Abstract
Aberrant sensory processing plays a fundamental role in the pathophysiology of dystonia; however, its underpinning neural mechanisms in relation to dystonia phenotype and genotype remain unclear. We examined temporal and spatial discrimination thresholds in patients with isolated laryngeal form of dystonia (LD), who exhibited different clinical phenotypes (adductor vs. abductor forms) and potentially different genotypes (sporadic vs. familial forms). We correlated our behavioral findings with the brain gray matter volume and functional activity during resting and symptomatic speech production. We found that temporal but not spatial discrimination was significantly altered across all forms of LD, with higher frequency of abnormalities seen in familial than sporadic patients. Common neural correlates of abnormal temporal discrimination across all forms were found with structural and functional changes in the middle frontal and primary somatosensory cortices. In addition, patients with familial LD had greater cerebellar involvement in processing of altered temporal discrimination, whereas sporadic LD patients had greater recruitment of the putamen and sensorimotor cortex. Based on the clinical phenotype, adductor form-specific correlations between abnormal discrimination and brain changes were found in the frontal cortex, whereas abductor form-specific correlations were observed in the cerebellum and putamen. Our behavioral and neuroimaging findings outline the relationship of abnormal sensory discrimination with the phenotype and genotype of isolated LD, suggesting the presence of potentially divergent pathophysiological pathways underlying different manifestations of this disorder.
Keywords: Sensory processing, Endophenotype, Brain imaging
Highlights
-
•
Abnormal temporal but not spatial discrimination is an LD mediational endophenotype.
-
•
Penetrance of abnormal TDT is higher in familial than sporadic LD.
-
•
No differences in penetrance between clinical phenotypes of LD
-
•
Distinct neural phenotype/genotype relations of TDT reflect divergent pathophysiology.
1. Introduction
Isolated focal dystonia is a multifactorial disorder with unclear causes and pathophysiology, which may affect various body regions, from eyelid to foot muscles. This phenotypical heterogeneity is further expanded by the task-specific forms of focal dystonia, which selectively affect similar muscle groups but lead to clinically distinct symptoms, such as writer's cramp vs. musician's hand dystonia or adductor vs. abductor laryngeal dystonia. Whether the different forms of dystonia have a common underlying pathophysiological mechanism and whether there are additional genetic or environmental factors that divert patients into different clinical phenotypes remain largely unknown. Contributing to this, gene discovery for isolated task-specific focal dystonias has been stagnant due, in part, to the small effect size of a risk allele on phenotypic variance, the lack of neural integrity markers of dystonia carriers, and poor understanding of their interplay with genes contributing to this disorder. However, several factors, including the family history of dystonia in up to 12% of patients with isolated focal dystonias (Chan et al., 1991, Friedman and Fahn, 1986, Grandas et al., 1988, Kirke et al., 2015, Maniak et al., 2003, Sheehy et al., 1988), suggest that genetic susceptibility or dominantly inherited genes with reduced penetrance may be involved in the etiopathophysiology of this disorder.
To this end, behavioral studies examining the underlying quantitative traits have recently hinted to the presence of the mediational endophenotypic markers of dystonia, which reflect gene expression and share common pathogenetic mechanisms with phenotype, thus linking genes with phenotype (Hutchinson et al., 2013). Specifically, the abnormal temporal discrimination threshold (TDT), a significantly extended time interval at which a subject perceives two stimuli as being asynchronous, has been proposed as a mediational endophenotype of dystonia (Hutchinson et al., 2013) based on the findings in writer's cramp, cervical dystonia, blepharospasm and generalized dystonia (Aglioti et al., 2003, Bradley et al., 2009, Bradley et al., 2012, Fiorio et al., 2003, Fiorio et al., 2008) as well as in up to 52% of unaffected relatives of patients with DYT1 and adult-onset cervical dystonias (Bradley et al., 2009, Fiorio et al., 2007, Kimmich et al., 2014). However, despite its possible importance in the pathophysiology of dystonia, our understanding of the relationships between abnormal sensory processing as a dystonia endophenotype and brain abnormalities underlying the pathophysiology of dystonia remains scarce.
In this study, we examined the visual temporal discrimination thresholds (TDT) in a large cohort of 84 patients with isolated laryngeal form of dystonia (LD), including patients with different clinical phenotypes (adductor vs. abductor forms) and possibly different genotypes (sporadic vs. familial forms), in order to determine phenotype- and putative genotype-specific features of abnormal temporal discrimination. We further investigated the relationships between TDT abnormalities, LD clinical symptoms, and brain structural and functional changes using voxel-based morphometry (VBM) of gray matter volume and functional MRI (fMRI) during both symptomatic speech production and the resting state. In addition, because the tactile spatial discrimination thresholds (SDT) have been previously reported to be altered in writer's cramp, blepharospasm, cervical dystonia but not in generalized DYT1 dystonia (Molloy et al., 2003), we assessed the SDT in the same cohort of LD patients. To establish the baseline measures, the TDT and SDT were also examined in 30 age- and gender-matched healthy individuals.
We hypothesized that both TDT and SDT will be significantly abnormal across the different groups of LD patients compared to controls. Because abnormal discrimination may represent a mediational endophenotype closer to genes than to clinical phenotype of dystonia (Hutchinson et al., 2013), we hypothesized that these alterations would be greater in familial than sporadic patients. Based on the prior reports of TDT abnormalities in unaffected first-degree relatives of cervical dystonia patients and asymptomatic carriers of DYT1 mutation (Kimmich et al., 2011, Kimmich et al., 2014), we hypothesized that abnormalities in discrimination would not significantly correlate with LD symptom duration or severity. However, because genes have an immediate impact on brain organization (Meyer-Lindenberg, 2010), we expected that abnormal sensory discrimination would establish significant correlations with the structure and function of brain regions, which likely contribute to dystonia pathophysiology (Neychev et al., 2011, Ramdhani and Simonyan, 2013, Zoons et al., 2011) and are related to abnormal speech motor control in patients with laryngeal dystonia. Specifically, we hypothesized that distinct patterns of correlations between sensorimotor, basal ganglia and cerebellar abnormalities and abnormal sensory discrimination would differ between sporadic and familial LD as well as adductor (ADLD) and abductor (ABLD) forms.
2. Methods
2.1. Subjects
We recruited 102 LD patients and 53 healthy controls. Our exclusion criteria included the presence of other forms of dystonia, left-handedness, bilingual non-native English speakers, past or present history of neurological, psychiatric, laryngeal or cognitive problems, impaired visual or tactile acuity, and known dystonia gene mutation. Based on these stringent exclusion criteria as well as study dropouts, the final subject groups comprised:
-
(1)
60 sporadic LD patients without family history of any form of dystonia, including 30 ADLD and 30 ABLD forms;
-
(2)
24 familial LD patients with one or more family members affected with LD or other forms of primary dystonia, including 17 ADLD and 7 ABLD;
-
(3)
30 healthy controls.
All final study participants were right-handed and monolingual native English speakers. None had any history of neurological (other than isolated LD in patients), psychiatric, or laryngeal problems. All subjects scored ≥ 27 points at the Mini-Mental State Examination, which is indicative of normal cognition (Table 1). Genetic testing performed on blood samples from all final study participants found no TOR1A (DYT1), THAP1 (DYT6), TUBB4A (DYT4) or GNAL (DYT25) mutations. None of the subjects had any conditions resulting in a loss of visual or tactile acuity, which may have interfered with the completion of experimental testing. The diagnosis of LD was confirmed by fiberoptic nasolaryngoscopy. The patients who received botulinum toxin injections participated in the study only when they were symptomatic, i.e., at the end of their treatment cycle at least 3–4 months after their last injection.
Table 1.
Demographic and clinical data.
| Sporadic |
Familial |
Controls |
|||
|---|---|---|---|---|---|
| ADLD |
ABLD |
ADLD |
ABLD |
||
| Number of subjects | 30 | 30 | 17 | 7 | 30 |
| Age (years; mean ± standard deviation) | 57.4 ± 10.4 | 53.1 ± 12.5 | 55.9 ± 15.9 | 58.1 ± 13.0 | 49.7 ± 9.5 |
| Gender (female/male) | 23/7 | 26/4 | 16/1 | 5/2 | 18/12 |
| Handedness (Edinburgh inventory) | Right | ||||
| Language | Monolingual native English | ||||
| Cognitive status | Mini-Mental State Examination ≥ 27 points | ||||
| Genetic status | Negative for DYT1, DYT4, DYT6 and DYT25 | ||||
| Disease duration (years; mean ± standard deviation) | 14.7 ± 9.6 | 12.2 ± 8.9 | 20.6 ± 13.9 | 24.7 ± 19.7 | N/A |
| Symptom severity (visual analog scale; mean ± standard deviation) | 7.2 ± 1.9 | 7.8 ± 1.9 | 7.0 ± 2.4 | 7.9 ± 1.5 | N/A |
TDT and SDT values did not show statistical differences between younger (< 50 years old) and older (> 50 years old) participants or between male and female participants (all p ≥ 0.05, corrected for multiple comparisons). There were no statistically significant differences between the groups in age or gender; the patient groups did not differ statistically in their symptom severity or disorder duration (all corrected p > 0.05).
All subjects provided written informed consents, which was approved by the Institutional Review Board of the Icahn School of Medicine at Mount Sinai.
2.2. Sensory testing
The visual TDT exam was performed using a custom-made device with two LED flashing lights according to a previously reported protocol (Bradley et al., 2009). The subject was instructed to focus on a reference focal point in the middle of the subject's field of view at a constant distance of 70 cm, while the device with two LED-flashing lights was positioned within the subject's left or right peripheral vision at a constant distance of 10 cm from the reference focal point. The left/right setup was randomized between the subjects, and both sites were tested in all subjects. While focusing on a focal point, all subjects were instructed to assess the flashing of the two LED lights, which were presented at 5-s intervals and illuminated for 5 ms, first appearing simultaneously and then gradually separating from each other in 5-ms steps. After the presentation of each stimulus pair, subjects verbally reported whether the LEDs appeared to flash synchronously or asynchronously. The same task was performed 6 times with the LED device positioned on each left and right site (total of 12 trials). The first of three correct consecutive responses, when the subject recognized the stimuli to be asynchronous, was considered as an interstimulus interval and defined as the subject-specific TDT. The median of these responses for 6 trials per each left and right sites was computed in order to account for the practice effect and then averaged to derive the TDT of the subject.
Tactile SDT was tested using a geometric series of (Semmes Weinstein) Von Frey monofilaments with the forces ranging from 2 to 0.008 g as described earlier (Belluscio et al., 2011). All subjects were blindfolded and paired presentations of an actual touch by the monofilament or a sham without a contact was randomly delivered to the glabrous part of the right hand. The stimuli were delivered in both directions of increasing and decreasing forces, with the initial choice of force direction randomized between the subjects. Subjects were asked to verbally identify the touch by the monofilament. The individual thresholds were calculated as the mean of the first of the three correct consecutive responses in each tested direction in each subject. Because the stimuli were delivered in both increasing and decreasing forces and because subjects were not made aware that one of the stimuli was a sham, subjects perceived finer monofilaments similar to shams, and vice versa. Conversely, at stronger forces, monofilaments were perceived as one stimulus with a definite distinction from a sham. We therefore acknowledge that this tactile stimulation fell in between tactile threshold detection and discrimination.
All TDT and SDT measures were converted into the standardized Z scores as follows:
Z-score = (patient's actual measure − control mean measure) / control standard deviation measure.
Z scores ≥ 2.0 were considered abnormal. Because Shapiro–Wilk tests found that data in some groups were not normally distributed (TDT: W ≤ 0.88, p ≤ 0.0005; SDT: W ≤ 0.86, p ≤ 0.004), we used non-parametric tests to assess the statistical differences between the groups while accounting for the variance differences in TDT/SDT measures. We conducted two a priori Kruskal–Wallis non-parametric analyses to estimate the overall group differences (1) between controls, sporadic and familial LD patients, and (2) between controls, ADLD and ABLD phenotypes, including TDT and SDT measures as dependent variables at p ≤ 0.025 to correct for multiple comparisons, which were followed by post hoc Mann–Whitney U tests to determine significant differences between the groups, wherever appropriate. ADLD and ABLD groups included both sporadic and familial cases because no significant statistical differences in either TDT or SDT measures were found in sporadic vs. familial ADLD or in sporadic vs. familial ABLD (all p ≥ 0.10). To estimate the accuracy of obtained statistical significance of each test, we performed nonparametric bootstrapping with replacement in 1000 samples to calculated 95% confidence interval (CI) for the differences between the groups. Analyses of the frequency rates of abnormal TDT between sporadic and familial groups and between ADLD and ABLD patients were carried out using Chi-square tests of association with bootstrap resampling with replacement in 1000 samples at an adjusted p ≤ 0.025 to correct for multiple comparisons. Finally, we used Pearson's correlation coefficients to examine the relationships between abnormal sensory discrimination and disorder duration and severity. LD severity was assessed using a visual analog scale that used 10 gradations along a 100-mm line with distance in mm used to indicate the degree of severity of LD-characteristic voice symptoms (i.e., breaks, effort) during the production of 20 sentences containing a high number of glottal stops before the vowels to elicit symptoms of ADLD and 20 sentences containing a high number of voiceless consonants (f/s/h/p/t/k) to elicit symptoms of ABLD (Ludlow et al., 2008).
2.3. Magnetic resonance imaging (MRI)
To determine the contribution of abnormal TDT to brain structure and function in LD, 23 patients (age 62.7 ± 5.9 years old; 17 females/6 males) with abnormal TDT underwent brain functional and structural MRI. Based on their LD characteristics, 8 patients had familial LD (4 ADLD/4ABLD; age 64.8 ± 4.8 years old; 6 females/2 males) and 15 patients had sporadic LD (8 ADLD/7 ABLD; age 61.7 ± 6.3 years old; 11 females/4 males). We examined gray matter volume, functional brain activation during symptomatic speech production and resting state in relation to abnormal TDT in all LD patients as well as in sporadic vs. familial and ADLD vs. ABLD patients, separately.
All patients were scanned on a 3 Tesla Philips scanner with an 8-channel head coil to obtain a high-resolution T1-weighted image as well as functional images during the resting state and symptomatic sentence production. To rule out structural lesions, anatomically reference fMRI data, and carry out volumetric measurements of gray matter, a high-resolution T1-weighted image was obtained using magnetization prepared rapid gradient echo (MPRAGE) sequence with repetition time (TR) = 7.5 ms, echo time (TE) = 3.4 ms, inversion time (TI) = 819 ms, flip angle (FA) = 8°, field of view (FOV) = 210 mm, 172 slices with 1-mm slice thickness. T1-weighted images were processed using VBM8 toolbox of SPM software running on MATLAB version 8.3. Images were bias-corrected for MRI inhomogeneities and noise, tissue-classified into gray matter using the unified segmentation approach (Ashburner and Friston, 2005) and refined by applying adaptive a posteriori estimations and a hidden Markov Random Field Model (Cuadra et al., 2005). Gray matter probability maps were non-linearly registered to the Montreal Neurological Institute (MNI) space using the diffeomorphic registration (DARTEL) algorithm to improve intersubject registration (Ashburner, 2007), modulated for the non-linear component only by the Jacobian determinant of the deformations to preserve tissue volume after warping, and smoothed using a 4-mm Gaussian kernel.
During resting-state fMRI, the participants were instructed to rest in the scanner with their eyes closed without falling asleep and to avoid thinking of anything in particular. Data were obtained using a single-shot echo-planar imaging (EPI) gradient echo sequence (TR = 2000 ms, TE = 30 ms, FA = 90°, FOV = 240 mm, voxel size = 3 × 3 × 3.5 mm with 33 slices covering the whole brain). A total of 150 volumes per subject were acquired in 5 min of the scan time. Resting-state fMRI data processing was performed using FSL software. After removal of the first four volumes due to potential T1 stabilization effects, images were motion corrected, high-pass filtered at 0.01 Hz as a cut-off frequency, smoothed using a Gaussian kernel full-width at half-maximum (FWHM) of 4 mm, registered to the individual's MPRAGE using a six-parameter rigid transformation, and normalized to the standard Talairach–Tournoux brain using a non-linear algorithm. Preprocessed images were then submitted to a multiple linear regression to control for the effect of white matter and CSF mean signals, as well as for the six motion parameters calculated during realignment of the functional volumes.
Functional images during sentence production were acquired using an event-related sparse-sampling design in order to minimize scanning artifacts due to orofacial movements and to neutralize the scanner noise interference with acoustic stimulus presentation as described earlier (Simonyan and Ludlow, 2010, Simonyan et al., 2013b). The experimental condition included production of AD- or AB-symptomatic sentences (e.g., “Are the olives large?”; “My father has a new car”) and a resting condition as a baseline. The subjects first listened to the auditory example of a task delivered through the MR-compatible headphones within a 3.6-s interval and then reproduced the same task within a 5-s interval, which was followed by a 2-s image acquisition while subjects silently fixated their attention on the black cross. Whole-brain functional images were acquired with a gradient-weighted EPI pulse sequence (TR = 2 s per volume and 10.6 s between volumes, TE = 30 ms, FA = 90, FOV = 240 mm, voxel size = 3.75 × 3.75 mm, 36 slices with 4-mm slice thickness). Each subject completed 4 functional runs; each functional run consisted of 24 tasks and 16 rest conditions. Functional data were analyzed using AFNI software. Following the standard image pre-processing and smoothing with a 4-mm FWHM Gaussian kernel, the task-related responses were analyzed using multiple linear regression with the task regressor convolved with a canonical hemodynamic response function at a scaled peak-to-peak height of 1.0; six motion parameters (x, y, z translations; pitch, roll, yaw rotations) to control for residual motion artifacts, and three polynomials to account for low-frequency component, such as scanner drift (Perrachione and Ghosh, 2013). The only contrast of interest was task versus rest as an implicit baseline.
All pre-processed functional and structural images were spatially transformed into the AFNI standard Talairach–Tournoux brain. To examine neural correlates of abnormal discrimination within and between LD groups, we computed whole-brain voxelwise Pearson's correlation coefficients to assess the relationships of abnormal discrimination measures with gray matter volume, functional activation during symptomatic sentence production, and low-frequency fluctuations during the resting state as described previously (Berman et al., 2013, Simonyan and Ludlow, 2012, Simonyan et al., 2013a). For this, we created a single volume for each of the VBM, resting-state fMRI, and speech-production fMRI datasets by concatenating the respective images (i.e., smoothed, modulated for the nonlinear components, DARTEL warped, segmented gray matter images; beta coefficients of functional activation during symptomatic sentence production, and beta estimates reflecting low-frequency fluctuations during the resting state) across all subjects for each imaging modality. Pearson's correlation coefficients were computed between each voxel in the concatenated datasets and the column of abnormal TDT values. The resultant maps were thresholded at an FWE-corrected p ≤ 0.05 (using Monte-Carlo simulations in the AlphaSim program of AFNI). The follow up conjunction analyses were performed to examine the extent of overlapping and distinct alteration in brain structure and function in relation to abnormal sensory discrimination in sporadic vs. familial and ADLD vs. ABLD patients.
3. Results
TDT and SDT values did not show statistical differences between younger (< 50 years old) and older (> 50 years old) participants or between males and females (all p ≥ 0.05, corrected for multiple comparisons) in either group. Similarly, there were no significant differences between the patient groups in respect to their symptom severity or disorder duration (all p ≥ 0.31, corrected for multiple comparisons).
3.1. Sensory testing in LD and controls
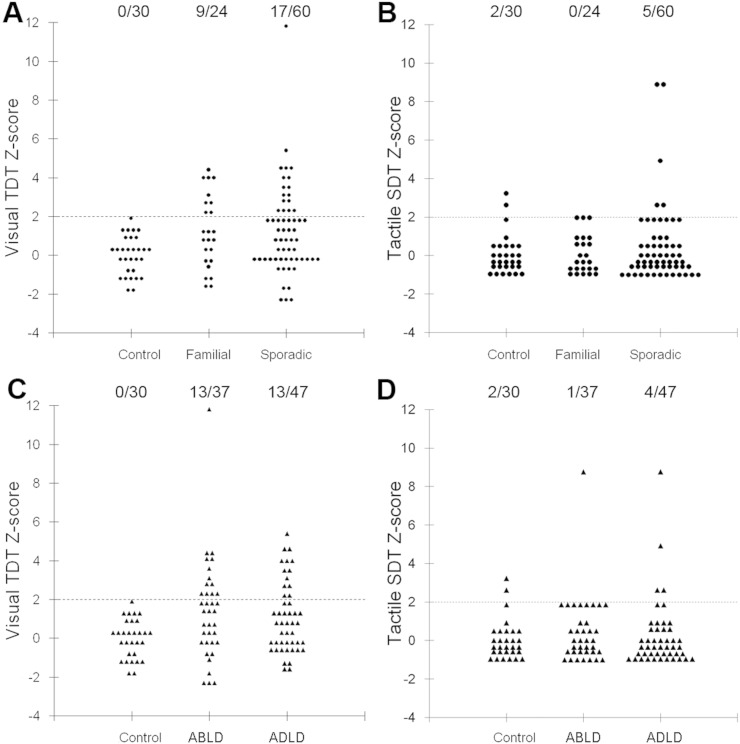
In 30 healthy controls, the visual TDT was 35.7 ± 10.7 ms (Z-score = 0.0 ± 1.00) and the tactile SDT was 0.16 ± 0.13 g (Z-score = 0.0 ± 1.04) (Table 2). None of the control subjects had abnormal TDT, but two healthy subjects (6.7%) had an abnormal SDT of 0.50 and 0.58 (Z-scores = 2.62 and 3.23, respectively) (Fig. 1, Table 2).
Table 2.
Temporal and spatial discrimination thresholds in LD patients and controls.
| Sensory testing modality | Mean ± s.d. | Mean Z-score | Z-score range | Group abnormal frequency | |
|---|---|---|---|---|---|
| Controls | TDT | 35.7 ± 10.1 | 0.0 | − 1.8 to 1.9 | 0/30 (0%) |
| SDT | 0.16 ± 0.13 | 0.0 | − 1.1 to 3.2 | 2/30 (6.7%) | |
| Patients | TDT | 48.4 ± 21.9 | 1.25 | − 2.3 to 11.8 | 26/84 (31.0%) |
| SDT | 0.19 ± 0.23 | 0.26 | − 1.1 to 8.8 | 5/84 (6.0%) | |
| Patient subgroups | |||||
| Sporadic LD | TDT | 48.6 ± 23.2 | 1.27 | − 2.3 to 11.8 | 17/60 (28.3%) |
| SDT | 0.21 ± 0.26 | 0.36 | − 1.1 to 8.8 | 5/60 (8.3%) | |
| Familial LD | TDT | 47.9 ± 19.0 | 1.21 | − 1.6 to 4.4 | 9/24 (37.5%) |
| SDT | 0.16 ± 0.13 | 0.01 | − 1.1 to 1.9 | 0/24 (0%) | |
| ADLD | TDT | 47.0 ± 18.1 | 1.11 | − 1.6 to 5.4 | 13/47 (27.7%) |
| SDT | 0.18 ± 0.23 | 0.18 | − 1.1 to 8.8 | 4/47 (8.5%) | |
| ABLD | TDT | 50.2 ± 26.1 | 1.44 | − 2.3 to 11.8 | 13/37 (35.1%) |
| SDT | 0.21 ± 0.23 | 0.37 | − 1.1 to 8.8 | 1/37 (2.7%) | |
Mean ± s.d. values of TDT are in ms; mean ± s.d. values of SDT are in g; s.d. — standard deviation.
Fig. 1.
(A, C) Visual temporal discrimination threshold (TDT) Z-scores and (B, D) tactile spatial discrimination threshold (SDT) Z-scores in healthy controls and patients with LD. Z-scores equal or greater than 2.0 were considered abnormal (indicated by a horizontal dotted line). The number of subjects with abnormal TDT Z-scores/the total number of subjects per each group is demonstrated at the top of each distribution plot. For the range of values, see Table 2.
Among all 84 LD patients, visual TDT was 48.4 ± 21.9 ms (Z-score = 1.25 ± 2.17) and the tactile SDT was 0.19 ± 0.23 g (Z-score = 0.26 ± 1.76) (Fig. 1, Table 2). Two sporadic LD patients had higher scores for discrimination (one ABLD: TDT Z-score = 11.8 and SDT Z-score = 8.8; one ADLD: SDT Z-score = 8.7), which were, however, similar to the range reported earlier across different forms of dystonia (Bradley et al., 2012).
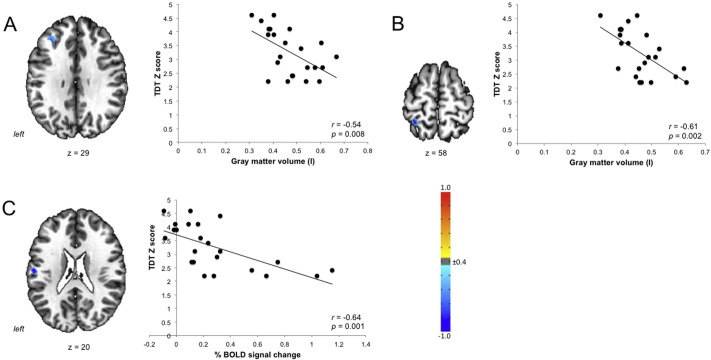
Compared to healthy controls, 26 LD patients (31%) had abnormal TDT and 5 patients (6%) had abnormal SDT. As hypothesized, these TDT and SDT abnormalities did not show significant relationships with LD severity or duration in any patient group (all p ≥ 0.31). However, at the neural level, abnormal TDT measures across all LD patients correlated with gray matter volume of the left middle frontal gyrus (r = − 0.54) and primary somatosensory cortex (r = − 0.61) as well as with brain activation in the left parietal operculum/primary somatosensory cortex (r = − 0.64) during symptomatic speech production (all FWE-corrected p ≤ 0.05) (Fig. 2). No significant relationships were found between abnormal TDT measures and resting-state brain activity in LD patients. Due to low frequency of SDT abnormalities in only 5% of LD patients and thus a low statistical power for further analysis, the correlations between abnormal SDT and brain function and structure were not performed.
Fig. 2.
Associations of abnormal TDT values with gray matter volume (A, B) and functional brain activation during speech production (C) across all LD patients. Z-scores equal or greater than 2.0 were considered abnormal. The color bar indicates the r values.
3.2. TDT and SDT measures based on a putative genotype of LD
Abnormal visual TDT was found in 9/24 (37.5%) familial LD patients and 17/60 (28.3%) sporadic LD patients (Fig. 1A; Table 2). Abnormal tactile SDT was found in 5/60 (8.3%) sporadic LD patients only (Fig. 1B; Table 2). An initial a priori Kruskal-Wallis test comparing TDT and SDT between familial, sporadic and control groups found a statistically significant group difference in visual TDT (χ2 = 11.5, p = 0.009, 95% CI = 0.58–1.31) but not tactile SDT (χ2 = 2.99, p = 0.39, 95% CI = − 0.09–0.50). The follow up Mann–Whitney U-tests determined that TDT was significantly increased in both patient groups compared to controls (familial LD vs. control: p = 0.02, 95% CI = 0.17–0.96; sporadic LD vs. control: p = 0.005; 95% CI = 0.45–1.28). A comparison of the frequency rate of abnormal TDT responses in sporadic and familial LD showed a significant difference between the two groups (χ2 = 115.5, p ≤ 0.005, 95% CI = 0.82–1.72).
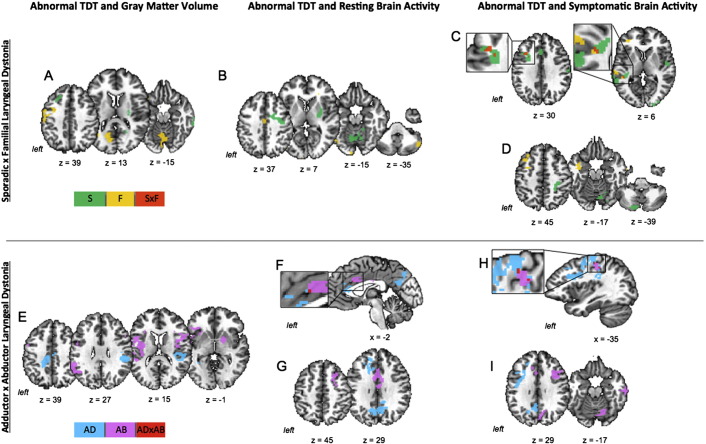
In both patient groups, abnormal TDT was negatively correlated with brain activity in the left middle frontal and superior temporal gyri (all peak r = − 0.79) during symptomatic speech production (Fig. 3C; Table 3). In addition, sporadic LD patients showed negative correlations in the right sensorimotor cortex and bilateral supplementary motor area (SMA) (r ≥ − 0.73) as well as positive correlations in the left anterior cingulate cortex (ACC) and bilateral cerebellum (left lobule VIIa and right lobule VI) (r ≥ 0.68). Familial LD patients had an additional positive correlation between abnormal TDT and symptomatic brain activation in the right superior temporal gyrus (r = 0.98).
Fig. 3.
Significant correlations of abnormal TDT with brain function and structure in LD patients. Top panel: In patients with sporadic and familial LD, abnormal TDT showed significant relationships with gray matter volume (A), resting-state brain activity (B), and brain activity during symptomatic speech production (C, D). Panel (C) shows regions of spatial overlap of these correlations between the two patient groups; Panel (D) shows additional regions of distinct correlations in each group. Bottom panel: In patients with ADLD and ABLD, abnormal TDT showed significant relationships with gray matter volume (E), resting-state brain activity (F, G), and brain activity during symptomatic speech production (H, I). Panels (F, H) show regions of spatial overlap of the corresponding correlations between the two patient groups. Panels (G, I) show additional regions of corresponding distinct correlations in each group. The color bars represent distinct correlations in sporadic (S), familial (F), ADLD (AD) and ABLD (AB) patients as well as common correlations between sporadic and familial patients (S × D) and between ADLD and ABLD patients (AD × AB). For the direction of correlations (positive and negative), see Table 3, Table 4.
Table 3.
Correlations of abnormal TDT with neuroimaging measures in sporadic and familial LD.
| Anatomical region | Cluster peak coordinates x, y, z | Cluster peak level r-value | Cluster size (voxels) |
|---|---|---|---|
| Abnormal TDT and brain activation during symptom production | |||
| Common to familial and sporadic LD | |||
| L middle frontal gyrus | |||
| Familial LD | − 33, 15, 38 | − 0.92 | 204 |
| Sporadic LD | − 37, 19, 30 | − 0.69 | 268 |
| Overlap in both groups | − 30, 20, 31 | − 0.79 | 12 |
| L superior temporal gyrus | |||
| Familial LD | − 51, − 25, 0 | − 0.93 | 283 |
| Sporadic LD | − 42, − 36, 8 | − 0.72 | 225 |
| Common to both groups | − 40, − 35, 4 | − 0.79 | 12 |
| Specific to sporadic LD | |||
| R precentral gyrus | 27, − 31, 44 | − 0.85 | 399 |
| R postcentral gyrus | 57, − 17, 28 | − 0.76 | 155 |
| L/R supplementary motor area | 1, − 23, 64 | − 0.73 | 207 |
| L anterior cingulate cortex | − 1, 25, 30 | 0.70 | 163 |
| L cerebellum (lobule VIIa) | − 23, − 77,− 36 | 0.77 | 125 |
| R cerebellum (lobule VI) | 11, − 70, − 15 | 0.68 | 172 |
| Specific to familial LD | |||
| R superior temporal gyrus | 57, − 21, 12 | 0.98 | 112 |
| Abnormal TDT and brain activation during the resting state | |||
| Specific to sporadic LD | |||
| R precentral gyrus | 37, − 7, 36 | 0.79 | 992* |
| R putamen/pallidum | 23, − 12, 6 | 0.81 | 992* |
| L/R cerebellum (lobule VI) | − 8, − 62, − 13 | 0.84 | 445 |
| Specific to familial LD | |||
| L middle cingulate cortex | − 2, − 6, 35 | 0.96 | 178 |
| L cerebellum (lobule VIIb) | − 38, − 54,− 49 | − 0.98 | 545 |
| R cerebellum (lobule VIIa) | 50, − 58, − 39 | − 0.92 | 110 |
| Abnormal TDT and gray matter volume | |||
| Specific to sporadic LD | |||
| L postcentral gyrus | − 50, − 18, 22 | − 0.79 | 471 |
| L superior frontal gyrus | − 21, 27, 36 | 0.74 | 276 |
| R middle temporal gyrus | 64, − 27, − 5 | 0.74 | 369 |
| R putamen/pallidum | 26, − 14, 12 | 0.59 | 232 |
| Specific to familial LD | |||
| L precentral gyrus | − 50, − 11, 40 | − 0.88 | 4111 |
| L/R cerebellum (lobule VI) | − 6, − 61, − 12 | − 0.97 | 4925 |
All r values are at an FWE-corrected p ≤ 0.05. The correlation peak coordinates are given in the AFNI standard Talairach − Tournoux space. L — left; R — right. The asterisk (*) denotes the clusters that span over two or more brain regions.
No common regions of correlation between sporadic and familial LD patients were identified for abnormal TDT values with either resting-state brain activity or gray matter volume (Fig. 3A, B). However, sporadic LD patients showed positive correlations between abnormal TDT measures and resting brain activity in the right precentral gyrus, putamen/pallidum and bilateral cerebellum (r ≥ 0.79) (Fig. 3B; Table 3). A negative correlation of abnormal TDT with gray matter volume was found in the left postcentral gyrus (r = − 0.79), while positive correlations were established in the left superior frontal gyrus, right middle temporal gyrus, and putamen (r ≥ 0.59) (Fig. 3A; Table 3).
On the other hand, abnormal TDT in familial LD patients had a positive relationship with resting brain activity in the left middle cingulate cortex (r = 0.96) and negative relationships in the bilateral cerebellum (lobule VII) (r ≥ − 0.92) (Fig. 3B; Table 3). Structurally, familial LD patients showed negative relationships between abnormal TDT scores and gray matter volume in the left precentral gyrus and bilateral cerebellum (r ≥ − 0.88) (Fig. 3A; Table 3).
3.3. TDT and SDT measures based on LD clinical phenotype
Abnormal TDT was found in 13/37 (35.1%) ABLD and 13/47 (27.7%) ADLD patients (Fig. 1C; Table 1), while SDT abnormalities were observed in 1/37 (2.7%) ABLD and 4/47 (8.5%) ADLD patients. An initial Kruskal–Wallis test of TDT and SDT Z-scores in ADLD, ABLD and control groups found a significant group effect in visual TDT (χ2 = 9.4, p = 0.009, 95% CI = 0.58–1.29) but not tactile SDT (χ2 = 0.40, p = 0.82, 95% CI = − 0.07–0.49). The follow up post hoc Mann–Whitney U-tests showed significantly increased TDT in both patient groups compared to controls (ADLD vs. control: p = 0.012, 95% CI = 0.32–1.04; ABLD vs. control: p = 0.004, 95% CI = 0.29–1.35). A comparison of the frequency rate of abnormal TDT responses in ADLD and ABLD patients showed a trend for significance between the two groups (χ2 = 39.7, p = 0.09, 95% CI = 0.4–1.55).
Common to both groups, abnormal TDT was correlated with brain activation during symptom production in the left primary sensorimotor cortex (r ≥ ± 0.68) and with resting brain activation in the left ACC (r = 0.64) (Fig. 3F, H; Table 4). In addition, ADLD patients showed negative correlations between abnormal TDT scores and symptom-related brain activation in the left middle/inferior frontal gyrus, posterior cingulate cortex and bilateral SMA (r ≥ − 0.70), while the left superior frontal gyrus and precuneus were positively correlated during the resting state (r ≥ 0.70) (Fig. 3G, I; Table 4). The ABLD group established positive relationships between abnormal TDT and symptomatic brain activation in the bilateral SMA, right insula/parietal operculum and cerebellum (lobule VI) (r ≥ 0.75), whereas the middle frontal gyrus was correlated with abnormal TDT during both resting state and speech production (Fig. 3G, I; Table 4).
Table 4.
Correlations of abnormal TDT with neuroimaging measures in ADLD and ABLD.
| Anatomical region | Cluster peak coordinates x, y, z | Cluster peak level r-value | Cluster size (voxels) |
|---|---|---|---|
| Abnormal TDT and brain activation during symptom production | |||
| Common to ADLD and ABLD | |||
| L sensorimotor cortex | |||
| ADLD | − 37, − 21, 54 | − 0.90 | 1211⁎ |
| ABLD | − 37, − 25, 42 | 0.89 | 240 |
| Overlap in both groups | − 31, − 21, 48 | ± 0.68# | 14 |
| Specific to ADLD | |||
| L middle frontal gyrus | − 23, 8, 46 | − 0.75 | 1211⁎ |
| L inferior frontal gyrus | − 32, 6, 29 | − 0.70 | 1211⁎ |
| L/R supplementary motor area | − 5, − 33, 56 | − 0.87 | 1511 |
| L posterior cingulate cortex | − 7, − 49, 26 | − 0.84 | 1011 |
| Specific to ABLD | |||
| L/R supplementary motor area | 1, 21, 40 | 0.86 | 287 |
| R insula/parietal operculum | 37, − 12, 17 | 0.75 | 321 |
| R middle frontal gyrus | 31, 19, 32 | 0.85 | 350 |
| R cerebellum (lobule VI) | 15, − 67, − 14 | 0.92 | 383 |
| Abnormal TDT and brain activation during the resting state | |||
| Common to ADLD and ABLD | |||
| L anterior cingulate cortex | |||
| ADLD | − 8, 24, 27 | 0.74 | 494⁎ |
| ABLD | − 10, 4, 31 | 0.84 | 258 |
| Overlap in both groups | − 1, 20, 26 | 0.64 | 2 |
| Specific to ADLD | |||
| L superior frontal gyrus | − 24, 32, 32 | 0.70 | 494⁎ |
| L precuneus | − 14, − 72, 39 | 0.97 | 711 |
| Specific to ABLD | |||
| L middle frontal gyrus | 24, 17, 44 | 0.77 | 248 |
| Abnormal TDT and gray matter volume | |||
| Specific to ADLD | |||
| L middle/posterior cingulate cortex | − 10, − 28, 33 | 0.94 | 1057 |
| R parietal operculum | 39, − 28, 25 | 0.85 | 1784 |
| Specific to ABLD | |||
| R insula/parietal operculum | 38, − 7, 13 | − 0.89 | 10,942* |
| L insula/parietal operculum | − 39, − 11, 17 | − 0.82 | 10,942* |
| L inferior frontal gyrus | − 44, 0, 21 | − 0.79 | 10,942* |
| L inferior parietal lobule | − 54, − 39, 25 | − 0.80 | 1710 |
| R putamen/pallidum | 18, 3, − 1 | − 0.79 | 2806 |
All r values are at an FWE-corrected p ≤ 0.05. The correlation peak coordinates are given in the AFNI standard Talairach–Tournoux space. L — left; R — right. The asterisk (*) denotes the clusters that span over two or more brain regions; the hash (#) denotes a positive correlation in one group and a negative correlation in the other group.
As in the case with sporadic and familial LD, the ADLD and ABLD patients did not show any common significant relationships between abnormal TDT measures and gray matter volume (Fig. 3E; Table 4). However, ADLD group-specific positive correlations were found in the left middle/posterior cingulate cortex and right parietal operculum (r ≥ 0.85), whereas ABLD group had negative correlations in the bilateral insula/parietal operculum, left inferior frontal gyrus and inferior parietal lobule as well as the right putamen/pallidum (r ≥ − 0.79).
4. Discussion
Our findings outline the relationships between abnormal sensory discrimination and phenotype/genotype interactions in isolated focal dystonia and elucidate the neural substrates underlying a possible endophenotype of this disorder. Specifically, our study demonstrates that patients with all forms of LD, including sporadic and familial LD as well as ADLD and ABLD, exhibit a range of abnormalities in temporal but not spatial discrimination, which is suggestive of greater sensitivity of the TDT measure in this disorder. Compared to sporadic LD patients, TDT abnormalities in familial LD had both greater frequency (i.e., 37.5% familial vs. 28.3% sporadic) and higher penetrance (i.e., 37.5% penetrance of abnormal TDT in familial LD vs. 12% penetrance of the familial phenotype (Kirke et al., 2015)). Conversely, abnormal TDT frequency rates did not differ significantly in clinically distinct ADLD and ABLD phenotypes. In line with this finding and as hypothesized, we did not observe any significant correlations between symptom duration or severity and TDT abnormalities, which points to abnormal TDT as an LD endophenotype with a closer, more upstream relationship to the underlying (albeit yet unknown) gene(s) than to the clinical phenotype. Further substantiating these findings, the presence of TDT abnormalities highlighted a separate group of LD patients, who exhibited structural and functional brain alterations in the middle frontal and somatosensory cortices. These findings are consistent with the previous report of TDT-associated brain activity in unaffected relatives of dystonia patients (Kimmich et al., 2014), hinting at a possibly causative nature of sensory alterations in dystonia pathophysiology. Importantly, fine-grained distinctions emerged in the comparisons between sporadic and familial LD and between ADLD and ABLD patients, which shed light on divergent, multifactorial pathophysiological pathways underlying distinct genotype and phenotype relationships in this disorder.
4.1. Neural correlates of abnormal TDT based on putative genotype of LD
Identification of both common and distinct neural correlates of TDT processing unifies different forms of LD and, at the same time, reflects the presence of possible differences in LD genotype/phenotype relations. During symptom production, both sporadic and familial LD patients established common negative correlations between abnormal TDT and brain activity in the left middle frontal and superior temporal gyri, indicating that an increase in discrimination threshold led to a decrease in activation in these brain regions. These findings suggest an importance of the executive frontal cortical network in LD pathophysiology, possibly through the aberrant basal ganglia-thalamo-cortical influences contributing to altered speech motor planning and execution (Alvarez and Emory, 2006, Bourguignon, 2014).
With regard to distinct features of TDT neural representation, familial LD patients extended their relationships between abnormal TDT and resting-state brain function to the middle cingulate cortex and cerebellum (lobules VIIa–VIIb), whereas sporadic LD patients showed additional correlations between abnormal TDT and brain function in the primary sensorimotor cortex, SMA, ACC, putamen/pallidum and cerebellum (lobules VI and VIIa). Differences in structural correlations between abnormal TDT and gray matter volume were found predominantly in the basal ganglia in sporadic LD and the cerebellum in familial LD. Such a disparity of neural representations of abnormal TDT processing across different forms of LD suggests greater involvement of the sensorimotor network during symptom generation in sporadic than familial LD. Within this circuitry, involvement of the putamen and pallidum in the sporadic group resonated well with previous studies reporting striatal volumetric changes as a contributing factor to the pathophysiology of focal dystonia (Black et al., 1998, Draganski et al., 2003, Etgen et al., 2006, Granert et al., 2011, Simonyan and Ludlow, 2012). Our findings further extend this knowledge by showing correlations between basal ganglia abnormalities and a potential mediational endophenotype of LD. Based on identified relationships of abnormal TDT with putamen/pallidal structure and resting-state activation in sporadic LD, we suggest that this region may also be involved in aberrant processing and integration of temporal aspects of sensory information with motor behavior in dystonia.
On the other hand, the microstructural changes (represented by gray matter volumetric abnormalities on VBM) in the cerebellum in the familial group not only signified its recently proposed role in dystonia pathophysiology (Prudente et al., 2014), but also suggested that this structure may be particularly critical in hereditary dystonias. Indeed, cerebellar influences on motor control were reported in a number of electrophysiological and imaging studies among various forms of hereditary dystonias, including DYT1 (Carbon and Eidelberg, 2009, Sadnicka et al., 2015) and DYT6 (Carbon et al., 2008), whereas the cerebello-thalamo-cortical projections have been shown to facilitate intracortical inhibition (Molinari et al., 2002) and to be associated with cortical plastic changes (Doyon et al., 1998). In a mouse model of dystonia, aberrant cerebellar relay to the basal ganglia was recently deemed to contribute to dystonia via the short latency cortico-striatal pathway (Chen et al., 2014, Ulug et al., 2011). In addition, the cerebellum establishes projections with several cortical regions, including prefrontal cortex, SMA and posterior parietal cortex (Akkal et al., 2007, Clower et al., 2001, Coffman et al., 2011), thus possibly exerting its direct or indirect influences on abnormal functional relationships between TDT and brain activity in the prefrontal cortex. Our findings suggest that cerebellar microstructural changes and outflow dysfunction in patients with hereditary dystonia may result, in part, from upstream altered sensory processing rather than represent a coexisting trait.
4.2. Neural correlates of abnormal TDT based on LD clinical phenotypes
Both ADLD and ABLD groups showed common correlations of abnormal TDT with symptomatic brain activation in the left primary sensorimotor cortex and with resting-state activation in the left ACC. The finding of sensorimotor involvement is consistent with previous reports of functional and microstructural alterations in this region (Ali et al., 2006, Haslinger et al., 2005, Simonyan and Ludlow, 2010, Simonyan and Ludlow, 2012, Simonyan et al., 2013a) and may underline the aberrant function of this region in processing and execution of motor task production. The contribution of the ACC in LD is less clear but may be important for controlling action–inhibition and perception as part of the executive resting-state network (Smith et al., 2009) via direct connections with the laryngeal/orofacial motor cortex (Simonyan and Jurgens, 2002, Simonyan and Jurgens, 2005).
Both ADLD and ABLD groups showed regions of distinct correlations with abnormal TDT similar to those in sporadic and familial LD patients. In addition, ADLD and ABLD groups established significant structural relationships with the insula and parietal operculum. While the parietal operculum is likely to be one of the structures on the afferent (sensory) pathway of LD, the insular cortex, along with the middle frontal cortex and ACC, may be responsible for the refinement of executive functions on the efferent pathway, upstream to the motor cortex, contributing to LD symptomatology.
5. Conclusions
Our study demonstrated that temporal discrimination is abnormal across different clinical phenotypes and putative genotypes of LD. We further showed that these abnormalities are related to alterations in brain function and structure, with both common and distinct patterns of abnormalities between sporadic and familial cases as well as between ADLD and ABLD clinical phenotypes. As a direction for future research, it is conceivable that these genetic influences are greater in familial LD patients, which may prime them to develop dystonia following a (yet unknown) trigger, whereas the dystonic cascade in sporadic patients may be provoked by motor entrainment coupled with abnormal sensory feedback. On the other hand, largely similar frequency rates of sensory discrimination abnormalities between ADLD and ABLD patients might suggest a role for additional influences (e.g., environmental (Schweinfurth et al., 2002, Tanner et al., 2011)) that may further divert these patients into different clinical phenotypes.
Conflicts of interest/financial disclosures
None.
Acknowledgments
We thank Aisling Fanning, Adriana Dabacan and Robert Whelan for their help with initial TDT setup, Beth Belluscio for her advice on SDT procedures, Amanda Pechman for patient recruitment, and Lazar Fleysher and Heather Alexander for imaging data acquisition. This work was supported by R01DC01180 grant to KS from the National Institute on Deafness and Other Communication Disorders, National Institutes of Health. PT was supported by a clinical fellowship training program from the Dystonia Medical Research Foundation.
References
- Aglioti S.M., Fiorio M., Forster B., Tinazzi M. Temporal discrimination of cross-modal and unimodal stimuli in generalized dystonia. Neurology. 2003;60:782–785. doi: 10.1212/01.wnl.0000046528.24693.5b. [DOI] [PubMed] [Google Scholar]
- Akkal D., Dum R.P., Strick P.L. Supplementary motor area and presupplementary motor area: targets of basal ganglia and cerebellar output. J. Neurosci. 2007;27:10659–10673. doi: 10.1523/JNEUROSCI.3134-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S.O., Thomassen M., Schulz G.M., Hosey L.A., Varga M., Ludlow C.L., Braun A.R. Alterations in CNS activity induced by botulinum toxin treatment in spasmodic dysphonia: an H215O PET study. J. Speech Lang. Hear. Res. 2006;49:1127–1146. doi: 10.1044/1092-4388(2006/081). [DOI] [PubMed] [Google Scholar]
- Alvarez J.A., Emory E. Executive function and the frontal lobes: a meta-analytic review. Neuropsychol. Rev. 2006;16:17–42. doi: 10.1007/s11065-006-9002-x. [DOI] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Belluscio B.A., Jin L., Watters V., Lee T.H., Hallett M. Sensory sensitivity to external stimuli in Tourette syndrome patients. Mov. Disord. 2011;26:2538–2543. doi: 10.1002/mds.23977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman B.D., Hallett M., Herscovitch P., Simonyan K. Striatal dopaminergic dysfunction at rest and during task performance in writer's cramp. Brain. 2013;136:3645–3658. doi: 10.1093/brain/awt282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black K.J., Ongur D., Perlmutter J.S. Putamen volume in idiopathic focal dystonia. Neurology. 1998;51:819–824. doi: 10.1212/wnl.51.3.819. [DOI] [PubMed] [Google Scholar]
- Bourguignon N.J. A rostro-caudal axis for language in the frontal lobe: the role of executive control in speech production. Neurosci. Biobehav. Rev. 2014;47:431–444. doi: 10.1016/j.neubiorev.2014.09.008. [DOI] [PubMed] [Google Scholar]
- Bradley D., Whelan R., Kimmich O., O'Riordan S., Mulrooney N., Brady P., Walsh R., Reilly R.B., Hutchinson S., Molloy F., Hutchinson M. Temporal discrimination thresholds in adult-onset primary torsion dystonia: an analysis by task type and by dystonia phenotype. J. Neurol. 2012;259:77–82. doi: 10.1007/s00415-011-6125-7. [DOI] [PubMed] [Google Scholar]
- Bradley D., Whelan R., Walsh R., Reilly R.B., Hutchinson S., Molloy F., Hutchinson M. Temporal discrimination threshold: VBM evidence for an endophenotype in adult onset primary torsion dystonia. Brain. 2009;132:2327–2335. doi: 10.1093/brain/awp156. [DOI] [PubMed] [Google Scholar]
- Carbon M., Eidelberg D. Abnormal structure–function relationships in hereditary dystonia. Neuroscience. 2009;164:220–229. doi: 10.1016/j.neuroscience.2008.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbon M., Kingsley P.B., Tang C., Bressman S., Eidelberg D. Microstructural white matter changes in primary torsion dystonia. Mov. Disord. 2008;23:234–239. doi: 10.1002/mds.21806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J., Brin M.F., Fahn S. Idiopathic cervical dystonia: clinical characteristics. Mov. Disord. 1991;6:119–126. doi: 10.1002/mds.870060206. [DOI] [PubMed] [Google Scholar]
- Chen C.H., Fremont R., Arteaga-Bracho E.E., Khodakhah K. Short latency cerebellar modulation of the basal ganglia. Nat. Neurosci. 2014;17:1767–1775. doi: 10.1038/nn.3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clower D.M., West R.A., Lynch J.C., Strick P.L. The inferior parietal lobule is the target of output from the superior colliculus, hippocampus, and cerebellum. J. Neurosci. 2001;21:6283–6291. doi: 10.1523/JNEUROSCI.21-16-06283.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman K.A., Dum R.P., Strick P.L. Cerebellar vermis is a target of projections from the motor areas in the cerebral cortex. Proc. Natl. Acad. Sci. U. S. A. 2011;108:16068–16073. doi: 10.1073/pnas.1107904108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadra M.B., Cammoun L., Butz T., Cuisenaire O., Thiran J.P. Comparison and validation of tissue modelization and statistical classification methods in T1-weighted MR brain images. IEEE Trans. Med. Imaging. 2005;24:1548–1565. doi: 10.1109/TMI.2005.857652. [DOI] [PubMed] [Google Scholar]
- Doyon J., Laforce R., Jr., Bouchard G., Gaudreau D., Roy J., Poirier M., Bedard P.J., Bedard F., Bouchard J.P. Role of the striatum, cerebellum and frontal lobes in the automatization of a repeated visuomotor sequence of movements. Neuropsychologia. 1998;36:625–641. doi: 10.1016/s0028-3932(97)00168-1. [DOI] [PubMed] [Google Scholar]
- Draganski B., Thun-Hohenstein C., Bogdahn U., Winkler J., May A. “Motor circuit” gray matter changes in idiopathic cervical dystonia. Neurology. 2003;61:1228–1231. doi: 10.1212/01.wnl.0000094240.93745.83. [DOI] [PubMed] [Google Scholar]
- Etgen T., Muhlau M., Gaser C., Sander D. Bilateral grey-matter increase in the putamen in primary blepharospasm. J. Neurol. Neurosurg. Psychiatry. 2006;77:1017–1020. doi: 10.1136/jnnp.2005.087148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorio M., Gambarin M., Valente E.M., Liberini P., Loi M., Cossu G., Moretto G., Bhatia K.P., Defazio G., Aglioti S.M., Fiaschi A., Tinazzi M. Defective temporal processing of sensory stimuli in DYT1 mutation carriers: a new endophenotype of dystonia? Brain. 2007;130:134–142. doi: 10.1093/brain/awl283. [DOI] [PubMed] [Google Scholar]
- Fiorio M., Tinazzi M., Bertolasi L., Aglioti S.M. Temporal processing of visuotactile and tactile stimuli in writer's cramp. Ann. Neurol. 2003;53:630–635. doi: 10.1002/ana.10525. [DOI] [PubMed] [Google Scholar]
- Fiorio M., Tinazzi M., Scontrini A., Stanzani C., Gambarin M., Fiaschi A., Moretto G., Fabbrini G., Berardelli A. Tactile temporal discrimination in patients with blepharospasm. J. Neurol. Neurosurg. Psychiatry. 2008;79:796–798. doi: 10.1136/jnnp.2007.131524. [DOI] [PubMed] [Google Scholar]
- Friedman A., Fahn S. Spontaneous remissions in spasmodic torticollis. Neurology. 1986;36:398–400. doi: 10.1212/wnl.36.3.398. [DOI] [PubMed] [Google Scholar]
- Grandas F., Elston J., Quinn N., Marsden C.D. Blepharospasm: a review of 264 patients. J. Neurol. Neurosurg. Psychiatry. 1988;51:767–772. doi: 10.1136/jnnp.51.6.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granert O., Peller M., Jabusch H.C., Altenmuller E., Siebner H.R. Sensorimotor skills and focal dystonia are linked to putaminal grey-matter volume in pianists. J. Neurol. Neurosurg. Psychiatry. 2011;82:1225–1231. doi: 10.1136/jnnp.2011.245811. [DOI] [PubMed] [Google Scholar]
- Haslinger B., Erhard P., Dresel C., Castrop F., Roettinger M., Ceballos-Baumann A.O. “Silent event-related” fMRI reveals reduced sensorimotor activation in laryngeal dystonia. Neurology. 2005;65:1562–1569. doi: 10.1212/01.wnl.0000184478.59063.db. [DOI] [PubMed] [Google Scholar]
- Hutchinson M., Kimmich O., Molloy A., Whelan R., Molloy F., Lynch T., Healy D.G., Walsh C., Edwards M.J., Ozelius L., Reilly R.B., O'Riordan S. The endophenotype and the phenotype: temporal discrimination and adult-onset dystonia. Mov. Disord. 2013;28:1766–1774. doi: 10.1002/mds.25676. [DOI] [PubMed] [Google Scholar]
- Kimmich O., Bradley D., Whelan R., Mulrooney N., Reilly R.B., Hutchinson S., O'Riordan S., Hutchinson M. Sporadic adult onset primary torsion dystonia is a genetic disorder by the temporal discrimination test. Brain. 2011;134:2656–2663. doi: 10.1093/brain/awr194. [DOI] [PubMed] [Google Scholar]
- Kimmich O., Molloy A., Whelan R., Williams L., Bradley D., Balsters J., Molloy F., Lynch T., Healy D.G., Walsh C., O'Riordan S., Reilly R.B., Hutchinson M. Temporal discrimination, a cervical dystonia endophenotype: penetrance and functional correlates. Mov. Disord. 2014;29:804–811. doi: 10.1002/mds.25822. [DOI] [PubMed] [Google Scholar]
- Kirke D.N., Frucht S.J., Simonyan K. Alcohol responsiveness in laryngeal dystonia: a survey study. J. Neurol. 2015;262(6):1548–1556. doi: 10.1007/s00415-015-7751-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlow C.L., Adler C.H., Berke G.S., Bielamowicz S.A., Blitzer A., Bressman S.B., Hallett M., Jinnah H.A., Juergens U., Martin S.B., Perlmutter J.S., Sapienza C., Singleton A., Tanner C.M., Woodson G.E. Research priorities in spasmodic dysphonia. Otolaryngol. Head Neck Surg. 2008;139:495–505. doi: 10.1016/j.otohns.2008.05.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniak S., Sieberer M., Hagenah J., Klein C., Vieregge P. Focal and segmental primary dystonia in north-western Germany—a clinico-genetic study. Acta Neurol. Scand. 2003;107:228–232. doi: 10.1034/j.1600-0404.2003.01362.x. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A. Intermediate or brainless phenotypes for psychiatric research? Psychol. Med. 2010;40:1057–1062. doi: 10.1017/s0033291709991929. [DOI] [PubMed] [Google Scholar]
- Molinari M., Filippini V., Leggio M.G. Neuronal plasticity of interrelated cerebellar and cortical networks. Neuroscience. 2002;111:863–870. doi: 10.1016/s0306-4522(02)00024-6. [DOI] [PubMed] [Google Scholar]
- Molloy F.M., Carr T.D., Zeuner K.E., Dambrosia J.M., Hallett M. Abnormalities of spatial discrimination in focal and generalized dystonia. Brain. 2003;126:2175–2182. doi: 10.1093/brain/awg219. [DOI] [PubMed] [Google Scholar]
- Neychev V.K., Gross R.E., Lehericy S., Hess E.J., Jinnah H.A. The functional neuroanatomy of dystonia. Neurobiol. Dis. 2011;42:185–201. doi: 10.1016/j.nbd.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrachione T.K., Ghosh S.S. Optimized design and analysis of sparse-sampling FMRI experiments. Front. Neurosci. 2013;7:55. doi: 10.3389/fnins.2013.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prudente C.N., Hess E.J., Jinnah H.A. Dystonia as a network disorder: what is the role of the cerebellum? Neuroscience. 2014;260:23–35. doi: 10.1016/j.neuroscience.2013.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramdhani R.A., Simonyan K. Primary dystonia: conceptualizing the disorder through a structural brain imaging lens. Tremor Other Hyperkinet Mov (N Y) 2013;3 doi: 10.7916/D8H70DJ7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadnicka A., Teo J.T., Kojovic M., Parees I., Saifee T.A., Kassavetis P., Schwingenschuh P., Katschnig-Winter P., Stamelou M., Mencacci N.E., Rothwell J.C., Edwards M.J., Bhatia K.P. All in the blink of an eye: new insight into cerebellar and brainstem function in DYT1 and DYT6 dystonia. Eur. J. Neurol. 2015;22(5):762–767. doi: 10.1111/ene.12521. [DOI] [PubMed] [Google Scholar]
- Schweinfurth J.M., Billante M., Courey M. Risk factors and demographics in patients with spasmodic dysphonia. Laryngoscope. 2002;112:220–223. doi: 10.1097/00005537-200202000-00004. [DOI] [PubMed] [Google Scholar]
- Sheehy M.P., Rothwell J.C., Marsden C.D. Writer's cramp. Adv. Neurol. 1988;50:457–472. [PubMed] [Google Scholar]
- Simonyan K., Berman B.D., Herscovitch P., Hallett M. Abnormal striatal dopaminergic neurotransmission during rest and task production in spasmodic dysphonia. J. Neurosci. 2013;33:14705–14714. doi: 10.1523/JNEUROSCI.0407-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonyan K., Herscovitch P., Horwitz B. Speech-induced striatal dopamine release is left lateralized and coupled to functional striatal circuits in healthy humans: a combined PET, fMRI and DTI study. Neuroimage. 2013;70:21–32. doi: 10.1016/j.neuroimage.2012.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonyan K., Jurgens U. Cortico-cortical projections of the motorcortical larynx area in the rhesus monkey. Brain Res. 2002;949:23–31. doi: 10.1016/s0006-8993(02)02960-8. [DOI] [PubMed] [Google Scholar]
- Simonyan K., Jurgens U. Afferent cortical connections of the motor cortical larynx area in the rhesus monkey. Neuroscience. 2005;130:133–149. doi: 10.1016/j.neuroscience.2004.08.031. [DOI] [PubMed] [Google Scholar]
- Simonyan K., Ludlow C.L. Abnormal activation of the primary somatosensory cortex in spasmodic dysphonia: an fMRI study. Cereb. Cortex. 2010;20:2749–2759. doi: 10.1093/cercor/bhq023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonyan K., Ludlow C.L. Abnormal structure–function relationship in spasmodic dysphonia. Cereb. Cortex. 2012;22:417–425. doi: 10.1093/cercor/bhr120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Fox P.T., Miller K.L., Glahn D.C., Fox P.M., Mackay C.E., Filippini N., Watkins K.E., Toro R., Laird A.R., Beckmann C.F. Correspondence of the brain's functional architecture during activation and rest. Proc. Natl. Acad. Sci. U. S. A. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner K., Roy N., Merrill R.M., Kimber K., Sauder C., Houtz D.R., Doman D., Smith M.E. Risk and protective factors for spasmodic dysphonia: a case-control investigation. J. Voice. 2011;25:e35–e46. doi: 10.1016/j.jvoice.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Ulug A.M., Vo A., Argyelan M., Tanabe L., Schiffer W.K., Dewey S., Dauer W.T., Eidelberg D. Cerebellothalamocortical pathway abnormalities in torsin A DYT1 knock-in mice. Proc. Natl. Acad. Sci. U.S.A. 2011;108:6638–6643. doi: 10.1073/pnas.1016445108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoons E., Booij J., Nederveen A.J., Dijk J.M., Tijssen M.A. Structural, functional and molecular imaging of the brain in primary focal dystonia—a review. Neuroimage. 2011;56:1011–1020. doi: 10.1016/j.neuroimage.2011.02.045. [DOI] [PubMed] [Google Scholar]