Abstract
Anti-Neutrophil Cytoplasmic Antibodies (ANCA)-associated vasculitides (AAV) are characterized by small vessel injury and in some cases granulomatous lesions and glomerular inflammation. The pathogenic bases of these clinical phenotypes are incompletely understood, but evidence from patients with AAV and other inflammatory diseases suggest a role for monocyte/macrophages in the perpetuation of tissue injury. Macrophage colony stimulating factor (M-CSF) is a promoter of monocyte recruitment and macrophage proliferation, involved in mesangial cell proliferation and experimental nephritis development. Serum concentrations of M-CSF mark and herald the onset of lupus nephritis. Plasma samples from 29 patients with AAV (18 granulomatosis with polyangiitis, GPA, 6 eosinophilic granulomatosis with polyangiitis, EGPA, and 5 microscopic polyangiitis, MPA) and from 10 healthy controls were collected together with clinical data. Patients with AAV had higher levels of M-CSF when compared to controls. M-CSF levels correlated positively with the BVAS, serum C-reactive protein and erythrocyte sedimentation rate, while haemoglobin correlated inversely with M-CSF. Patients with active renal disease had significantly higher levels of M-CSF when compared to the other subgroups. M-CSF levels did not differ between ANCA subserotypes and were not associated with the involvement of other organs. In conclusion, M-CSF is higher in patients with AAV and active nephritis and could contribute to the pathogenesis of these diseases. In addition, M-CSF could behave as a useful marker of renal involvement in AAV.
Keywords: M-CSF, ANCA, Vasculitis, Glomerulonephritis, Macrophage, Granulomatosis with polyangiitis, Microscopic polyangiitis, Eosinophilic granulomatosis with polyangiitis
1. Introduction
Renal involvement represents the most severe manifestation of ANCA-associated vasculitides (AAV) and the leading cause of chronic damage accrual [1], despite the lack of specific biomarkers to guide the Clinician’s diagnostic and therapeutic decisions [2]. Macrophage colony stimulating factor (M-CSF) is a promoter of monocyte recruitment and macrophage proliferation [3], [4]. Circulating levels of M-CSF are increased in patients with chronic renal failure, possibly as a consequence of immune dysregulation during uraemia, since M-CSF metabolism is independent of the renal function [5]. Furthermore, M-CSF is involved in mesangial cell proliferation and acts in synergy with LPS to prompt macrophage recruitment from the bloodstream in experimental nephritis [3], [4]. Previous studies also documented increased levels of M-CSF in sera and renal biopsies of patients with proliferative glomerulonephritis (GN) and in particular lupus nephritis (LN) [6], [7]. In addition, recent works showed that serum concentrations of M-CSF marked and heralded the onset of LN in two large lupus cohorts [6]. By contrast, no specific evidence is available to date about a role of M-CSF in AAV, despite pathogenic similarities between AAV and other forms of kidney inflammation, such as LN [8]. In this study, we thus aimed at evaluating a role of M-CSF as a biomarker of disease activity and organ involvement in AAV.
2. Methods
Upon informed consent, plasma samples from 29 patients with AAV (18 granulomatosis with polyangiitis, GPA, 6 eosinophilic granulomatosis with polyangiitis, EGPA, and 5 microscopic polyangiitis, MPA) and from 10 healthy controls were collected together with data regarding clinical history, disease activity (as estimated by the Birmingham Vasculitis Activity Score, BVAS) at the time of venepuncture, routine laboratory parameters and markers of renal involvement such as increased 24 h proteinuria and active urinary sediment. According to the clinical phenotype, AAV patients were divided in patients with active renal disease, patients with active disease without renal involvement, patients in remission with past history of renal involvement, patients in remission with no history of renal involvement. M-CSF plasma concentration was assessed by using a commercial assay (Human M-CSF Quantikine® ELISA Kit, R&D Systems, Minneapolis, MN). M-CSF levels were also standardized for GFR by defining a specific variable resulting from the product M-CSF*ln(GFR). Differences in normally distributed continuous variables (creatinine; glomerular filtration rate, GFR) among groups were measured by employing the ANOVA with Bonferroni’s correction, whereas non-normally distributed continuous variables (M-CSF) were analysed by using the Mann–Whitney U-test or the Kruskall–Wallis test for multiple comparisons. Correlations between continuous variables were assessed by using the Spearman’s test. Data are expressed as median (interquartile range), unless specifically indicated.
3. Results
The clinical and serological phenotypes of patients with AAV were equally distributed among disease groups (Table 1).
Table 1.
Clinical characteristics according to disease activity and renal involvement.
| Active renal disease | Active disease without renal involvement | History of renal involvement | No history of renal involvement | Total AAV | |
|---|---|---|---|---|---|
| Anti-PR3+ | 6 | 1 | 8 | 3 | 18 |
| Anti-MPO+ | 3 | 3 | 1 | 4 | 11 |
| GPA (N) | 6 | 1 | 8 | 3 | 18 |
| EGPA (N) | 1 | 2 | 0 | 3 | 6 |
| MPA (N) | 2 | 1 | 1 | 1 | 5 |
| Mean±SEM serum creatinine (mg/dl) | 2.40±0.64 | 0.83±0.06 | 1.21±0.24 | 0.88±0.11 | 1.47±0.25 |
| Mean±SEM glomerular filtration rate (ml/min) | 38.55±7.11⁎ | 92.78±9.85⁎ | 72.78±11.32 | 90.75±9.63⁎ | 68.49±6.34 |
p<0.05.
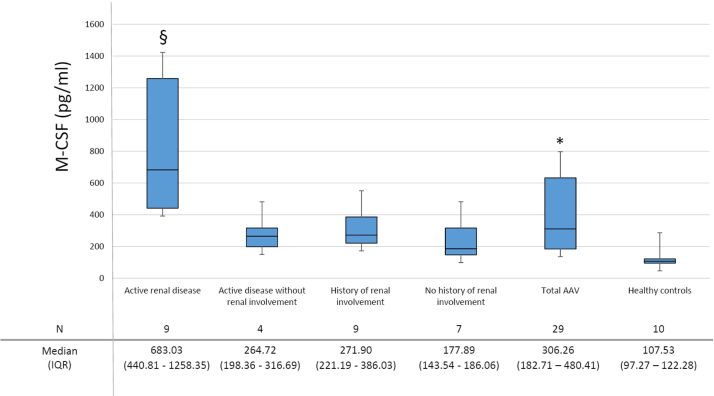
Patients with AAV had higher levels of M-CSF when compared to controls (Fig. 1). The levels of M-CSF correlated positively with erythrocyte sedimentation rate (ρ=0.521; p=0.022) and serum C-reactive protein (ρ=0.764; p<0.001) and negatively with haemoglobin (ρ=−0.552; p=0.012). Furthermore, M-CSF levels correlated positively with the BVAS (ρ=0.472; p=0.010).
Fig. 1.
Plasma M-CSF in AAV and healthy controls. M-CSF levels discriminate between patients with AAV and healthy controls. M-CSF levels are higher in patients with active renal disease. SEM: standard error of mean. ⁎p<0.001, significantly different from controls; §: p=0.014, p=0.019, p=0.002 and p<0.001, significantly different from patients with active disease without renal involvement, from patients in remission with a history of renal involvement, from patients in remission without a history of renal involvement and from healthy controls respectively.
Patients with active renal disease had significantly higher levels of M-CSF when compared to patients with active disease and no kidney involvement, with patients in remission with or without a history of renal involvement and with healthy controls (p=0.014; p=0.019; p=0.002; p<0.001 respectively; Fig. 1). Patients with renal involvement and in particular those with active renal disease had also higher creatinine levels and lower GFR (p<0.05 towards patients with active disease without renal involvement and patients in remission without history of renal involvement), as expected (Table 1). However, when M-CSF levels were standardized for GFR, the differences among groups were not significantly affected (p=0.001 between AAV patients and controls; p=0.064, p=0.031, p=0.007; p<0.001 when comparing AAV patients with active renal disease and patients with active disease without renal involvement, patients in remission with a history of renal involvement, patients in remission without a history of renal involvement and healthy controls respectively). Patients with active urinary sediment, significant 24 h proteinuria and typical findings at renal biopsy had also higher M-CSF levels although these findings did not reach statistical significance (Table 2).
Table 2.
M-CSF levels according to clinical features.
| Groups | N | M-CSF (pg/ml) | p |
|---|---|---|---|
| Vasculitis serotype | |||
| Anti-PR3 – vasculitis | 18 | 289.08 (197.36–622.47) | NS |
| Anti-MPO – vasculitis | 11 | 327.13 (159.49–432.75) | |
| Vasculitis phenotype | |||
| GPA | 18 | 289.08 (197.36–622.47) | NS |
| EGPA | 6 | 159.49 (107.71–325.95) | |
| MPA | 5 | 385.09 (327.13–480,41) | |
| Histological findingsa | |||
| Diagnostic renal biopsy | 8 | 460.61 (313.32–868.86) | NS |
| Diagnostic biopsy in non-renal tissues | 6 | 205.30 (121.00–284.99) | |
| Non-diagnostic biopsy | 7 | 231.75 (204.77–607.15) | |
| Urinary sediment in patients with renal involvement | |||
| Active | 3 | 1258.35 (251.77–581.72) | NS |
| Non active | 11 | 386.03 (792.74–1429.48) | |
| Presence of proteinuria in patients with renal involvement | |||
| Proteinuria present | 7 | 480.41 (383.97–970.69) | NS |
| Proteinuria absent | 7 | 271.90 (226.42–644.33) | |
| Pulmonary phenotypes | |||
| Alveolar haemorrhage | 3 | 231.65 (161.52–308.37) | NS |
| Nodular lesions without alveolitis | 11 | 386.03 (247.84–909.62) | |
In eight cases no biopsy was performed.
M-CSF levels did not discriminate AAV serotype (anti-PR3/MPO positive vasculitis) or phenotype (GPA, EGPA, MPA; Table 2), were similar between genders and did not identify any other type of organ involvement. In particular, circulating M-CSF in patients with vasculitic lung involvement were comparable to those of patients with nodular lesions only (Table 2).
Patients receiving prednisone alone at time of sample collection (all in the induction phase while waiting to start an immune suppressant) had significantly higher M-CSF than those taking prednisone in combination with immune suppressants (p=0.033). By contrast, M-CSF levels did not correlate with prednisone dose at venepuncture and were not significantly different among patients taking different immunosuppressive agents either for induction and maintenance of remission. Although the study was not designed for prospective evaluation, we also recorded data about disease activity at 6 months from venepuncture: patients with persistent non-remission or disease flare had slightly higher M-CSF levels, although the datum did not reach statistical significance (Table 3).
Table 3.
M-CSF levels according to therapeutic regimens and outcomes.
| Groups | N | M-CSF (pg/ml) | p |
|---|---|---|---|
| Prednisone at venepuncture | |||
| Steroid therapy | 21 | 297.69 (189.42–440.81) | NS |
| Steroid free | 8 | 350.42 (166.76–638.16) | |
| Therapeutic regimen at venepuncture | |||
| Prednisone+Immune suppressants | 22 | 251.77 (157.57–370.60) | 0.033 |
| Prednisone only | 4 | 869.38 (418.25–1343.92) | |
| Rituximab | 3 | 150.80 (121.09–211.35) | NS |
| Methotrexate | 9 | 221.19 (136.27–306.26) | |
| Azathioprine | 8 | 279.39 (187.74–399.73) | |
| Others (cyclophosphamide, mycophenolate) | 2 | 534.06 (459.57–608.54) | |
| Any therapy | 26 | 284.80 (179.10–427.12) | NS |
| No therapy | 3 | 902.63 (638.16–1232.80) | |
| Outcome at 6 monthsa | |||
| Remission | 20 | 251.82 (171.12–460.28) | NS |
| No remission/flare | 8 | 373.54 (278.57–545.07) | |
One patient lost at follow up.
4. Discussion
In this brief report, we first describe the presence of higher M-CSF levels in patients with AAV. M-CSF correlated with clinical and biochemical markers of systemic inflammation and showed a trend towards a higher expression in patients who eventually experienced a disease flare or who did not reach remission 6 months after sample collection. The highest M-CSF levels were detected in patients with active renal disease. Consistently, we also observed a trend towards a higher M-CSF expression in patients with active urinary sediment, significant 24 h proteinuria and typical histological findings at renal biopsy. Unfortunately, these data did not reach statistical significance, possibly because of the limited sample size. Patients with active renal disease had also signs of impaired renal function, as documented by lower GFR levels. When M-CSF levels were standardized for GFR only minimal differences were observed, which possibly reflect the common influence of renal inflammation on both variables [5]. Previous reports have in fact repeatedly described a general rise in circulating M-CSF in patients with renal disease [5] and a significantly higher local and systemic expression of M-CSF in patients with proliferative GN, class III and IV LN in particular [6], [7]. In this latter context, extensive studies, both in vitro and in vivo, dissected the precise role of M-CSF and its isoforms in prompting the recruitment of circulating monocytes into the glomerulus to cause GN [9].
Unfortunately, less is known about the pathogenic role of M-CSF in AAV. However, evidence from AAV disease models suggest that: (a) M-CSF plays a non-redundant role in experimental inflammatory (LPS-induced) nephritis [4]; (b) monocytes recruitment (possibly through M-CSF released from tubular epithelial cells) from the bloodstream is a shared pathogenic event in LN and AAV [8], [9]. We thus propose that M-CSF could play a pathogenic and clinical role as a marker in AAV with nephritic manifestations. We acknowledge the limitations of our study, in particular the small sample size, which warrants the validations of our data in another larger cohort. We are also aware that a prospective and multiparametric (i.e. including a pool of candidate cytokines in parallel) would better prove the specificity of M-CSF as a marker of renal inflammation in AAV, confirming and extending our current data.
5. Conclusions
To the best of our knowledge, this is the first study to explore M-CSF expression in AAV. This cytokine plays a fundamental role in macrophage recruitment at sites of renal inflammation, a crucial event in the development of proliferative GN. Our data, in line with those reported in similar pathological conditions, suggest a selective expression of M-CSF in AAV with active nephritis and a potential role of M-CSF as a universal marker of proliferative GN. Larger studies are warranted to confirm this hypothesis and further investigate the role of M-CSF in the pathogenesis of this group of diseases.
Conflict of interest statement
The authors declare that there is no conflict of interest in connection with this paper.
Funding sources
This work was supported by the Italian Ministry of Health (Ministero della Salute) through the Research Programme “Ricerca Finalizzata” (to A.A.M. and P.R.Q.).
References
- 1.Kallenberg C.G. Key advances in the clinical approach to ANCA-associated vasculitis. Nat. Rev. Rheumatol. 2014;10(8):484–493. doi: 10.1038/nrrheum.2014.104. Aug. [DOI] [PubMed] [Google Scholar]
- 2.Luqmani R.A. State of the art in the treatment of systemic vasculitides. Front. Immunol. 2014:5. doi: 10.3389/fimmu.2014.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamilton T.A., Zhao C., Pavicic P.G., Jr., Datta S. Myeloid colony-stimulating factors as regulators of macrophage polarization. Front. Immunol. 2014;5:554. doi: 10.3389/fimmu.2014.00554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Utsunomiya Y., Omura K., Yokoo T., Imasawa T., Kawamura T., Abe A. Macrophage-colony stimulating factor (M-CSF) enhances proteinuria and recruitment of macrophages into the glomerulus in experimental murine nephritis. Clin. Exp. Immunol. 1996;106(2):286–296. doi: 10.1046/j.1365-2249.1996.d01-831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le Meur Y., Fixe P., Aldigier J.C., Leroux-Robert C., Praloran V. Macrophage colony stimulating factor involvement in uremic patients. Kidney Int. 1996;50(3):1007–1012. doi: 10.1038/ki.1996.402. [DOI] [PubMed] [Google Scholar]
- 6.Menke J., Amann K., Cavagna L., Blettner M., Weinmann A., Schwarting A. Colony Stimulating Factor 1: a potential biomarker for lupus nephritis. J. Am. Soc. Nephrol. 2014 doi: 10.1681/ASN.2013121356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Isbel N.M., Nikolic-Paterson D.J., Hill P.A., Dowling J., Atkins R.C. Local macrophage proliferation correlates with increased renal M-CSF expression in human glomerulonephritis. Nephrol. Dial. Transplant.: Off. Publ. Eur. Dial. Transpl. Assoc. – Eur. Ren. Assoc. 2001;16(8):1638–1647. doi: 10.1093/ndt/16.8.1638. [DOI] [PubMed] [Google Scholar]
- 8.Hamano Y., Abe M., Matsuoka S., Zhang D., Kondo Y., Kagami Y. Susceptibility quantitative trait loci for pathogenic leucocytosis in SCG/Kj mice, a spontaneously occurring crescentic glomerulonephritis and vasculitis model. Clin. Exp. Immunol. 2014;177(1):353–365. doi: 10.1111/cei.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Menke J., Rabacal W.A., Byrne K.T., Iwata Y., Schwartz M.M., Stanley E.R. Circulating CSF-1 promotes monocyte and macrophage phenotypes that enhance lupus nephritis. J. Am. Soc. Nephrol. 2009;20(12):2581–2592. doi: 10.1681/ASN.2009050499. [DOI] [PMC free article] [PubMed] [Google Scholar]