Abstract
Coarctation of the aorta is a relatively common form of congenital heart disease, with an estimated incidence of approximately 3 cases per 10000 births. Coarctation is a heterogeneous lesion which may present across all age ranges, with varying clinical symptoms, in isolation, or in association with other cardiac defects. The first surgical repair of aortic coarctation was described in 1944, and since that time, several other surgical techniques have been developed and modified. Additionally, transcatheter balloon angioplasty and endovascular stent placement offer less invasive approaches for the treatment of coarctation of the aorta for some patients. While overall morbidity and mortality rates are low for patients undergoing intervention for coarctation, both surgical and transcatheter interventions are not free from adverse outcomes. Therefore, patients must be followed closely over their lifetime for complications such as recoarctation, aortic aneurysm, persistent hypertension, and changes in any associated cardiac defects. Considerable effort has been expended investigating the utility and outcomes of various treatment approaches for aortic coarctation, which are heavily influenced by a patient’s anatomy, size, age, and clinical course. Here we review indications for intervention, describe and compare surgical and transcatheter techniques for management of coarctation, and explore the associated outcomes in both children and adults.
Keywords: Coarctation of the aorta, Cardiac surgery, Cardiac catheterization, Balloon angioplasty, Stents
Core tip: This review explores both surgical and transcatheter approaches for the treatment of coarctation of the aorta and examines outcomes of these techniques in children and adults.
INTRODUCTION
Coarctation of the aorta was first described by Morgagni in 1760[1], and in its simplest form refers to congenital narrowing of the proximal thoracic aorta. While aortic coarctation most commonly occurs as a discrete stenosis in the juxtaductal position, it may also be associated with long segment narrowing, hypoplasia of the transverse aortic arch, or stenosis of the abdominal aorta[2-4]. Coarctation of the aorta accounts for 5%-7% of all congenital heart disease[5], with an incidence of approximately 3 cases per 10000 births[6]. Coarctation may be seen in isolation or with additional cardiac lesions, such as bicuspid aortic valve, ventricular septal defect, patent ductus arteriosus, transposition of the great arteries, atrioventricular canal defects, or left-sided obstructive heart defects, including hypoplastic left heart syndrome[7-11]. Crafoord was the first to perform a successful surgical repair of aortic coarctation in 1944[12]. Since then, various surgical and transcatheter approaches have been developed, allowing patients to have significantly improved outcomes. Here, we briefly review the presentation and diagnosis of aortic coarctation and then focus on surgical and transcatheter approaches with their most recent associated outcomes.
DIAGNOSIS
Clinical presentation
Coarctation can present at any age. Neonates with ductal dependent or “critical coarctation” often present with heart failure, acidosis, and shock following closure of the ductus arteriosus. Without prompt medical resuscitation and surgical intervention, death may occur rapidly[13,14]. Prenatal diagnosis can prevent these sequelae by allowing for intervention before ductal closure. However, prenatal diagnosis of coarctation is challenging due to the presence of the ductus arteriosus and limited blood flow across the aortic isthmus in utero[13]. In the United States, fewer than 1 in 4 patients with isolated coarctation requiring neonatal intervention are diagnosed prenatally[15,16]. Moreover, approximately 30% of neonates with coarctation remain undiagnosed upon discharge after delivery[17]. For these reasons, many physicians advocate for newborn pulse oximetry screening programs, which increase the likelihood of detecting lesions like coarctation before ductal closure[18,19]. Additionally, coarctation must be suspected in infants with other left-sided obstructive heart lesions and may be diagnosed in infants with chromosomal defects, especially those with Turner syndrome and Jacobsen syndrome[20].
Patients with less severe coarctation may not be diagnosed until later in childhood when a murmur is heard or hypertension noted. In these patients, collateral vessels develop from the internal thoracic and subclavian arteries, thyrocervical trunks, and vertebral and anterior spinal arteries to supply blood to the descending aorta[21,22]. For those who enter adulthood undiagnosed, hypertension is the most common presenting symptom[23]. Others may complain of frequent headaches or claudication of the lower extremities with exertion. In these patients, the most telling exam findings suggestive of coarctation are diminished and/or delayed lower extremity pulses and a systolic pressure gradient between the upper and lower extremities[13,23]. However, for patients with extensive collateral blood flow, femoral pulses and lower extremity blood pressures may only be minimally diminished[24].
Evaluation
Chest X-ray is often nonspecific in young patients. In older patients, an anterior-posterior film may show indentation of the aorta at the site of coarctation with pre- and post-stenotic dilation of the aorta, creating the classic “3 sign”. Notching of the posterior fourth to eighth ribs due to dilated intercostal arteries may also be seen in older patients[24,25]. Electrocardiogram is typically normal in infants, but in older children and adults, left ventricular hypertrophy is common due to ventricular pressure overload[24].
Transthoracic echocardiography can assess the presence and severity of aortic coarctation and any associated cardiac defects and is the diagnostic gold standard in neonates and infants (Figure 1). Although transthoracic echocardiogram remains the initial test of choice for coarctation, in larger children and adults[24], echocardiographic windows may be suboptimal. When this is the case, a computed tomography scan or magnetic resonance imaging (MRI) provides excellent anatomic detail at the site of coarctation, and these modalities are commonly used to create three-dimensional images for interventional planning (Figure 2). MRI has the additional benefit of defining and quantifying collateral vessel flow. Although cardiac catheterization was frequently used for diagnosis of coarctation in the past, it is now typically reserved for therapeutic intervention or in those cases where hemodynamic data is additive to the diagnostic evaluation[24,25].
Figure 1.
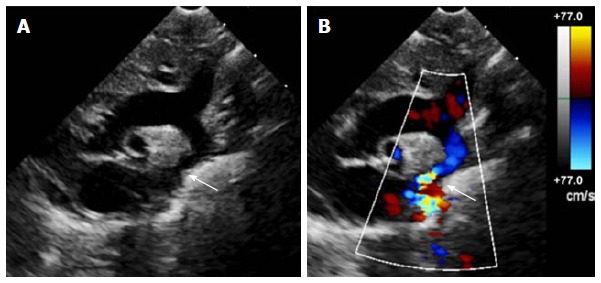
Echocardiogram of coarctation. A: Two-dimensional transthoracic echocardiogram image obtained from the suprasternal notch in an 11-day-old infant demonstrating discrete coarctation (arrow); B: Color Doppler of the same image with aliasing of flow at the site of coarctation (arrow).
Figure 2.
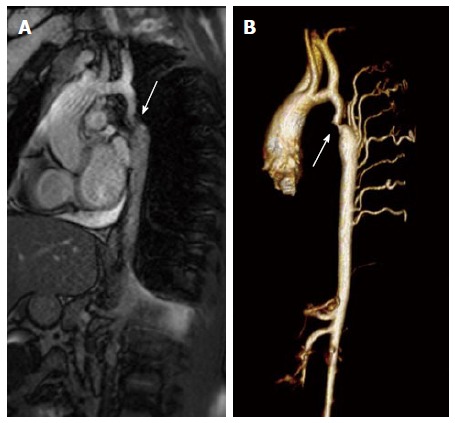
Magnetic resonance imaging of coarctation. A: Magnetic resonance image (steady-state free precession) in a sagittal projection demonstrating transverse arch hypoplasia and long segment coarctation of the aorta distal to the left subclavian artery (arrow) in a 12-year-old male; B: Three-dimensional reconstruction of a gated contrasted angiogram for the same patient, which demonstrates transverse arch hypoplasia, coarctation at the aorta at the distal transverse aortic arch and isthmus (arrow), and dilated intercostal arteries (collaterals).
TREATMENT
Without intervention, the outcome for patients with coarctation of the aorta is poor. In his classic 1970 natural history study, Campbell examined autopsy and clinical records of 465 patients with coarctation who survived beyond one year of age. The mean age of death was 34 years, with 75% mortality at 43 years of age. Causes of death included congestive heart failure (26%), aortic rupture (21%), bacterial endocarditis (18%), and intracranial hemorrhage (12%)[26]. Fortunately, several treatment options are now available, including surgical and transcatheter interventions. Guidelines regarding indications for intervention exist for both children and adults with coarctation (Tables 1 and 2), which include a peak-to-peak gradient ≥ 20 mmHg or lesser gradients when there is significant anatomic evidence of narrowing on imaging with extensive collateral flow[24,27]. Other factors that may be considered include the presence of systemic hypertension, additional cardiac defects and/or single ventricle physiology, left ventricular hypertrophy, or elevated left ventricular end diastolic pressure[24,27-29].
Table 1.
Notable studies and guideline statements in the treatment and outcome of coarctation in adults and children
| Ref. | n | Follow-up | Outcome |
| Cowley et al[60] | 36 | Mean 14 yr | Randomized trial comparing BA and surgery for native coarctation in children. Aortic aneurysm developed in 35% of BA patients and none of the surgical patients |
| Carr[81] | 846 | Mean 36 mo for catheter-based group and 7.8 yr for surgical group | Meta-analysis comparing catheter vs surgical intervention for adults with coarctation. Higher risk of restenosis and need for reintervention found in catheter-based group |
| Forbes et al[68] | 578 | Median 12 mo | Retrospective multicenter analysis at intermediate follow-up after stent placement for coarctation. Exceeding a balloon:coarct ratio of 3.5 and prestent BA increased risk of aortic wall injury |
| Warnes et al[24] | - | - | ACC/AHA guidelines for management of coarctation in adults |
| Holzer et al[67] | 302 | 3-60 mo | Prospective analysis of acute, intermediate, and long-term follow-up after stent placement for coarctation using CCISC registry. At long-term follow-up, recoarctation in 20% of patients, 4% required unplanned reintervention, and 1% had aortic wall injury |
| Feltes et al[27] | - | - | AHA guidelines for transcatheter intervention in children with coarctation |
| Forbes et al[69] | 350 | Mean 1.7 yr | Multicenter observational study comparing surgery, BA, and stent placement for native coarctation in children using CCISC registry. Significantly lower acute complication rates in stent group but higher planned reintervention rates. Hemodynamic and arch imaging outcomes superior in stent and surgical patients compared to BA group |
| Harris et al[55] | 130 | 3-60 mo | Prospective, multicenter analysis of short and intermediate outcomes for BA in native and recurrent coarctation in children. Trend toward increased acute aortic wall injury and restenosis in native coarctation patients |
| Sohrabi et al[75] | 120 | Mean 31.1 mo | Randomized clinical trial comparing covered and bare CP stents for native coarctation in adolescents and adults. Trend of increased rates of restenosis and lower rates of pseudoaneurysm in bare stent group |
| Meadows et al[70] | 105 | 2 yr | Prospective, multicenter, single-arm study assessing safety and efficacy of CP stent in children and adults with coarctation. Two year follow-up of 86% showed 23 fractured stents with no significant clinical effects, 6 aortic aneurysms, 19 repeat catheter interventions, and no surgical interventions |
BA: Balloon angioplasty; ACC: American College of Cardiology; AHA: American Heart Association; CCISC: Congenital Cardiovascular Interventional Study Consortium; CP: Cheatham platinum.
Table 2.
Executive summary on the diagnosis and treatment of coarctation in children and adults
| Diagnosis |
| Accounts for 5%-7% of congenital heart disease diagnoses |
| Neonates often present with heart failure, acidosis, and shock with critical coarctation |
| Less severe coarctation often detected during evaluation for hypertension or murmur in the older child or adult |
| Diminished or delayed lower extremity pulses and a systolic pressure gradient between the upper and lower extremities are the most useful exam findings |
| Transthoracic echocardiogram is initial test of choice; CT and MRI useful if echocardiogram inconclusive and for surgical planning |
| Treatment |
| Surgical repair |
| Extended end-to-end anastomosis typically preferred surgical method, as it avoids prosthetic material, allows resection of the coarctation, and has a wider incision that is less prone to restenosis |
| Surgical repair typically preferred over transcatheter approaches in the infant and young child with native coarctation, patients requiring repair of associated cardiac defects, or in those with complex coarctation anatomy |
| Balloon angioplasty |
| Often the preferred intervention for recurrent coarctation |
| Concern for recoarctation and aneurysm formation in native coarctation |
| Endovascular stent |
| Provides structural support and decreased rates of aortic wall injury and aneurysm compared to balloon angioplasty |
| Covered stents may protect against shear stress and subsequent restenosis, though care must be taken to avoid overlying vital branch vessels |
| Use of stents in small children controversial due to need for large sheath size and limitations in accommodating for somatic growth |
| Patient follow-up |
| Lifelong follow-up with at least annual cardiology visits and repeat imaging every 5 yr to assess coarctation site |
| High suspicion and aggressive treatment of baseline and exercise- induced hypertension |
| Future perspectives |
| Further long-term data analysis needed to determine optimal intervention based on patient anatomy, size, and age |
CT: Computed tomography; MRI: Magnetic resonance imaging.
Surgical repair
The first surgical technique described for coarctation of the aorta was resection with end-to-end anastomosis by Crafoord in 1944[30] (Figure 3A). Early studies showed recoarctation rates in over half of patients with this technique, and the use of a circumferential suture line was thought to be a major contributor[31,32].
Figure 3.
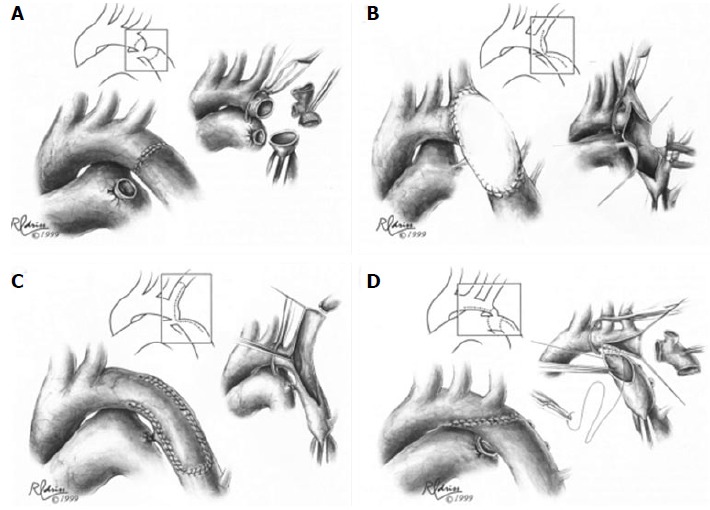
Surgical techniques in coarctation repair. A: Resection and simple end-to-end anastomosis. The coarctation is resected, and an end-to-end, circumferential anastomosis is created; B: Patch aortoplasty. An incision is extended across the coarctation, and a patch is sutured in place to enlarge the stenotic region; C: Subclavian flap aortoplasty. The left subclavian artery is ligated and divided. A longitudinal incision is extended from the proximal left subclavian artery beyond the area of coarctation, and the proximal left subclavian stump is folded down to enlarge the area of coarctation; D: Resection with extended end-to-end anastomosis. The coarctation is resected using a broad, longitudinal incision, and an oblique anastomosis is constructed between the undersurface of the transverse arch and the descending thoracic aorta. Figures adapted and reprinted with permission from the Journal of Cardiac Surgery[30].
Vosschulte described prosthetic patch aortoplasty as an alternative technique for coarctation repair in 1961. In this approach, the ductal tissue is ligated and divided, a longitudinal incision across the coarctation is made, and a prosthetic patch is sutured in place to enlarge the stenotic region (Figure 3B). This technique can be applied to longer segments of coarctation, avoids a circumferential suture line, and minimizes aortic mobilization and ligation of intercostal arteries[33]. While recoarctation rates of 5%-12%[34] were lower compared to the resection and end-to-end anastomosis technique, aortic aneurysm was a long-term concern, with rates between 18%-51% of patients[35-38]. Using more distensible polytetralfluroethylene patch material instead of Dacron was initially promising[34] but still showed a 7% risk for aortic aneurysm and a 25% risk of recoarctation[39].
Subclavian flap aortoplasty was a modified approach reported by Waldhausen and Nahrwold in 1966. Here, the left subclavian artery is ligated and divided, and a longitudinal incision down the proximal left subclavian artery is extended beyond the area of coarctation. The proximal left subclavian stump is then folded down to enlarge the area of coarctation (Figure 3C). This technique avoids a circumferential suture line and the use of prosthetic material, which may allow for improved growth, and it can be used for repair of long segment coarctation[40,41]. Although still occasionally used by surgeons, one of the main reservations of this approach has been the need to sacrifice the left subclavian artery. This can create a subclavian steal phenomenon, with retrograde flow down the vertebral artery, and it has been associated with decreased length and muscle bulk of the left upper extremity, as well as claudication with exercise[42,43].
Coarctation resection and replacement with an interposition graft was described by Gross[44] in 1951. After aortic cross clamp, the stenotic tissue is excised, and either a homograft or Dacron tube graft is sutured into the aorta. This approach is rarely used in the current era, as it is not ideal for pediatric patients due to growth limitations. However, occasionally it is an appropriate technique for adult patients with coarctation, especially those with aneurysm, long segment coarctation, or recoarctation after primary repair[45].
In 1977, Amato et al[46] described a modification to Crafoord’s resection and end-to-end anastomosis technique, where a broader, longitudinal incision and anastomosis are created across the proximal aorta (Figure 3D). The extended end-to-end anastomosis still avoids the use of prosthetic material and allows resection of the coarctation and residual ductal tissue, but the wider incision is less prone to restenosis and enables enlargement of the transverse aorta, which is particularly helpful in neonates[46,47]. In the present era, extended end-to-end anastomosis is typically the preferred technique for surgical repair, especially in small children, due to low mortality rates and low rates of restenosis, ranging between 4%-11%[47-50].
Balloon angioplasty: Native coarctation
Surgical therapy was the only treatment option for coarctation until 1982, when the use of balloon angioplasty was described by Lock et al[51]. Several studies since then have shown balloon angioplasty to be a relatively effective acute intervention for native coarctation, with rates of recoarctation ranging from 8%-32%[52-54]. In a report from the prospective, multicenter Congenital Cardiovascular Interventional Study Consortium (CCISC), 34 patients undergoing balloon angioplasty for native coarctation had adequate intermediate follow-up data at 18 to 60 mo post-intervention. In these patients, the rate of recoarctation was 15%[55]. A second concern with native coarctation angioplasty is aneurysm formation. Histologic and intravascular ultrasound studies have demonstrated the mechanism of angioplasty involves tearing of the intima and media[56-59]. Although some of these tears may heal, disruption of vascular integrity is believed to contribute to a relatively high incidence of aneurysm formation. This was demonstrated in a single center randomized trial comparing balloon angioplasty vs surgical repair of coarctation in older children (ages 3 years to 10 years). In this study with mean follow-up of 14 years after intervention, 35% of the balloon angioplasty patients developed aneurysm, compared to none of the surgical patients[60]. Similarly, the 2014 CCISC observational study showed 24% of patients with native coarctation developed aortic aneurysm at intermediate follow-up after balloon angioplasty[55].
Balloon angioplasty: Recurrent coarctation
In contrast to native coarctation, balloon angioplasty is often the preferred intervention for recurrent coarctation in children[27]. Acute success rates for this procedure range from 80%-93%[61]. Reported rates of aortic wall injury are low (1%-2%), and the longer term risk of aneurysm is believed to be ameliorated by scar tissue at the site of the recoarctation, which limits the degree of vascular disruption. However recoarctation rates remain a concern, with a broad range between 6%-53% described[62,63].
Likely the most fragile patient population to develop recurrent coarctation is children with hypoplastic left heart syndrome or other single right ventricle lesions. These patients are at risk for significant morbidity and mortality with recoarctation due to exacerbation of atrioventricular valve regurgitation and ventricular dysfunction[64]. The Pediatric Heart Network Single Ventricle Reconstruction trial was a large, multicenter, prospective study examining the outcome of infants with single right ventricle lesions after randomization to either right ventricle-pulmonary artery shunt or a modified Blalock-Taussig shunt at the time of Norwood procedure[65]. The incidence and timing of intervention for recoarctation in the first 12 mo after randomization was assessed, and 97 of 549 patients (18%) underwent intervention for recoarctation, which was most commonly performed at the time of pre-stage II cardiac catheterization by balloon angioplasty. Balloon angioplasty achieved adequate short term results, but 39% of patients required reintervention for recoarctation, compared to 5% of the patients who underwent surgical reintervention. Reassuringly, with catheter or surgical intervention, the presence of recoarctation did not affect survival rates in this tenuous patient population[64].
Endovascular stent placement
In 1991, the use of endovascular stents for the treatment of coarctation was first reported[66], adding another dimension to the utility of transcatheter treatment for coarctation. Endovascular stents are inserted using balloon catheters but do not require overdilation of the vessel wall. Stents also offer structural support, thereby decreasing the rates of aortic wall injury and restenosis observed with balloon angioplasty alone[5] (Figure 4). Several studies have assessed the utility of endovascular stent placement in the treatment of coarctation[67]. Retrospective analysis of acute procedural data from 17 institutions from 1989 to 2005 showed successful stent placement without a significant residual gradient or serious complication in 553 of 565 (97.9%) patients. The overall complication rate was 14.3%, with aortic wall complications (aneurysm, intimal tear, or dissection) contributing 3.9%[68]. In a subsequent study, acute and long-term data regarding endovascular stent placement for coarctation were obtained from the CCISC. Here, the acute results of stent placement were successful without a significant blood pressure gradient or need for reintervention in 249/260 (96%) of cases. During follow-up spanning between 3 to 60 mo, recoarctation was seen in 20% of the 164 patients with follow-up imaging, and 4% of patients required unplanned repeat interventions. Aortic wall complications consisting of dissection or aneurysm were seen in 1% of the 302 total cases[67].
Figure 4.
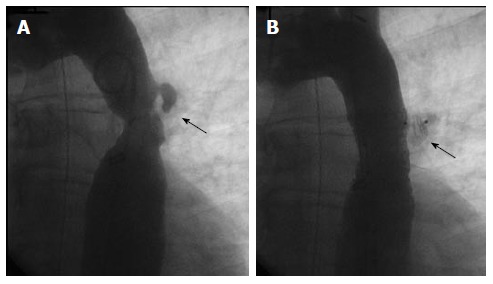
Endovascular stent placement for coarctation. A: Angiogram (LAO 30°, caudal 30°) demonstrating a discrete coarctation and intercostal aneurysm (arrow) in a 45-year-old male; B: Angiogram in the same projection after endovascular bare metal stent placement showing no significant residual stenosis. The intercostal aneurysm was successfully occluded with an Amplatzer Vascular Plug II (arrow).
In a multicenter observational study comparing the outcomes of surgical, balloon angioplasty alone, and endovascular stent placement for coarctation using the CCISC registry, patients undergoing stent placement had significantly lower complication rates compared to balloon angioplasty or surgical patients. There was no significant difference among treatment groups for unplanned reintervention rates (4%-7%) at a mean of 1.7 years of follow-up, but those who underwent stent placement were more likely to undergo planned reintervention. Aortic wall injury was more likely to occur in patients who underwent balloon angioplasty alone, at a rate of 9.8% compared to none in the endovascular stent group[69]. In an attempt to more rigorously assess the effectiveness and safety of endovascular stent placement vs surgical repair, a Cochrane review was attempted in 2012, but it was determined that there was insufficient data available to identify the best treatment modality[5].
The Coarctation of the Aorta Stent Trial (COAST) has been an influential prospective study examining the effectiveness and safety of endovascular stent placement in children and adults with coarctation. Currently, no endovascular stent has been granted FDA approval for use in the aorta, and initially biliary stents were used off-label for treatment of coarctation. NuMED (Hopkinton, NY) created the Cheatham Platinum (CP) stent for specific use in the aorta, and in 2007, the COAST trial was designed as a prospective, multicenter, single-arm clinical study to assess the safety and efficacy of this stent for the treatment of coarctation[6].
Results for up to two years from CP stent placement are currently available from the COAST trial. CP stent implantation was attempted in 105 patients ranging from 8 to 52 years of age. All but one implantation was successfully completed, with no significant adverse events acutely. No patient had a significant gradient between the ascending and descending aorta after stent placement, and 99% had a gradient < 20 mmHg at one month. There was 89% follow-up at one year after stent placement and 86% follow-up at two years. At two years, 90% of patients maintained a blood pressure gradient < 20 mmHg between the upper and lower extremities. To date, 23 fractured stents have been identified, though none have led to decreased stent integrity, stent migration, aortic wall injury, or hemodynamic obstruction. Aortic aneurysm was diagnosed in 6 patients, one of which resolved without intervention. No patient has required surgical reintervention, and a total of 19 patients have undergone repeat transcatheter intervention, either due to aortic wall injury or dilation of the initial stent[70].
Thus far, the CP stent is felt to be a safe and effective treatment option for coarctation in older children and adults with native or recurrent coarctation. Stent fracture is common but thus far has been clinically insignificant. Reintervention is also common, either due to planned dilation of smaller stents or aortic wall injury. Follow-up for the COAST trial is planned for up to 60 mo after stent placement and will provide further insight regarding these issues[6].
The use of endovascular stents in small children remains controversial due to the challenges in accommodating for significant somatic growth and the requirement for relatively larger sheath sizes. The ideal stent would be deployed through a small sheath but retain the ability to be dilated to an adult vessel diameter. This technology does not yet exist and still would require multiple interventions for stent dilation throughout a patient’s lifetime. Additionally, neonates often have transverse arch hypoplasia, which does not easily lend itself to endovascular stent placement. Some small, single center studies have had positive short-term results with endovascular stent placement in young children, but further follow-up and investigation is needed[71,72].
Covered endovascular stents represent the latest transcatheter innovation and were first used for the treatment of coarctation in 1999. The fabric within the stent provides additional structural support, creates a protective barrier at the site of stent placement, and can help decrease shear stress. All of these factors theoretically reduce the risk of acute vascular trauma as well as longer term aneurysm formation. When aortic aneurysm or stent fracture occurs with bare stent placement, covered stents are often used as a rescue therapy. Covered stents may also be the initial transcatheter intervention of choice, especially in the setting of complex anatomy of the coarctation or in older patients with more friable and calcified aortic wall tissue. However, covered stents require larger sheath sizes, which limits their use in small children. Additionally, care must be taken to avoid stent occlusion of significant aortic branches, including paraspinal branches off of the descending aorta, which can be difficult to identify[73].
Initial smaller studies examining the use of covered stent placement in aortic coarctation were promising, with no reported aneurysms[73,74]. More recently, a randomized clinical trial was performed comparing bare CP stent vs covered CP stent placement in 120 patients. Both groups had no acute procedural complications, and at an average follow-up of 31.1 mo, the bare CP stent group had a statistically nonsignificant increase in the rate of recoarctation (6.7% vs 0%) and a nonsignificant lower incidence of pseudoaneurysm (0% vs 3.3%), compared to the covered CP stent cohort. The two cases of pseudoaneurysm in the covered stent group occurred at the proximal end of the stent, and both were successfully treated with a second covered stent with no further complications[75]. Further investigation into the safety and efficacy of covered stents is on-going with the COAST II trial. This trial was initiated in 2010, hoping to provide information that will support FDA pre-market approval of the covered CP stent in preventing aortic wall injury in high risk patients with coarctation as well as treatment of existing aortic wall injury related to complications from previous interventions for coarctation. Results are expected in the near future, but at this time, covered CP stents are not available for the treatment of coarctation in the United States outside of use in the COAST II trial[70].
MANAGEMENT ALGORITHM
With many different options, deciding on the optimal treatment strategy for coarctation can be complicated, and there is no comprehensive evidence-based standard of care or algorithm. Guidelines from the American College of Cardiology and the American Heart Association provide some insight, but the level of evidence supporting these recommendations is suboptimal (Level B or C for all recommendations)[24,27]. In general, management is dictated by the age at presentation, complexity of the coarctation, and whether or not the coarctation represents a native vs recurrent obstruction. For the infant or young child presenting with native coarctation, most centers prefer surgical repair due to the long-term risk of aneurysm after balloon angioplasty, the need for redilation with stent placement, and the limitations imposed by small arteries unable to accommodate larger sheath sizes[60]. However, balloon angioplasty can be considered as a palliative strategy to stabilize neonates presenting in extremis and considered too sick for immediate surgical intervention[27]. Surgical repair may also be more appropriate in patients with complex coarctation anatomy, including those with transverse arch obstruction, tortuous segments of recoarctation, distortion of adjacent arterial branches, or when repair of associated cardiac defects is required[24].
In the older child, adolescent, or adult presenting with a simple, juxtaductal, native coarctation, stent placement is considered a reasonable approach, offering a less invasive alternative to surgical intervention and good long-term outcomes[24,27,76]. Only stents expandable to an adult size should be used, in an effort to avoid later surgical intervention[27].
For recurrent coarctation in the younger child, it is reasonable to consider initial balloon angioplasty, as aneurysm is less of a long-term concern than with native coarctation[27]. Balloon angioplasty is variably successful, and surgical reintervention may be required when there is incomplete relief of obstruction[55]. Stent placement can also be considered for recoarctation in older children and adolescents when the stent can be dilated to near adult size, thus avoiding the need for multiple redilations[27].
PATIENT FOLLOW-UP
Patients with repaired or unrepaired coarctation must be followed by a cardiologist throughout their lifetime. For those who have undergone repair, this follow-up should be at least annually, with specific attention paid to baseline or exercise-induced hypertension[24]. Hypertension is endemic in patients with aortic coarctation, even if no residual coarctation exists, and it must be appropriately treated[77,78]. The etiology of such high rates of baseline and exercise-induced hypertension remains unclear but may be due to any combination of underlying arteriopathy, decreased aortic wall compliance, abnormal streaming of blood flow, or renal abnormalities[77].
Additionally, imaging of the repaired coarctation should be performed at least every 5 years, or sooner based on original anatomy and symptoms, to assess the coarctation repair site for complications like aortic aneurysm or recurrent stenosis[24]. For repaired patients with a normal upper to lower extremity blood pressure gradient, normotension at rest and with exercise, and no evidence of aneurysm or associated heart defects, exercise is encouraged, and only activities with a large static component should be avoided[79]. Finally, per the 2007 American Heart Association guidelines, endocarditis prophylaxis is not routinely recommended beyond the first six months after surgical or transcatheter intervention, barring a previous history of infectious endocarditis[80].
CONCLUSION
In the seventy years since the first description of surgical intervention for aortic coarctation by Crafoord, tremendous progress has been made in the treatment and outcomes for these patients. Modifications of various surgical techniques have led to low mortality and morbidity rates, even in the smallest patients. Transcatheter balloon angioplasty and subsequently endovascular stent placement have expanded treatment options and provided less invasive approaches for repair in some patients. Still, both surgical and transcatheter approaches retain risks for adverse events and subsequent patient morbidity. As examined in this review, much effort has been spent investigating the intervention which yields the best patient outcomes, but further long-term data assessment is needed. The aortic coarctation patient population is a fascinating and heterogeneous one, and considering an individual patient’s clinical presentation, anatomy, size, and age will most certainly continue to heavily influence treatment approach.
Footnotes
Conflict-of-interest statement: Fleming GA is the site principal investigator for the Covered Cheatham Platinum Stents for the Prevention or Treatment of Aortic Wall Injury Associated With Coarctation of the Aorta (COAST II) trial at Duke University Medical Center. There are no other conflicts of interest to disclose.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: June 1, 2015
First decision: August 6, 2015
Article in press: September 30, 2015
P- Reviewer: Bernhard R, Chu D, Iacoviello M, Petretta M S- Editor: Qiu S L- Editor: A E- Editor: Wu HL
References
- 1.Zani A, Cozzi DA. Giovanni Battista Morgagni and his contribution to pediatric surgery. J Pediatr Surg. 2008;43:729–733. doi: 10.1016/j.jpedsurg.2007.12.065. [DOI] [PubMed] [Google Scholar]
- 2.Ho SY, Anderson RH. Coarctation, tubular hypoplasia, and the ductus arteriosus. Histological study of 35 specimens. Br Heart J. 1979;41:268–274. doi: 10.1136/hrt.41.3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Price TP, Whisenhunt AK, Policha A, Ayad MT, Gardiner GA, Abai B, DiMuzio PJ, Salvatore DM. Middle aortic coarctation. Ann Vasc Surg. 2014;28:1314.e15–1314.e21. doi: 10.1016/j.avsg.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 4.Mullen MJ. Coarctation of the aorta in adults: do we need surgeons? Heart. 2003;89:3–5. doi: 10.1136/heart.89.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pádua LM, Garcia LC, Rubira CJ, de Oliveira Carvalho PE. Stent placement versus surgery for coarctation of the thoracic aorta. Cochrane Database Syst Rev. 2012;5:CD008204. doi: 10.1002/14651858.CD008204.pub2. [DOI] [PubMed] [Google Scholar]
- 6.Ringel RE, Gauvreau K, Moses H, Jenkins KJ. Coarctation of the Aorta Stent Trial (COAST): study design and rationale. Am Heart J. 2012;164:7–13. doi: 10.1016/j.ahj.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Anderson RH, Lenox CC, Zuberbuhler JR. Morphology of ventricular septal defect associated with coarctation of aorta. Br Heart J. 1983;50:176–181. doi: 10.1136/hrt.50.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shinebourne EA, Tam AS, Elseed AM, Paneth M, Lennox SC, Cleland WP. Coarctation of the aorta in infancy and childhood. Br Heart J. 1976;38:375–380. doi: 10.1136/hrt.38.4.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shone JD, Sellers RD, Anderson RC, Adams P, Lillehei CW, Edwards JE. The developmental complex of “parachute mitral valve,” supravalvular ring of left atrium, subaortic stenosis, and coarctation of aorta. Am J Cardiol. 1963;11:714–725. doi: 10.1016/0002-9149(63)90098-5. [DOI] [PubMed] [Google Scholar]
- 10.Warnes CA. Bicuspid aortic valve and coarctation: two villains part of a diffuse problem. Heart. 2003;89:965–966. doi: 10.1136/heart.89.9.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Becker AE, Becker MJ, Edwards JE. Anomalies associated with coarctation of aorta: particular reference to infancy. Circulation. 1970;41:1067–1075. doi: 10.1161/01.cir.41.6.1067. [DOI] [PubMed] [Google Scholar]
- 12.Kvitting JP, Olin CL. Clarence Crafoord: a giant in cardiothoracic surgery, the first to repair aortic coarctation. Ann Thorac Surg. 2009;87:342–346. doi: 10.1016/j.athoracsur.2008.08.072. [DOI] [PubMed] [Google Scholar]
- 13.Rosenthal E. Coarctation of the aorta from fetus to adult: curable condition or life long disease process? Heart. 2005;91:1495–1502. doi: 10.1136/hrt.2004.057182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ward KE, Pryor RW, Matson JR, Razook JD, Thompson WM, Elkins RC. Delayed detection of coarctation in infancy: implications for timing of newborn follow-up. Pediatrics. 1990;86:972–976. [PubMed] [Google Scholar]
- 15.Gómez-Montes E, Herraiz I, Mendoza A, Escribano D, Galindo A. Prediction of coarctation of the aorta in the second half of pregnancy. Ultrasound Obstet Gynecol. 2013;41:298–305. doi: 10.1002/uog.11228. [DOI] [PubMed] [Google Scholar]
- 16.Quartermain MD, Pasquali SK, Hill KD, Goldberg DJ, Huhta JC, Jacobs JP, Jacobs ML, Kim S, Ungerleider RM. Variation in Prenatal Diagnosis of Congenital Heart Disease in Infants. Pediatrics. 2015:In press. doi: 10.1542/peds.2014-3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liberman RF, Getz KD, Lin AE, Higgins CA, Sekhavat S, Markenson GR, Anderka M. Delayed diagnosis of critical congenital heart defects: trends and associated factors. Pediatrics. 2014;134:e373–e381. doi: 10.1542/peds.2013-3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahle WT, Martin GR, Beekman RH, Morrow WR. Endorsement of Health and Human Services recommendation for pulse oximetry screening for critical congenital heart disease. Pediatrics. 2012;129:190–192. doi: 10.1542/peds.2011-3211. [DOI] [PubMed] [Google Scholar]
- 19.Mahle WT, Newburger JW, Matherne GP, Smith FC, Hoke TR, Koppel R, Gidding SS, Beekman RH, Grosse SD. Role of pulse oximetry in examining newborns for congenital heart disease: a scientific statement from the American Heart Association and American Academy of Pediatrics. Circulation. 2009;120:447–458. doi: 10.1161/CIRCULATIONAHA.109.192576. [DOI] [PubMed] [Google Scholar]
- 20.McBride KL, Zender GA, Fitzgerald-Butt SM, Koehler D, Menesses-Diaz A, Fernbach S, Lee K, Towbin JA, Leal S, Belmont JW. Linkage analysis of left ventricular outflow tract malformations (aortic valve stenosis, coarctation of the aorta, and hypoplastic left heart syndrome) Eur J Hum Genet. 2009;17:811–819. doi: 10.1038/ejhg.2008.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steffens JC, Bourne MW, Sakuma H, O’Sullivan M, Higgins CB. Quantification of collateral blood flow in coarctation of the aorta by velocity encoded cine magnetic resonance imaging. Circulation. 1994;90:937–943. doi: 10.1161/01.cir.90.2.937. [DOI] [PubMed] [Google Scholar]
- 22.Leschka S, Alkadhi H, Wildermuth S. Images in cardiology. Collateral circulation in aortic coarctation shown by 64 channel multislice computed tomography angiography. Heart. 2005;91:1422. doi: 10.1136/hrt.2004.054346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strafford MA, Griffiths SP, Gersony WM. Coarctation of the aorta: a study in delayed detection. Pediatrics. 1982;69:159–163. [PubMed] [Google Scholar]
- 24.Warnes CA, Williams RG, Bashore TM, Child JS, Connolly HM, Dearani JA, del Nido P, Fasules JW, Graham TP, Hijazi ZM, et al. ACC/AHA 2008 Guidelines for the Management of Adults with Congenital Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to develop guidelines on the management of adults with congenital heart disease) Circulation. 2008;118:e714–e833. doi: 10.1161/CIRCULATIONAHA.108.190690. [DOI] [PubMed] [Google Scholar]
- 25.Karaosmanoglu AD, Khawaja RD, Onur MR, Kalra MK. CT and MRI of aortic coarctation: pre- and postsurgical findings. AJR Am J Roentgenol. 2015;204:W224–W233. doi: 10.2214/AJR.14.12529. [DOI] [PubMed] [Google Scholar]
- 26.Campbell M. Natural history of coarctation of the aorta. Br Heart J. 1970;32:633–640. doi: 10.1136/hrt.32.5.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feltes TF, Bacha E, Beekman RH, Cheatham JP, Feinstein JA, Gomes AS, Hijazi ZM, Ing FF, de Moor M, Morrow WR, et al. Indications for cardiac catheterization and intervention in pediatric cardiac disease: a scientific statement from the American Heart Association. Circulation. 2011;123:2607–2652. doi: 10.1161/CIR.0b013e31821b1f10. [DOI] [PubMed] [Google Scholar]
- 28.Rao PS. Coarctation of the aorta. Curr Cardiol Rep. 2005;7:425–434. doi: 10.1007/s11886-005-0060-0. [DOI] [PubMed] [Google Scholar]
- 29.Vergales JE, Gangemi JJ, Rhueban KS, Lim DS. Coarctation of the aorta - the current state of surgical and transcatheter therapies. Curr Cardiol Rev. 2013;9:211–219. doi: 10.2174/1573403X113099990032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dodge-Khatami A, Backer CL, Mavroudis C. Risk factors for recoarctation and results of reoperation: a 40-year review. J Card Surg. 2000;15:369–377. doi: 10.1111/j.1540-8191.2000.tb01295.x. [DOI] [PubMed] [Google Scholar]
- 31.Kappetein AP, Zwinderman AH, Bogers AJ, Rohmer J, Huysmans HA. More than thirty-five years of coarctation repair. An unexpected high relapse rate. J Thorac Cardiovasc Surg. 1994;107:87–95. [PubMed] [Google Scholar]
- 32.Williams WG, Shindo G, Trusler GA, Dische MR, Olley PM. Results of repair of coarctation of the aorta during infancy. J Thorac Cardiovasc Surg. 1980;79:603–608. [PubMed] [Google Scholar]
- 33.Vossschulte K. Surgical correction of coarctation of the aorta by an “isthmusplastic” operation. Thorax. 1961;16:338–345. doi: 10.1136/thx.16.4.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Backer CL, Paape K, Zales VR, Weigel TJ, Mavroudis C. Coarctation of the aorta. Repair with polytetrafluoroethylene patch aortoplasty. Circulation. 1995;92:II132–II136. doi: 10.1161/01.cir.92.9.132. [DOI] [PubMed] [Google Scholar]
- 35.Rheuban KS, Gutgesell HP, Carpenter MA, Jedeikin R, Damman JF, Kron IL, Wellons J, Nolan SP. Aortic aneurysm after patch angioplasty for aortic isthmic coarctation in childhood. Am J Cardiol. 1986;58:178–180. doi: 10.1016/0002-9149(86)90270-5. [DOI] [PubMed] [Google Scholar]
- 36.Clarkson PM, Brandt PW, Barratt-Boyes BG, Rutherford JD, Kerr AR, Neutze JM. Prosthetic repair of coarctation of the aorta with particular reference to Dacron onlay patch grafts and late aneurysm formation. Am J Cardiol. 1985;56:342–346. doi: 10.1016/0002-9149(85)90861-6. [DOI] [PubMed] [Google Scholar]
- 37.Ala-Kulju K, Heikkinen L. Aneurysms after patch graft aortoplasty for coarctation of the aorta: long-term results of surgical management. Ann Thorac Surg. 1989;47:853–856. doi: 10.1016/0003-4975(89)90018-0. [DOI] [PubMed] [Google Scholar]
- 38.Parks WJ, Ngo TD, Plauth WH, Bank ER, Sheppard SK, Pettigrew RI, Williams WH. Incidence of aneurysm formation after Dacron patch aortoplasty repair for coarctation of the aorta: long-term results and assessment utilizing magnetic resonance angiography with three-dimensional surface rendering. J Am Coll Cardiol. 1995;26:266–271. doi: 10.1016/0735-1097(95)00127-l. [DOI] [PubMed] [Google Scholar]
- 39.Walhout RJ, Lekkerkerker JC, Oron GH, Hitchcock FJ, Meijboom EJ, Bennink GB. Comparison of polytetrafluoroethylene patch aortoplasty and end-to-end anastomosis for coarctation of the aorta. J Thorac Cardiovasc Surg. 2003;126:521–528. doi: 10.1016/s0022-5223(03)00030-8. [DOI] [PubMed] [Google Scholar]
- 40.Waldhausen JA, Nahrwold DL. Repair of coarctation of the aorta with a subclavian flap. J Thorac Cardiovasc Surg. 1966;51:532–533. [PubMed] [Google Scholar]
- 41.Pierce WS, Waldhausen JA, Berman W, Whitman V. Late results of the subclavian flap procedure in infants with coarctation of the thoracic aorta. Circulation. 1978;58:I78–I82. [PubMed] [Google Scholar]
- 42.Pandey R, Jackson M, Ajab S, Gladman G, Pozzi M. Subclavian flap repair: review of 399 patients at median follow-up of fourteen years. Ann Thorac Surg. 2006;81:1420–1428. doi: 10.1016/j.athoracsur.2005.08.070. [DOI] [PubMed] [Google Scholar]
- 43.van Son JA, van Asten WN, van Lier HJ, Daniëls O, Vincent JG, Skotnicki SH, Lacquet LK. Detrimental sequelae on the hemodynamics of the upper left limb after subclavian flap angioplasty in infancy. Circulation. 1990;81:996–1004. doi: 10.1161/01.cir.81.3.996. [DOI] [PubMed] [Google Scholar]
- 44.Gross RE. Treatment of certain aortic coarctations by homologous grafts; a report of nineteen cases. Ann Surg. 1951;134:753–768. doi: 10.1097/00000658-195110000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Charlton-Ouw KM, Codreanu ME, Leake SS, Sandhu HK, Calderon D, Azizzadeh A, Estrera AL, Safi HJ. Open repair of adult aortic coarctation mostly by a resection and graft replacement technique. J Vasc Surg. 2015;61:66–72. doi: 10.1016/j.jvs.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 46.Amato JJ, Rheinlander HF, Cleveland RJ. A method of enlarging the distal transverse arch in infants with hypoplasia and coarctation of the aorta. Ann Thorac Surg. 1977;23:261–263. doi: 10.1016/s0003-4975(10)64121-5. [DOI] [PubMed] [Google Scholar]
- 47.Kaushal S, Backer CL, Patel JN, Patel SK, Walker BL, Weigel TJ, Randolph G, Wax D, Mavroudis C. Coarctation of the aorta: midterm outcomes of resection with extended end-to-end anastomosis. Ann Thorac Surg. 2009;88:1932–1938. doi: 10.1016/j.athoracsur.2009.08.035. [DOI] [PubMed] [Google Scholar]
- 48.Thomson JD, Mulpur A, Guerrero R, Nagy Z, Gibbs JL, Watterson KG. Outcome after extended arch repair for aortic coarctation. Heart. 2006;92:90–94. doi: 10.1136/hrt.2004.058685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burch PT, Cowley CG, Holubkov R, Null D, Lambert LM, Kouretas PC, Hawkins JA. Coarctation repair in neonates and young infants: is small size or low weight still a risk factor? J Thorac Cardiovasc Surg. 2009;138:547–552. doi: 10.1016/j.jtcvs.2009.04.046. [DOI] [PubMed] [Google Scholar]
- 50.Wright GE, Nowak CA, Goldberg CS, Ohye RG, Bove EL, Rocchini AP. Extended resection and end-to-end anastomosis for aortic coarctation in infants: results of a tailored surgical approach. Ann Thorac Surg. 2005;80:1453–1459. doi: 10.1016/j.athoracsur.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 51.Lock JE, Bass JL, Amplatz K, Fuhrman BP, Castaneda-Zuniga W. Balloon dilation angioplasty of aortic coarctations in infants and children. Circulation. 1983;68:109–116. doi: 10.1161/01.cir.68.1.109. [DOI] [PubMed] [Google Scholar]
- 52.Tynan M, Finley JP, Fontes V, Hess J, Kan J. Balloon angioplasty for the treatment of native coarctation: results of Valvuloplasty and Angioplasty of Congenital Anomalies Registry. Am J Cardiol. 1990;65:790–792. doi: 10.1016/0002-9149(90)91389-n. [DOI] [PubMed] [Google Scholar]
- 53.Mendelsohn AM, Lloyd TR, Crowley DC, Sandhu SK, Kocis KC, Beekman RH. Late follow-up of balloon angioplasty in children with a native coarctation of the aorta. Am J Cardiol. 1994;74:696–700. doi: 10.1016/0002-9149(94)90312-3. [DOI] [PubMed] [Google Scholar]
- 54.Fawzy ME, Fathala A, Osman A, Badr A, Mostafa MA, Mohamed G, Dunn B. Twenty-two years of follow-up results of balloon angioplasty for discreet native coarctation of the aorta in adolescents and adults. Am Heart J. 2008;156:910–917. doi: 10.1016/j.ahj.2008.06.037. [DOI] [PubMed] [Google Scholar]
- 55.Harris KC, Du W, Cowley CG, Forbes TJ, Kim DW. A prospective observational multicenter study of balloon angioplasty for the treatment of native and recurrent coarctation of the aorta. Catheter Cardiovasc Interv. 2014;83:1116–1123. doi: 10.1002/ccd.25284. [DOI] [PubMed] [Google Scholar]
- 56.Ho SY, Somerville J, Yip WC, Anderson RH. Transluminal balloon dilation of resected coarcted segments of thoracic aorta: histological study and clinical implications. Int J Cardiol. 1988;19:99–105. doi: 10.1016/0167-5273(88)90195-7. [DOI] [PubMed] [Google Scholar]
- 57.Ino T, Kishiro M, Okubo M, Akimoto K, Nishimoto K, Yabuta K, Kawasaki S, Hosoda Y. Dilatation mechanism of balloon angioplasty in children: assessment by angiography and intravascular ultrasound. Cardiovasc Intervent Radiol. 1998;21:102–108. doi: 10.1007/s002709900224. [DOI] [PubMed] [Google Scholar]
- 58.Lock JE, Castaneda-Zuniga WR, Bass JL, Foker JE, Amplatz K, Anderson RW. Balloon dilatation of excised aortic coarctations. Radiology. 1982;143:689–691. doi: 10.1148/radiology.143.3.6210934. [DOI] [PubMed] [Google Scholar]
- 59.Sohn S, Rothman A, Shiota T, Luk G, Tong A, Swensson RE, Sahn DJ. Acute and follow-up intravascular ultrasound findings after balloon dilation of coarctation of the aorta. Circulation. 1994;90:340–347. doi: 10.1161/01.cir.90.1.340. [DOI] [PubMed] [Google Scholar]
- 60.Cowley CG, Orsmond GS, Feola P, McQuillan L, Shaddy RE. Long-term, randomized comparison of balloon angioplasty and surgery for native coarctation of the aorta in childhood. Circulation. 2005;111:3453–3456. doi: 10.1161/CIRCULATIONAHA.104.510198. [DOI] [PubMed] [Google Scholar]
- 61.Saxena A. Recurrent coarctation: interventional techniques and results. World J Pediatr Congenit Heart Surg. 2015;6:257–265. doi: 10.1177/2150135114566099. [DOI] [PubMed] [Google Scholar]
- 62.Yetman AT, Nykanen D, McCrindle BW, Sunnegardh J, Adatia I, Freedom RM, Benson L. Balloon angioplasty of recurrent coarctation: a 12-year review. J Am Coll Cardiol. 1997;30:811–816. doi: 10.1016/s0735-1097(97)00228-3. [DOI] [PubMed] [Google Scholar]
- 63.Reich O, Tax P, Bartáková H, Tomek V, Gilík J, Lisy J, Radvansky J, Matejka T, Tláskal T, Svobodová I, et al. Long-term (up to 20 years) results of percutaneous balloon angioplasty of recurrent aortic coarctation without use of stents. Eur Heart J. 2008;29:2042–2048. doi: 10.1093/eurheartj/ehn251. [DOI] [PubMed] [Google Scholar]
- 64.Hill KD, Rhodes JF, Aiyagari R, Baker GH, Bergersen L, Chai PJ, Fleming GA, Fudge JC, Gillespie MJ, Gray RG, et al. Intervention for recoarctation in the single ventricle reconstruction trial: incidence, risk, and outcomes. Circulation. 2013;128:954–961. doi: 10.1161/CIRCULATIONAHA.112.000488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ohye RG, Sleeper LA, Mahony L, Newburger JW, Pearson GD, Lu M, Goldberg CS, Tabbutt S, Frommelt PC, Ghanayem NS, et al. Comparison of shunt types in the Norwood procedure for single-ventricle lesions. N Engl J Med. 2010;362:1980–1992. doi: 10.1056/NEJMoa0912461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.O’Laughlin MP, Perry SB, Lock JE, Mullins CE. Use of endovascular stents in congenital heart disease. Circulation. 1991;83:1923–1939. doi: 10.1161/01.cir.83.6.1923. [DOI] [PubMed] [Google Scholar]
- 67.Holzer R, Qureshi S, Ghasemi A, Vincent J, Sievert H, Gruenstein D, Weber H, Alday L, Peirone A, Zellers T, et al. Stenting of aortic coarctation: acute, intermediate, and long-term results of a prospective multi-institutional registry--Congenital Cardiovascular Interventional Study Consortium (CCISC) Catheter Cardiovasc Interv. 2010;76:553–563. doi: 10.1002/ccd.22587. [DOI] [PubMed] [Google Scholar]
- 68.Forbes TJ, Garekar S, Amin Z, Zahn EM, Nykanen D, Moore P, Qureshi SA, Cheatham JP, Ebeid MR, Hijazi ZM, et al. Procedural results and acute complications in stenting native and recurrent coarctation of the aorta in patients over 4 years of age: a multi-institutional study. Catheter Cardiovasc Interv. 2007;70:276–285. doi: 10.1002/ccd.21164. [DOI] [PubMed] [Google Scholar]
- 69.Forbes TJ, Kim DW, Du W, Turner DR, Holzer R, Amin Z, Hijazi Z, Ghasemi A, Rome JJ, Nykanen D, et al. Comparison of surgical, stent, and balloon angioplasty treatment of native coarctation of the aorta: an observational study by the CCISC (Congenital Cardiovascular Interventional Study Consortium) J Am Coll Cardiol. 2011;58:2664–2674. doi: 10.1016/j.jacc.2011.08.053. [DOI] [PubMed] [Google Scholar]
- 70.Meadows J, Minahan M, McElhinney DB, McEnaney K, Ringel R. Intermediate Outcomes in the Prospective, Multicenter Coarctation of the Aorta Stent Trial (COAST) Circulation. 2015;131:1656–1664. doi: 10.1161/CIRCULATIONAHA.114.013937. [DOI] [PubMed] [Google Scholar]
- 71.Bentham J, Shettihalli N, Orchard E, Westaby S, Wilson N. Endovascular stent placement is an acceptable alternative to reoperation in selected infants with residual or recurrent aortic arch obstruction. Catheter Cardiovasc Interv. 2010;76:852–859. doi: 10.1002/ccd.22586. [DOI] [PubMed] [Google Scholar]
- 72.Mohan UR, Danon S, Levi D, Connolly D, Moore JW. Stent implantation for coarctation of the aorta in children & lt; 30 kg. JACC Cardiovasc Interv. 2009;2:877–883. doi: 10.1016/j.jcin.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 73.Tzifa A, Ewert P, Brzezinska-Rajszys G, Peters B, Zubrzycka M, Rosenthal E, Berger F, Qureshi SA. Covered Cheatham-platinum stents for aortic coarctation: early and intermediate-term results. J Am Coll Cardiol. 2006;47:1457–1463. doi: 10.1016/j.jacc.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 74.Bruckheimer E, Dagan T, Amir G, Birk E. Covered Cheatham-Platinum stents for serial dilation of severe native aortic coarctation. Catheter Cardiovasc Interv. 2009;74:117–123. doi: 10.1002/ccd.21923. [DOI] [PubMed] [Google Scholar]
- 75.Sohrabi B, Jamshidi P, Yaghoubi A, Habibzadeh A, Hashemi-Aghdam Y, Moin A, Kazemi B, Ghaffari S, Abdolahzadeh Baghayi MR, Mahmoody K. Comparison between covered and bare Cheatham-Platinum stents for endovascular treatment of patients with native post-ductal aortic coarctation: immediate and intermediate-term results. JACC Cardiovasc Interv. 2014;7:416–423. doi: 10.1016/j.jcin.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 76.Kische S, D’Ancona G, Stoeckicht Y, Ortak J, Elsässer A, Ince H. Percutaneous treatment of adult isthmic aortic coarctation: acute and long-term clinical and imaging outcome with a self-expandable uncovered nitinol stent. Circ Cardiovasc Interv. 2015;8:pii: e001799. doi: 10.1161/CIRCINTERVENTIONS.114.001799. [DOI] [PubMed] [Google Scholar]
- 77.Morgan GJ, Lee KJ, Chaturvedi R, Bradley TJ, Mertens L, Benson L. Systemic blood pressure after stent management for arch coarctation implications for clinical care. JACC Cardiovasc Interv. 2013;6:192–201. doi: 10.1016/j.jcin.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 78.Hager A, Kanz S, Kaemmerer H, Schreiber C, Hess J. Coarctation Long-term Assessment (COALA): significance of arterial hypertension in a cohort of 404 patients up to 27 years after surgical repair of isolated coarctation of the aorta, even in the absence of restenosis and prosthetic material. J Thorac Cardiovasc Surg. 2007;134:738–745. doi: 10.1016/j.jtcvs.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 79.Graham TP, Driscoll DJ, Gersony WM, Newburger JW, Rocchini A, Towbin JA. Task Force 2: congenital heart disease. J Am Coll Cardiol. 2005;45:1326–1333. doi: 10.1016/j.jacc.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 80.Wilson W, Taubert KA, Gewitz M, Lockhart PB, Baddour LM, Levison M, Bolger A, Cabell CH, Takahashi M, Baltimore RS, et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2007;116:1736–1754. doi: 10.1161/CIRCULATIONAHA.106.183095. [DOI] [PubMed] [Google Scholar]
- 81.Carr JA. The results of catheter-based therapy compared with surgical repair of adult aortic coarctation. J Am Coll Cardiol. 2006;47:1101–1107. doi: 10.1016/j.jacc.2005.10.063. [DOI] [PubMed] [Google Scholar]


