Abstract
Acute and severe anemia of inflammation (AI) is a common complication of various clinical syndromes, including fulminant infections, critical illness with multiorgan failure, and exacerbations of autoimmune diseases. Building on recent data showing beneficial results with isocitrate treatment for chronic low-grade AI in a rat model, we used a mouse model of acute and severe AI induced by intraperitoneal heat-killed Brucella abortus to determine if isocitrate would be effective in this more stringent application. Inflamed mice treated with isocitrate developed an early but transient improvement in hemoglobin compared to solvent-treated controls, with a robust improvement on day 7, and only a trend towards improvement by day 14. Reticulocyte counts were increased in treated mice transiently, with no significant difference by day 21. Serum erythropoietin (EPO) levels were similar in treated versus control mice, indicating that isocitrate increased sensitivity to EPO. Serum and tissue iron levels showed no significant differences between the treated and control mice, ruling out improved iron availability as the cause of the increased response to endogenous EPO. Compared to the milder rat model, much higher doses of isocitrate were required for a relatively modest benefit.
INTRODUCTION
Anemia of inflammation (AI) is a feature of a broad spectrum of inflammatory disorders, including connective tissue diseases, infections, certain malignancies, and chronic kidney disease [1]. The phenotype of AI is typically a normocytic normochromic anemia with a shortened erythrocyte lifespan and suppressed erythropoiesis despite adequate levels of circulating erythropoietin (EPO) [2]. Perhaps the most characteristic feature of AI is a dysregulation of systemic iron homeostasis characterized by hypoferremia despite intact iron stores [1], resulting in decreased availability of iron for hemoglobin synthesis and erythrocyte production. Evolutionarily, this inflammatory response limited iron availability to microbes during infections.
The principal regulator of iron homeostasis is hepcidin, a 25-amino acid peptide hormone produced primarily by hepatocytes [3]. Hepcidin acts by binding to ferroportin, the sole known cellular iron exporter that is expressed on the surface of macrophages, hepatocytes, and the basolateral membranes of enterocytes. Hepcidin binding causes ferroportin endocytosis and degradation [4], inhibiting enteral iron absorption and the efflux of cellular iron necessary for erythropoiesis. Excessive hepcidin production causes hypoferremia and iron sequestration in macrophages, as seen in transgenic mice with hepcidin overexpression [5] and in the human genetic syndrome iron-refractory iron-deficiency anemia (IRIDA) that occurs due to mutations in matriptase-2/TMPRSS6 [6]. During inflammation or infection, hepcidin expression is strongly stimulated, largely by IL-6 [7] via the JAK-STAT pathway [8–10].
Our group recently published a detailed characterization of a mouse model of acute and severe inflammation that served as the platform for the current studies [11]. With a single intraperitoneal injection of Brucella abortus (BA), mice develop a severe anemia with a hemoglobin nadir at day 14, iron restriction despite increased tissue iron stores, erythropoietic suppression, and a shortened erythrocyte lifespan. Hepcidin ablation causes a partial correction of the resulting anemia, accompanied by an accelerated recovery [11]. In short, this model displays all the major characteristics seen in AI, and is an effective vehicle for testing any potential interventions for acute and severe AI.
Recent in vitro and murine studies have shown beneficial results with isocitrate treatment for anemia of inflammation. In particular, Richardson et al. showed a significant and persistent attenuation of anemia in a rat arthritis model of moderate chronic AI with a short course of isocitrate treatment [12]. Building on these promising data, the current study uses the BA mouse model to investigate the potential therapeutic use of isocitrate in the context of a very acute and severe inflammation. We examined the effects of isocitrate treatment on hemoglobin levels and erythropoiesis, as well as the mechanistic contributions of EPO production, changes in iron status, and inflammation.
METHODS
Animal models
All animal studies were approved by the Animal Research Committee at University of California, Los Angeles (UCLA). Only male mice were used in the study to avoid the effects of gender-related differences in iron parameters and hepcidin [13]. C57BL/6J mice were obtained from Charles River Laboratories (Wilmington, MA) or The Jackson Laboratories (Bar Harbor, ME). Wild-type (WT) mice were maintained on a standard chow (approx. 270 ppm iron, Harlan Teklad, Indianopolis, IN) until 6 weeks of age and they were then switched to an iron-adequate diet (50 ppm iron, Harlan Teklad) for 14–15 days before injection of BA. The same iron-adequate diet was continued through the remainder of the experiment. The dietary conditioning was applied because the high iron content of standard chow maximally stimulates hepcidin expression and renders it unresponsive to inflammatory stimuli [7], and because dietary iron absorption in humans accounts for ~5–10% of the daily iron fluxes but as much as ~50% in mice fed standard chow [14]. High dietary iron absorption in mice may obscure the contribution of iron recycling by macrophages [15] and lead to progressive iron loading. Reducing the dietary iron content of mouse chow was designed to model iron fluxes of human homeostasis.
To induce AI, animals were injected intraperitoneally (IP) with 5 × 108 particles/mouse of heat-killed Brucella abortus (BA; lots 5-1101 and 5-1304; US Department of Agriculture, Animal and Plant Health Inspection Service, National Veterinary Services Laboratories) as previously described [16]. Daily IP injections of 100 mg/kg/d of trisodium isocitrate (Sigma-Aldrich; Saint Louis, MO) dissolved in 200 μL 0.9% saline, or equivalent volumes of 0.9% saline solution, were initiated 3 days prior to Brucella abortus injection and continued for a total of 9 days). Mice were euthanized at 3 time points (7 d, 14 d, 21 d) and blood, liver, and spleen were collected at necropsy. See Supplementary Figure 1 for the experimental timeline diagram. For each time point, 8 isocitrate-treated and 8 saline-treated mice came from the same cohort and were processed simultaneously to minimize cohort-to-cohort variability. A second set of 8 isocitrate-injected and 8 saline-injected mice was analyzed at 7 d to confirm the significant hematologic differences seen at that time point. A final set of 16 mice was analyzed in order to evaluate the effect of isocitrate in uninflamed mice. Saline-injected mice were treated with either isocitrate or saline, then analyzed at day 7.
Hematologic studies
Complete blood counts were obtained with a HemaVet blood analyzer (Drew Scientific; Waterbury, CT). To assess iron-restricted erythropoiesis, zinc protoporphyrin (ZPP) was measured using a hematofluorometer (AVIV; Lakewood, NJ).
Reticulocytes were counted by flow cytometry. Blood (5 μl) was added to 1 ml of thiazole orange in phosphate buffered saline with 0.1% sodium azide (PBS-azide, BD Bioscience; San Jose, CA) and incubated at room temperature for 1–3 h. As an unstained control, blood was added to PBS-azide without thiazole orange. The percentage of red-fluorescent reticulocytes (Retic %) was measured by flow cytometry at the UCLA Jonsson Comprehensive Cancer Center (JCCC) and Center for AIDS Research Flow Cytometry Core Facility that is supported by National Institutes of Health awards CA-16042 and AI-28697, and by the JCCC, the UCLA AIDS Institute, and the David Geffen School of Medicine at UCLA. Unstained controls were used to establish a gate to exclude background fluorescence. The results are expressed as the reticulocyte count (M/μL) = RBC (M/μL) X Retic %. Spleen weight measurements were also obtained from a large subset of the mice (7–12 mice per treatment group per time point) as a marker of extramedullary erythropoiesis.
Measurement of iron parameters, serum EPO, and serum lactate dehydrogenase (LDH)
Serum iron and liver and spleen non-heme iron concentrations were determined by a colorimetric assay for iron quantification (Sekisui Diagnostics; Lexington, MA) as previously described [14]. Serum EPO was measured from a subset of the mice (7–8 mice per treatment group per time point) using a solid-phase enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions (R&D; Minneapolis, MN). Serum LDH was measured from a subset of the mice (4–6 mice per treatment group per time point) at our Diagnostic Lab Services core facilities (Division of Laboratory Animal Medicine; University of California, Los Angeles).
RNA isolation and real-time quantitative PCR
Total liver RNA was isolated from a subset of the mice (7–8 mice per treatment group per time point), and analyzed by real-time RT-PCR as described previously [17]. Primers are listed in Supplementary Table 1.
Statistics
SigmaStat 12.5 was used for all statistical analyses (Systat Software; Point Richmond, CA). Normally distributed data were compared using Student’s t-test. We used the resulting one-tailed p values to evaluate the data, as our experiments were specifically designed to test whether we could replicate the beneficial effects of isocitrate seen in the Goldfarb study [12] in a mouse model of acute inflammation. Measurements that were not normally distributed were compared by the nonparametric Mann-Whitney rank sum test. P<0.05 was considered statistically significant.
RESULTS
Isocitrate treatment improves anemia and increases reticulocytosis in a mouse model of acute and severe inflammation
We employed our previously characterized BA mouse model of AI in order to investigate the effects of isocitrate on the anemia of acute and severe inflammation [11]. For the current studies, groups of BA-injected mice were treated with multiple injections of isocitrate or saline, then sacrificed and analyzed at various time points. A pilot trial was performed using 3 injections of isocitrate 400 mg/kg/d, double the weight-based dose used in the previous Richardson et al. study that showed anemia attenuation with isocitrate in a rat model of chronic inflammation [12]. As this pilot trial yielded no increase in hemoglobin (Hgb) or reticulocyte counts [data not shown], we proceeded with a more intensive treatment regimen using 9 daily injections of isocitrate 1000 mg/kg/d that began 3 days prior to BA injection.
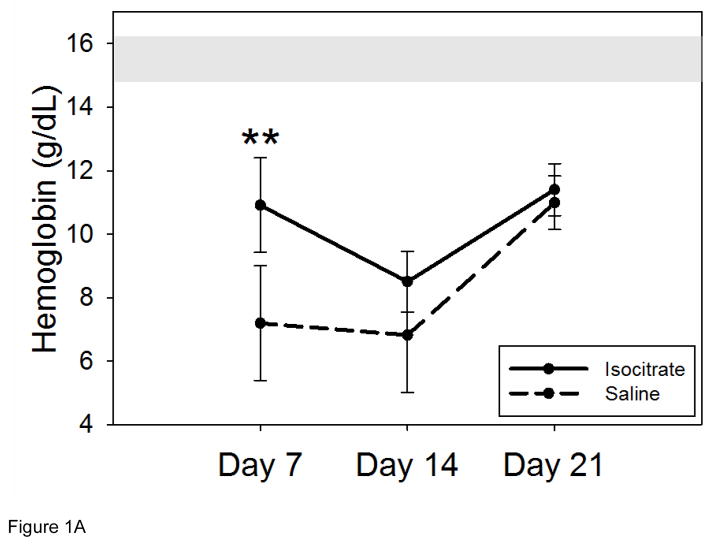
Inflamed mice treated with this regimen of isocitrate developed a milder anemia at 7 d than saline-treated mice (Figure 1A, mean isocitrate Hgb 10.9 g/dL vs. saline Hgb 7.2 g/dL; p<0.001). This benefit was transient and no longer significant by 14 d, although there was still a trend towards milder anemia at 14 d (median isocitrate Hgb 8.6 g/dL vs. saline Hgb 7.0 g/dL; p=0.094). After the expected Hgb nadir of both groups at 14 d, both treated and untreated groups had partially recovered their Hgb levels by 21 d (mean isocitrate Hgb 11.4 g/dL vs. saline Hgb 11.0 g/dL). In order to explore the possibility that isocitrate alone could affect Hgb without inflammation, we repeated the 7 d experiment using mice that were not injected with BA. These saline-injected mice were then treated with either isocitrate or saline and analyzed at 7 d. The treatment groups had no difference in Hgb levels (Supplementary Figure 2), eliminating the possibility that isocitrate may have an effect on erythropoiesis in the absence of inflammation.
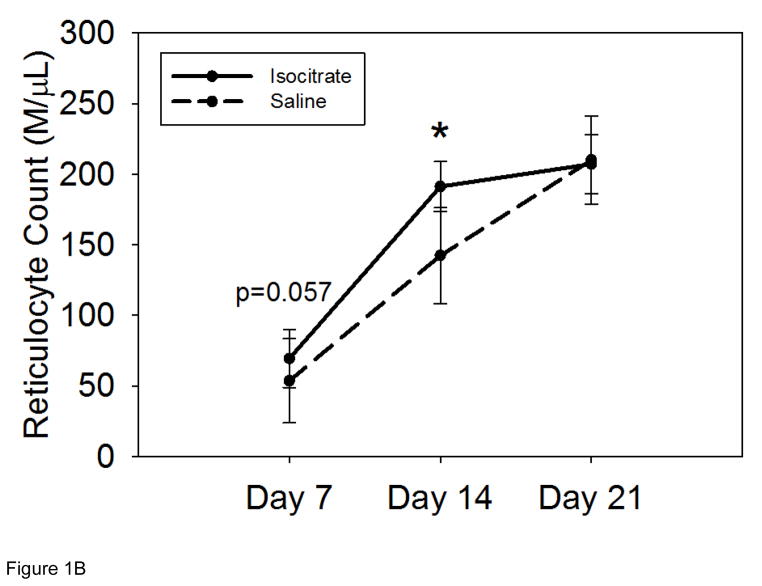
Figure 1. Isocitrate treatment improves anemia and increases reticulocytosis in a mouse model of acute and severe inflammation.
Compared to BA-injected saline treated mice, BA-injected isocitrate-treated mice develop: (A) an early and transient increase in hemoglobin at day 7, with an eventual loss of the attenuation of anemia by day 21; (B) a trend towards increased reticulocytosis at day 7, followed by a transient increase in reticulocytosis at day 14. The gray band in (A) indicates the expected baseline hemoglobin range based on the age, diet, and gender of the mice. Saline and isocitrate groups each included 7–14 evaluable male mice per time point. Means ± SD are shown, **p<0.001 for comparison of treated and control groups; p by t-test or Mann-Whitney rank sum test.
Reticulocyte measurements were obtained to quantify the production of new erythrocytes in isocitrate-treated and saline-control mice. Isocitrate treatment yielded increased reticulocytosis at 14 d (Figure 1B, mean isocitrate reticulocyte count 191 M/μL vs. saline reticulocyte count 142 M/μL; p<0.05), with a trend towards increased reticulocytosis at 7 d (Figure 1B, mean isocitrate 69 M/μL vs. mean saline 53 M/μL; p=0.057) and no significant difference between the treatment and control groups at the later time point of 21 d. At 7 d, the reticulocyte counts of both groups had begun responding to the fall in Hgb. The reticulocyte counts then rose steadily until 21 d (mean isocitrate 207 M/μL vs. saline 210 M/μL), contributing to the recovery from anemia seen in both groups at this time point.
In order to explore the potential contribution of erythrocyte destruction to the difference in Hgb of saline control and isocitrate-treated mice, we measured serum LDH levels of treated and untreated inflamed mice at each time point. LDH levels were increased in all mice as compared to normal [18], although there were no significant differences between control and isocitrate-treated mice at any time point (Supplementary Figure 3). This lack of difference does not confirm the contribution of shortened erythrocyte lifespan to the early anemia improvement in treated mice, but the increase in LDH could also be secondary to increased turnover of other cell types, including leukocytes and hepatocytes [19, 20].
The early attenuation of anemia seen in isocitrate-treated mice is not due to increased serum EPO levels
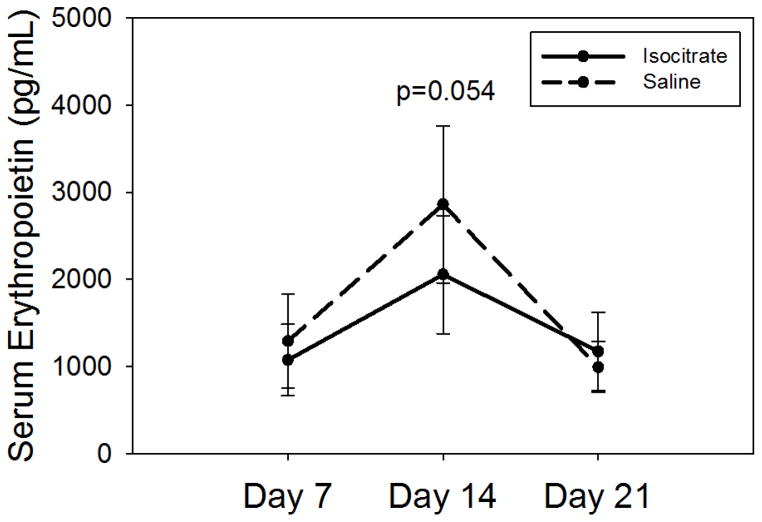
In order to investigate potential etiologies of increased reticulocytosis and increased Hgb in treated mice, we measured serum EPO levels. There was no significant difference in the EPO levels of the two groups at any time point, and certainly no indication that EPO levels were increased in the isocitrate group. At 14 d, saline-treated mice showed a weak trend towards increased EPO levels (Figure 2, median isocitrate EPO 1891 pg/mL vs. saline EPO 2458 pg/mL; p=0.054), which is likely a response to their lower Hgb as compared to treated mice. The absence of an increase in serum EPO levels in isocitrate-treated mice effectively eliminates increased EPO levels as a significant contributor to the increased erythropoiesis seen in treated mice.
Figure 2. Isocitrate-treated mice do not have increased serum erythropoietin levels.
Compared to control mice, isocitrate-treated mice do not have elevated serum EPO levels at any time point, with a trend towards decreased EPO levels at day 14. Saline and isocitrate groups each included 7–8 male mice per time point. Means ± SD are shown, p by t-test or Mann-Whitney rank sum test.
Spleen weight measurements of treated and untreated mice were also performed at each time point as a marker of extramedullary erythropoiesis. While both groups had increased spleen weights at the later time points, likely as a result of increased erythropoiesis in response to anemia, there was no significant difference between the isocitrate-treated and untreated groups at each time point (Supplementary Figure 4).
Isocitrate treatment does not significantly alter iron status in this BA mouse model
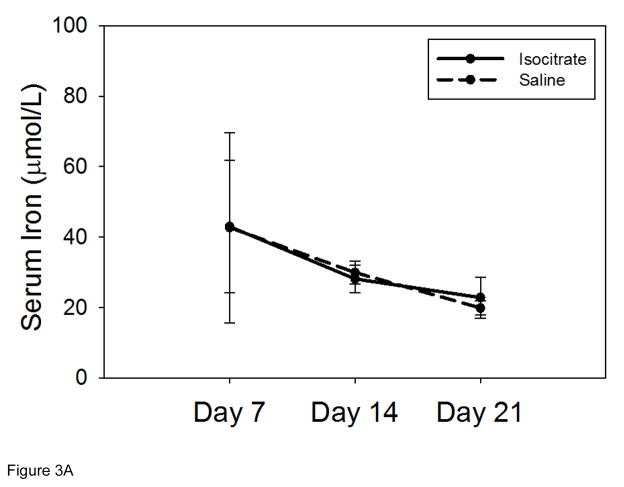
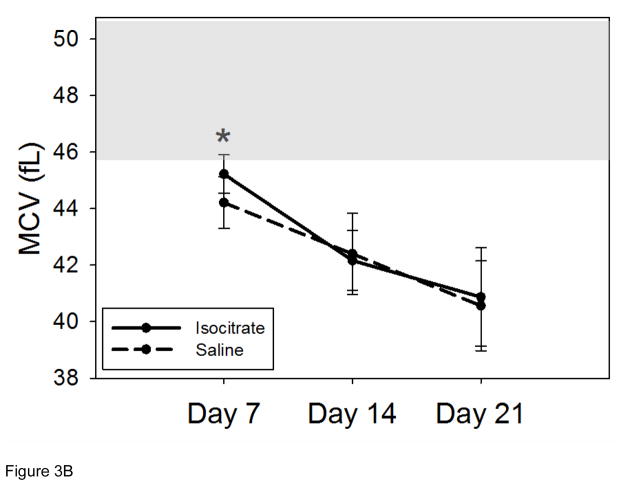
Serum iron concentrations in the isocitrate-treated and saline-treated groups remained similar at all time points (Figure 3A). Mean corpuscular volume (MCV) measurements were obtained to evaluate for microcytosis as a marker of iron restriction [21]. Aside from a negligible increase in MCV in treated mice at day 7 (Figure 3B, mean isocitrate MCV 45.2 fL vs. saline MCV 44.2 fL; p<0.05), there was no significant difference in the MCV measurements of the isocitrate and control groups. The early small increase in MCV of isocitrate-treated mice is likely secondary to the subtle increase in reticulocytosis. Mean corpuscular hemoglobin (MCH) measurements of peripheral red blood cells showed no significant difference between treatment groups at any time point (Supplementary Figure 5). Given the negligible difference between the mean hemoglobin content of peripheral reticulocytes (CHr) and the MCH of mature erythrocytes [22], the increased MCV is most consistent with an increase in the larger reticulocyte population rather than a change in erythrocyte iron content.
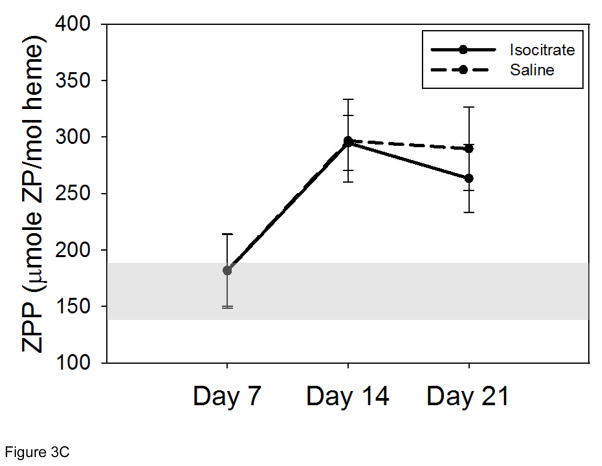
Figure 3. Isocitrate treatment does not affect circulating iron levels.
(A) Isocitrate mice and control mice have similar serum iron levels at all time points. (B) Isocitrate mice and control mice have similar mean corpuscular volumes, with an insignificant difference at day 7. (C) Isocitrate mice and control mice have similar zinc protoporphyrin levels at all time points. The light gray bands in (B) and (C) indicate the expected baseline ranges based on the age, diet, and gender of the mice. Saline and isocitrate groups each included 7–16 evaluable male mice per time point. Means ± SD are shown, *p<0.05 for comparison of treated and control groups; p by t-test or Mann-Whitney rank sum test.
Erythrocyte ZPP levels were measured to further assess iron-restricted erythropoiesis (Figure 3C). When insufficient iron is available for erythropoiesis, increased amounts of zinc are substituted and incorporated into the protoporphyrin ring [23]. The treated and control groups had similar ZPP levels at all time points, confirming that available circulating iron levels are unchanged with isocitrate treatment.
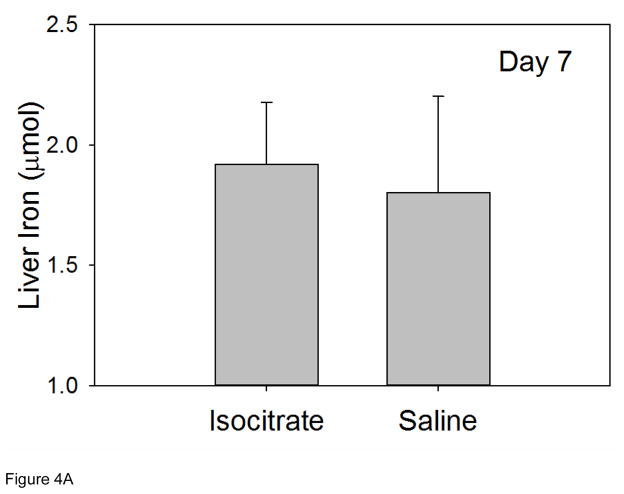
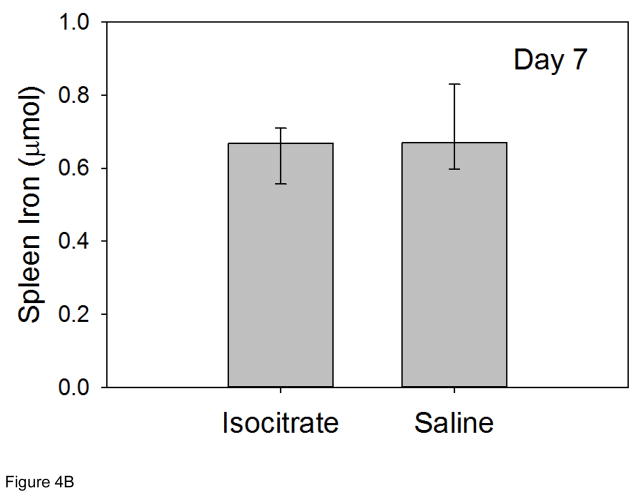
Tissue iron levels were measured in mouse livers and spleens at 7 d in order to assess iron stores at the time point with the most significant isocitrate-related improvement in anemia. Liver iron levels at 7 d were similar in isocitrate and saline groups, indicating no change in hepatocyte iron storage with isocitrate treatment (Figure 4A). Spleen iron levels at 7 d were also unchanged with isocitrate treatment, indicating no difference in macrophage iron retention (Figure 4B). The lack of any significant difference in either circulating iron levels or tissue iron stores indicates that iron status is not a significant contributor to the early improvement of AI that we see in this mouse model.
Figure 4. Isocitrate treatment does not affect tissue iron stores.
At day 7, treated and control mice have similar levels of (A) liver iron; (B) spleen iron. Saline and isocitrate groups each included 15–16 evaluable male mice per time point. Panel A: Means ± SD are shown, p by t-test; Panel B: Medians ± 75th/25th percentile are shown, p by Mann-Whitney rank sum test.
Changes in hepcidin expression and/or inflammation are not responsible for the early partial reversal of AI with isocitrate treatment
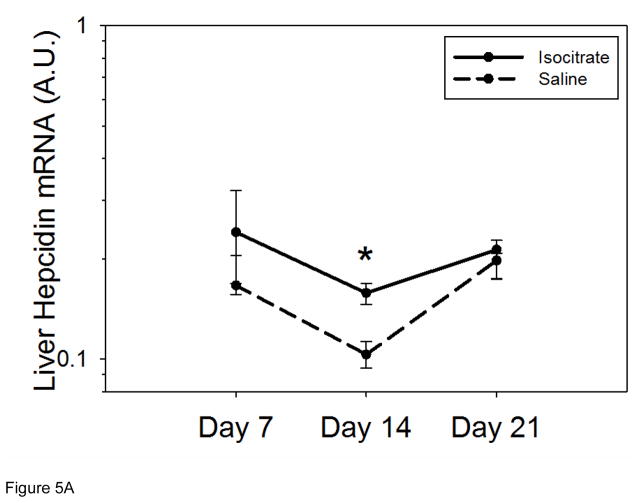
Liver hepcidin mRNA was measured in order to investigate whether suppressed hepcidin expression, and thus increased iron availability for Hgb synthesis, could be contributing to the anemia attenuation seen with isocitrate treatment. At 7 d and 21 d, the treated and untreated groups had similar hepcidin mRNA levels (Figure 5A). At 14 d, the saline control mice had lower hepcidin expression (median isocitrate hepcidin mRNA 0.158 vs. saline hepcidin mRNA 0.103; p<0.05). Thus, the early increase in Hgb with isocitrate treatment is not secondary to suppressed hepcidin expression and increased iron availability.
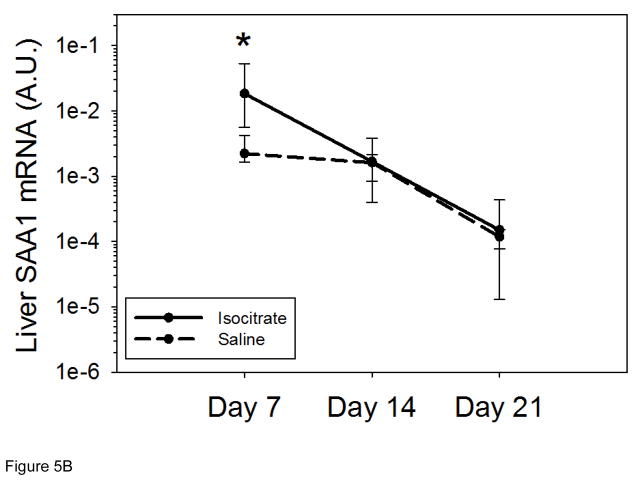
Figure 5. Isocitrate treatment does not suppress hepcidin or SAA1 expression.
(A) Isocitrate mice do not develop decreased hepcidin expression compared to control mice, and have a small increase at day 14. (B) Isocitrate-treated mice have an early and transient increase in SAA1 expression at day 7, as compared to saline-treated mice. Saline and isocitrate groups each included 7–8 male mice per time point. Medians ± 75th/25th percentile are shown, *p<0.05 for comparison of treated and control groups; p by t-test or Mann-Whitney rank sum test.
We also measured serum amyloid A1 (SAA1) mRNA, a marker of the acute phase response which like hepcidin, is regulated by IL-6 [24]. As erythropoietic suppression by IL-6 and other cytokines has been shown to contribute to AI in other mouse models of inflammation [25, 26], we measured this inflammatory marker to examine whether isocitrate affected the intensity of the cytokine response. At 14 d and 21 d, the treated and untreated groups had similar levels of SAA1 mRNA (Figure 5B). At 7 d, isocitrate-treated mice actually had increased SAA1 expression (median isocitrate SAA1 mRNA 0.0184 vs. saline SAA1 mRNA 0.0022; p<0.05), possibly secondary to multiple high-dose injections of an exogenous compound causing mild irritation and inflammation. Consistent with possibly causing a stimulatory effect of isocitrate on early inflammation, the isocitrate-treated mice had increased white blood cell (WBC) counts and neutrophil percentages on day 7, as compared to control mice (Supplementary Figure 6A,B). In contrast, isocitrate-treated mice had decreased percentages of blood monocytes, which have a smaller role in the initial acute inflammatory response (Supplementary Figure 6C). The increased hepcidin mRNA levels seen in isocitrate-treated mice on day 14 is consistent with an early rise in IL-6, and thus SAA1, activity and inflammation subsequently promoting hepcidin expression. Thus, isocitrate-mediated suppression of inflammation or hepcidin expression does not appear to be the mechanism of the early increase in Hgb seen in treated mice.
Discussion
Anemia of inflammation is a mild to moderate anemia seen in the context of infections, critical illness, and systemic inflammatory disorders, including rheumatologic disorders, inflammatory bowel diseases, malignant neoplasms, and chronic kidney disease [1]. The pathogenesis of AI is multifactorial, including hepcidin-mediated iron restriction, impaired proliferation of erythroid precursors, resistance to EPO, and shortened erythrocyte lifespan [1, 27], and may be further exacerbated by blood loss and iron deficiency.
Recent studies reported that the enzyme aconitase and its substrate isocitrate play a role in erythropoiesis and AI. Aconitase, an iron-sulfur cluster containing protein, interconverts the metabolites citrate and isocitrate [28], and its inactivation leads to decreased isocitrate production and an inhibition of erythroid growth and differentiation [29]. Bullock et al. found that exogenous isocitrate administration in vivo abrogated anemia in iron-deprived mice [29]. They postulated that iron restriction destabilizes the aconitase iron-sulfur clusters and induces the assembly of a signaling complex that involves protein kinase C (PKC) hyperactivation and impairs EPO responsiveness, and that isocitrate rescue may stabilize the iron-sulfur clusters and prevent the assembly of this inhibitory signaling complex [29]. In another paper, they found that in vitro aconitase inhibition caused a blockade in EPO signal transmission via ERK, likely by disturbing the ERK-aconitase physical interaction, leading to impaired erythroid proliferation and maturation [30].
Richardson et al. recently used a rat arthritis model of moderately severe chronic AI to test the effects of isocitrate treatment [12]. This group found that a short 3-day course of isocitrate injections caused a significant and persistent improvement in anemia. Treatment of iron restricted primary human progenitors with isocitrate conferred resistance to the inhibitory effects of IFN-γ and TNF-α on differentiation and proliferation. PU.1 is a key transcription factor in promoting myeloid differentiation at the expense of erythroid lineage commitment [31] during times of inflammation. Richardson et al. found that the combination of inflammation and iron restriction upregulated PU.1 and that isocitrate treatment prevented this upregulation.
As isocitrate treatment appeared to have a beneficial effect on a rat model of chronic AI, we investigated its effects in acute and severe inflammation in a recently characterized mouse model of AI induced with heat-killed Brucella abortus [11]. As expected, saline-treated mice developed a severe anemia by 7 d, with a Hgb nadir at 14 d followed by significant but partial recovery by 21 d. Our pilot study using double the weight-based dosing regimen of Richardson et al. [12] demonstrated no effects of isocitrate on erythropoiesis in our model, and an eventual dose of 15-fold the dosing regimen used by Richardson et al. was required to observe a significant effect in our AI model. We did not test any higher doses as we reached the point of toxicity as evidenced by increased inflammation in the isocitrate-treated mice. Isocitrate treatment caused an early increase in Hgb of almost 4 g/dL, but in contrast to the persistent improvement seen in the rat model of chronic arthritis, this was a transient effect. Although there was still a trend towards increased Hgb in treated mice at 14 d, the difference between isocitrate-treated and saline-treated mice completely dissipated by 21 d. Reticulocyte measurements showed a trend towards an increase in the number of immature erythrocytes in isocitrate-treated mice at 7 d with a significant increase by 14 d, but the lack of difference in Hgb concentrations at day 21 indicates that the increased reticulocytosis was not sufficient to cause sustained Hgb improvement. Our previous characterization of the BA model revealed a shortened erythrocyte lifespan, partially secondary to microangiopathic hemolysis, and a second group implicated increased hemophagocytosis as one etiology of this RBC destruction [32]. Peripheral blood smears showed that hemolysis peaked at 14 d, demonstrated by increased schistocytosis and erythrocyte membrane irregularities indicative of membrane damage. Thus, the robust erythrocyte destruction at 14 d may be counteracting the persistent increase in reticulocytosis in our isocitrate-treated mice, resulting in a dampening of the Hgb improvement by this time point. While our mouse serum LDH levels did not differ between treated and untreated groups, it remains possible that any difference in LDH related to erythrocyte destruction is masked by the overall increased turnover of other cell types.
As serum EPO levels were unchanged in the isocitrate versus saline groups, EPO responsiveness rather than EPO production may play a role in this enhanced erythropoiesis. Our group previously showed that the BA model is characterized by depressed erythropoiesis, as evidenced by a transient suppression of reticulocyte counts despite adequate levels of circulating EPO [11]. This blunted response of the bone marrow has been seen in other inflammatory mouse models, including a turpentine-induced sterile abscess model [26] and a chronic IFN-γ overproduction model [25]. Isocitrate could have transiently improved AI in our mouse model by reversing the dampened erythropoietic response that occurs in the context of inflammation. The lack of difference in spleen weights of treated and untreated mice that we see in our study could indicate that the increased erythropoiesis is predominantly an intramedullary process, or that the increased extramedullary erythropoiesis could have occurred at an unexamined time point.
Our study showed no significant differences in either the circulating iron levels or the tissue iron stores of the treated and untreated mice, which indicates that iron status is not a contributor to the Hgb differences seen with isocitrate treatment. Consistent with this notion, hepcidin expression was not suppressed in isocitrate-treated mice, and thus was not augmenting iron availability for Hgb synthesis. SAA1 measurements were also obtained to rule out a decrease in inflammation as a potential driver of improved Hgb in isocitrate-treated mice, which actually showed an early increase in this acute phase reactant with treatment. WBC measurements confirmed this early and transient increase in inflammation in isocitrate mice, with increased leukocytosis and neutrophil percentages. Interestingly, previous studies had shown that PU.1 overexpression increased the commitment of progenitor cells to the myeloid lineage at the expense of erythroid development [33–35], perhaps as an evolutionary immune response to combat infections. Thus, in our model the increased inflammation caused by high-dose isocitrate injections likely overrode the effects that isocitrate had on PU.1 suppression and myeloid production.
As compared to the previous study of isocitrate treatment in a rat model of chronic inflammation, our data show that much higher doses of isocitrate were required to see even a transient Hgb improvement in our BA mouse model. It remains to be seen whether the need for high doses of isocitrate may limit the applicability of this therapy in humans.
In summary, the early beneficial effects of isocitrate on anemia in our model appear to be secondary to an increase in erythropoiesis that is not caused by effects on EPO levels, iron status, hepcidin, or inflammation, consistent with increased responsiveness of erythroid precusors to the effects of EPO. Ultimately, the effect of erythrocyte destruction in this mouse model of acute and severe inflammation appears to overwhelm the erythropoietic benefit of isocitrate.
Acute and severe AI is an important clinical problem with limited therapeutic options. Further research is necessary to elucidate its key mechanisms and potential therapies.
Supplementary Material
Acknowledgments
This study was supported by research funding from Keryx Biopharmaceuticals to T.G. and E.N. We are also grateful to Drs. Chante Richardson and Adam Goldfarb for their valuable input in establishing the specifics of our isocitrate dosing regimen. We also thank Drs. Tara Arvedson, Barbra Sasu and Keegan Cooke for introducing us to the Brucella abortus mouse model used in this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Airie Kim, Email: airiekim@mednet.ucla.edu.
Eileen Fung, Email: fung.eileen@gmail.com.
Sona G. Parikh, Email: sonagparikh@gmail.com.
Victoria Gabayan, Email: vgabayan@mednet.ucla.edu.
Elizabeta Nemeth, Email: enemeth@mednet.ucla.edu.
Tomas Ganz, Email: tganz@mednet.ucla.edu.
References
- 1.Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352(10):1011–23. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 2.Adamson JW. The anemia of inflammation/malignancy: mechanisms and management. Hematology Am Soc Hematol Educ Program. 2008:159–65. doi: 10.1182/asheducation-2008.1.159. [DOI] [PubMed] [Google Scholar]
- 3.Park CH, et al. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem. 2001;276(11):7806–10. doi: 10.1074/jbc.M008922200. [DOI] [PubMed] [Google Scholar]
- 4.Nemeth E, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306(5704):2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 5.Roy CN, et al. Hepcidin antimicrobial peptide transgenic mice exhibit features of the anemia of inflammation. Blood. 2007;109(9):4038–4044. doi: 10.1182/blood-2006-10-051755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Falco L, et al. Iron refractory iron deficiency anemia. Haematologica. 2013;98(6):845–53. doi: 10.3324/haematol.2012.075515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nemeth E, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113(9):1271–1276. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wrighting DM, Andrews NC. Interleukin-6 induces hepcidin expression through STAT3. Blood. 2006;108(9):3204–3209. doi: 10.1182/blood-2006-06-027631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pietrangelo A, et al. STAT3 is required for IL-6-gp130-dependent activation of hepcidin in vivo. Gastroenterology. 2007;132(1):294–300. doi: 10.1053/j.gastro.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 10.Verga Falzacappa MV, et al. STAT3 mediates hepatic hepcidin expression and its inflammatory stimulation. Blood. 2007;109(1):353–358. doi: 10.1182/blood-2006-07-033969. [DOI] [PubMed] [Google Scholar]
- 11.Kim A, et al. A mouse model of anemia of inflammation: complex pathogenesis with partial dependence on hepcidin. Blood. 2014;123(8):1129–36. doi: 10.1182/blood-2013-08-521419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richardson CL, et al. Isocitrate ameliorates anemia by suppressing the erythroid iron restriction response. J Clin Invest. 2013;123(8):3614–23. doi: 10.1172/JCI68487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Courselaud B, et al. Strain and gender modulate hepatic hepcidin 1 and 2 mRNA expression in mice. Blood Cells Molecules, and Diseases. 2004;32(2):283–289. doi: 10.1016/j.bcmd.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Ramos E, et al. Evidence for distinct pathways of hepcidin regulation by acute and chronic iron loading in mice. Hepatology. 2011;53(4):1333–1341. doi: 10.1002/hep.24178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Z, et al. Ferroportin1 in hepatocytes and macrophages is required for the efficient mobilization of body iron stores in mice. Hepatology. 2012;56(3):961–71. doi: 10.1002/hep.25746. [DOI] [PubMed] [Google Scholar]
- 16.Sasu BJ, et al. Antihepcidin antibody treatment modulates iron metabolism and is effective in a mouse model of inflammation-induced anemia. Blood. 2010;115(17):3616–3624. doi: 10.1182/blood-2009-09-245977. [DOI] [PubMed] [Google Scholar]
- 17.Goodnough JB, et al. Inhibition of hepcidin transcription by growth factors. Hepatology. 2012;56(1):291–9. doi: 10.1002/hep.25615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adams V, Myles MH. Multiplex fluorescent immunoassay for detection of mice infected with lactate dehydrogenase elevating virus. J Am Assoc Lab Anim Sci. 2013;52(3):253–8. [PMC free article] [PubMed] [Google Scholar]
- 19.Kotoh K, et al. Lactate dehydrogenase production in hepatocytes is increased at an early stage of acute liver failure. Exp Ther Med. 2011;2(2):195–199. doi: 10.3892/etm.2011.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kato GJ, et al. Lactate dehydrogenase as a biomarker of hemolysis-associated nitric oxide resistance, priapism, leg ulceration, pulmonary hypertension, and death in patients with sickle cell disease. Blood. 2006;107(6):2279–85. doi: 10.1182/blood-2005-06-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brugnara C. Reticulocyte cellular indices: a new approach in the diagnosis of anemias and monitoring of erythropoietic function. Crit Rev Clin Lab Sci. 2000;37(2):93–130. doi: 10.1080/10408360091174196. [DOI] [PubMed] [Google Scholar]
- 22.d’Onofrio G, et al. Simultaneous measurement of reticulocyte and red blood cell indices in healthy subjects and patients with microcytic and macrocytic anemia. Blood. 1995;85(3):818–23. [PubMed] [Google Scholar]
- 23.Wong SS, et al. Detection of iron-deficiency anemia in hospitalized patients by zinc protoporphyrin. Clin Chim Acta. 1996;244(1):91–101. doi: 10.1016/0009-8981(95)06200-9. [DOI] [PubMed] [Google Scholar]
- 24.Hagihara K, et al. IL-6 plays a critical role in the synergistic induction of human serum amyloid A (SAA) gene when stimulated with proinflammatory cytokines as analyzed with an SAA isoform real-time quantitative RT-PCR assay system. Biochem Biophys Res Commun. 2004;314(2):363–9. doi: 10.1016/j.bbrc.2003.12.096. [DOI] [PubMed] [Google Scholar]
- 25.Libregts SF, et al. Chronic IFN-gamma production in mice induces anemia by reducing erythrocyte life span and inhibiting erythropoiesis through an IRF-1/PU.1 axis. Blood. 2011;118(9):2578–88. doi: 10.1182/blood-2010-10-315218. [DOI] [PubMed] [Google Scholar]
- 26.Prince OD, et al. Late stage erythroid precursor production is impaired in mice with chronic inflammation. Haematologica. 2012;97(11):1648–56. doi: 10.3324/haematol.2011.053397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cartwright GE. The anemia of chronic disorders. Semin Hematol. 1966;3(4):351–75. [PubMed] [Google Scholar]
- 28.Tong WH, Rouault TA. Metabolic regulation of citrate and iron by aconitases: role of iron-sulfur cluster biogenesis. Biometals. 2007;20(3–4):549–64. doi: 10.1007/s10534-006-9047-6. [DOI] [PubMed] [Google Scholar]
- 29.Bullock GC, et al. Iron control of erythroid development by a novel aconitase-associated regulatory pathway. Blood. 2010;116(1):97–108. doi: 10.1182/blood-2009-10-251496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Talbot AL, et al. Aconitase regulation of erythropoiesis correlates with a novel licensing function in erythropoietin-induced ERK signaling. PLoS One. 2011;6(8):e23850. doi: 10.1371/journal.pone.0023850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wontakal SN, et al. A core erythroid transcriptional network is repressed by a master regulator of myelo-lymphoid differentiation. Proc Natl Acad Sci U S A. 2012;109(10):3832–7. doi: 10.1073/pnas.1121019109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gardenghi S, et al. Distinct roles for hepcidin and interleukin-6 in the recovery from anemia in mice injected with heat-killed Brucella abortus. Blood. 2014;123(8):1137–45. doi: 10.1182/blood-2013-08-521625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leddin M, et al. Two distinct auto-regulatory loops operate at the PU.1 locus in B cells and myeloid cells. Blood. 2011;117(10):2827–38. doi: 10.1182/blood-2010-08-302976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McIvor Z, et al. Transient expression of PU.1 commits multipotent progenitors to a myeloid fate whereas continued expression favors macrophage over granulocyte differentiation. Exp Hematol. 2003;31(1):39–47. doi: 10.1016/s0301-472x(02)01017-2. [DOI] [PubMed] [Google Scholar]
- 35.Nerlov C, Graf T. PU.1 induces myeloid lineage commitment in multipotent hematopoietic progenitors. Genes Dev. 1998;12(15):2403–12. doi: 10.1101/gad.12.15.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.