Abstract
Background:
Vitellaria paradoxa is a traditional medicinal plant of Cameroon. Several studies on this plant have focused on the cosmetic profile of its fruits. The present study focuses on the anti-inflammatory potency of stem barks extract of this plant.
Objective:
The objective was to evaluate the effect of methanolic extract of V. paradoxa (VPME) stem barks on inflammatory response in rats.
Materials and Methods:
Anti-inflammatory effects of VPME were evaluated in acute and chronic (28 days) inflammation induced in Wistar albino rats. The effects on hyperalgesia and locomotors activity were also quantified. The relative weight of lymphoid organs was obtained as well as some hematological parameters.
Results:
In the carrageenan-induced inflammation, VPME (75 mg/kg) exhibited a significant (66.67%) inhibition after 1 h. On the complete Freund's adjuvant-induced rheumatoid arthritis, VPME showed a significant protective effect with 8.12% inflammation against 25.00% for the control group after 2 days of the treatment. The extract (75 and 150 mg/kg) significantly reduced the score of arthritis with a maximum obtained on day 19th of the experimentation. There was a significant increase in the reaction time of rats on the hot plate as well as the exploratory activities of the animals in the open field. This extract significantly prevented weight, hemoglobin and red blood cells losses, and spleen hypertrophy. A protective action against skin destruction and cartilage erosion was evident. Liquid chromatography-mass spectrometry analysis of the extract revealed the presence of catechins.
Conclusions:
These findings suggested that V. paradoxa may contribute to the reduction of the inflammatory response.
Keywords: Arthritis, catechin, hematological parameters, hyperalgesia, inflammation, Vitellaria paradoxa
INTRODUCTION
Inflammation is a complex biological response of vascular tissues to harmful stimuli such as pathogens, damaged cells or irritants (physical or chemical). It is a defense mechanism aimed to remove the injurious stimuli and initiate the tissue healing process.[1] It involves the participation of various cell types expressing and reacting to diverse mediators along a very precise sequence of events.[2] However, prolonged inflammation can lead to numerous diseases including rheumatoid arthritis (RA), psoriasis, and inflammatory bowel disease. RA, a chronic inflammatory disease that afflicts 1–2% of the global population, is characterized by immune-mediated inflammatory sinusitis, which leads to hyperplasia, vasculogenesis, cartilage and bone destruction, joint malformation, functional impairment. All these symptoms markedly reduce the quality-of-life and can lead to depression. Arthritis, as a bilateral disease mainly in the ankles, causes continued swelling around the joint, pain, synovial hyperplasia, pannus formation, and morphological changes.[3,4] Inflammatory reaction occurs through the production of specific soluble pro-inflammatory mediators such as cytokines or chemokines, prostaglandins, leukotrienes, and the modulation of their production may be an effective therapy for the treatment of inflammation.
To fight against pain and inflammatory disorders, several drugs such as cyclooxygenase (COX) inhibitors are often used. However, their use is limited by some side effects such as dyspepsia, increase in high blood pressure, liver and kidney problems, heart failure, aggravation of infection, if no antibiotic is associated.[5] Many developing countries are facing the problem of the availability of essential drugs at low cost. In these countries, the most common generic drugs are available only at 42% in the public hospitals and 64% in the private medical sector. In the private pharmacies, generic drugs are 10 times more expensive than their international reference price and pharmaceutical specialties are generally more expensive.[6] To overcome these obstacles, people in rural areas simply and systematically use traditional herbal medicines.
Vitellaria paradoxa C. F. Gaertn, recorded in the pharmacopeia of Cameroon, is used in traditional medicine for the treatment of various ailments, including dysentery, stomach ailments, cutaneous infection, inflammation, diarrhea, dysentery, microbial infection, and fever.[7,8] A plant from the Sapotaceae family, V. paradoxa commonly known as shea is generally protected and venerated because of the economic value of the shea butter extracted from the fermented kernel.[9,10] Recent studies have shown that triterpene alcohols contained in shea butter in large quantities possesses anti-inflammatory activity by inhibiting pro-inflammatory cytokines, inducible nitric oxide synthase (iNOS), and COX-2 expression.[11] However, poor people in rural areas are not aware of theses scientific discoveries. They naturally continue to use alcoholic and aqueous extracts of the bark of this tree for the treatment of inflammatory diseases among others. The WHO strategy for health improvement in developing country includes the development of research on the safety and efficiency of traditional medicines.[12] But, so far as we know, there are no scientific data to validate this use of V. paradoxa. Therefore, this study was undertaken to examine whether stem bark of Me-OH extract of V. paradoxa (VPME) possesses anti-inflammatory and anti-rheumatismal activities in rats using the oral administration of methanolic extracts.
MATERIALS AND METHODS
Plant material
Vitellaria paradoxa stem bark were collected at Garoua (North Region, Cameroon) in July 2012, and identified by Mr. Bamba Jean Paul, a plant taxonomist of the National School of Fauna, Garoua, Voucher specimen was deposited under the number HEFGN 6276 for further verification.
Extraction procedure
The plant material was cut in small pieces, air-dried in the shade for 3 weeks. The dried barks of V. paradoxa were reduced to a very fine powder and 5000 g soxhlet-extracted, with 8 L of methanol for 24 h. The resulting VPME solution was concentrated in vacuum using a rotavapor to obtain a brown powder (500 g).
Preliminary phytochemical screening and high-resolution mass spectra
The preliminary phytochemical screening of the VPME stem bark was carried out according to the method described by Harborne.[13]
High-resolution mass spectra were recorded using a linear trap quadrupole orbitrap spectrometer equipped with an atmospheric pressure chemical ionization ion source (Ion Max) operating in positive mode. The spectrometer was equipped with a surveyor HPLC system consisting of LC-pump, photodiode array detector, and auto-sampler (injection volume 10 μL). Nitrogen was employed both as the sheath (40 arbitrary units) and auxiliary (10 arbitrary units) gas. The capillary temperature was set to 190°C. The vaporizer temperature was set to 400°C. The separations were performed using a Phenomenex Synergi Fusion RP column (4 μm, 3 mm × 150 mm) with water (+0.1% HCOOH/+10 mM NH4AC) (A) acetonitrile (+0.1% HCOOH) (B) gradient (flow rate 500 μL/min). Samples were analyzed using a gradient program as follows: 80% A isocratic for 4 min, linear gradient to 0% A over 20 min, after 100% B isocratic for 10 min, the system returned to its initial condition (80% A) within 1-min, and was equilibrated for 5 min.
Animals
Male Wistar rats weighing 120–180 g at the start of the experiment were used. The animals were housed in a temperature and light-controlled room (25°C, natural 12-h cycle starting at 6:00 h) and were fed and allowed to drink water ad libitum. Rats were treated in accordance with the guidelines of the Cameroonian Bioethics Committee (Reg. No. FWA-IRB00001954) and in accordance with NIH-Care and Use of Laboratory Animals Manual (8th Edition).
Chemicals
Diclofenac was used as the reference drug (positive control, a product of Merck, Darmstadt, Germany). Complete Freund's adjuvant (CFA) was purchased from Sigma Chemical Co., (St. Louis, MO, USA). All other chemicals and reagents, unless specified otherwise, were from VWR BDH Prolabo, (Fontenay-sous-Bois, France).
Pharmacological studies
Carrageenan-induced acute inflammation
The method of carrageenan-induced paw edema in rats described by Winter et al.[14] was used to evaluate the anti-inflammatory activity. The treatment was done 30 min before the injection of 100 μl of carrageenan (100 μg) (kappa carrageenan type III) into the rat hind paw plantar surface. Paw volume was measured plethysmometrically 30 min after and at 1 h intervals after carrageenan injection for 6 h. The treatments were undertaken orally for rat at the doses of 75, 150, and 300 mg/kg of VPME. These 3 doses of extract were selected after a dose-response screening. The negative control group was treated with 5% dimethyl sulfoxide (DMSO)-saline solution (10 ml/kg, p.o.) while the positive control group received diclofenac (10 mg/kg, p.o.).
Completed Freund's adjuvant-induced arthritis
Thirty-six animals were divided into six groups of six animals each as follows:
-
Nonarthritic animals
- Group I: Normal animals: Received aqueous solution of 5% DMSO (10 ml/kg, p.o.)
-
Arthritic animals
- Group II: Control animals: Received aqueous solution of 5% DMSO (10 ml/kg, p.o.)
- Group III: Drug treated animals: Received VPME (75 mg/kg, p.o.)
- Group IV: Drug treated animals: Received VPME (150 mg/kg, p.o.)
- Group V: Drug treated animals: Received VPME (300 mg/kg, p.o.)
- Group VI: Drug treated animals: Received diclofenac (4 mg/kg, p.o.).
Arthritis was induced by the injection of 100 μl of CFA, (containing 1 mg/mL of heat-killed Mycobacterium tuberculosis in paraffin oil and mannide monooleate) into the sub plantar region of right hind paw of rat. The day of CFA injection was considered as day 1. The oral administration of the VPME, diclofenac of vehicle of all the groups started from day 14, once daily until day 28. Anti-arthritic activity of VPME was evaluated on joint diameter, paw volume, pain withdrawal latency, and arthritic score on day 0, 4, 7, 10, 12, 14, 19, 21, 24, and day 28. The last day, all the animals were sacrificed under ether anesthesia and the blood obtained in the heparin tubes for hematological parameters. The joints were also cut for histology.
Behavioral assessment
Arthritic score and joint diameter
The degree of arthritis was observed daily and scored visually by a single observer on a scale of 0–4 per paw for a maximal score of 8 per rat. The score assignment criteria were: Normal paw = 0, mild swelling and erythema of digits = 1, swelling and erythema of the digits = 2, severe swelling and erythema = 3, gross deformity and inability to use the limb = 4 on respective days. The joint diameters of right hind paw were measured using an electronic Vernier caliper (Fischer Scientific, CON3417) on the above-mentioned testing days after induction of arthritis.[15]
Locomotory activity test (open field test)
The open field arena was made of white poly wood (72 cm × 72 cm × 36 cm) with red lines drawn on the floor and clearly visible through the clear plexiglass floor. Each animal was placed into the center of the open field and allowed to explore the apparatus for 5 min during with ambulation (the number of squares crossed with all four paws) and rearing (frequency with which the mice stood on their hind legs in the maze) were recorded.[16] After the 5 min test, rats were returned to their home cages, and the open field cleaned with 70% ethanol and permitted to dry between tests.
Anti-hyperalgesic activity
The apparatus consists of a hot plate apparatus maintained at 55°C ± 0.5°C on which the rats were placed for testing (Eddy's hot plate method). Pain threshold was determined by the latency for nociceptive response (reaction time for the animal to lick the paw or jump from the hot plate) with a maximum cut-off time of 15 s for every animal.[17]
Determination of hematological parameters
At the end of the experiment, blood was collected with anticoagulant (heparin) tubes. White blood cell (WBC) number, the plasma level of hemoglobin (Hb) and hematocrit were measured by the Sysmex KX-2IN automated hematology analyzer with specific software for rat blood samples.
Histological study
For histological analyses, the paws of two animals per group were collected, and the skin was removed. Tissues were fixed in 10% neutral-buffered formalin, decalcified in 5% formic acid, and embedded in paraffin. Sections (5 μm) were stained with hematoxylin-eosin and masson trichrome.
Statistical analysis
The results are presented as the mean ± standard error of the mean of 5–6 animals, except for the hematological parameters, which are presented as the means of a blood sample of three animals per group. The percentages of inflammation are reported as the mean obtained for each individual experiment. Statistical comparison of the data was performed by one-way analysis of variance followed by Newman–Keuls multiple comparison test, using GraphPad Prism version 5.00 for Windows, GraphPad Software, San Diego California USA, www.graphpad.com. P < 0.05 (P < 0.05 or less) were considered significant.
RESULTS
Preliminary phytochemical screening
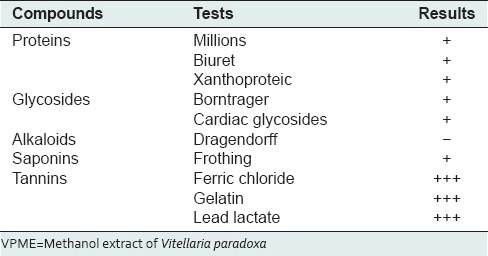
The phytochemical and liquid chromatography-mass spectrometry analysis of the VPME revealed that this plant extract was very rich in flavonoids of the type catechins [Figure 1], tannins, saponins, glycosides, and proteins but not alkaloids [Table 1].
Figure 1.
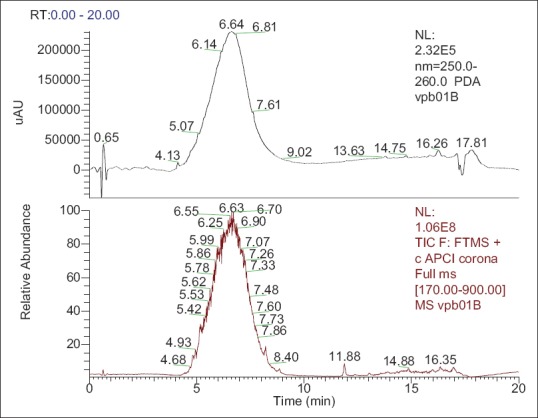
Liquid chromatography (LC)-ultraviolet, LC-mass spectrometry of the methanol extract of Vitellaria paradoxa stern bark showing abundance of flavonoids of the type catechin
Table 1.
List of compounds present in VPME
Effects of methanol extract of Vitellaria paradoxa on carrageenan-induced edema
The subcutaneous injection of the carrageenan on the hind paw of animal induced a topical and rapid inflammation of the hind paw with a maximum volume observed after 3 h. The pretreatment of animals with VPME (75, 150, and 300 mg/kg) and diclofenac (10 mg/kg) significantly inhibited the carrageenan-induced inflammation. With the dose of 75 mg/kg, a significant inhibition effect was observed 60 min after the plant extract administration (P ˂ 0.05). This dose also inhibited the second phase of inflammation with a maximum effect at the 5th h [Figure 2].
Figure 2.
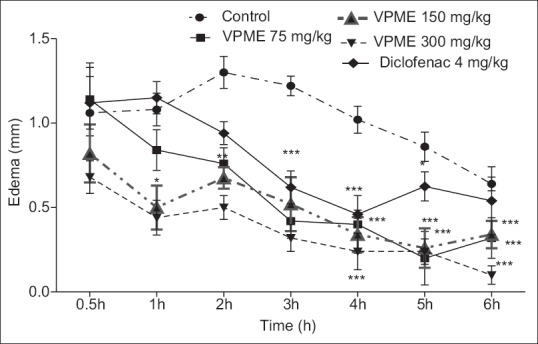
Effect of methanol extract of Vitellaria paradoxa oral administration on carrageenan-induced paw edema. Data are expressed as mean ± standard error of the mean from 5 rats and analyzed by one-way analysis of variance followed by Newman–Keuls posttest. *P < 0.05, **P < 0.01, ***P < 0.001 as compared to control group animals
Completed Freund's adjuvant-induced arthritis
Effect of the methanol extract of Vitellaria paradoxa on join diameter
Two days after, the beginning of treatment, VPME (75 and 150 mg/kg) treated groups exhibited remarkable and significant (P 0˂ 0.05) decline in the joint diameter when compared to that of control arthritic group while diclofenac-treated animals showed a significant (P ˂ 0.05) result some days later (24 days) [Figure 3].
Figure 3.
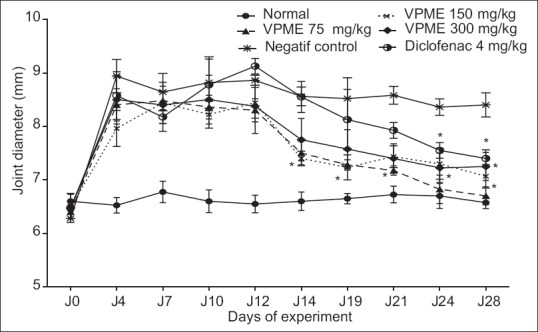
Effect of methanol extract of Vitellaria paradoxa oral administration on joint diameter. Data represent mean of five animals. *P < 0.05, **P < 0.01 as compared to control group animals
Effects of the methanol extract of Vitellaria paradoxa on arthritic score
Subplantar administration of CFA results in significant increased (P < 0.001) in arthritic score in all CFA treated rats as compared to normal rats. There was a decrease in the arthritic score from day 4 to 10, however, this change was not significant. The arthritic score of the control group remained high till the end of the experiment, that is, up to the 28th day as compared to normal rats. Rats treated with VPME (150 mg/kg) showed significant decreased in arthritic score from day 14 to day 21 (P < 0.05) and from day 24 onward till the end of the study (P < 0.01) as compared to control rats [Figure 4].
Figure 4.
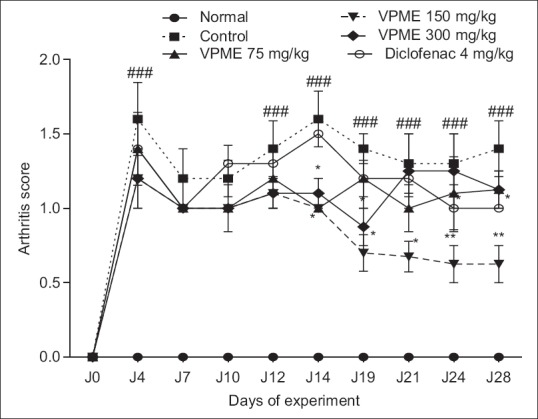
Effect of oral administration of methanol extract of Vitellaria paradoxa on the arthritic score. Data represent mean ± standard error of the mean of five animals. *P < 0.05, **P < 0.01 as compared to control group animals and ###P < 0.001 as compared to normal animals
Effects of the methanol extract of Vitellaria paradoxa on nociceptive threshold
Seven days after the CFA injection, inflammatory swelling was observed in all animals with a reduction of latency response in the hot plate test. The latency time of all animals decreased significantly until the 14th day. From day 14 to day 28, there was a stabilization of latency response in control animals and a nonsignificant increase of the latency time in the normal group. VPME at different doses (75, 150, and 300 mg/kg) caused a significant increase in the latency time compared to the control group (P < 0.001, on days 21, 24, and 28) while the diclofenac treatment (4 mg/kg) resulted in a nonsignificant increase in latency time [Figure 5].
Figure 5.
Effect of methanol extract of Vitellaria paradoxa oral administration on rearing behavior. Data represent mean ± standard error of the mean of five animals. *P < 0.05, **P < 0.01 as compared to control group animals
Effects of methanol extract of Vitellaria paradoxa on the locomotory activity and on rearing behavior
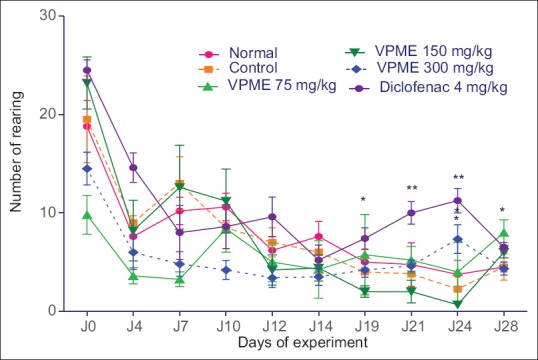
In the open field test (OFT), the number of lines crossed and the number of rearing behavior were improved by VPME treatment. From the 19th day, there was an increase in the rearing behavior of diclofenac-treated animals till the end of the experiment. In animals treated with VPME, there was a significant increase in this behavior at the dose of 300 mg/kg on day 21 and 75 mg/kg on day 28 [Figure 6]. Subsequent Neuman–Keuls multiple comparison test demonstrated that the VPME induced a significant increase (P < 0.05) in locomotory activity at the dose of 75 mg/kg. The effect of diclofenac was more prominent in this parameter [Figure 7].
Figure 6.
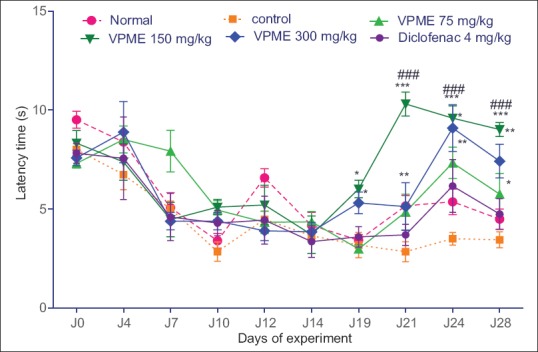
Effect of methanol extract of Vitellaria paradoxa oral administration on the nociceptive threshold at the hot plate test. Data represent mean ± standard error of the mean of five animals. *P < 0.05, **P < 0.01, ***P < 0.001 as compared to control group animals
Figure 7.
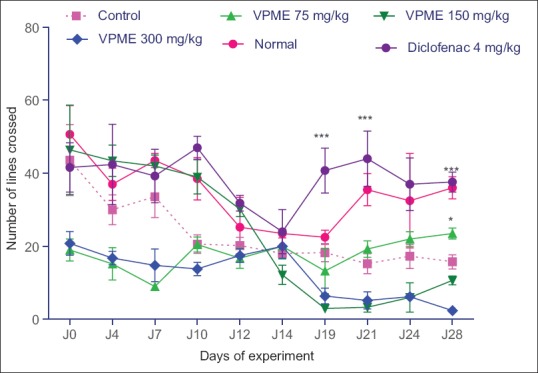
Effect of methanol extract of Vitellaria paradoxa oral administration on locomotor activity. Data represent mean ± standard error of the mean of five animals. *P < 0.05, **P < 0.01, ***P < 0.001 as compared to control group animals
Effect of methanol extract of Vitellaria paradoxa on body weight
The body weight in the normal group gradually and significantly increased during the experiment (P < 0.05), while in the control group, a significant decrease (P < 0.05) in animals weight was observed from day 10 to day 28. With the treatment of the animals by VPME, the body weight of animal slowly increased but this was significant only at the dose of 75 mg/kg. Changes in body weight were not significant in diclofenac-treated group (results not shown).
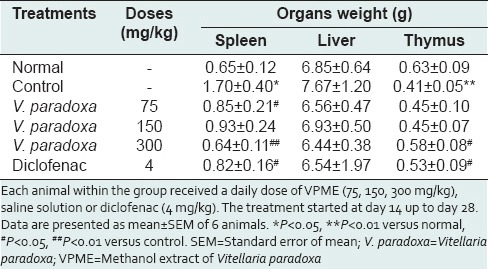
Effect of methanol extract of Vitellaria paradoxa on various organ weights
There was a significant increase (P < 0.05) in spleen weight whereas it was observed a significant decrease (P < 0.05) in thymus weight of control rats as compared to normal rats. Rise in spleen weight was significantly (P < 0.05) inhibited in animals treated with VPME (75–300 mg/kg) as compared to control rats. However, when compared to control rats, treatment with VPME (300 mg/kg) significantly (P < 0.05) attenuated thymus atrophy induced by CFA. The increase in liver weight by CFA was not significant, and VPME treatment (75–300 mg/kg) seems to maintain the weight of this organ around its normal value [Table 2].
Table 2.
Effect of oral administration of VPME on, spleen weight, liver weight, and thymus weight
Effect of methanol extract of Vitellaria paradoxa oral administration on white blood cell, hemoglobin, and hematocrit
At the end of the study, there was significant increase (P < 0.05) in the total WBC, and significant decrease (P < 0.05) along the Hb and hematocrit level of control animals as compared to normal rats. However, the treatment with VPME (150 and 300 mg/kg) showed a significant increase in the Hb (P < 0.001) and hematocrit (P < 0.05) when compared to control group. At the same time, the VPME (150 and 300 mg/kg) significantly attenuated the increase of WBC level and at the end, their values were close to that of normal animals [Figure 8]. The effect of diclofenac on the WBC level was not significant.
Figure 8.
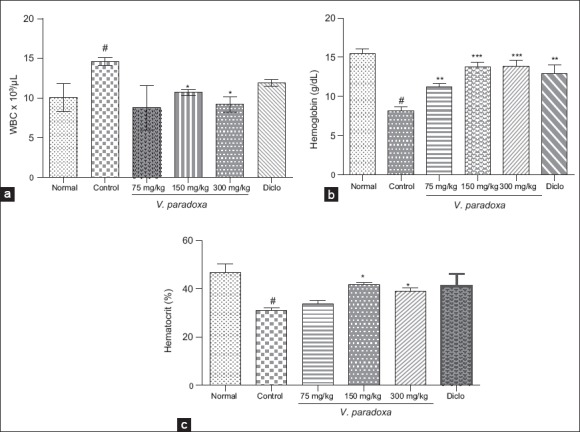
Effect of methanol extract of Vitellaria paradoxa oral administration on white blood cell, hemoglobin, and hematocrit. Data are presented as mean ± ESM (n = 4). *P < 0.05; **P < 0.01; ***P < 0.001 versus control; #P < 0.05; versus normal
Effect of methanol extract of Vitellaria paradoxa oral administration on paw tissues evaluated by light microscopy
The histological assessment of tissue sections from rat foot skin and ankles was performed on day 28 of treatment. The histopathology of paw tissue [Figure 9] revealed that adjuvant-induced arthritic rats showed severe edema formation, cells infiltration, vascular dilatation and a disorganization of fibroblasts and collagen fibers. Diclofenac-treated group shows moderate edema formation and cellular infiltration and the VPME-treated groups showed a relative good organization of the skin structure without edema formation and vasodilatation. The joint histological architecture of rats nontreated with CFA was normal [Figure 10a]. This joint architecture was markedly abnormal in the RA model group rats [Figure 10b], which synovial hyperplasia, extensive erosive with an increase of the joint space, chondrocytes destruction, and changes in the cartilage. However, the treatment with the VPME has a protective effect on the joint structure and tends to maintain the normal histological architecture with a chondrocytes multiplication and cartilage protection [Figure 10c-e].
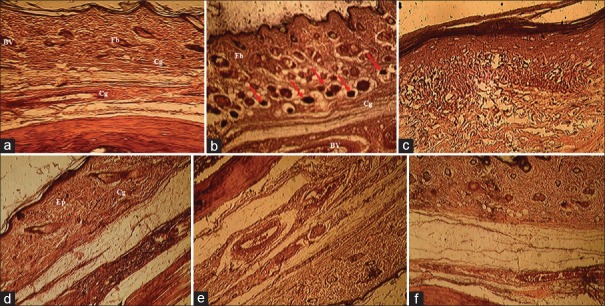
Figure 9.
Histopathological study of paw tissue (H and E, ×400). Group I - normal rats (a). Group II - arthritis, which shows severe edema formation and severe cells infiltration and vascular dilatation (b), Group III - arthritis + methanol extract of Vitellaria paradoxa (VPME) 75 mg/kg, shows skin disorganization (fibroblasts and collagen fiber) and cells infiltrations (c), Group IV - arthritis + VPME 150 mg/kg, shows mild edema formation fibroblasts are well-arranged (d), Group V - arthritis + VPME 300 mg/kg, shows mild edema formation and cells organization without vasodilatation (e) and Group VI - arthritis + diclofenac-treated group (f)
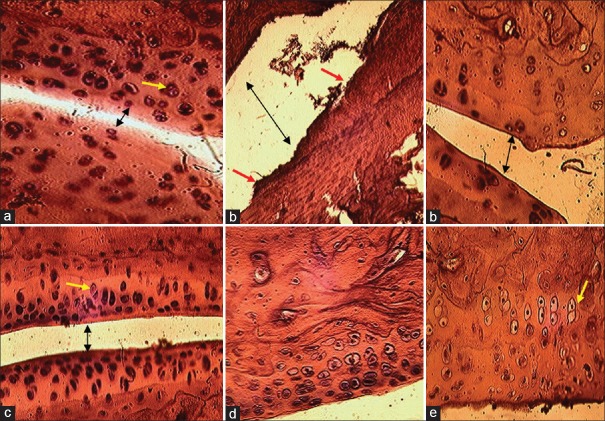
Figure 10.
Histological structures of joint and cartilage (H and E, ×400) from treated and nontreated rats. The normal control rat (a); the arthritic rat (b); methanol extract of Vitellaria paradoxa treated rat 150 mg/kg (c), 300 mg/kg (d) and diclofenac-treated rat (e). The black arrow (in a-c) showed the joint space variation, the yellow arrow showed the chondrocytes in the cartilage tissue and red arrow showed cartilage erosion
DISCUSSION
In the present study, the anti-inflammatory effects of the VPME on acute inflammatory processes and RA have been established. Inflammation is a very complex series of interactions between numerous soluble factors and cells that can arise in any tissue in response to traumatic, infectious, postischemic, toxic or autoimmune injury. When acute inflammation is no more in control, it may cause serious sepsis, which is the leading cause of death.[18] Moreover, the acute inflammation may lead to persistent damage and switch to chronic inflammation, which is often associated with many pathophysiological processes and diseases, such as cancer, atherosclerosis, osteoarthritis and Alzheimer's disease,[19] RA, chronic asthma, multiples sclerosis, inflammatory bowel disease, and psoriasis.[20]
All the above-cited diseases are common worldwide. Inflammation and inflammation-mediated illnesses are the biggest challenges in current conventional medicine and understanding or resolving inflammation is therefore currently one of the main targets in medical science.
Carrageenan-induced rat paw edema assay is accepted as useful phlogistic agent for the investigation of new anti-inflammatory drugs.[21] The acute inflammatory responses induced after carrageenan injection involves three different phases through the sequential release of several mediators. The initial phase (1.5 h) involves the release of serotonin and histamine while the second phase (1.5–2.5 h) is mediated by bradykinin. The third phase is known to be mediated by prostaglandins and occurs between 2.5 and 6 h and is the most important process in the inflammatory response.[22,23] The maximal vascular response, closely associated with leukocyte migration to the inflamed area, also reaches its maximum level in the final phase. It is well-known that prostaglandins, modulators of inflammatory responses, play an important role in the inflammatory mechanism[24] with COX acting as the key enzyme that synthesizes prostaglandins and thromboxane from arachidonic acid.
Inappropriate COX-2 expression is known to be involved in the development of inflammatory pathogenesis seen in diseases of gastrointestinal tract, central nervous systems, ischemia and lung inflammation and fibrosis.[25,26] Our results clearly indicated that in the rats treated by VPME, there was a significant suppression of paw edema 0.5–5 h after carrageenan injection. Suggesting that VPME exerted an anti-inflammatory action by inhibiting the releases of mediators or enzyme involved in this process.
Any exacerbation of inflammatory stimuli can lead to chronic inflammation such as RA. RA is a chronic and debilitating pathology that affects between 0.3% and 1% people in the world. It is estimated that 9.6% of men and 18% of women aged over 60 years suffer from this disease. It limits the movement of 80% affected persons while 25% are unable to perform the daily living tasks.[27,28] This type of inflammation also involves the release of number of mediators. These mediators are responsible for the pain, destruction of bone and cartilage that can lead to severe disability.[29]
The CFA is an antigenic solution containing attenuated M. tuberculosis. It mobilizes the immune system by stimulating cellular mediators and potentiating the production of certain immunoglobulins.[30] RA induced by CFA results in a localized inflammation of the joints associated with hypertrophy of the synovial membrane and the progressive and irreversible destruction of the cartilage.[31,32] CFA chronic inflammation is a biphasic process.[33]
The first and acute phase (0–10 days) is caused by various mediators such as histamine, serotonin, kinins, and prostaglandins, released by leukocytes that migrate to the affected region and provoked a vasculo-exsudatifs phenomena responsible for edema.[33,34] The second and chronic phase (10–28 days) is due to cellular inflammatory mediators such as cytokines (interleukin-1β [IL-1β], IL-6, tumor necrosis factor [TNF-α]), granulocyte-macrophage colony-stimulating factor), interferon-g and prostaglandins. These substances are responsible to the synovial hyperplasia; cartilage destruction, nociceptive stimulation and pro-inflammatory mediator's production.[33,35] In this study, animals were treated from day 12 to day 28, corresponding to the second phase of the arthritis and VPME-treated animals showed significant inhibition of joint diameter inflammation as well as arthritic scores compared to control rats. On these two main parameters, the efficiency of VPME at 150 mg/kg was more than that of diclofenac (4 mg/kg), the positive control drug used in our study. Inflammatory pain is a complex process with multiple modulators including neurotransmitters, receptors, and ion channels. In addition, diverse pain behaviors including spino-bulbospinal jumping, abdominal stretching reflexes, vocalization, scratching, biting, and licking. Nociceptive behavioral responses to a stimulus can be evaluated through a variety of pain models such as formalin, carrageenan, CFA, and many others models.[36] The CFA pain model is characterized by an inflammation-induced hyperalgesia and allodynia as the consequence of alterations in transduction sensitivity of high threshold nociceptors.[37] The conversion of prostaglandins into arachidonic acid mainly contributed to this amplification. No pain modulator or neurotransmitter was measured in this study, but it is well-known that in the CFA-inflammatory model, the injection of CFA increased the COX-2 expression.
In the present study, we reported that VPME at all the doses used significantly increased the reaction time of animal on the hot plate test. With the dose of 150 mg/kg, the increase was 5 times compared to the control group. Moreover the reaction time at this dose was better than that of the normal rats, suggesting a powerful anti-inflammatory and/or central analgesic activity of this extract. The inhibition of the production or release of the pro-inflammatory cytokines TNF-α, IL-1, and IL-6 has been associated with anti-nociceptive effects.[38] Our results showed that treatment with VPME significantly increases the pain threshold. It may thus be suggested that this effect may be through the inhibition of the pro-inflammatory cytokines IL-1, IL-6, and TNF-α following CFA-induced inflammation in rats. Supplementary analgesic analysis is, however, necessary to confirm this. The suppression of pain sensation by the VPME was directly reflected in the locomotory ability of the treated animals. The VPME (150 and 300 mg/kg) as well as diclofenac significantly ameliorated the locomotory ability (number of line crossed) in the OFT while the number of rearing was also improved. In this behavioral paradigm, the rearing is a vertical movement considered as an index of locomotory activity.[39,40]
The spleen is an important lymphoid organ involved in immune responses against all types of antigens that appears in the circulation and it provides a readily available source of cells known to be involved in adjuvant arthritis. The increase in spleen weight of the CFA induced arthritic rats has been reported to be associated with splenomegaly, generalized lymphadenopathy, and altered hepatic function.[15] Sub plantar administration of CFA significantly increased weight of the spleen and decreased the weight of thymus, and this is in accordance with the findings of the previous studies.[41,42] The significant decrease in spleen weight and the increase in thymus weight might thus be attributed to the treatment of rats with VPME. 12 days after the CFA-injection, the Hb value significantly decreased while the WBC level increased. We also noticed a significant decrease in the hematocrit which is an anemia index due to the destruction of premature red blood cell, or as a sign of iron deficiency, inflammation, and intestinal malabsorption.[43] The decrease in the hematocrit value is thus common in chronic inflammatory condition. 14 days later, in the VPME-treated group, all these hematologic parameters returned to their normal value, especially for the animals treated with the dose of 150 mg/kg, indicating the VPME improvement on these hematological parameters. These results strongly suggest that the inhibitory action of VPME on inflammatory mediators may have an effect on the lymphoid organ weights stabilization as well as on the inhibition of the modifications of blood parameters on VPME treated animals.
The hallmarks of adjuvant arthritis model are reliable onset and progression of polyarticular inflammation, marked bone resorption, periosteal bone proliferation, and cartilage destruction. In this study, the histopathological examination of skin and knee joints showed that the VPME effectively protect the skin and cartilage damage against the cocktail of pro-inflammatory mediators release after CFA injection. This gives supplementary argument on the anti-arthritic and anti-inflammatory propensity of this extract on the acute and chronic animal models of inflammation used in this study.
The major bioactive compound in our extract was flavonol of the type catechins. These findings are in accordance with the pharmacological profile of the extract since flavonoids were reported to inhibit the COX and lipoxygenase pathways of arachidonate metabolism[44] while catechins have many biological functions, including anti-inflammatory, anti-oxidative, and anti-carcinogenic effects. These effects are induced by the suppression of inflammatory factors including nuclear factor-kappa B, a multipotential promoter of iNOS, adhesion molecules (intercellular adhesion molecule-1 and vascular cell adhesion molecule-1) and cytokines (TNF-α, IL-6 and IL-8), but also by enhancing the production of IL-4 and IL-10.[45,46]
CONCLUSIONS
VPME has significant anti-inflammatory and anti-arthritic effects in carrageenan-induced inflammation and CFA-induced arthritic animal model, respectively. Furthermore, we have shown that VPME inhibited both the hyperalgesia due to CFA-injection and some hematological disorders associated with the arthritic condition. These results suggest that VPME may be a potent candidate for anti-inflammatory and anti-rheumatic drug. Further experimentation is needed in order to understand the precise mechanism of action of this extract.
Footnotes
Source of Support: Nil
Conflicts of Interest: None declared.
REFERENCES
- 1.Maldini M, Sosa S, Montoro P, Giangaspero A, Balick MJ, Pizza C, et al. Screening of the topical anti-inflammatory activity of the bark of Acacia cornigera Willdenow, Byrsonima crassifolia Kunth, Sweetia panamensis Yakovlev and the leaves of Sphagneticola trilobata Hitchcock. J Ethnopharmacol. 2009;122:430–3. doi: 10.1016/j.jep.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Babu NP, Pandikumar P, Ignacimuthu S. Anti-inflammatory activity of Albizia lebbeck Benth. an ethnomedicinal plant, in acute and chronic animal models of inflammation. J Ethnopharmacol. 2009;125:356–60. doi: 10.1016/j.jep.2009.02.041. [DOI] [PubMed] [Google Scholar]
- 3.Butler SH, Godefroy F, Besson JM, Weil-Fugazza J. A limited arthritic model for chronic pain studies in the rat. Pain. 1992;48:73–81. doi: 10.1016/0304-3959(92)90133-V. [DOI] [PubMed] [Google Scholar]
- 4.Wang Q, Kuang H, Su Y, Sun Y, Feng J, Guo R, et al. Naturally derived anti-inflammatory compounds from Chinese medicinal plants. J Ethnopharmacol. 2013;146:9–39. doi: 10.1016/j.jep.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 5.Guo D, Xu L, Cao X, Guo Y, Ye Y, Chan CO, et al. Anti-inflammatory activities and mechanisms of action of the petroleum ether fraction of Rosa multiflora Thunb. hips. J Ethnopharmacol. 2011;138:717–22. doi: 10.1016/j.jep.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 6.Genève: WHO; 2011. WHO. World Health Statistics; pp. 18–20. [Google Scholar]
- 7.Ziba L, Yameogo F. The benefits of shea for rural populations, communities and countries. Proceedings of the workshop organized by the Food and Agriculture Organization of the United Nations, the Common Fund for Commodity and Ecological Monitoring Centre. 2002:80. [Google Scholar]
- 8.Ayankunle AA, Kolawole OT, Adesokan AA, Akübinu MO. Antibacterial activity and sub-chronic toxicity studies of Vitellaria paradoxa stem back extract. J Pharmacol Toxicol. 2012;7:298–304. [Google Scholar]
- 9.Matig OE, Ndoye O, Kengue J, Awono A. Cotonou, Benin: IPGRI Regional Office for West and Central Africa; 2006. The forest edible fruits of Cameroon; pp. 150–22. [Google Scholar]
- 10.Sanou H, Lamien N. Vitellaria paradoxa Shea: Conservation and sustainable use of genetic resources of food tree species in sub-Saharan Africa. Saforgen. 2011:2–4. [Google Scholar]
- 11.Verma N, Chakrabarti R, Das RH, Gautam HK. Anti-inflammatory effects of shea butter through inhibition of iNOS, COX-2, and cytokines via the Nf-?B pathway in LPS-activated J774 macrophage cells. J Complement Integr Med. 2012;9:1–11. doi: 10.1515/1553-3840.1574. [DOI] [PubMed] [Google Scholar]
- 12.WHO. World Health Organization strategy of Traditional Medicine for 2002-2005. WHO/EDM/TRM/2002.1; 2002-2005. :65. [Google Scholar]
- 13.Harborne JB. London: Chapman and Hall, Ltd; 1973. Phytochemical Methods; pp. 49–188. [Google Scholar]
- 14.Winter CA, Risley EA, Nuss GW. Carrageenin-induced edema in hind paw of the rat as an assay for antiinflammatory drugs. Proc Soc Exp Biol Med. 1962;111:544–7. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- 15.Patil MV, Kandhare AD, Bhise SD. Anti-arthritic and anti-inflammatory activity of Xanthium strumarium L. ethanolic extract in Freund's complete adjuvant induced arthritis. Biomed Aging Pathol. 2012;2:6–15. [Google Scholar]
- 16.Carrey N, McFadyen MP, Brown RE. Effects of subchronic methylphenidate hydrochloride administration on the locomotor and exploratory behavior of prepubertal mice. J Child Adolesc Psychopharmacol. 2000;10:277–86. doi: 10.1089/cap.2000.10.277. [DOI] [PubMed] [Google Scholar]
- 17.Anderson GD, Hauser SD, McGarity KL, Bremer ME, Isakson PC, Gregory SA. Selective inhibition of cyclooxygenase (COX)-2 reverses inflammation and expression of COX-2 and interleukin 6 in rat adjuvant arthritis. J Clin Invest. 1996;97:2672–9. doi: 10.1172/JCI118717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–10. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Vasto S, Candore G, Balistreri CR, Caruso M, Colonna-Romano G, Grimaldi MP, et al. Inflammatory networks in ageing, age-related diseases and longevity. Mech Ageing Dev. 2007;128:83–91. doi: 10.1016/j.mad.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 20.Gautam R, Jachak SM. Recent developments in anti-inflammatory natural products. Med Res Rev. 2009;29:767–820. doi: 10.1002/med.20156. [DOI] [PubMed] [Google Scholar]
- 21.Sindhu G, Ratheesh M, Shyni GL, Nambisan B, Helen A. Anti-inflammatory and antioxidative effects of mucilage of Trigonella foenum graecum (Fenugreek) on adjuvant induced arthritic rats. Int Immunopharmacol. 2012;12:205–11. doi: 10.1016/j.intimp.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 22.Sharma US, Sharma UK, Sutar N, Sing A, Shukla DK. Anti-inflammatory activity of Cordia dichotoma forstf. Seeds extract. Int J Pharm Anal. 2010;2:1–4. [Google Scholar]
- 23.Saha S, Subrahmanyam EV, Chandrashekar KS, Chastry CS. In vivo study for anti-inflammatory activity of Bauhinia variegate L. leaves. Pharm Crops. 2011;2:70–3. [Google Scholar]
- 24.Zhang W, Dai SM. Mechanisms involved in the therapeutic effects of Paeonia lactiflora Pallas in rheumatoid arthritis. Int Immunopharmacol. 2012;14:27–31. doi: 10.1016/j.intimp.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Keskek M, Gocmen E, Kilic M, Gencturk S, Can B, Cengiz M, et al. Increased expression of cyclooxygenase-2 (COX-2) in radiation-induced small bowel injury in rats. J Surg Res. 2006;135:76–84. doi: 10.1016/j.jss.2006.03.031. [DOI] [PubMed] [Google Scholar]
- 26.Ansari N, Khodagholi F, Ramin M, Amini M, Irannejad H, Dargahi L, et al. Inhibition of LPS-induced apoptosis in differentiated-PC12 cells by new triazine derivatives through NF-kB-mediated suppression of COX-2. Neurochem Int. 2010;57:958–68. doi: 10.1016/j.neuint.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 27.London: Royal College of Physicians; 2009. National Collaborating Centre for Chronic Conditions. Rheumatoid arthritis: National clinical guideline for management and treatment in adults. [PubMed] [Google Scholar]
- 28.Gibofsky A. Overview of epidemiology, pathophysiology, and diagnosis of rheumatoid arthritis. Am J Manag Care. 2012;18(13 Suppl):S295–302. [PubMed] [Google Scholar]
- 29.Griswold DE, Adams JL. Constitutive cyclooxygenase (COX-1) and inducible cyclooxygenase (COX-2): Rationale for selective inhibition and progress to date. Med Res Rev. 1996;16:181–206. doi: 10.1002/(SICI)1098-1128(199603)16:2<181::AID-MED3>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 30.Wang L, Zhao D, Di L, Xu T, Lin X, Yang B, et al. The analgesic and anti-rheumatic effects of Thladiantha dubia fruit crude polysaccharide fraction in mice and rats. J Ethnopharmacol. 2011;137:1381–7. doi: 10.1016/j.jep.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 31.Roberts L, McColl GJ. Tumour necrosis factor inhibitors: Risks and benefits in patients with rheumatoid arthritis. Intern Med J. 2004;34:687–93. doi: 10.1111/j.1445-5994.2004.00710.x. [DOI] [PubMed] [Google Scholar]
- 32.Klinkhoff A. Biological agents for rheumatoid arthritis: Targeting both physical function and structural damage. Drugs. 2004;64:1267–83. doi: 10.2165/00003495-200464120-00001. [DOI] [PubMed] [Google Scholar]
- 33.Tripathy S, Sahoo SP, Pradham D, Sahoo S, Satapathy DK. Evaluation of anti-arthritic potential of Hybathus enneaspermus. Afr J Pharm Pharmacol. 2010;3:96–100. [Google Scholar]
- 34.Zaringhalam J, Akbari A, Tekieh E, Manaheji H, Rezazadeh S. Achillea santolina reduces serum interleukin-6 level and hyperalgesia during complete Freund's adjuvant-induced inflammation in male Wistar rats. Zhong Xi Yi Jie He Xue Bao. 2010;8:1180–9. doi: 10.3736/jcim20101211. [DOI] [PubMed] [Google Scholar]
- 35.Dhanalakshmi M, Jayasree T, Shaik S. Antiarthritic activity of leaves of Michelia champaca 1 by complete freund's adjuvant. Int J Pharmacol Toxicol. 2012;4:4607–15. [Google Scholar]
- 36.Mogil JS. Nat Rev Neurosci. 2009. Animal models of pain: Progress and challenges; pp. 283–94. [DOI] [PubMed] [Google Scholar]
- 37.Fang JF, Liang Y, Du JY, Fang JQ. Transcutaneous electrical nerve stimulation attenuates CFA-induced hyperalgesia and inhibits spinal ERK1/2-COX-2 pathway activation in rats. BMC Complement Altern Med. 2013;13:134. doi: 10.1186/1472-6882-13-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.da Silva KA, Paszcuk AF, Passos GF, Silva ES, Bento AF, Meotti FC, et al. Activation of cannabinoid receptors by the pentacyclic triterpene α,β-amyrin inhibits inflammatory and neuropathic persistent pain in mice. Pain. 2011;152:1872–87. doi: 10.1016/j.pain.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 39.Vogel HG. 2nd ed. New York: Springer; 2002. Drug discovery and evaluation, pharmacological Assay; p. 670. [Google Scholar]
- 40.Foyet HS, Tsala DE, Bouba AA, Hritcu L. Anxiolytic and antidepressant-like effects of the aqueous extract of Alafia multiflora stem barks in rodents. Adv Pharmacol Sci 2012. 2012:912041. doi: 10.1155/2012/912041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Geboes L, De Klerck B, Van Balen M, Kelchtermans H, Mitera T, Boon L, et al. Freund's complete adjuvant induces arthritis in mice lacking a functional interferon-gamma receptor by triggering tumor necrosis factor alpha-driven osteoclastogenesis. Arthritis Rheum. 2007;56:2595–607. doi: 10.1002/art.22791. [DOI] [PubMed] [Google Scholar]
- 42.Ismail MF, El-Maraghy SA, Sadik NA. Study of the immunomodulatory and antiinflammatory effects of evening primrose oil in adjuvant arthritis. Afr J Biochem Res. 2008;2:74–80. [Google Scholar]
- 43.Barca M, Baconi DL, Ciobanu A, Burcea GT, Balalau C. Comparative evaluation of methotrexate toxicity as solution for injection and liposomes following a short-term treatment in a murine model of arthritis. Haematological and biochemical evaluation. Farmacia. 2013;61:220–8. [Google Scholar]
- 44.Arumugam P, Priya NG, Subathra M, Ramesh A. Anti-inflammatory activity of four solvent fractions of ethanol extract of Mentha spicata L. investigated on acute and chronic inflammation induced rats. Environ Toxicol Pharmacol. 2008;26:92–5. doi: 10.1016/j.etap.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 45.Suzuki J, Ogawa M, Futamatsu H, Kosuge H, Sagesaka YM, Isobe M. Tea catechins improve left ventricular dysfunction, suppress myocardial inflammation and fibrosis, and alter cytokine expression in rat autoimmune myocarditis. Eur J Heart Fail. 2007;9:152–9. doi: 10.1016/j.ejheart.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 46.Nakanishi T, Mukai K, Yumoto H, Hirao K, Hosokawa Y, Matsuo T. Anti-inflammatory effect of catechin on cultured human dental pulp cells affected by bacteria-derived factors. Eur J Oral Sci. 2010;118:145–50. doi: 10.1111/j.1600-0722.2010.00714.x. [DOI] [PubMed] [Google Scholar]