Abstract
HIV-1 co-infection with human parasitic diseases is a growing public health problem worldwide. Leishmania parasites infect and replicate inside macrophages, thereby subverting host signaling pathways, including the response mediated by PKR. The HIV-1 Tat protein interacts with PKR and plays a pivotal role in HIV-1 replication. This study shows that Tat increases both the expression and activation of PKR in Leishmania-infected macrophages. Importantly, the positive effect of Tat addition on parasite growth was dependent on PKR signaling, as demonstrated in PKR-deficient macrophages or macrophages treated with the PKR inhibitor. The effect of HIV-1 Tat on parasite growth was prevented when the supernatant of HIV-1-infected macrophages was treated with neutralizing anti-HIV-1 Tat prior to Leishmania infection. The addition of HIV-1 Tat to Leishmania-infected macrophages led to inhibition of iNOS expression, modulation of NF-kB activation and enhancement of IL-10 expression. Accordingly, the expression of a Tat construct containing mutations in the basic region (49–57aa), which is responsible for the interaction with PKR, favored neither parasite growth nor IL-10 expression in infected macrophages. In summary, we show that Tat enhances Leishmania growth through PKR signaling.
Diseases caused by the protozoan parasite Leishmania spp. can be impacted by human immunodeficiency virus (HIV)-1 co-infection, particularly in countries with a high incidence of both pathogens1,2. Leishmania spp. is one of the most common parasites worldwide and causes approximately 0.2 to 0.4 million episodes of visceral leishmaniasis and 0.9 to 1.2 million cases of cutaneous leishmaniasis each year3. Episodes of Leishmania/HIV-1 coinfection have been reported in tropical and Mediterranean countries and are frequently associated with a poor clinical prognosis for both diseases4,5. Leishmania modulates cellular signaling pathways, including increasing the expression and activation of the double-stranded RNA (dsRNA)-activated protein kinase R (PKR)6. PKR is notorious for its pivotal role in the antiviral response via the expression of type I interferons7,8. Recently, our group and others reported a novel role for PKR-mediated signaling in the outcome of infections with intracellular protozoan parasites such as Leishmania and Toxoplasma gondii6,9,10. Depending on the Leishmania species involved in the infection, PKR activation exacerbates the intracellular growth of the parasite due to the production of interleukin (IL)-10 and interferon (IFN)-β10,11.
PKR activity can be regulated by the HIV-1 Tat protein, which is responsible for the transactivation of the HIV-LTR through binding to the trans-activation response element (TAR) in nascent viral transcripts12. The Tat protein is released by HIV-1-infected cells, is abundant in the serum and tissues of infected individuals and plays several modulatory roles in addition to regulating HIV-1 replication. Once in the extracellular milieu, HIV-1 Tat interacts with host cells and modulates signaling pathways by binding to external membrane receptors, such as TLR413. The Tat protein can be internalized by different cells including macrophages, and interacts with intracellular signaling kinases, such as PKR14. The PKR-Tat interaction can culminate in the activation of the transcription factor NF-κB and the expression of cytokines such as TNF-α and IL-10 15. Additionally, Tat interacts with and is phosphorylated by PKR16,17, whose kinase activity on Tat increases HIV-1 long terminal repeat (LTR) transcription activity, thereby up-modulating viral production18. In addition, NF-κB activation by Tat relies on PKR kinase activity19, and so the Tat-induced expression of IL-10 20. Therefore, it is plausible to propose that HIV-1 infection and/or the HIV-1 Tat protein can interfere with intracellular Leishmania growth via PKR activity. We previously demonstrated that the exogenous addition of Tat increases the infection load of intracellular Leishmania amazonensis in macrophages21, and in the present work we investigated the role of PKR in the aggravation of Leishmania infection driven by Tat. Our data implicate PKR as a key molecule bridging the effect of the HIV-1 protein on parasite infection.
Material and Methods
Reagents
Phorbol-12 myristate-13 acetate (PMA) was purchased from Sigma-Aldrich (St. Louis, MO, USA) and PKR-inhibitor CAS 608512-97-6 was purchased from Millipore (Darmstadt, Germany). The HIV-1 Tat protein was obtained from Dr. J. Brady (National Cancer Institute, National Institutes of Health) and the rabbit antiserum to HIV-1 Tat was obtained from B. Cullen (Duke University Medical Center) through the AIDS Research and Reference Reagent Program (Division of AIDS, National Institute of Allergy and Infectious Diseases, N.I.H.). The lyophilized 86-aa Tat was reconstituted in PBS containing 0.1 mmol/L dithiothreitol (DTT) (Invitrogen, Carlsbad, CA, USA) and 1 mg/mL bovine serum albumin (Promega Corp., Madison, WI, USA). Oxidized Tat was prepared as previously described21.
Cell lines and culture
The human monocytic leukemia cell line THP-1 (ATCC:TIB202TM) was maintained in DMEM medium with high glucose (Vitrocell Embriolife, Campinas, SP, Brazil) supplemented with 10% heat-inactivated fetal bovine serum (Sigma-Aldrich, St. Louis, MO, USA). THP-1 cells were differentiated into macrophages by incubating with 40 ng/mL of PMA for 3 days. Then, the cells were washed 3 times with PBS and incubated with fresh medium for an additional 3 days. RAW 264.7 cells expressing either empty vector (RAW-WT-Bla cells) or a dominant-negative PKR K296R (RAW-DN-PKR cells) were provided by Dr. Aristóbolo Silva (Federal University of Minas Gerais, Brazil). Monocyte-derived macrophages were obtained from peripheral blood mononuclear cells (PBMCs) isolated from buffy coat preparations of human healthy blood donors as previously described21.Thioglycolate-elicited peritoneal macrophages from wild-type or PKR-knockout 129 Sv/Ev mice were obtained by injecting 8 mL of serum-free DMEM into the peritoneal cavity. After five days, the cells were washed in PBS one time and then plated in in DMEM medium supplemented with 10% FBS on glass coverslips at a density of 2 × 105/well in 24-well polystyrene plates for subsequent Leishmania infection assays. All experimental procedures involving human cells were approved by the Oswaldo Cruz Foundation/Fiocruz Research Ethics Committee (Rio de Janeiro, RJ, Brazil), under the number 397-07. The experimental protocols using mice were approved by the Federal University of Rio de Janeiro Committee for Animal Use (permit numbers: IMPPG 024 and IBCCF171.
Parasites, culture conditions and infection
Leishmania (L.) amazonensis (WHOM/BR/75/Josefa) was used in this study. Promastigote forms were grown at 26 °C in Schneider’s Insect Medium (Sigma-Aldrich) with 10% fetal bovine serum and were used at the stationary growth phase (5–6 days). Macrophages were infected with Leishmania promastigotes with a parasite: cell ratio of 5:1 at 37 °C. In some experiments, THP-1 cells were treated with 300 nM of the PKR inhibitor. Recombinant HIV-1 Tat (100 ng/mL) was added to the cell cultures after Leishmania infection (the use of this concentration was based on our previous published work21). Infected macrophages were counted by light microscopy to assess the infection index, which was calculated by multiplying the percentage of infected macrophages by the average number of parasites per macrophage in Giemsa-stained slides. Promastigote production derived from infected cells was evaluated as follows: after 3 days of infection, non-adherent cells were removed and the adherent cells were washed 3 times with 1x PBS. The adherent cells were added to the wells in 0.5 mL of Schneider medium containing 20% FBS. After five to seven days at 26 °C, the growth of extracellular motile promastigotes originating from the infected macrophages was assessed by counting in a Neubauer chamber.
Safety of Leishmania treatment with Tat and PKR inhibitor
Leishmania promastigotes were treated with Tat or oxidized-Tat (both at 100 ng/mL), or with 300 nM of the PKR-inhibitor for 1 h. Then, the promastigotes were washed with PBS and incubated with peritoneal macrophages (obtained as described above) at a 1:5 parasite: cell ratio for 1 h or 48 h at 35 °C in 5% CO2. The infection index was calculated as described above and used to determine parasite survival.
HIV-1 isolate and co-infection
THP-1 cells were infected with the monocytotropic CCR5-dependent HIV-1 isolate Ba-L using viral suspensions containing 5–10 ng/mL of HIV-1 p24 antigen. Excess virus was washed out after 24 hours and then fresh medium was added back. The cells were maintained under standard culture conditions for 10 days. Subsequently, macrophages were infected with L. amazonensis promastigotes with a parasite: cell ratio of 5:1 at 37 °C overnight. Non-internalized promastigotes were washed out, fresh medium was added, and the cultures were maintained at 37 °C in 5% CO2 for an additional 72 hours for counting by light microscopy to estimate the infection index. For HIV-1 Tat neutralization, rabbit anti-HIV-1 Tat or the IgG isotype control were employed as follows: the supernatant of HIV-1 infected macrophages was removed and treated with anti-Tat, the IGg1 isotype or iPKR. Fresh medium was added and the promastigotes were allowed to interact with the cultures for one hour. After this period of time, the treated supernatant was added to the infected macrophages and the infection index was estimated as described above.
Nitric Oxide Production
The Griess reaction was employed to analyze the nitrite (NO2−) content as an indicator of NO production in the supernatant of RAW 264.7 cell lines accordingly the manufacturer’s instructions (Sigma-Aldrich, St. Louis, MO, USA). The reaction was read at 540 nm and the NO2- concentration was determined with reference to a standard curve generated using sodium nitrite. The results were expressed as micromolar concentrations of nitrite.
Immunoblotting
THP-1 cells (1 × 106 cells) were washed twice with ice-cold PBS and then lysed in 100 μl of lysis buffer (50 mM Tris-HCl, pH 7.5, 5 mM EDTA, 10 mM EGTA, 50 mM NaF, 20 mM β-glycerophosphate, 250 mM NaCl, 0.1% Triton X-100, 1 μg/ml BSA and a 1:100 dilution of protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA) for total protein extraction. For nuclear protein extraction, after infection and/or treatment, the cells were washed twice with 1X PBS and then lysed with 100 μL of buffer A (HEPES 10 mM pH 7.9, 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 0.25% NP-40 (v/v), and a protease inhibitor cocktail for 10 minutes on ice. Then, the lysed cells were centrifuged for 14.000 g for 1 minute at 4 °C and the pellet was resuspended in 60 μL of buffer C (20 mM HEPES pH 7.9, 0.4 M NaCl, 1 mM EDTA, 1 mM EGTA, 20% glycerol, and protease inhibitor cocktail) and incubated on ice for 20 minutes. The lysed cells were centrifuged at 14.000 g for 5 minutes, and the supernatant containing nuclear proteins was collected in a new tube. Protein extracts were subjected to electrophoresis in 10% SDS-polyacrylamide gels and transferred to a nitrocellulose membrane (Amersham Biosciences, Piscataway, NJ, USA). After blocking with 5% nonfat dry milk in TBS with 0.1% Tween-20 (TBS-T), the blots were incubated overnight with antibodies against PKR, phospho-eIF2α Ser51, α-Tubulin, GAPDH, Lamin A/C (Cell Signaling Technology, Danvers, MA, USA), phospho-PKR Th451 (Millipore, Darmstadt, Germany), NFκB-p65, iNOS or β-actin (Santa Cruz Biotechnology, Dallas, TX, U.S.A), followed by anti-rabbit or anti-mouse horseradish peroxidase-conjugate IgG (1:4000). Then, the membranes were washed 3 times with 0.1% TBS-T after each incubation and the proteins were detected by the ECL chemiluminescent detection system (Amersham Biosciences).
Quantitative Real Time RT-PCR
Total RNA from THP-1, wild-type RAW 264.7 and DN-PKR cells (1 × 106 cells) was extracted with the Invitrap® Spin Cell RNA mini kit (STRACTEC Molecular GmbH, Berlin, Germany). A 1 μg aliquot was reverse transcribed to first-strand cDNA with ImProm-II (Promega) and the oligo(dT) primer according to the manufacturer’s instructions. The DNA sequences of the primers used in this work are: Hu-PKR-F: 5’-GACCTTCCTGACATGAAAGAA-3′ and Hu-PKR-R: 5′-AACTATTTTTGCTGTTCTCAGG-3′; GAPDH-F: 5′-TGCACCACCAACTGCTTAGC-3′ and GAPDH-R: 5′-GGCATGGACTGTGGTCATGAG-3′; Mu-iNOS-F: 5′-CAGCTGGGCTGTACAAACCTT- 3′ and Mu-iNOS-R: 5′-CATTGGAAGTGAAGCGTTTCG-3′; and Hu-IL10-F: 5′-AGAACCTGAAGACCCTCAGGC -3′ and Hu-IL10-R: 5′-CCACGGCCTTGCTCTTGTT-3′. Amplicon specificity was carefully verified by the presence of a single melting temperature peak in the dissociation curves run after real-time RT-PCR, and the detection of a single band of the expected size was examined by electrophoresis. Real-time quantitative RT-PCR (qRT-PCR) was performed via the Applied Biosystems StepOneTM detection system (Applied Biosystems) using the GoTaq® qPCR Master Mix (Promega Corp., Madison, WI, USA). All qRT-PCR experiments were performed at least 3 times. qRT-PCR data from the experiments were normalized using GAPDH as an endogenous control. All expression ratios were computed via the analysis of the relative gene expression using the ΔΔCt method through StepOne software version 2.0 (Applied Biosystems).
Luciferase assays
RAW 264.7 cells (1 × 105 cells) were plated in 48-well polystyrene plates and transfected with 1 μg of reporter plasmids using the Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA). THP-1 cells (2 × 106) were transfected with 500 ng of luciferase reporter plasmids using NucleofectorTM Technology (Lonza, Basel, Switzerland) according to the manufacturer’s instructions. The PKR-Luc promoter plasmid used in the assays was kindly provided by Dr. Charles E. Samuel (University of California, Santa Barbara, USA). For the measurement of NF-kB transcriptional activity, the p6kB-Luc construct was used in our assays (kindly provided by Dr. Patrick Baeuerle). The iNOS promoter luciferase reporter plasmids were provided by Dr. David Geller of the University of Pittsburgh (PA, USA). After infection and treatment, the cells were washed with PBS, lysed according to the Dual Luciferase System protocol (Promega), and analyzed in the GloMax®-Multi (Promega Corp., Madison, WI, USA). The plasmid pBlue3’LTR-luc was provided by Dr. Reink Jeeninga and Dr. Ben Berkhout through the NIH AIDS Reagent Program (Division of AIDS, NIAID, NIH)22,23.
Plasmids for Tat overexpression
Cloned plasmids with the Tat gene isoforms were kindly provided by Dr. Mauro Giacca (ICGEB - International Centre for Genetic Engineering and Biotechnology, Italy). The group provided two isoforms: Tat-86 wild-type and Tat86 R(49–57)A, which is a basic-domain mutant19. The sequences were amplified by PCR and subcloned into pEGFP-N2 (ClonTech) between the HindIII and EcoRI restriction sites. The constructs were transfected into THP-1 cells (2 × 106) using 500 ng of DNA with the NucleofectorTM Technology (Lonza, Basel, Switzerland) according to the manufacturer’s instructions.
Statistical analyses
Data were analyzed by One-way ANOVA for independent samples using Prism 5 software. Data are expressed as the average of three independent determinations, and significant differences are indicated by p < 0.05.
Ethics Statement
The methods carried out in this work are in accordance with the guidelines approved by the Ethical Committee of biological Research Experimentation, Federal University of Rio de Janeiro, Brazil. All the experimental protocols were approved by Oswaldo Cruz Foundation/Fiocruz Research Ethics Committee (Rio de Janeiro, RJ, Brazil), under the number 397–07 and Federal University of Rio de Janeiro Committee for Animal Use (permit numbers: IMPPG 024 and IBCCF171.
Results
Tat enhances PKR expression in macrophages infected with L. amazonensis
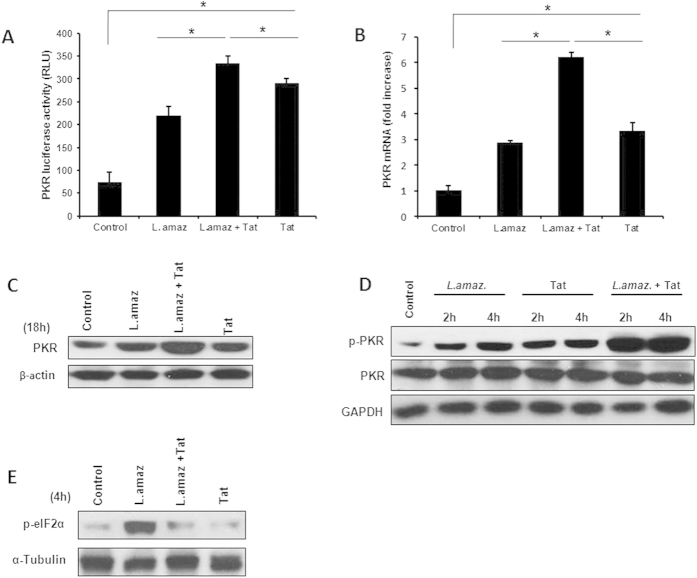
L. amazonensis induces the expression of PKR and triggers PKR signaling in macrophages6,10. Because PKR signaling plays a pivotal role in the host macrophage-Leishmania interaction, we investigated whether the HIV-1 Tat protein would enhance the expression and the levels of activated PKR in Leishmania-infected macrophages. We used a luciferase gene reporter construct containing inducible elements of the PKR promoter to test the role of Tat in Leishmania-infected macrophages. As observed in Fig. 1A, the addition of Tat increased the activity of the PKR promoter and enhanced the luciferase levels induced by parasite infection. Accordingly, PKR expression was highly increased in infected macrophages treated with Tat (Fig. 1B,C). The pre-incubation of HIV-1 Tat with Leishmania did not affect the parasite association with macrophages or the parasite load 48 hours post-infection, thereby ruling out a direct effect of Tat on the parasite (Supplementary Figure 1). Importantly, PKR underwent an increase in phosphorylation in Leishmania-infected cells treated with Tat (Fig. 1D), as revealed by the use of anti-pPKR (Thr451). Therefore, we investigated whether the main PKR substrate, the initiation factor eIF2α, was phosphorylated. Figure 1E shows that eIF2α was phosphorylated in infected macrophages. However, the addition of Tat remarkably reduced eIF2α phosphorylation, suggesting that Tat may be a PKR substrate and compete with eIF2α, as described elsewhere14. Taken together, these results indicate that HIV-1Tat is able to induce an increase in PKR levels and phosphorylation upon Leishmania infection.
Figure 1. HIV-Tat protein induces PKR expression in infected macrophages.
(A) RAW 264.7 cells were transiently transfected with a PKR-promoter-luciferase reporter plasmid, then treated with Tat (100 ng/mL) and/or infected with L. amazonensis 24 h after transfection. Whole-cell lysates were analyzed for luciferase activity 24 hours later. (B) THP-1 cells were infected with L. amazonensis for one hour, non-internalized promastigotes were washed out, fresh medium was added and then the cells were treated for an additional three hours with Tat (100 ng/mL). Total RNA was extracted and a quantitative real-time RT-PCR was performed. Western blot was performed using anti-PKR (C), anti-phospho-PKR (D) or anti-phospho-eIF2α (E) antibodies in infected and/or treated THP-1 cells as indicated. The results are representative of three independent experiments. *P < 0.05.
PKR is critical for the Tat-induced enhancement of intracellular Leishmania growth
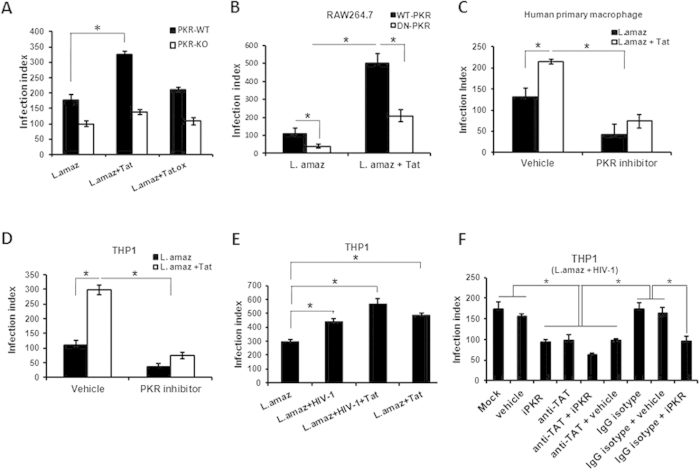
The above results prompted us to investigate the impact of the Tat-mediated enhancement of PKR activation on Leishmania intracellular growth. To this end, we used a RAW 264.7 cell line stably expressing a dominant-negative (DN)-PKR protein24 or PKR-KO macrophages. As depicted in Fig. 2A,B, Tat-induced growth of Leishmania was impaired in PKR-KO macrophages and DN-PKR RAW 264.7 cell line, thus suggesting that PKR is crucial for the Tat effect on intracellular parasite replication. To corroborate these results, we treated the Leishmania-infected THP-1 macrophage cell line or primary human macrophages with the PKR inhibitor CAS 608512-97-6 in the presence of Tat. Accordingly, PKR inhibition strongly reduced the effect of Tat on parasite growth (Fig. 2C,D). Moreover, the pre-incubation of iPKR with Leishmania did not affect the parasite association with macrophages or the parasite load 48 hours post-infection, thereby ruling out a direct effect of iPKR on the parasite (Supplementary Figure 1). Next, we addressed the effect of Tat in the context of HIV-1 and Leishmania co-infection. As expected, HIV-1 infection stimulated parasite growth, and additional treatment with Tat almost doubled parasite replication (Fig. 2E). Then, we investigated whether cells exposed to both HIV-1 and L. amazonensis exhibited enhanced parasite growth in a TAT/PKR-dependent manner. Figure 2F shows that PKR inhibition reduced parasite growth in HIV-1-/Leishmania co-infected cells. The same effect was observed when neutralizing anti-Tat antibody was added to the cultures. Moreover, the parasite load was further reduced when human macrophages were treated with iPKR and anti-Tat was added to the infected cells. Taken together, this set of results strongly suggests that Tat requires host cell PKR signaling to promote the growth of amastigotes in macrophages.
Figure 2. HIV-1 Tat protein induces Leishmania multiplication in macrophages in a PKR-dependent manner.
The infection index was measured in (A) Peritoneal macrophages (wild-type or PKR knockout) and (B) RAW 264.7 cells stably transfected with either the empty vector (RAW-WT-Bla PKR cells) or the dominant-negative PKR K296R (RAW-DN-PKR cells) infected with the promastigote forms of L. amazonensis for 24 hours, then treated with Tat (100 ng/mL) for an additional 48 hours. (C) Human primary macrophages and (D) THP-1 cells were also infected with L. amazonensis and treated with Tat (100 ng/mL) and 300 nM of the PKR inhibitor prior to amastigote quantification. (E) THP-1 cells were infected with HIV-1 and L. amazonensis promastigotes and treated with recombinant Tat (100 ng/mL). (F) Alternatively, the co-infection was treated with anti-Tat serum plus PKR inhibitor. The results are representative of three independent experiments. *P < 0.05.
L. amazonensis infection down regulates Tat-induced NF-κB activation downstream of PKR signaling
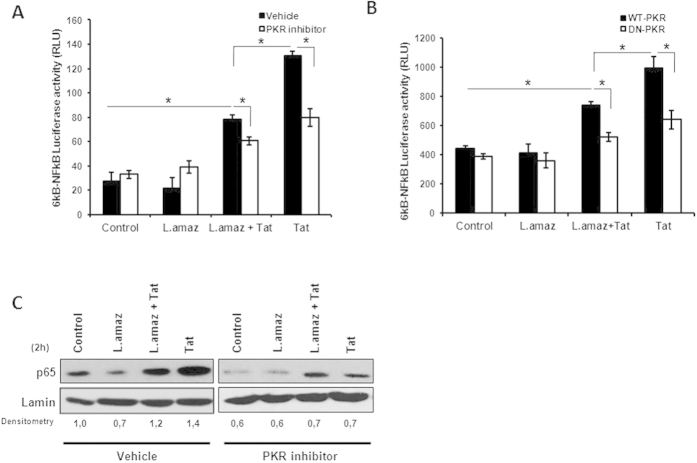
The transcription factor NF-κB is activated in viral and parasitic infections25. Briefly, the heterodimer RelA/p50 is translocated to the nuclei of infected cells and regulates the expression of a number of cytokines26. RelA/p50 is also important for HIV-1 LTR expression and may be induced by bacterial co-infections27 or by HIV-1-encoded proteins such as Tat28. We investigated the activation of NF-κB in L. amazonensis-infected macrophages treated with Tat and the PKR inhibitor through gene reporter assays (Fig. 3A). The NF-κB reporter construct was induced by Tat, and the inhibition of PKR partially prevented this effect. Importantly, Tat treatment increased NF-κB activation in uninfected cells, while Leishmania infection diminished this activation. These results were corroborated by employing the DN-PKR-RAW 264.7 cell line, thereby strengthening the hypothesis that Tat-mediated NF-κB activation was dependent on PKR signaling and that Leishmania infection reduced this effect (Fig. 3B). Next, we investigated the nuclear levels of RelA (p65) induced by Tat and the dependence of this phenomenon on PKR. Figure 3C shows that Tat increased the nuclear levels of RelA and that PKR inhibition prevented this effect. Taken together, these observations corroborate previous results on the role of Tat in NF-κB activation29 and demonstrate the importance of PKR signaling in this event. Importantly, L. amazonensis infection per se did not induce the canonical NF-κB p65/50 dimer, as previously described30.
Figure 3. L. amazonensis and Tat induced NF-kB activation downstream of PKR signaling.
(A) THP-1 and (B) RAW 264.7 cells stably transfected with either the empty vector (RAW-WT-Bla PKR cells) or the dominant-negative PKR K296R (RAW-DN-PKR cells) were transiently transfected with the 6kB-Luciferase consensus vector. Then, 24 h post-transfection the cells were infected with L. amazonensis for 18 h and/or treated with Tat (100 ng/mL). After twenty-four hours, whole-cell lysates were analyzed for luciferase activity. (C) Western blot was performed for THP-1 cell nuclear extracts. The cells were treated with Tat and/or PKR two hours post-infection. Nuclear proteins were extracted and incubated with the anti-p65 antibody in PVDF membranes. The results are representative of three independent experiments. *P < 0.05.
Leishmania infection reduces PKR-dependent Tat-induced iNOS expression
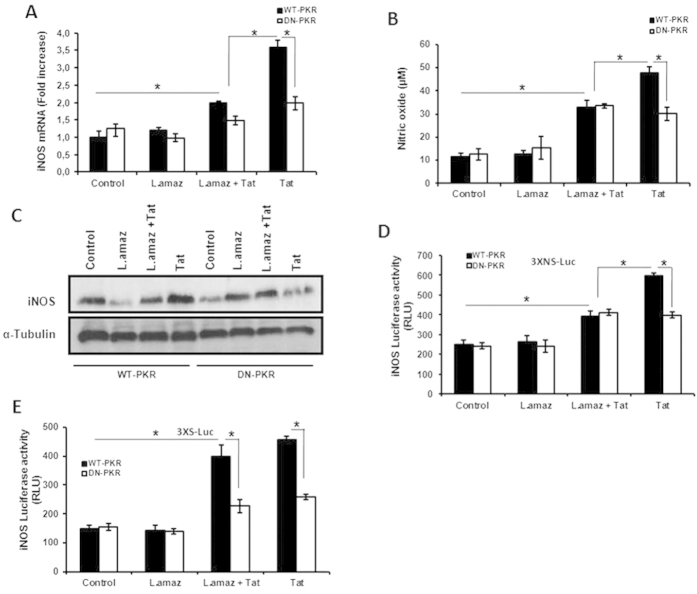
The production of nitric oxide (NO) is associated with the activation of macrophages and can reduce the number of Leishmania amastigotes31. Because HIV-1 Tat has been demonstrated to induce iNOS32, we investigated whether L. amazonensis interferes with iNOS expression and NO production in the context of the Tat-PKR axis. We found that Tat induced iNOS expression in a PKR-dependent fashion (Fig. 4A). The presence of Leishmania reduced this effect, suggesting that Leishmania impaired the induction of iNOS by Tat. Accordingly, NO production followed the same pattern (Fig. 4B). These results were corroborated by immunoblot assays. Figure 4C clearly shows that Tat induced iNOS expression via PKR and that this effect was reduced in the presence of the parasite. To evaluate the effect of Tat on the NF-κB/STAT1 regulatory elements present in the iNOS promoter33, we performed luciferase reporter assays. Sole infection by L. amazonensis did not induce the constructs carrying either the NF-κB/STAT1 or STAT1 regulatory element (Fig. 4D,E, respectively). Importantly, Tat treatment resulted in the activation of both reporter constructs, but Leishmania infection reduced this effect on the NF-κB/STAT1 construct. Based on these results, we suggest that L. amazonensis reduced the expression of iNOS induced by HIV1 Tat.
Figure 4. L. amazonensis reduces iNOS expression levels induced by treatment with Tat in a PKR-dependent manner.
(A) RAW 264.7 cells stably transfected with either the empty vector (RAW-WT-Bla PKR) or the dominant-negative PKR K296R (RAW-DN-PKR) were infected with L. amazonensis. Non-internalized promastigotes were washed out and fresh medium was added, and then the cultures were treated for an additional three hours with Tat (100 ng/mL). Total RNA was extracted and a quantitative real-time RT-PCR for iNOS was performed. (B) RAW 264.7 cell lines were infected with L. amazonensis and/or treated with Tat (100 ng/mL) for 24 h. Then, the supernatant was analyzed for the presence of nitric oxide through the Griess reaction. (C) RAW 264.7 cell lines were infected for one hour with L. amazonensis. Non-internalized promastigotes were washed out and fresh medium was added, and then the cultures were treated for an additional five hours with Tat (100 ng/mL). Western blot analysis of iNOS protein expression was performed. RAW 264.7 cell lines were transiently transfected with the 3XNS-Luc (D) or 3XS-Luc (E) vectors. Then, 24 h post-transfection the cells were infected with L. amazonensis for 18 h and/or treated with Tat (100 ng/mL). After twenty-four hours, the whole cell lysates were analyzed for luciferase activity. The results are representative of three independent experiments. *P < 0.05.
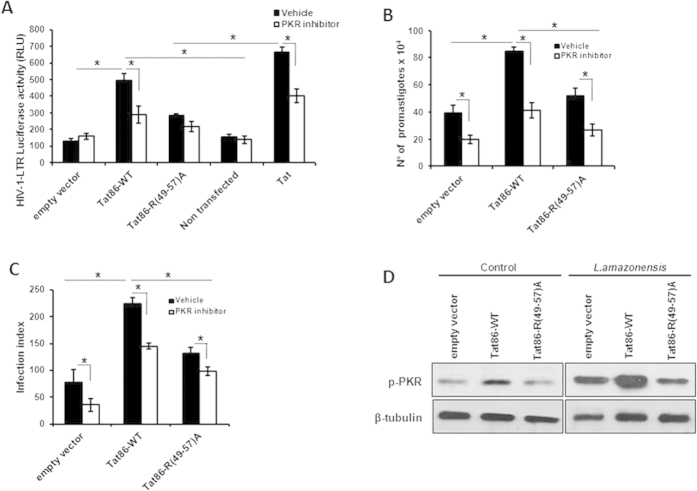
The Tat basic domain (49–57) is required for PKR activation and parasite growth enhancement in macrophages
Tat binds to PKR through a cognate basic domain (49–57 aa)16. To address whether the Tat effect on the modulation of parasite infection requires the Tat-basic domain, we used a Tat mutant construct with the arginine (R) residues replaced by alanine (A) (Tat86-R(49–57)A) to evaluate whether both Tat constructs could activate the HIV-1-LTR reporter construct in THP-1 macrophages. As predicted, Tat86-R (49–57)A could not induce HIV-1 LTR activity (Fig. 5A), while Tat86-WT strongly stimulated the LTR regulatory elements. Importantly, the pharmacological inhibition of PKR decreased LTR induction. These results prompted us to evaluate the effect of Tat86-R(49–57)A on intracellular parasite growth. As shown in Fig. 5B,C, Tat86-R(49–57)A did not promote the enhancement of the parasite load. Then, we analyzed the role of Tat in PKR activation in macrophages infected with Leishmania infection. As observed in Fig. 5D, the expression of Tat86-R(49–57)A in Leishmania-infected macrophages did not activate PKR, while the Tat86-WT isoform potentiated pPKR levels. Taken together, our results indicate that Tat favors L. amazonensis growth in a manner that is dependent on the Tat basic region and is possibly mediated through PKR.
Figure 5. The basic domain of Tat is required for PKR activation in L. amazonensis-infected macrophages.
THP-1 cells were transiently transfected with the empty vector, Tat86-WT or Tat86 R(49–57)A plasmids as previously described. Twenty-four hours post-transfection, the cells were differentiated into macrophages with PMA treatment for 4 days. (A) The cells were co-transfected with the LTR-Luciferase vector and treated with or without PKR inhibitor and/or Tat (100 ng/mL). Whole-cell lysates were analyzed for luciferase activity 24 hours later. (B) The cells were infected with L. amazonensis for 48 h, and the number of parasites that emerged from the infected cells was counted in the supernatants. (C) Alternatively, the intracellular infection index was measured. (D) Western blotting was performed for THP-1 transfected cells infected with L. amazonensis with anti-phospho-PKR at the indicated time points. The results are representative of three independent experiments. *P < 0.05.
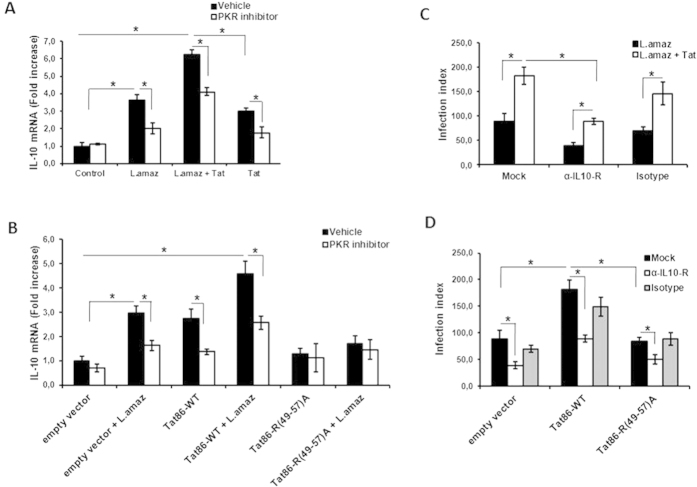
Tat favors L. amazonensis growth through PKR-dependent IL-10 expression
We previously reported that PKR activation induces IL-10, which in turn favors L. amazonensis infection6. Because HIV-1 Tat may also induce IL-10 expression, we studied the expression of IL-10 in infected macrophages treated with Tat and tested the role of PKR on IL-10 induction. Figure 6A shows that Tat potentiates the ability of L. amazonensis to induce IL-10 expression in macrophages in a PKR-dependent fashion. Similar results were obtained in infected DN-PKR RAW 264.7 macrophages (Supplementary Figure 2). Next, we tested the requirement for the Tat basic domain for IL-10 induction in infected macrophages. Our results showed that the Tat86 construction with the mutated basic domain was unable to induce IL-10 and, as predicted, it could not synergize with the L. amazonensis infection (Fig. 6B). To further characterize the effect of the axis formed by Tat/IL-10 on the exacerbation of parasite growth, we assessed the parasite growth in infected cells treated with Tat in the presence of an anti-IL-10 receptor neutralizing antibody. Blockage of the IL-10 receptor abolished the effect of Tat on parasite growth (Fig. 6C). Moreover, the enhancement of Leishmania growth by Tat86-WT was also dependent on IL-10, whereas the mutant construct Tat86-R (49–57)A could not exacerbate parasite growth (Fig. 6D).
Figure 6. IL-10 signaling is necessary for Leishmania growth induced by PKR activation via Tat.
THP-1 cells were treated with Tat (A) or transfected with Tat86-WT or Tat86-R(49-57)R (B) and then infected with L. amazonensis for 4 h. Total RNA was extracted and analyzed for IL-10 transcripts using quantitative real-time PCR. The infection index was evaluated for THP-1 infected cells treated with neutralizing anti-IL-10 R antibody or the IgG isotype (C) and in THP-1 cells transfected with Tat86-WT or Tat86-R(49-57)R (D). The results are representative of three independent experiments. *P < 0.05.
Discussion
The soluble HIV-1 Tat protein can be found in in the serum of HIV-1 infected patients as well as in the tissues34. Once in the extracellular milieu, Tat can be taken up by bystander host cells35. Inside the cells, Tat is able to interact and activate signaling pathways36, which can result in the induction of cytokines such as IL-10 13 and TGF-β37. Tat was previously reported to induce the uptake of Leishmania parasites38. We also demonstrated that Tat increased the intracellular growth of Leishmania amazonensis and revealed the importance of secreted factors in the Leishmania-exacerbation effect mediated by Tat21. However, neutralization of two soluble factors (Prostaglandin E2 and TGF-β) did not completely abolish the up-modulating effect of Tat on Leishmania21, suggesting that other mediators (or even interference with a cellular signaling pathway) could participate together with these secreted molecules. The dsRNA-activated kinase PKR can be targeted and activated by Tat14,17. Importantly, previous work demonstrated a pivotal role for PKR in the growth of intracellular parasites6,10,11. These observations strengthened our hypothesis that Tat could also affect the cell signaling cascade without requiring prior cytokine secretion. In this work, we tested the hypothesis that Tat could modulate the growth of intracellular parasites in infected macrophages via PKR signaling and IL-10 expression.
Our results clearly showed that Tat potentiated PKR activation resulting from Leishmania infection. The PKR binding sequence of the main PKR substrate (the translation initiation factor eIF2α) shares amino acid sequence similarities with the phosphorylation region of HIV-1 Tat. Our results confirmed that L. amazonensis infection leads to PKR activation and the phosphorylation of eIF2α. This effect was prevented by the addition of HIV-1 Tat. HIV-1Tat 86 can be phosphorylated by PKR, thus competing with the PKR substrate eIF2α14. Importantly, the PKR promoter construct was activated by Tat treatment or Leishmania infection alone, and the association of both conditions increased the stimulation of the PKR promoter, probably through the induction of ISRE elements present in the construct. We cannot rule out a direct effect of HIV-1 Tat on the activation of these responsive elements. However, we observed the induction of IFN1β expression in Tat-treated macrophages (data not shown), which could lead to the activation of the PKR promoter.
As expected, based on our previous studies21, HIV-1 Tat enhanced amastigote growth in macrophages and now we show that this phenomenon requires PKR signaling. Importantly, the neutralization of HIV-1 Tat produced by HIV-1-infected macrophages reduced the parasite load, and this effect was even more accentuated when anti-HIV-1 Tat was added to iPKR-treated Leishmania-infected macrophages. PKR and Tat act together to induce IL-10 expression39 and our results reveal an important aspect of Leishmania and HIV-1 co-infection: HIV-1 Tat protein is able to induce IL-10 secretion in Leishmania-infected macrophages through PKR activation. This conclusion is supported by our results showing that IL-10 expression was highly induced in Tat-treated Leishmania-infected macrophages, while there was reduced expression of IL-10 in the infected DN-PKR RAW 264.7 cell line and human macrophages treated with iPKR. The blockage of the IL-10 receptor completely abrogated the Tat effect in promoting the intracellular growth of Leishmania, thus corroborating the notion that IL-10 is an essential effector of the Tat-PKR axis during co-infection. Moreover, we expressed in infected macrophages a Tat mutant construct in which the basic region was altered, which resulted in a protein that was unable to interact with PKR19. The expression of Tat86-R (49–57)A in infected macrophages did not enhance parasite growth or up-modulate IL-10 expression and PKR activation. We have not pursued co-immunoprecipitation studies to verify the direct binding of HIV-1 Tat 86 to PKR. However, our results are in accordance with previous reports that showed and mapped the minimum sequences involved in the direct interaction of Tat with PKR.
Considering the cascade of signals, HIV-1 Tat could induce IL-10 production even from non-infected cells, and this secreted IL-10 could act in nearby Leishmania-infected cells40. HIV-1 Tat can activate the canonical NF-κB pathway, leading to the nuclear translocation of the RelA/p50 heterodimer41, which may contribute to the expression of pro-inflammatory cytokines and iNOS. Our results show that Tat indeed promotes iNOS expression and NO production. However, both effects were reduced in Leishmania-infected macrophages. Accordingly, L. amazonensis subverts canonical NF-κB activation in macrophages and promotes the formation of the repressor homodimer p50/50 30, which silences the nk regulatory elements observed in the iNOS promoter. These results show that L. amazonensis interferes with cell host signaling induced by HIV-1 Tat, thereby reducing iNOS production and modulating NF-κB dimer activation. Taken together, we envisage a scenario in which released Tat enters the cell, interacts with PKR, and regulates its activation. This complex promotes nuclear translocation of NF-κB and IL-10 induction. Despite the fact that PKR activation could induce a harmful environment for Leishmania survival, the presence of the parasite overrides the effect of PKR action on iNOS expression, thereby permitting parasite survival and growth. Altogether, our data support the notion that Tat plays a pivotal role in favoring L. amazonensis infection through PKR signaling and IL-10 expression. Our results unveil the basic molecular mechanisms by which Leishmania-HIV-1 co-infection may result in the clinical aggravation of leishmaniasis symptoms, and may contribute to the development of novel treatment procedures.
Additional Information
How to cite this article: Vivarini, C. et al. HIV-1 Tat protein enhances the intracellular growth of Leishmania amazonensis via the ds-RNA induced protein PKR. Sci. Rep. 5, 16777; doi: 10.1038/srep16777 (2015).
Supplementary Material
Acknowledgments
We thank the Hemotherapy Service of the Hospital Clementino Fraga Filho (Federal University of Rio de Janeiro, Brazil) for providing buffy-coats from healthy blood donors. This study was supported by the Brazilian funding agencies CNPq and FAPERJ, through grants awarded to the authors EMS, UGL and DCBH.
Footnotes
Author Contributions A.C.V.: designed and carried out experiments, he also interpreted the data and wrote the manuscript; R.M.S.P.: performed experiments and interpreted the data; V.B.S.: performed initial experiments for the study; J.R.T.: carried out experiments and also discussed the results; E.M.S.: designed experiments and analyzed the data; A.M.S.: provided reagents and discussed the results; D.C.S.: performed experiments and interpreted the data and discussed the results; D.C.B.-H.: provided input for experimental design and interpretation, elaborated experiments and provided reagents; U.G.L.: directed the study obtained resources, interpreted the data, provided reagents and wrote the manuscript.
References
- Karp C. L. & Auwaerter P. G. Coinfection with HIV and tropical infectious diseases. II. Helminthic, fungal, bacterial, and viral pathogens. Clin Infect Dis 9, 1214–20 (2007). [DOI] [PubMed] [Google Scholar]
- Andreani G., Lodge R., Richard D. & Tremblay M. J. Mechanisms of interaction between protozoan parasites and HIV. Curr Opin HIV AIDS 3, 276–82 (2012). [DOI] [PubMed] [Google Scholar]
- Alvar. J. et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS One 7, e35671 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecílio P. et al. Deception and manipulation: the arms of Leishmania, a successful parasite. Front Immunol 5, 480 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cota G. F. et al. Leishmania-HIV co-infection: clinical presentation and outcomes in an urban area in Brazil. PLoS Negl Trop Dis 4, e2816 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira R. M. et al. Novel role for the double-stranded RNA-activated protein kinase PKR: modulation of macrophage infection by the protozoan parasite Leishmania. FASEB J 2, 617–26 (2010). [DOI] [PubMed] [Google Scholar]
- Yim H. C. & Williams B. R. Protein kinase R and the inflammasome. J Interferon Cytokine Res 6, 447–54 (2014). [DOI] [PubMed] [Google Scholar]
- Cachat A. et al. Alpha interferon restricts human T-lymphotropic virus type 1 and 2 de novo infection through PKR activation. J Virol 24, 13386–96 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogolla P. S. et al. The protein kinase double-stranded RNA-dependent (PKR) enhances protection against disease caused by a non-viral pathogen. PLoS Pathog 8, e1003557 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivarini A. C. et al. Human cutaneous leishmaniasis: interferon-dependent expression of double-stranded RNA-dependent protein kinase (PKR) via TLR2. FASEB J 12, 4162–73 (2011). [DOI] [PubMed] [Google Scholar]
- Faria M. S. et al. Role of protein kinase R in the killing of Leishmania major by macrophages in response to neutrophil elastase and TLR4 via TNFα and IFNβ. FASEB J 7, 3050–63 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. Y. et al. NUCKS1, a novel Tat coactivator, plays a crucial role in HIV-1 replication by increasing Tat-mediated viral transcription on the HIV-1 LTR promoter. Retrovirology 11(1), 67 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Haij N., Leghmari K., Planès R., Thieblemont N. & Bahraoui E. HIV-1 Tat protein binds to TLR4-MD2 and signals to induce TNF-α and IL-10. Retrovirology 10, 123 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand S. R., Kobayashi R. & Mathews M. B. The Tat protein of human immunodeficiency virus type 1 is a substrate and inhibitor of the interferon-induced, virally activated protein kinase, PKR. J Biol Chem 272, 8388–95 (1997). [DOI] [PubMed] [Google Scholar]
- Li J. C., Lee D. C., Cheung B. K. & Lau A. S. Mechanisms for HIV Tat upregulation of IL-10 and other cytokine expression: kinase signaling and PKR-mediated immune response. FEBS Lett 579, 3055–62 (2005). [DOI] [PubMed] [Google Scholar]
- McMillan N. A. et al. HIV-1 Tat directly interacts with the interferon-induced, double-stranded RNA-dependent kinase, PKR. Virology 213, 413–24 (1995). [DOI] [PubMed] [Google Scholar]
- Endo-Munoz L., Warby T., Harrich D. & McMillan N. A. Phosphorylation of HIV Tat by PKR increases interaction with TAR RNA and enhances transcription. Virol J 2, 17 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meurs E. F., McMillan N., Williams B. R., Hovanessian A. G. & Southern P. J. Human PKR transfected into murine cells stimulates expression of genes under control of the HIV1 or HTLV-ILTR. Virology 214, 653–9 (1995). [DOI] [PubMed] [Google Scholar]
- Demarchi F., Gutierrez M. I. & Giacca M. Human immunodeficiency virus type 1 tat protein activates transcription factor NF-kappaB through the cellular interferon-inducible, double-stranded RNA-dependent protein kinase, PKR. J Virol 73, 7080–6 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leghmari K., Bennasser Y. & Bahraoui E. HIV-1 Tat protein induces IL-10 production in monocytes by classical and alternative NF-kappaB pathways. Eur J Cell Biol 87, 947–62 (2008). [DOI] [PubMed] [Google Scholar]
- Barreto-de-Souza V. et al. Increased Leishmania replication in HIV-1-infected macrophages is mediated by tat protein through cyclooxygenase-2 expression and prostaglandin E2 synthesis. J Infect Dis 194, 846–54 (2006). [DOI] [PubMed] [Google Scholar]
- Jeeninga R. E. et al. Functional differences between the long terminal repeat transcriptional promoters of human immunodeficiency virus type 1 subtypes A through G. J Virol 74, 3740–3751 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klave B. & Berkhout B. Comparison of the 5′ and 3′ LTR promoter function in the human immunodeficiency virus. J Virol 68, 3830–3840 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva A. M. et al. Protein kinase R (PKR) interacts with and activates mitogen-activated protein kinase kinase 6 (MKK6) in response to double-stranded RNA stimulation. J Biol Chem 279, 37670–37676 (2004). [DOI] [PubMed] [Google Scholar]
- Tato C. M. & Hunter C. A. Host-pathogen interactions: subversion and utilization of the NF-kappa B pathway during infection. Infect Immun 70, 3311–7 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tak P. P. & Firestein G. S. NF-kappaB: key role in inflammatory diseases. J Clin Invest 107, 7–11 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira V. H. et al. Endometrial epithelial cell responses to coinfecting viral and bacterial pathogens in the genital tract can activate the HIV-1 LTR in an NF-κB-and AP-1-dependent manner. J Infect Dis 204, 299–308 (2011). [DOI] [PubMed] [Google Scholar]
- Fiume G. et al. Human immunodeficiency virus-1 Tat activates NF-κB via physical interaction with IκB-α and p65. Nucleic Acids Res 40, 3548–62 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demarchi F., d’Adda-di-Fagagna F., Falaschi A. & Giacca M. Activation of transcription factor NF-kappaB by the Tat protein of human immunodeficiency virus type 1. J Virol 70, 4427–4437 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calegari-Silva T. C. et al. NF-kappaB-mediated repression of iNOS expression in Leishmania amazonensis macrophage infection. Immunol. Lett 127, 19–26 (2009). [DOI] [PubMed] [Google Scholar]
- Mukbel R. M. et al. Macrophage killing of Leishmania amazonensis amastigotes requires both nitric oxide and superoxide. Am J Trop Med Hyg 76, 669–75 (2007). [PubMed] [Google Scholar]
- Liu X. et al. Human immunodeficiency virus type 1 (HIV-1) Tat induces nitric-oxide synthase in human astroglia. J Biol Chem 277, 39312–9 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z., Shao L., Du Q., Park K. S. & Geller D. A. Identification of a classic cytokine-induced enhancer upstream in the human iNOS promoter. FASEB J 21, 535–42 (2007). [DOI] [PubMed] [Google Scholar]
- Goldstein G. HIV-1 Tat protein as a potential AIDS vaccine. Nat Med 1, 960–4 (1996). [DOI] [PubMed] [Google Scholar]
- Ben-Dov N. & Korenstein R. The uptake of HIV Tat peptide proceeds via two pathways which differ from macropinocytosis. Biochim Biophys Acta 1848, 869–877 (2014). [DOI] [PubMed] [Google Scholar]
- Herbein G., Gras G., Khan K. A. & Abbas W. Macrophage signaling in HIV-1 infection. Retrovirology 7, 34 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zauli G. et al. Tat protein stimulates production of transforming growth factor-beta 1 by marrow macrophages: a potential mechanism for human immunodeficiency virus-1-induced hematopoietic suppression. Blood 80, 3036–43 (1992). [PubMed] [Google Scholar]
- Lodge R. et al. HIV-1 promotes intake of Leishmania parasites by enhancing phosphatidylserine-mediated, CD91/LRP-1-dependent phagocytosis in human macrophages. PLoS One 7, e32761 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahraoui E., Briant L. & Chazal N. E5564 inhibits immunosuppressive cytokine IL-10 induction promoted by HIV-1 Tat protein. Virol J 11, 214 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz T. et al. T cell-derived IL10 determines leishmaniasis disease outcome and is suppressed by a dendritic cell based vaccine. PLoS Pathog 9, e1003476 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott M., Lovett J. L., Mueller L. & Verdin E. Superinduction of IL-8 in T cells by HIV-1 Tat protein is mediated through NF-kappaB factors. J Immunol 160, 2872–80 (1998). [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.