SUMMARY
We describe the case of an eight-year-old boy with X-linked agammaglobulinemia who developed mild varicella despite regular intravenous immunoglobulin (IVIG) therapy. He maintained protective antibody levels against varicella and the previous batches of IVIG that he received had adequate varicella-specific IgG levels. The case illustrates that IVIG may not prevent VZV infection.
Keywords: Chickenpox, Immunoglobulin, Intravenous, Agammaglobulinemia, Immunodeficiency disorders
RESUMO
Relatamos o caso de uma criança com agamaglobulinemia ligada ao X, sexo masculino, oito anos de idade, que desenvolveu quadro de varicela leve, apesar do tratamento regular com imunoglobulina intravenosa (IVIG). O paciente mantinha níveis adequados de imunoglobulina (IgG) contra varicela, assim como, os últimos lotes de IVIG por ele recebido também apresentavam níveis adequados do anticorpo específico. O caso ilustra que o tratamento regular com IVIG não é suficiente para prevenir a infecção pelo vírus da varicela-zoster.
INTRODUCTION
Patients who have immunodeficiency disorders rely on immunoglobulin prophylaxis to prevent common infectious diseases. Intravenous immunoglobulin (IVIG) products are prepared from pools of plasma collected from a large number of healthy donors and, therefore, contain antibodies against many infectious agents preventing a variety of bacterial and viral infections, such as varicella, in patients with impaired antibody production1 , 4 , 12.
X-linked agammaglobulinemia (XLA) is a hereditary immunodeficiency, characterized by absence of mature B cells, resulting in very low levels of all immunoglobulin isotypes. The mainstay of therapy for these patients is immunoglobulin replacement9 , 13 , 16.
Varicella is a disease caused by the primary infection with the varicella-zoster virus (VZV). Clinical manifestations of the disease depend on age, immune and vaccination status, and type of exposure 2. As a result of immunization, in some countries, chickenpox is no longer a common illness11. In Brazil, however, this disease is still endemic.
The majority of primary VZV infections involve uncomplicated chickenpox. However, in newborns and inadequately protected immunosuppressed patients, exposure to VZV can lead to severe illness2 , 11 , 17.
In this study, we report a case of chickenpox in a child with XLA who is being treated with regular intravenous immunoglobulin. Although the patient developed a mild disease, this case shows that regular intravenous immunoglobulin therapy did not effectively prevent chickenpox.
CASE REPORT
An eight-year-old boy was diagnosed with XLA at the age of four, and presented 0.5% of B lymphocytes and serum IgG, IgA and IgM levels of 149 mg/dL, 1 mg/dL and 11 mg/dL, respectively. T lymphocyte numbers were normal. He started treatment with IVIG (600 mg/kg, every four weeks) with excellent compliance and good outcome, keeping IgG levels over 600 mg/dL.
He was admitted to our clinic with a one-day history of skin lesions on trunk and abdomen that had started 19 days after his last IVIG infusion and was diagnosed with a mild varicella (less than 50 lesions at various stages - red papules, vesicles and broken vesicles leaving a crust). There was no history of fever or other symptoms. He was treated with oral acyclovir for five days and received an extra dose of standard IVIG. He recovered without any complication.
Total serum IgG levels, varicella-zoster IgG levels, and avidity, assessed on day 1 of varicella, are presented in Table 1. VZV antibodies and VZV IgG avidity were also assessed from serum samples collected five, ten, sixteen and twenty months before varicella (Table 1). Varicella-specific IgG levels from the batch of IVIG that was administered to the patient for the last three months before varicella were determined (Table 1).
Table 1. a Varicella-specific IgG levels and its avidity, and total IgG levels obtained from the serum of the patient. b Varicella-specific IgG levels from the batch of IVIG that patient received over the last three months before VZV infection. Samples were tested for varicella-specific IgG levels by ELISA5,14 .
| Varicella-specific IgG titer (IU/mL) | Varicella-specific IgG avidity (%) | IgG (mg/dL) | |
|---|---|---|---|
| Day 1 of varicellaa | 2.03 | 70.3 | 718 |
| Five months before varicellaa | 1.2 | 62.4 | |
| Ten months before varicellaa | 0.48 | 72.3 | |
| Sixteen months before varicellaa | 0.79 | 66.9 | |
| Twenty months before varicellaa | 0.87 | 70.9 | |
| IVIGb | 12.4 | -- |
Protective IgG levels are considered when higher than 0.1 IU/mL8,9.
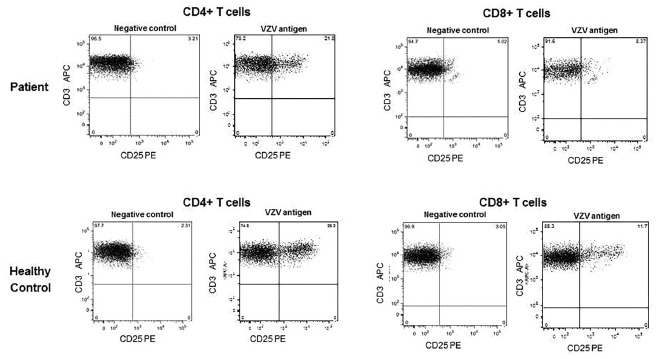
At the time of varicella infection, the patient's CD4+ and CD8+ T lymphocyte counts were 2388 cells/mm3 and 1293 cells/mm3, respectively. Fifteen days after infection, both CD4+ and CD8+ T cells expressed CD25 upon in vitro stimulation with VZV specific antigens18 (Fig. 1).
Fig. 1. - CD25 expression on CD4+ and CD8+ T lymphocytes after incubation with VZV specific antigens. The final value of positive cells was obtained by subtracting the percentage of cells stimulated with cell supernatant of non-VZV-infected cells (negative control) from the percentage of cells from the culture in presence of the stimulus (VZV antigen). Adapted from VIANA et al., 201018.
DISCUSSION
The aim of IVIG replacement therapy in hypogammaglobulinemic patients is to protect them from potentially preventable infections.
However, it is well known that many factors may have an impact on the quality and quantity of antibodies in IVIG preparations, and there are differences in immunoglobulin content from brand to brand as well as from batch to batch1 , 3 , 4 , 6 , 11 , 12.
Assessment of current IVIG preparations showed that they contain high levels of VZV specific IgG, despite the changing epidemiology of varicella due to vaccine introduction11 , 12. A study published in 2000 showed that patients receiving monthly IVIG at 400 mg/kg may be protected against varicella and probably do not require varicella-zoster immune globulin (VZIG) if the last dose of IVIG was given three weeks or less before exposure8. That is in agreement with the fact that antibodies for some virus and bacteria in IVIG preparations seems to have a half-life longer than 20 days10.
In 2009, we had two other patients, one with common variable immunodeficiency (CVI) and another with XLA who also presented with mild varicella while on regular IVIG replacement therapy, similar to the patient in this case. In this current report, we showed that the previous-batches of IVIG that the patient received had adequate varicella-specific IgG levels and our patient maintained protective antibody levels against varicella during the previous months (Table 1).
Specific antibody levels have been correlated with protection against several diseases. Antibody levels that correlate with protection are generally derived from studies with healthy population wherein observations have been shown that individuals with a certain level of antibody are always or nearly always protected from disease15. However, these protective levels are usually determined after active immunization. There has been no study that has defined what level of varicella-specific antibody provides adequate protection in passive prophylaxis usage. What would be the protective antibody levels for a patient who does not produce antibodies?
Humoral immunity is very important for VZV neutralization of the cell-free virus and cellular immunity is essential for limiting the extent of primary infection with VZV2 , 7. XLA patients have normal T cells function and these cells from our patient responded to VZV (Fig. 1) which could explain his favorable evolution. However, certain groups of patients, such as those with impaired T cell-mediated immunity or with antibody deficiency but taking immunosuppressive drugs can develop severe complications7 and must be protected.
Although the efficacy of IVIG in the prevention of several diseases is well established1 , 4, we have to be aware that this therapy cannot be totally effective for some diseases in certain situations. Maybe IVIG is effective in modifying varicella infection but not in preventing the disease.
Footnotes
FUNDINGNone.
ETHICAL APPROVALNot required.
REFERENCES
- 1.Albin S, Cunningham-Rundles C. An update on the use of immunoglobulin for the treatment of immunodeficiency disorders. Immunotherapy. 2014;6:1113–1126. doi: 10.2217/imt.14.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Academy of Pediatrics Varicella-zoster infectionsPickering LK, Baker CJ, Kimberlin DW, Long SS.Red Book: 2012 report of the Committee on Infectious Diseases thElk Grove Village: American Academy of Pediatrics; 2012774–789. [Google Scholar]
- 3.Audet S, Virata-Theimer ML, Beeler JA, Scott DE, Frazier DJ, Mikolajczyk MG. Measles-virus-neutralizing antibodies in intravenous immunoglobulins. J Infect Dis. 2006;194:781–789. doi: 10.1086/506363. [DOI] [PubMed] [Google Scholar]
- 4.Berger M. Principles of and advances in immunoglobulin replacement therapy for primary immunodeficiency. Immunol Allergy Clin North Am. 2008;28:413–437. doi: 10.1016/j.iac.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Ory F, Echevarría JM, Kafatos G, Anastassopoulou C, Andrews N, Backhouse J. European seroepidemiology network 2: standardization of assays for seroepidemiology of varicella zoster virus. J Clin Virol. 2006;36:111–118. doi: 10.1016/j.jcv.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 6.Gelfand EW. Differences between IGIV products:impact on clinical outcome. Int Immunopharmacol. 2006;6:592–599. doi: 10.1016/j.intimp.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Gershon AA, Gershon MD, Breuer J, Levin MJ, Oaklnder AL, Griffiths PD. Advances in the understanding of the pathogenesis and epidemiology of herpes zoster. J Clin Virol. 2010;48(Suppl 1):S2–S7. doi: 10.1016/S1386-6532(10)70002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keller MA, Stiehm ER. Passive immunity in prevention and treatment of infectious diseases. Clin Microbiol Rev. 2000;13:602–614. doi: 10.1128/cmr.13.4.602-614.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maarschalk-Ellerbroek LJ, Hoepelman IM, Ellerbroek PM. Immunoglobulin treatment in primary antibody deficiency. Int J Antimicrob Agents. 2011;37:396–404. doi: 10.1016/j.ijantimicag.2010.11.027. [DOI] [PubMed] [Google Scholar]
- 10.Mankarious S, Lee M, Fischer S, Pyun KH, Ochs HD, Oxelius VA. The half-lives of IgG subclasses and specific antibodies in patients with primary immunodeficiency who are receiving intravenously administered immunoglobulin. J Lab Clin Med. 1988;112:634–640. [PubMed] [Google Scholar]
- 11.Maranich AM, Rajnik M. Varicella-specific immunoglobulin G titers in commercial intravenous immunoglobulin preparations. Pediatrics. 2009;124:e484–e488. doi: 10.1542/peds.2009-0047. [DOI] [PubMed] [Google Scholar]
- 12.Nobre FA, Gonzalez IGS, Simão RM, Moraes-Pinto MI, Costa-Carvalho BT. Antibody levels to tetanus, diphtheria, measles and varicella in patients with primary immunodeficiency undergoing intravenous immunoglobulin therapy: a prospective study. BMC Immunol. 2014;15:26–26. doi: 10.1186/1471-2172-15-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Notarangelo LD. Primary immunodeficiencies. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S182–S194. doi: 10.1016/j.jaci.2009.07.053. [DOI] [PubMed] [Google Scholar]
- 14.Ono E, Lafer MM, Weckx LY, Granato C, de Moraes-Pinto MI. A simple and cheaper in house varicella zoster virus antibody indirect ELISA. Rev Inst Med Trop Sao Paulo. 2004;46:165–168. doi: 10.1590/s0036-46652004000300008. [DOI] [PubMed] [Google Scholar]
- 15.Pichichero ME. Booster vaccinations: can immunologic memory outpace disease pathogenesis? Pediatrics. 2009;124:1633–1641. doi: 10.1542/peds.2008-3645. [DOI] [PubMed] [Google Scholar]
- 16.Plebani A, Soresina A, Rondelli R, Amato GM, Azzari C, Cardinale F. Clinical, immunological, and molecular analysis in a large cohort of patients with X-linked agammaglobulinemia: an Italian multicenter study. Clin Immunol. 2002;104:221–230. doi: 10.1006/clim.2002.5241. [DOI] [PubMed] [Google Scholar]
- 17.Sartori AMC. A review of the varicella vaccine in immunocompromised individuals. Int J Infect Dis. 2004;8:259–270. doi: 10.1016/j.ijid.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Viana PO, Ono E, Miyamoto M, Salomao R, Costa-Carvalho BT, Weckx LY. Humoral and cellular immune responses to measles and tetanus: the importance of elapsed time since last exposure and the nature of the antigen. J Clin Immunol. 2010;30:574–582. doi: 10.1007/s10875-010-9420-7. [DOI] [PubMed] [Google Scholar]


