Abstract
Background:
Sargassum species (phaeophyceae) are economically important brown algae in southern parts of Iran. Sargassum is mainly harvested as a row material in alginate production industries and is a source of plant foods or plant bio-stimulants even a component of animal foods.
Objective:
In this study, Sargassum glaucescens, collected from the seashore of Chabahar, was employed for phytochemical and biological evaluations.
Materials and Methods:
For that purpose, the dried algae was extracted by methanol and subjected to different chromatographic separation methods.
Results:
Six sterols, fucosterol (1), 24(S)-hydroxy-24-vinylcholesterol (2), 24(R)-hydroxy-24-vinylcholesterol (3), stigmasterol (4), β-sitosterol (5) and cholesterol (6) were identified by spectroscopic methods including 1H-NMR, 13C-NMR and mass spectroscopy. In vitro alpha-amylase inhibitory test was performed on the methanolic extract and the results revealed a potent inhibition (IC50 = 8.9 ± 2.4 mg/mL) of the enzyme compared to acarbose as a positive control.
Conclusion:
Various biological activities and distribution of sterols in Sargassum genus have been critically reviewed here. The results concluded that these algae are a good candidate for further anti-diabetic investigations in animals and human.
Keywords: Alpha-amylase inhibitor, Brown algae, Sargassum glaucescens, Sterol
INTRODUCTION
Sargassum (Phaeophyceae family) is a genus of brown algae, well-known as macroalgae or seaweeds, distributed throughout the temperate and tropical oceans in the world. Moreover, this genus is known for its planktonic species. Interestingly, Sargassum species are found to grow in temperate water opposite of other species belonging to Phaeophyceae that are predominantly cold water organisms.[1] These algae are generally observed in brown or dark green colors and commonly grow sub-tidally attached to the corals, rocks or shells in moderately exposed or sheltered rocky areas. Sargassum is mainly harvested as a row material in alginate production industries and is a source of plant foods or plant bio-stimulants even a component of animal foods. These are also employed as nutraceuticals and pharmaceuticals due to the presence of fucoidan and other bioactive compounds in their extracts.[2,3]
A bibliography revealed that Sargassum may be the source of several bioactive compounds including, steroids,[4,5] flavonoids,[4,5,6] polysaccharides,[7,8] terpenoids,[9,10] fatty acids,[11] plastoquinones[12] and tannins.[13] Among them, sterols are the major bioactive secondary metabolites in these algae. Recently, several sterols have been isolated and identified from brown algae, of which fucosterol and saringosterol were separated from S. pallidum.[5] Various Δ5-3 β-sterols possessing carbon numbers ranged from C19-C23 to C26-C30 have been reported from the extract of S. muticum.[14] Furthermore, S. oligocystum has been reported for isolation and identification of 22-dehydrocholesterol, cholesterol, fucosterol, 29-hydroperoxystigmasta-5,24 (28)-dien-3 β-ol, 24-hydroperoxy-24-vinylcholesterol, 24(S) and 24(R)-hydroxy-24-vinylcholesterol, as well as ostreasterol.[15] A number of sterols reported from various species of Sargassum are exhibited in Table 1.
Table 1.
Distribution of sterols in Sargassum genus
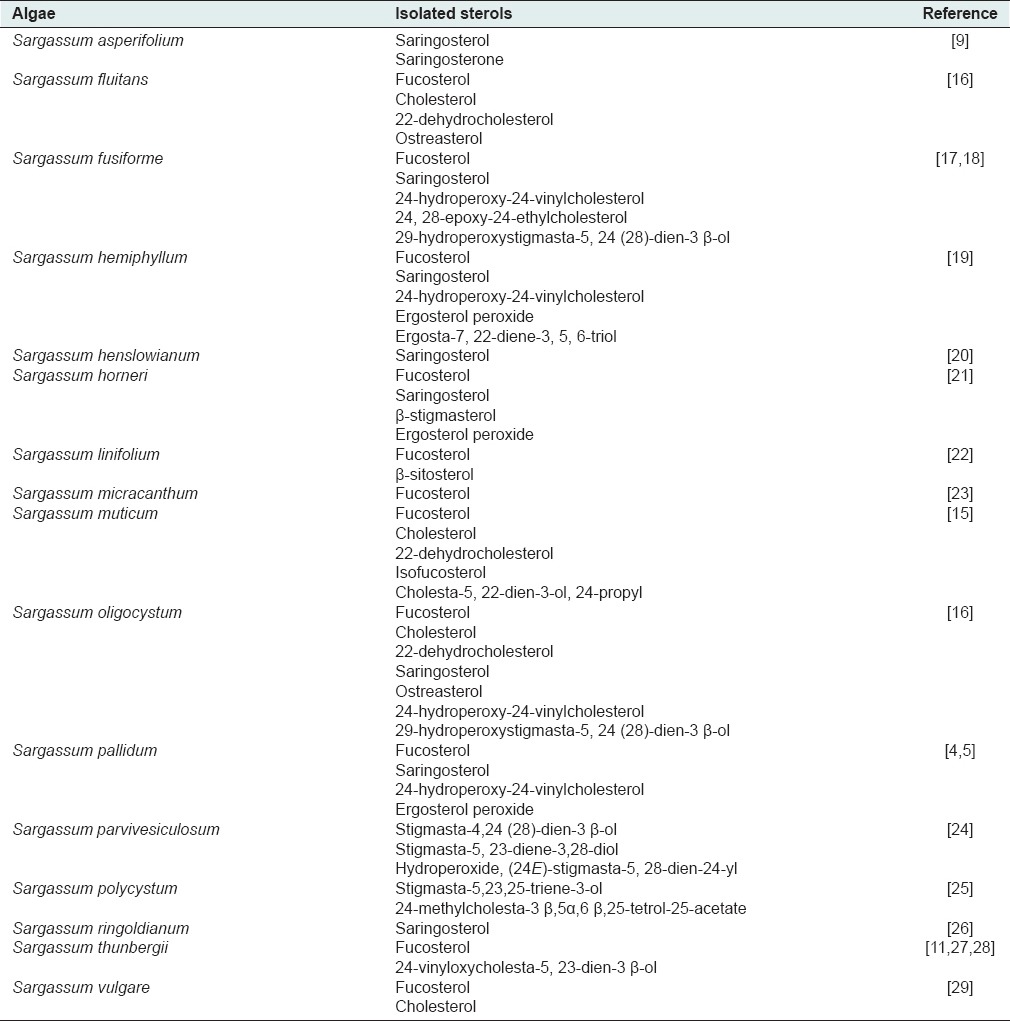
Here in this study, we focused on S. glaucescens as one of the most abundant brown algae distributed in Persian Gulf and Oman Sea. As far as we could ascertain there is no report on its sterol composition. Therefore, we aimed to report the isolation and structural elucidation of the sterols from S. glaucescens methanolic extract for the first time.
MATERIALS AND METHODS
Instruments and materials
All the chemicals used in the biochemical assay were purchased from Sigma-Aldrich Chemie Gmbh (Germany) and Merck (Germany) companies. The chemicals were of analytical grade. The enzyme (EC 3.2.1.1) was purchased from Sigma (Germany) that extracted from soy bean source. α-amylase activity was determined by measuring the absorbance of the mixtures at 540 nm in Elisa stat fax 2100 (Awarness Technology Inc., FL, USA). 1H-NMR and13C-NMR spectra were recorded on a Brucker Avance 500 DRX spectrometer® (Germany) with tetramethylsilane as an internal standard and chemical shifts are given in d (ppm). Multiple-pulse experiments (HSQC, HMBC, and H-H COSY) were performed using the standard Bruker® programs. Silica gel 60 F254 and Silica gel 60 RP-18 F254 S pre-coated plates (Merck®, Germany) were used for thin layer chromatography. The spots were detected by spraying with anisaldehyde-H2SO4 reagent (Mahestan Shimi, Iran), followed by heating.
Plant materials
The whole parts of Sargassum glaucescens were collected from the seashore of Chabahar, Sistan and Baluchestan Province in the Southeast of Iran in 2011, washed with distilled water, dried at room temperature and Identified by Mr. B. M. Gharanjik. A voucher specimen (No. P 50-16) was deposited at the Research Center of Persian Gulf Biotechnology (Qeshm Island, Iran).
Extraction and isolation process
Dried algae, Sargassum glaucescens, were cut into small pieces (2 kg) and extracted with methanol at room temperature by percolation method for 48 h and 3 times. The solvent was evaporated by rotary evaporator. The methanolic extract (60 g) was fractionated by silica gel column chromatography (CC) with hexane: chloroform (2:8), chloroform: ethyl acetate (8:2, 5:5) and ethyl acetate, respectively, to yield seven fractions (A-G). Fraction C (6.5 g) were chosen and subjected to silica gel CC with chloroform: ethyl acetate (19:1, 8:2 and 0:1) to obtain six main fractions (C1-C6). Fraction C2 (1100 mg) was submitted to silica gel CC with chloroform: ethyl acetate (19:1) to yield compound 1 (22 mg). Fraction C4 (610 mg) was submitted to silica gel CC with chloroform: ethyl acetate (9:1) to result five fractions. Third fraction (52 mg) was subjected to sephadex LH20 to yield three other main fractions. First fraction (26 mg) was fractionated on silica gel (chloroform: ethyl acetate, 8:2) to gain compound 2 and 3 (8 and 5 mg, respectively). Fraction D (330 mg) was submitted to silica gel CC with chloroform: methanol (98:2) to result four parts (D1-D4). D2 (83 mg) was fractionated on sephadex LH20 with methanol to purify compound 4 and 5. From Fraction E (710 mg) after chromatographing on silica gel eluted with chloroform: ethyl acetate (8:2, 6:4), five parts resulted. Compound 6 (12 mg) was purified from a third part (69 mg) after loading on sephadex LH20 CC eluted with methanol.
Alpha-Amylase inhibitory assay
The α-amylase inhibition assay was performed by some modification in the method proposed by Giancarlo et al.[30] The starch solution (1% w/v) was obtained by boiling and stirring 1 g of potato starch in 100 mL of sodium phosphate buffer for 30 min. The enzyme (EC 3.2.1.1) solution (50 U/1 mL) was prepared by mixing 0.01 g of α-amylase in 10 mL of sodium phosphate buffer (PH 6.9) containing 0.0006 mM sodium chloride. The extracts were dissolved in dimethyl sulfoxide (DMSO) to give concentrations from 5 to 15 mg/mL (5, 10 and 15 mg/mL). The color reagent was a solution containing 0.1 g of 3, 5-dinitrosalicylic acid plus 2.99 g sodium potassium tartrate in 0.16 g sodium hydroxide and phosphate buffer (10 mL).
Totally, 50 microliter of each algae extract, and 150 μL of starch solution, as well as 10 μL of enzyme, were mixed in a 96 well plate and incubated at 37°C for 30 min. Then, 20 μL of sodium hydroxide and 20 μL of color reagent were added, and the closed plate placed into a 100°C water bath. After 20 min, the reaction mixture was removed from the water bath and cooled, thereafter α-amylase activity was determined by measuring the absorbance of the mixture at 540 nm using Elisa. Blank samples were used to correct the absorption of the mixture in which the enzyme was replaced with buffer solution. Furthermore, a control reaction was used, in which the algae extract was replaced with 50 μ of DMSO, and the maximum enzyme activity was determined. Acarbose solution at the concentrations (5, 10, 15 mg/mL) was used as a positive standard. The inhibition percentage of α-amylase was assessed by the following formula:
I α–Amylase % =100 × (ΔAcontrol–ΔAsample)/ΔAcontrol
ΔAcontrol = Atest – ABlank
ΔAsample = Atest – ABlank
I: Inhibitory activity; A: Absorbance at 540 nm.
Statistical analysis
Statistical analysis was performed using the SPSS version 21.0 (IBM Corporation, 2012). The IC50 values were estimated by nonlinear curve and presented as their respective 95% confidence limits. Probit analysis of variance was used to assess the presence of significant differences (P < 0.05) between the extracts.
RESULTS AND DISCUSSION
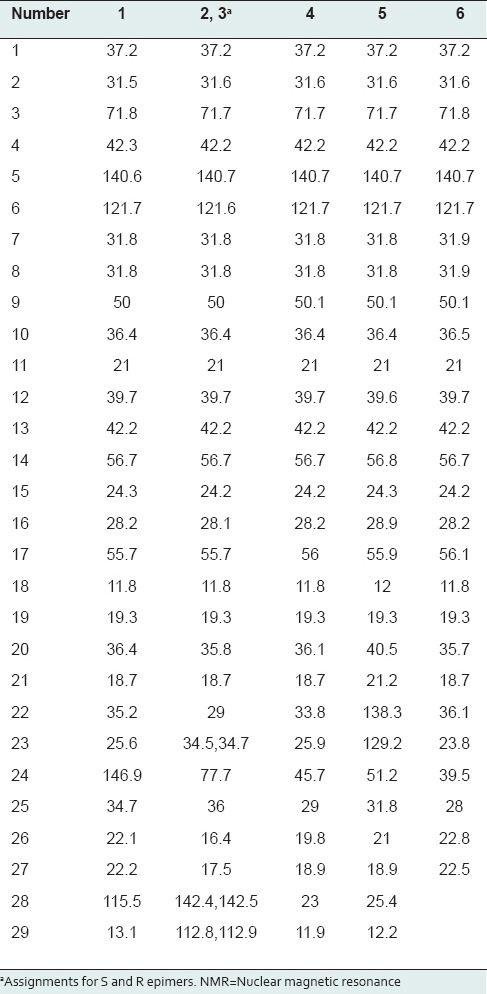
Methanolic extract of marine algae, S. glaucescens, was used for the separation of sterols. Isolation and purification of the main compounds were carried out on silica gel and sephadex LH20 CC to obtain six pure compounds. Structural elucidation of these compounds was based on the data obtained from 1H-NMR, 13C-NMR studies. Separated compounds from S. glaucescens were identified as fucosterol (1),[31] a mixture of 24(S)-hydroxy-24-vinylcholesterol (2) and 24(R)-hydroxy-24-vinylcholesterol (3),[15] stigmasterol (4),[32,33] β-sitosterol (5)[34,35] and cholesterol (6)[36] compared to the spectral data reported in the literatures [Figure 1]. 13C NMR data of sterols isolated from S. glaucescens are summarized in Table 2.
Figure 1.
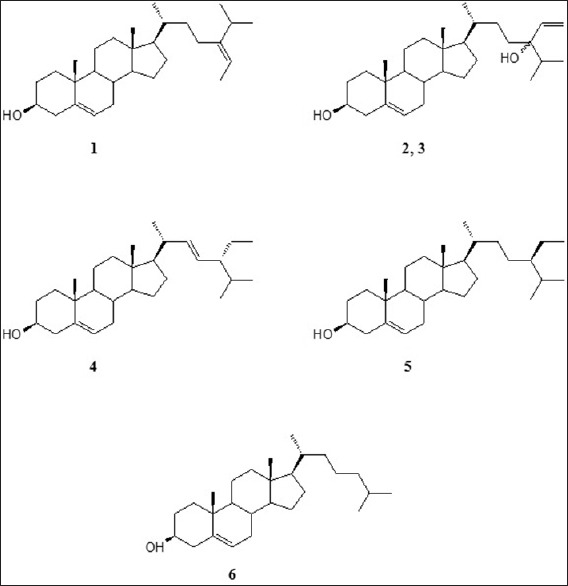
Chemical structures of isolated sterols from Sargassum glaucescens
Table 2.
13C-NMR data (δ ppm) of isolated sterols from Sargassum glaucescens in CDCl3
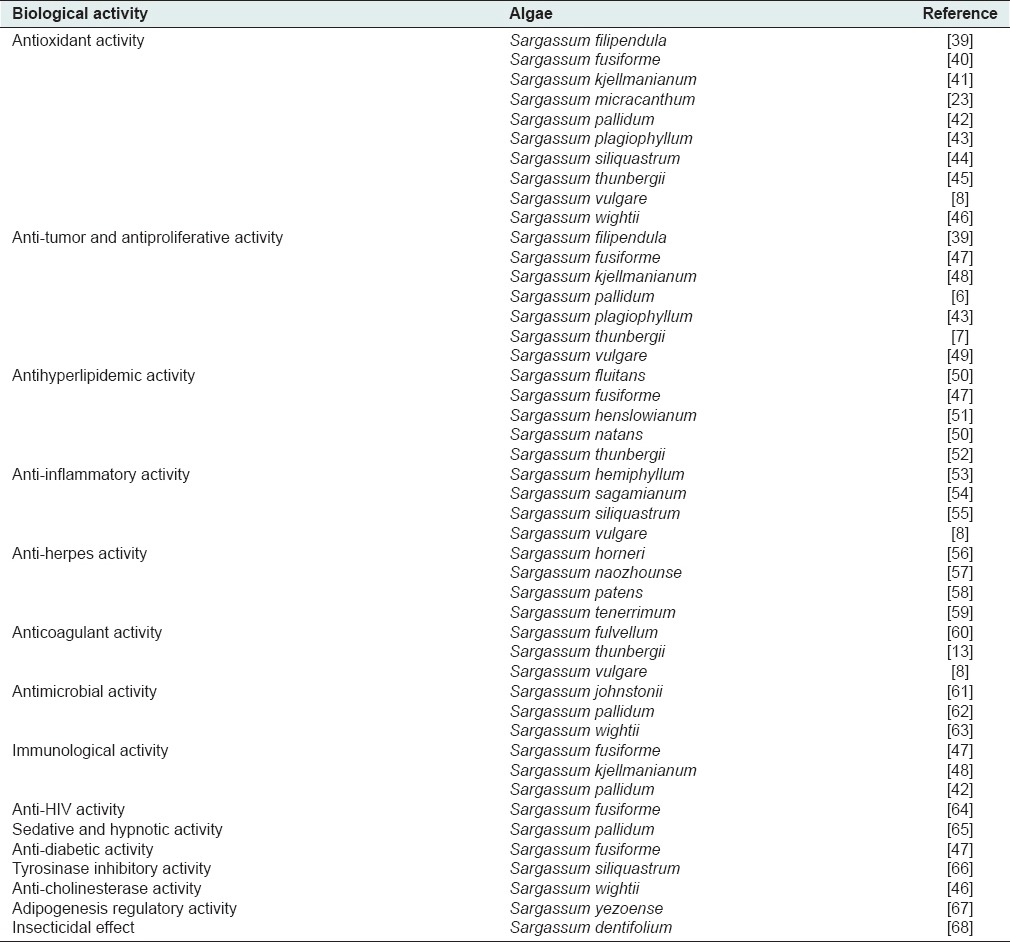
Besides cholesterol and fucosterol as the main and characteristic sterols in brown algae, hydroperoxy sterols like 24-hydroperoxy 24-vinyl cholesterol are predominantly found in those algae.[36,37,38] A literature review demonstrates that different species of Sargassum exhibited various biological activities, which are summarized in Table 3.
Table 3.
A number of biological activities reported from various species of Sargassum
Among the isolated compounds from the algae, sitosterol is reported to possess several biological activities including anti-inflammatory, hypocholesterolemic, analgesic, chemoprotective, immunomodulatory, and anthelmintic activities. The most important effects on Benign prostatic hyperplasia and prostatic cancer treatment, as well as anti-diabetic and antioxidant activities, are well-documented.[69] Furthermore, another compound stigmasterol has been frequently reported for its anti-inflammatory, hypocholesterolemic, ameliorating, antiperoxidative, thyroid inhibitory, and hypoglycemic properties.[69] In addition, fucosterol is a major compound of this algae and well-known for its anti-diabetic, antioxidant and hepatoprotective effects as well as histamine and acetylcholine esterase inhibitory activities.[69]
As far as we could ascertain, 24-hydroxy-24-vinylcholesterol (saringasterol), the most aboundant sterol in Sargassum species, are reported for a variety of biological activities. For instance, 24 R-saringasterol exhibited the proliferation activity (under 1000 nM after 24 h incubation) and concentration-dependent proliferation activity (under 300 nM after 72 h incubation) significantly. Furthermore, this compound could have inhibitory activity against bone-resorbent metabolic bone disorders including osteoporosis and periodontitis.[70] Saringasterol is also evaluated for its probable cytotoxic activity and showed weak inhibitory effects on LNCaP cells (IC50 = 41.60 ± 4.26 μM) and was inactive on DU145 and PC3 cells (IC50 > 50 μM).[71] In addition, saringasterol possesses in vitro antitrypanosomal activity with IC50 value of 3.2 ± 1.2 μM.[72] Finally, inhibition of Mycobacterium tuberculosis growth has been reported by saringosterol while no significant toxicity was observed from this compound against Vero cells.[73]
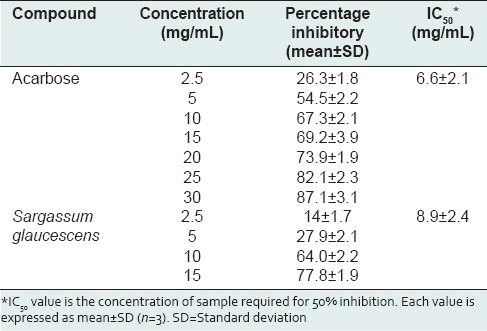
In the present study, the results of biochemical assay showed that this algal species exhibited a potent inhibitory activity on α-amylase enzyme (IC50 = 8.9 ± 2.4 mg/mL) compared the positive standard, acarbose (IC50 = 6.6 ± 2.1 mg/mL) [Table 4]. A concentration dependent inhibition was observed for various concentrations of this algal extract. The highest inhibitory activity for S. glaucescens was found to be 77.8 ± 1.9 (at 15 mg/mL) while the percentage inhibitory activity for acarbose at the same concentration was observed as 69.2 ± 3.9.
Table 4.
α-Amylase inhibitory activities and IC50 values of different concentrations of Sargassum glaucescens in comparison to acarbose as a positive standard
A literature review shows that fucosterol isolated from Pelvetia siliquosa was reported for its anti-diabetic activity. In this study, fucosterol was administered orally (30 mg/kg) in streptozotocin-induced diabetic rats which showed a significant reduction in serum glucose levels and inhibited the sorbitol accumulations in the lenses. It also caused an inhibition of blood glucose level and glycogen degradation at 300 mg/kg in epinephrine-induced diabetic rats. The authors concluded that fucosterol is a main anti-diabetic principle in P. siliquosa.[74] Furthermore, the anti-diabetic potential of fucosterol has been reported by evaluating the ability of this compound to inhibit rat lens aldose reductase (RLAR), human recombinant aldose reductase (HRAR), protein tyrosine phosphatase 1B (PTP1B), and α-glucosidase. The investigators revealed that it exhibited a moderate inhibitory activity against RLAR, HRAR, and PTP1B, while a weak or no activity against advanced glycation end formation and α-glucosidase.[75] On the other hand, β-sitosterol is also well-documented for anti-diabetic activity. For instance, in a recent study, three doses of this compound (10, 15 and 20 mg/kg, orally) resulted in decreasing the glycated hemoglobin, serum glucose, and nitric oxide, with concomitant increases in serum insulin levels. In addition, it could increase pancreatic antioxidant levels, with a concomitant decrease in thiobarbituric acid-reactive substances.[76] Moreover, in silico studies exhibited the potent inhibition for β-sitosterol on human pancreatic alpha-amylase. The inhibition constant (Ki) for this compound was estimated as 269.35 nmol with two hydrogen bond interactions, although there is no report on the evaluation of fucosterol and β-sitosterol against pancreatic α-amylase activity so far.[77] Taking together, anti-diabetic sterols of this algae may be responsible at least in part for anti-diabetic activity of Sargassum species.[46] However, this activity is supposed to be performed via different mechanisms, of which α-amylase inhibitory activity is one of the most critical ones.
CONCLUSION
Taking together, S. glaucescens the brown algae from Southern Iran was subjected for isolation and identification of the main sterols resulting in separation of fucosterol, 24(S)-hydroxy-24-vinylcholesterol, 24(R)-hydroxy-24-vinylcholesterol, stigmasterol, β-sitosterol and cholesterol. The α-amylase inhibitory activity of this algae was evaluated compared to acarbose and exhibited a potent inhibition against this enzyme in an in vitro assay.
ACKNOWLEDGMENT
This research has been supported by Tehran University of Medical Sciences and Health Services Grant (No. 17619). The authors wish to thank Dr. Bayram Gharanjic for his kind help in algae identification.
Footnotes
Source of Support: Nil
Conflicts of Interest: None declared.
REFERENCES
- 1.Hogan MC. Brown algae. In: Monosson E, Cleveland CJ, editors. Encyclopedia of Earth. Washington, DC: National Council for Science and the Environment; 2011. [Google Scholar]
- 2.Boaden PJ. The adventives seaweed Sargassum muticum (Yendo) Fensholt in Sterangford Lough, Northen Ireland. Ir Nat J. 1995;25:111–3. [Google Scholar]
- 3.Cannel RJ. Algae as a source of biologically active products. Pestic Sci. 2006;39:143–53. [Google Scholar]
- 4.Xu FQ, Feng YY, Guo L, Guo GL, Yan BL. On chemical constitutes from Sargassum pallidum. Anhui Nongye Kexue. 2013;41:6658–9. [Google Scholar]
- 5.Liu X, Wang CY, Shao CL, Wei YX, Wang BG, Sun LL, et al. Chemical constituents from Sargassum pallidum (Turn). C. Agardh Biochem Syst Ecol. 2009;37:127–9. [Google Scholar]
- 6.Guo L, Shao C, Liu X, Fang Y, Wei Y, Sun L, et al. Chemical composition of Sargassum pallidum and its antitumor activity in vitro. Zhong Cao Yao. 2009;40:1879–82. [Google Scholar]
- 7.Itoh H, Noda H, Amano H, Zhuaug C, Mizuno T, Ito H. Marine algal polysaccharide, GIV-A from Sargassum thunbergii markedly inhibited the growth of Ehrlich ascites carcinoma at the dose of 20 mg/kg per day X10 with no sign of toxicity in mice. Anticancer Res. 1993;13:2045–52. [PubMed] [Google Scholar]
- 8.Dore CM, das C Faustino Alves MG, Will LS, Costa TG, Sabry DA, de Souza Rêgo LA, et al. A sulfated polysaccharide, fucans, isolated from brown algae Sargassum vulgare with anticoagulant, antithrombotic, antioxidant and anti-inflammatory effects. Carbohydr Polym. 2013;91:467–75. doi: 10.1016/j.carbpol.2012.07.075. [DOI] [PubMed] [Google Scholar]
- 9.Ayyad SE, Sowellim SZ, el-Hosini MS, Abo-Atia A. The structural determination of a new steroidal metabolite from the brown alga Sargassum asperifolium. Z Naturforsch C. 2003;58:333–6. doi: 10.1515/znc-2003-5-607. [DOI] [PubMed] [Google Scholar]
- 10.Reddy P, Urban S. Meroditerpenoids from the southern Australian marine brown alga Sargassum fallax. Phytochemistry. 2009;70:250–5. doi: 10.1016/j.phytochem.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 11.Qin M, Li X, Yin S, Wang C, Wang B. Chemical constituents from Sargassum thunbergii. Haiyang Kexue. 2007;31:47–50. [Google Scholar]
- 12.Kim MC, Kwon HC, Kim SN, Kim HS, Um BH. Plastoquinones from Sargassum yezoense; chemical structures and effects on the activation of peroxisome proliferator-activated receptor gamma. Chem Pharm Bull (Tokyo) 2011;59:834–8. doi: 10.1248/cpb.59.834. [DOI] [PubMed] [Google Scholar]
- 13.Wei Y, Wang C, Li J, Qi H, Guo Q. Effects of high-molecular-weight phlorotannins from Sargassum thunbergii Kuntze on anticoagulation activity and platelet cytosolic calcium level. Zhongguo Yaoke Da Xue Xue Bao. 2008;39:257–61. [Google Scholar]
- 14.Wang P, Xu G, Bian L, Zhang S, Song F. Study on sterols from brown algae (Sargassum muticum) Chin Sci Bull. 2006;51:2520–8. [Google Scholar]
- 15.Permeh P, Saeidnia S, Mashinchian-Moradi A, Gohari AR. Sterols from Sargassum oligocystum, a brown algae from the Persian Gulf, and their bioactivity. Nat Prod Res. 2012;26:774–7. doi: 10.1080/14786419.2010.548812. [DOI] [PubMed] [Google Scholar]
- 16.Smith LL, Dhar AK, Gilchrist JL, Lin YY. Sterol metabolism. XXVII. Sterols of the brown alga Sargassum fluitans. Phytochemistry. 1973;12:2727–32. [Google Scholar]
- 17.Wang W, Li H, Wang Y, Xia X, Okada Y, Okuyama T. Chemical constituents from brown alga Sargassum fusiforme. Zhong Cao Yao. 2008;39:657–61. [Google Scholar]
- 18.Xu S, Cen Y, Cai L, Li Y, Xu S. Studies on the chemical components from Sargassum fusiform. Zhong Yao Cai. 2001;24:491–2. [PubMed] [Google Scholar]
- 19.Liu H, Cui Z, Li Y, Yin J, Dong Y, Ding W. Chemical constituents of Sargassum hemiphyllum. Zhongguo Yaoxue Za Zhi. 1998;33:464–6. [Google Scholar]
- 20.Wei M, Li S, Chen J, Lin Y. Chemical constituents of the brown alga Sargassum henslowianum collected from the South China Sea. Chem Nat Compd. 2012;48:677–8. [Google Scholar]
- 21.Yuan Q, Fu L. Study on the chemical constituents of brown alga Sargassum horneri. Guangdong Huagong. 2006;33:42–3. [Google Scholar]
- 22.Sallam LA, Hamdy AA, Naim N, El-Refai AH, Karawya MS. Chemical composition of some Egyptian marine algae. 1-Sterol constituents. Egyp J Pharm Sci. 1984;23:179–86. [Google Scholar]
- 23.Ham YM, Kim KN, Lee WJ, Lee NH, Hyun CG. Chemical constituents from Sargassum micracanthum and antioxidant activity. Int J Pharmacol. 2010;6:147–51. [Google Scholar]
- 24.Qi SH, Zhang S, Huang JS, Xiao ZH, Wu J, Long LJ. Glycerol derivatives and sterols from Sargassum parvivesiculosum. Chem Pharm Bull (Tokyo) 2004;52:986–8. doi: 10.1248/cpb.52.986. [DOI] [PubMed] [Google Scholar]
- 25.Xu SH, Ding LS, Wang MK, Peng SL, Liao X. Studies on the chemical constituents of the algae Sargassum polycystum. Youji Huaxue. 2002;22:138–40. [Google Scholar]
- 26.Ikekawa N, Tsuda K, Morisaki N. Saringosterol: A new sterol from brown algae. Chem Ind. 1966;27:1179–80. [Google Scholar]
- 27.Jiang Q, Liu D, Yang JB, Yan PC, Huang KX, Lin WH. Chemical constituents from marine alga Sargassum thunbergii. Zhongguo Yaoxue Za Zhi. 2012;47:948–52. [Google Scholar]
- 28.Kobayashi M, Hasegawa A, Mitsuhashi H. Marine sterols. XV. Isolation of 24-vinyloxycholesta-5,23-dien-3b-ol from the brown alga Sargassum thumbergii. Chem Pharm Bull. 1985;33:4012–3. [Google Scholar]
- 29.Halket JM, Lisboa BP, Pinheiro-Joventino F. The major sterols of Sargassum vulgare C. Agardh investigated by mass chromatography. Arq Cien Mar. 1976;16:117–22. [Google Scholar]
- 30.Giancarlo S, Rosa LM, Nadjafi F, Francesco M. Hypoglycaemic activity of two spices extracts: Rhus coriaria L. and Bunium persicum Boiss. Nat Prod Res. 2006;20:882–6. doi: 10.1080/14786410500520186. [DOI] [PubMed] [Google Scholar]
- 31.Saeidnia S, Permeh P, Gohari AR, Mashinchian-Moradi A. Gracilariopsis persica from Persian Gulf contains bioactive sterols. Iran J Pharm Res. 2012;11:845–9. [PMC free article] [PubMed] [Google Scholar]
- 32.Saeidnia S, Gohari AR, Malmir M, Moradi-Afrapoli F, Ajani Y. Tryptophan and sterols from Salvia limbata. J Med Plants. 2011;10:41–4. [Google Scholar]
- 33.Saeidnia S, Ghamarinia M, Gohari AR, Shakeri A. Terpenes from the root of Salvia hypoleuca Benth. Daru. 2012;20:66. doi: 10.1186/2008-2231-20-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shahani S, Monsef-Esfahani HR, Saeidnia S, Saniee P, Siavoshi F, Foroumadi A, et al. Anti-Helicobacter pylori activity of the methanolic extract of Geum iranicum and its main compounds. Z Naturforsch C. 2012;67:172–80. [PubMed] [Google Scholar]
- 35.Gohari AR, Saeidnia S, Hadjiakhoondi A, Honda G. Isolation and identification of four sterols from Oud. J Med Plants. 2008;7:47–55. [Google Scholar]
- 36.Nasir M, Saeidnia S, Mashinchian-Moradi A, Gohari AR. Sterols from the red algae, Gracilaria salicornia and Hypnea flagelliformis, from Persian Gulf. Pharmacogn Mag. 2011;7:97–100. doi: 10.4103/0973-1296.80663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moghadam MH, Firouzi J, Saeidnia S, Hajimehdipoor H, Jamili S, Rustaiyan A, et al. A cytotoxic hydroperoxy sterol from the brown alga, Nizamuddinia zanardinii. Daru. 2013;21:24. doi: 10.1186/2008-2231-21-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kamenarska ZG, Dimitrova-Konaklieva SD, Stefanov KL, Popov SS. A comparative study on the sterol composition of some brown algae from the Black Sea. J Serbian Chem Soc. 2003;68:269–75. [Google Scholar]
- 39.Costa LS, Fidelis GP, Telles CB, Dantas-Santos N, Camara RB, Cordeiro SL, et al. Antioxidant and antiproliferative activities of heterofucans from the seaweed Sargassum filipendula. Mar Drugs. 2011;9:952–66. doi: 10.3390/md9060952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li C, Wang C, Wang S, Qian G, Zhu Q, Liu Y, et al. Optimization of ultrasonic-assisted extraction technology of Sargassum fusiforme polysaccharides and evaluation of their antioxidant activity. Food Sci Technol Res. 2013;19:157–62. [Google Scholar]
- 41.Wei Y, Li Z, Hu Y, Xu Z. Inhibition of mouse liver lipid peroxidation by high molecular weight phlorotannins from Sargassum kjellmanianum. J Appl Phycol. 2003;15:507–11. [Google Scholar]
- 42.Zhang RL, Luo WD, Bi TN, Zhou SK. Evaluation of antioxidant and immunity-enhancing activities of Sargassum pallidum aqueous extract in gastric cancer rats. Molecules. 2012;17:8419–29. doi: 10.3390/molecules17078419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suresh V, Senthilkumar N, Thangam R, Rajkumar M, Anbazhagan C, Rengasamy R, et al. Separation, purification and preliminary characterization of sulfated polysaccharides from Sargassum plagiophyllum and its in vitro anticancer and antioxidant activity. Process Biochem. 2013;48:364–73. [Google Scholar]
- 44.Lee JI, Seo Y. Chromanols from Sargassum siliquastrum and their antioxidant activity in HT 1080 cells. Chem Pharm Bull (Tokyo) 2011;59:757–61. doi: 10.1248/cpb.59.757. [DOI] [PubMed] [Google Scholar]
- 45.Seo Y, Park KE, Kim YA, Lee HJ, Yoo JS, Ahn JW, et al. Isolation of tetraprenyltoluquinols from the brown alga Sargassum thunbergii. Chem Pharm Bull (Tokyo) 2006;54:1730–3. doi: 10.1248/cpb.54.1730. [DOI] [PubMed] [Google Scholar]
- 46.Syad AN, Shunmugiah KP, Kasi PD. Antioxidant and anti-cholinesterase activity of Sargassum wightii. Pharm Biol. 2013;51:1401–10. doi: 10.3109/13880209.2013.793721. [DOI] [PubMed] [Google Scholar]
- 47.Chen Q, Xu N. Active principles and pharmacological effect of Sargassum fusiforme (Harv.) Setchell. Zhongguo Yaoye. 2005;14:95–6. [Google Scholar]
- 48.Ma WW, Li L, Zhou GF. In vitro immunoregulatory and antitumor activity of sulfated polysaccharides from Sargassum kjellmanianum. Shipin Kexue. 2013;34:270–4. [Google Scholar]
- 49.Guerra Dore CM, Faustino Alves MG, Santos ND, Cruz AK, Câmara RB, Castro AJ, et al. Antiangiogenic activity and direct antitumor effect from a sulfated polysaccharide isolated from seaweed. Microvasc Res. 2013;88:12–8. doi: 10.1016/j.mvr.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 50.Yunev OA, Markova GA. Extraction of Steroids with a hypocholesterolemic effect from Brown Algae. Ekol Morsk Org Mater Vses Nauchno Tekh Konf. 1981:74–8. [Google Scholar]
- 51.Chen S, Wang W, Liu H, Li C, Liu C. Purification and lowing hyperlipidemia activity of fucoidan from Sargassum henslowianum. Shipin Yu Fajiao Gongye. 2010;36:28–31. [Google Scholar]
- 52.Kim DH, Kim KB, Kim MJ, Sunwoo C, Jung SA, Kim HJ, et al. Effects of heat, pH, and gamma irradiation treatments on lipase inhibitory activity of Sargassum thunbergii ethanol extract. Han’guk Sikp’um Yongyang Kwahak Hoechi. 2012;41:566–70. [Google Scholar]
- 53.Hwang PA, Chien SY, Chan YL, Lu MK, Wu CH, Kong ZL, et al. Inhibition of lipopolysaccharide (LPS)-induced inflammatory responses by Sargassum hemiphyllum sulfated polysaccharide extract in RAW 264.7 macrophage cells. J Agric Food Chem. 2011;59:2062–8. doi: 10.1021/jf1043647. [DOI] [PubMed] [Google Scholar]
- 54.Chang HW, Jang KH, Lee D, Kang HR, Kim TY, Lee BH, et al. Monoglycerides from the brown alga Sargassum sagamianum: Isolation, synthesis, and biological activity. Bioorg Med Chem Lett. 2008;18:3589–92. doi: 10.1016/j.bmcl.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 55.Heo SJ, Yoon WJ, Kim KN, Oh C, Choi YU, Yoon KT, et al. Anti-inflammatory effect of fucoxanthin derivatives isolated from Sargassum siliquastrum in lipopolysaccharide-stimulated RAW 264.7 macrophage. Food Chem Toxicol. 2012;50:3336–42. doi: 10.1016/j.fct.2012.06.025. [DOI] [PubMed] [Google Scholar]
- 56.Preeprame S, Hayashi K, Lee JB, Sankawa U, Hayashi T. A novel antivirally active fucan sulfate derived from an edible brown alga, Sargassum horneri. Chem Pharm Bull (Tokyo) 2001;49:484–5. doi: 10.1248/cpb.49.484. [DOI] [PubMed] [Google Scholar]
- 57.Peng Y, Xie E, Zheng K, Fredimoses M, Yang X, Zhou X, et al. Nutritional and chemical composition and antiviral activity of cultivated seaweed Sargassum naozhouense Tseng et Lu. Mar Drugs. 2012;11:20–32. doi: 10.3390/md11010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhu W, Ooi VE, Chan PK, Ang PO., Jr Isolation and characterization of a sulfated polysaccharide from the brown alga Sargassum patens and determination of its anti-herpes activity. Biochem Cell Biol. 2003;81:25–33. doi: 10.1139/o02-169. [DOI] [PubMed] [Google Scholar]
- 59.Sinha S, Astani A, Ghosh T, Schnitzler P, Ray B. Polysaccharides from Sargassum tenerrimum: Structural features, chemical modification and anti-viral activity. Phytochemistry. 2010;71:235–42. doi: 10.1016/j.phytochem.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 60.De Zoysa M, Nikapitiya C, Jeon YJ, Jee Y, Lee J. Anticoagulant activity of sulfated polysaccharide isolated from fermented brown seaweed Sargassum fulvellum. J Appl Phycol. 2008;20:67–74. [Google Scholar]
- 61.Rao PP, Rao PS, Karmarkar SM. Antibacterial substances from brown algae. II. Efficiency of solvents in the evaluation of antibacterial substances from Sargassum johnstonii Setchell et Gardner. Bot Mar. 1986;29:503–7. [Google Scholar]
- 62.Gerasimenko NI, Martyyas EA, Logvinov SV, Busarova NG. Biological activity of lipids and photosynthetic pigments of Sargassum pallidum C. Agardh. Appl Biochem Microbiol. 2014;50:73–81. doi: 10.7868/s0555109914010036. [DOI] [PubMed] [Google Scholar]
- 63.Arunkumar K, Selvapalam N, Rengasamy R. The antibacterial compound sulphoglycerolipid 1-0 palmitoyl-3-0 (6’- sulpho-α-quinovopyranosyl)-glycerol from Sargassum wightii greville (Phaeophyceae) Bot Mar. 2005;48:441–5. [Google Scholar]
- 64.Paskaleva EE, Lin X, Duus K, McSharry JJ, Veille JC, Thornber C, et al. Sargassum fusiforme fraction is a potent and specific inhibitor of HIV-1 fusion and reverse transcriptase. Virol J. 2008;5:8. doi: 10.1186/1743-422X-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ji A, Yao Y, Che O, Wang B, Sun L, Li X, et al. Isolation and characterization of sulfated polysaccharide from the Sargassum pallidum (Turn.) C. Ag And its sedative/hypnotic activity. J Med Plants Res. 2011;5:5240–6. [Google Scholar]
- 66.Kim SJ, Woo S, Yun H, Yum S, Choi E, Do JR, et al. Total phenolic contents and biological activities of Korean seaweed extracts. Food Sci Biotechnol. 2005;14:798–802. [Google Scholar]
- 67.Kim SN, Choi HY, Lee W, Park GM, Shin WS, Kim YK. Sargaquinoic acid and sargahydroquinoic acid from Sargassum yezoense stimulate adipocyte differentiation through PPAR alpha/gamma activation in 3T3-L1 cells. FEBS Lett. 2008;582:3465–72. doi: 10.1016/j.febslet.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 68.Aboutabl EA, Saleh MM, El-Sakhawy F, Afifi MS, Moawad SS, El-Rafei HA. Constituents and biological activity of Sargassum dentifolium (Agardh) towards cotton leaf worm. Bull Fac Pharm. 2002;40:63–72. [Google Scholar]
- 69.Saeidnia S, Manayi A, Gohari AR, Abdollahi M. The story of beta-sitosterol-A review. Eur J Med Plants. 2014;4:590–609. [Google Scholar]
- 70.Huh GW, Lee DY, In SJ, Lee DJ, Park SY, Yi TH, et al. Fucosterols from Hizikia fusiformis and their proliferation activities on osteosarcoma-derived cell MG63. J Korean Soc Appl Biol Chem. 2012;55:551–5. [Google Scholar]
- 71.Zhang JL, Tian HY, Li J, Jin L, Luo C, Ye WC, et al. Steroids with inhibitory activity against the prostate cancer cells and chemical diversity of marine alga Tydemania expeditionis. Fitoterapia. 2012;83:973–8. doi: 10.1016/j.fitote.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 72.Hoet S, Pieters L, Muccioli GG, Habib-Jiwan JL, Opperdoes FR, Quetin-Leclercq J. Antitrypanosomal activity of triterpenoids and sterols from the leaves of Strychnos spinosa and related compounds. J Nat Prod. 2007;70:1360–3. doi: 10.1021/np070038q. [DOI] [PubMed] [Google Scholar]
- 73.Wächter GA, Franzblau SG, Montenegro G, Hoffmann JJ, Maiese WM, Timmermann BN. Inhibition of Mycobacterium tuberculosis growth by saringosterol from Lessonia nigrescens. J Nat Prod. 2001;64:1463–4. doi: 10.1021/np010101q. [DOI] [PubMed] [Google Scholar]
- 74.Lee YS, Shin KH, Kim BK, Lee S. Anti-diabetic activities of fucosterol from Pelvetia siliquosa. Arch Pharm Res. 2004;27:1120–2. doi: 10.1007/BF02975115. [DOI] [PubMed] [Google Scholar]
- 75.Jung HA, Islam MN, Lee CM, Oh SH, Lee S, Jung JH, et al. Kinetics and molecular docking studies of an anti-diabetic complication inhibitor fucosterol from edible brown algae Eisenia bicyclis and Ecklonia stolonifera. Chem Biol Interact. 2013;206:55–62. doi: 10.1016/j.cbi.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 76.Gupta R, Sharma AK, Dobhal MP, Sharma MC, Gupta RS. Antidiabetic and antioxidant potential of ß-sitosterol in streptozotocin-induced experimental hyperglycemia. J Diabetes. 2011;3:29–37. doi: 10.1111/j.1753-0407.2010.00107.x. [DOI] [PubMed] [Google Scholar]
- 77.Rathinavelusamy P, Mazumder PM, Sasmal D, Jayaprakash V. Evaluation of in silico, in vitro a-amylase inhibition potential and antidiabetic activity of Pterospermum acerifolium bark. Pharm Biol. 2014;52:199–207. doi: 10.3109/13880209.2013.823551. [DOI] [PubMed] [Google Scholar]


