D.B. Multiplex measurement of cytokine/receptor gene polymorphisms and interaction between interleukin-10 (-1082) genotype and chorioamnionitis in extreme preterm delivery. J Soc Gynecol Investig. 2006;13(5):350–356. doi: 10.1016/j.jsgi.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 112.Bhandari V., Bizzarro M.J., Shetty A., Neonatal Genetics Study Group Familial and genetic susceptibility to major neonatal morbidities in preterm twins. Pediatrics. 2006;117(6):1901–1906. doi: 10.1542/peds.2005-1414. [DOI] [PubMed] [Google Scholar]
- 113.Floros J., Londono D., Gordon D. IL-18R1 and IL-18RAP SNPs may be associated with bronchopulmonary dysplasia in African-American infants. Pediatr Res. 2012;71(1):107–114. doi: 10.1038/pr.2011.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ali S., Hirschfeld A.F., Mayer M.L. Functional genetic variation in NFKBIA and susceptibility to childhood asthma, bronchiolitis, and bronchopulmonary dysplasia. J Immunol. 2013;190(8):3949–3958. doi: 10.4049/jimmunol.1201015. [DOI] [PubMed] [Google Scholar]
- 115.Wang H., St Julien K.R., Stevenson D.K. A genome-wide association study (GWAS) for bronchopulmonary dysplasia. Pediatrics. 2013;132(2):290–297. doi: 10.1542/peds.2013-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hagood J.S. Beyond the genome: epigenetic mechanisms in lung remodeling. Physiology (Bethesda) 2014;29(3):177–185. doi: 10.1152/physiol.00048.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Park J., Wick H.C., Kee D.E. Finding novel molecular connections between developmental processes and disease. PLoS Comput Biol. 2014;10(5):e1003578. doi: 10.1371/journal.pcbi.1003578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hoffmann T.J., Shaw G.M., Stevenson D.K. Copy number variation in bronchopulmonary dysplasia. Am J Med Genet A. 2014;164A(10):2672–2675. doi: 10.1002/ajmg.a.36659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Stouch A.N., Zaynagetdinov R., Barham W.J. IkappaB kinase activity drives fetal lung macrophage maturation along a non-M1/M2 paradigm. J Immunol. 2014;193(3):1184–1193. doi: 10.4049/jimmunol.1302516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lingappan K., Srinivasan C., Jiang W. Analysis of the transcriptome in hyperoxic lung injury and sex-specific alterations in gene expression. PLoS One. 2014;9(7):e101581. doi: 10.1371/journal.pone.0101581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sorensen G.L., Dahl M., Tan Q. Surfactant protein-D-encoding gene variant polymorphisms are linked to respiratory outcome in premature infants. J Pediatr. 2014;165(4):683–689. doi: 10.1016/j.jpeds.2014.05.042. [DOI] [PubMed] [Google Scholar]
- 122.Bhattacharya S., Zhou Z., Yee M. The genome-wide transcriptional response to neonatal hyperoxia identifies Ahr as a key regulator. Am J Physiol Lung Cell Mol Physiol. 2014;307(7):L516–L523. doi: 10.1152/ajplung.00200.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ambalavanan N., Cotten C.M., Page G.P. Integrated genomic analyses in bronchopulmonary dysplasia. J Pediatr. 2015;166(3):531–537.e13. doi: 10.1016/j.jpeds.2014.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Carrera P., Di Resta C., Volonteri C. Exome sequencing and pathway analysis for identification of genetic variability relevant for bronchopulmonary dysplasia (BPD) in preterm newborns: a pilot study. Clin Chim Acta. 2015 doi: 10.1016/j.cca.2015.01.001. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 125.Li J., Yu K.H., Oehlert J. Exome sequencing of neonatal blood spots identifies genes implicated in bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2015;192(5):589–596. doi: 10.1164/rccm.201501-0168OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dowling D.J., Levy O. Ontogeny of early life immunity. Trends Immunol. 2014;35(7):299–310. doi: 10.1016/j.it.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol. 2007;7(5):379–390. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- 128.Hillman N.H., Moss T.J., Nitsos I. Toll-like receptors and agonist responses in the developing fetal sheep lung. Pediatr Res. 2008;63(4):388–393. doi: 10.1203/PDR.0b013e3181647b3a. [DOI] [PubMed] [Google Scholar]
- 129.Ivarsson M.A., Loh L., Marquardt N. Differentiation and functional regulation of human fetal NK cells. J Clin Invest. 2013;123(9):3889–3901. doi: 10.1172/JCI68989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bruder D., Srikiatkhachorn A., Enelow R.I. Cellular immunity and lung injury in respiratory virus infection. Viral Immunol. 2006;19(2):147–155. doi: 10.1089/vim.2006.19.147. [DOI] [PubMed] [Google Scholar]
- 131.Adkins B., Leclerc C., Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol. 2004;4(7):553–564. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- 132.Marchant A., Goldman M. T cell-mediated immune responses in human newborns: ready to learn? Clin Exp Immunol. 2005;141(1):10–18. doi: 10.1111/j.1365-2249.2005.02799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhao Y., Dai Z.P., Lv P. Phenotypic and functional analysis of human T lymphocytes in early second- and third-trimester fetuses. Clin Exp Immunol. 2002;129(2):302–308. doi: 10.1046/j.1365-2249.2002.01920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Schultz C., Reiss I., Bucsky P. Maturational changes of lymphocyte surface antigens in human blood: comparison between fetuses, neonates and adults. Biol Neonate. 2000;78(2):77–82. doi: 10.1159/000014253. [DOI] [PubMed] [Google Scholar]
- 135.Ballow M., Cates K.L., Rowe J.C. Peripheral blood T-cell subpopulations in the very low birth weight (less than 1,500-g) infant. Am J Hematol. 1987;24(1):85–92. doi: 10.1002/ajh.2830240111. [DOI] [PubMed] [Google Scholar]
- 136.Series I.M., Pichette J., Carrier C. Quantitative analysis of T and B cell subsets in healthy and sick premature infants. Early Hum Dev. 1991;26(2):143–154. doi: 10.1016/0378-3782(91)90018-x. [DOI] [PubMed] [Google Scholar]
- 137.Kotiranta-Ainamo A., Apajasalo M., Pohjavuori M. Mononuclear cell subpopulations in preterm and full-term neonates: independent effects of gestational age, neonatal infection, maternal pre-eclampsia, maternal betamethason therapy, and mode of delivery. Clin Exp Immunol. 1999;115(2):309–314. doi: 10.1046/j.1365-2249.1999.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Suursalmi P., Kopeli T., Korhonen P. Very low birthweight bronchopulmonary dysplasia survivors show no substantial association between lung function and current inflammatory markers. Acta Paediatr. 2015;104(3):264–268. doi: 10.1111/apa.12837. [DOI] [PubMed] [Google Scholar]
- 139.Kuypers E., Collins J.J., Kramer B.W. Intra-amniotic LPS and antenatal betamethasone: inflammation and maturation in preterm lamb lungs. Am J Physiol Lung Cell Mol Physiol. 2012;302(4):L380–L389. doi: 10.1152/ajplung.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rosen D., Lee J.H., Cuttitta F. Accelerated thymic maturation and autoreactive T cells in bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2006;174(1):75–83. doi: 10.1164/rccm.200511-1784OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ryan R.M., Ahmed Q., D'Angelis C.A. Pediatric Academic Society; 2009. CD8+ T-lymphocytes in infants with bronchopulmonary dysplasia (BPD)http://www.abstracts2view.com/pasall/index.php E-PAS2009: 3858.137.Available at: Accessed September 9, 2015. [Google Scholar]
- 142.Ballabh P., Simm M., Kumari J. Lymphocyte subpopulations in bronchopulmonary dysplasia. Am J Perinatol. 2003;20(8):465–475. doi: 10.1055/s-2003-45387. [DOI] [PubMed] [Google Scholar]
- 143.Turunen R., Vaarala O., Nupponen I. Activation of T cells in preterm infants with respiratory distress syndrome. Neonatology. 2009;96(4):248–258. doi: 10.1159/000220764. [DOI] [PubMed] [Google Scholar]
- 144.Berrington J.E., Barge D., Fenton A.C. Lymphocyte subsets in term and significantly preterm UK infants in the first year of life analysed by single platform flow cytometry. Clin Exp Immunol. 2005;140(2):289–292. doi: 10.1111/j.1365-2249.2005.02767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Luciano A.A., Yu H., Jackson L.W. Preterm labor and chorioamnionitis are associated with neonatal T cell activation. PLoS One. 2011;6(2):e16698. doi: 10.1371/journal.pone.0016698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Scheible K.M., Emo J., Yang H. Developmentally determined reduction in CD31 during gestation is associated with CD8+ T cell effector differentiation in preterm infants. Clin Immunol. 2015 doi: 10.1016/j.clim.2015.07.003. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Duggan P.J., Maalouf E.F., Watts T.L. Intrauterine T-cell activation and increased proinflammatory cytokine concentrations in preterm infants with cerebral lesions. Lancet. 2001;358(9294):1699–1700. doi: 10.1016/s0140-6736(01)06723-x. [DOI] [PubMed] [Google Scholar]
- 148.Misra R.S., Shah S., Fowell D.J. Preterm cord blood CD4(+) T cells exhibit increased IL-6 production in chorioamnionitis and decreased CD4(+) T cells in bronchopulmonary dysplasia. Hum Immunol. 2015;76(5):329–338. doi: 10.1016/j.humimm.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kallapur S.G., Presicce P., Senthamaraikannan P. Intra-amniotic IL-1beta induces fetal inflammation in rhesus monkeys and alters the regulatory T cell/IL-17 balance. J Immunol. 2013;191(3):1102–1109. doi: 10.4049/jimmunol.1300270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sunday M.E., Yoder B.A., Cuttitta F. Bombesin-like peptide mediates lung injury in a baboon model of bronchopulmonary dysplasia. J Clin Invest. 1998;102(3):584–594. doi: 10.1172/JCI2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Scher H., Miller Y.E., Aguayo S.M. Urinary bombesin-like peptide levels in infants and children with bronchopulmonary dysplasia and cystic fibrosis. Pediatr Pulmonol. 1998;26(5):326–331. doi: 10.1002/(sici)1099-0496(199811)26:5<326::aid-ppul4>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 152.Culley F.J., Pollott J., Openshaw P.J. Age at first viral infection determines the pattern of T cell-mediated disease during reinfection in adulthood. J Exp Med. 2002;196(10):1381–1386. doi: 10.1084/jem.20020943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tregoning J.S., Yamaguchi Y., Harker J. The role of T cells in the enhancement of respiratory syncytial virus infection severity during adult reinfection of neonatally sensitized mice. J Virol. 2008;82(8):4115–4124. doi: 10.1128/JVI.02313-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tasker L., Lindsay R.W., Clarke B.T. Infection of mice with respiratory syncytial virus during neonatal life primes for enhanced antibody and T cell responses on secondary challenge. Clin Exp Immunol. 2008;153(2):277–288. doi: 10.1111/j.1365-2249.2008.03591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.You D., Ripple M., Balakrishna S. Inchoate CD8+ T cell responses in neonatal mice permit influenza-induced persistent pulmonary dysfunction. J Immunol. 2008;181(5):3486–3494. doi: 10.4049/jimmunol.181.5.3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gern J.E., Brooks G.D., Meyer P. Bidirectional interactions between viral respiratory illnesses and cytokine responses in the first year of life. J Allergy Clin Immunol. 2006;117(1):72–78. doi: 10.1016/j.jaci.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 157.Friedlander S.L., Jackson D.J., Gangnon R.E. Viral infections, cytokine dysregulation and the origins of childhood asthma and allergic diseases. Pediatr Infect Dis J. 2005;24(11 Suppl):S170–S176. doi: 10.1097/01.inf.0000187273.47390.01. [discussion: S174–5] [DOI] [PubMed] [Google Scholar]
- 158.Wang E.E., Law B.J., Robinson J.L. PICNIC (Pediatric Investigators Collaborative Network on Infections in Canada) study of the role of age and respiratory syncytial virus neutralizing antibody on respiratory syncytial virus illness in patients with underlying heart or lung disease. Pediatrics. 1997;99(3):E9. doi: 10.1542/peds.99.3.e9. [DOI] [PubMed] [Google Scholar]
- 159.DeVincenzo J.P., Wilkinson T., Vaishnaw A. Viral load drives disease in humans experimentally infected with respiratory syncytial virus. Am J Respir Crit Care Med. 2010;182(10):1305–1314. doi: 10.1164/rccm.201002-0221OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Houben M.L., Coenjaerts F.E., Rossen J.W. Disease severity and viral load are correlated in infants with primary respiratory syncytial virus infection in the community. J Med Virol. 2010;82(7):1266–1271. doi: 10.1002/jmv.21771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bosis S., Esposito S., Osterhaus A.D. Association between high nasopharyngeal viral load and disease severity in children with human metapneumovirus infection. J Clin Virol. 2008;42(3):286–290. doi: 10.1016/j.jcv.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Utokaparch S., Marchant D., Gosselink J.V. The relationship between respiratory viral loads and diagnosis in children presenting to a pediatric hospital emergency department. Pediatr Infect Dis J. 2011;30(2):e18–e23. doi: 10.1097/INF.0b013e3181ff2fac. [DOI] [PubMed] [Google Scholar]
- 163.Barnes M.J., Powrie F. Regulatory T cells reinforce intestinal homeostasis. Immunity. 2009;31(3):401–411. doi: 10.1016/j.immuni.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 164.Biswas A., Wilmanski J., Forsman H. Negative regulation of Toll-like receptor signaling plays an essential role in homeostasis of the intestine. Eur J Immunol. 2011;41(1):182–194. doi: 10.1002/eji.201040479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Jacquot A., Neveu D., Aujoulat F. Dynamics and clinical evolution of bacterial gut microflora in extremely premature patients. J Pediatr. 2011;158(3):390–396. doi: 10.1016/j.jpeds.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 166.Namba K., Hatano M., Yaeshima T. Effects of Bifidobacterium longum BB536 administration on influenza infection, influenza vaccine antibody titer, and cell-mediated immunity in the elderly. Biosci Biotechnol Biochem. 2010;74(5):939–945. doi: 10.1271/bbb.90749. [DOI] [PubMed] [Google Scholar]
- 167.Warner B.B., Hamvas A. Lungs, microbes and the developing neonate. Neonatology. 2015;107(4):337–343. doi: 10.1159/000381124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tracy M., Cogen J., Hoffman L.R. The pediatric microbiome and the lung. Curr Opin Pediatr. 2015;27(3):348–355. doi: 10.1097/MOP.0000000000000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bisgaard H., Hermansen M.N., Bonnelykke K. Association of bacteria and viruses with wheezy episodes in young children: prospective birth cohort study. BMJ. 2010;341:c4978. doi: 10.1136/bmj.c4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lohmann P., Luna R.A., Hollister E.B. The airway microbiome of intubated premature infants: characteristics and changes that predict the development of bronchopulmonary dysplasia. Pediatr Res. 2014;76(3):294–301. doi: 10.1038/pr.2014.85. [DOI] [PubMed] [Google Scholar]
- 171.Mourani P.M., Harris J.K., Sontag M.K. Molecular identification of bacteria in tracheal aspirate fluid from mechanically ventilated preterm infants. PLoS One. 2011;6(10):e25959. doi: 10.1371/journal.pone.0025959. [DOI] [PMC free article] [PubMed] [Google Scholar]