Abstract
Background:
Recently, we have reported antihypertensive activity of oleanolic acid (OA) in glucocorticoid-induced hypertension with restoration of nitric oxide (NO) level. However, the involvement of NO-releasing action of OA was unclear.
Objective:
To explore antihypertensive activity of OA in Nω-nitro-L-arginine methyl ester (L-NAME) hypertensive rats wherein NO is completely blocked, which would allow exploring the possibility of involvement of NO-releasing action of OA.
Materials and Methods:
Five groups of rats were investigated as normal control, L-NAME (40 mg/kg/day), L-NAME + enalapril (15 mg/kg/day), L-NAME + l-arginine (100 mg/kg/day), and L-NAME + OA (60 mg/kg/day) for 4 weeks. The systolic blood pressure, body weight, and heart rate were measured weekly for 4 weeks. Serum nitrate/nitrite (NOx) level, urine electrolytes concentration, cardiac mass index, and serum creatinine level were determined followed by organ histopathology.
Results:
OA and enalapril delayed the rise in blood pleasure following L-NAME administration. Decreased serum NOx level was not significantly increased with any of the treatment. OA produced a small, though nonsignificant, increase in the NOx level. L-NAME administration did not affect cardiac mass index. There was an increase in serum creatinine upon L-NAME administration which was prevented by OA. Decreased urine volume, urine sodium and potassium were reversed by OA.
Conclusion:
These results suggest that the antihypertensive effect of OA in L-NAME hypertension is due to diuresis and nephroprotection. However, OA has nonsignificantly affected the NO levels.
Keywords: Endothelial dysfunction, Invasive blood pressure, Nitric oxide, Triterpenoid, Viscum
INTRODUCTION
Oleanolic acid (OA), 3 β-hydroxy-olea-12-en-28-oic acid is one of the best known bioactive ubiquitous pentacyclic triterpenoids with minimal toxicity in medicinal herbs, and is integral part of the human diet.[1] A variety of novel pharmacological effects produced by OA have been reported, including their beneficial effects on cardiovascular systems, interaction with cytochrome P450s) and other biological activities such as anti-HIV, diuretic, nephroprotective, anti-diabetic, antioxidant and hepatoprotective activity.[2,3,4,5,6,7,8]
In vitro studies have found that OA evokes relaxation by release of nitric oxide (NO).[9] In aortic segments from normotensive and hypertensive rats, OA evokes endothelium-dependent relaxations sensitive to inhibition of endothelial nitric oxide synthase.[10] In our previous study, OA has prevented dexamethasone-induced hypertension with the restoration of NO level.[2] But as the NO-redox imbalance is important in the dexamethasone-induced hypertension, the restoration of NO level was thought may be due to the anti-oxidant effect of OA in preventing overproduction of reactive oxygen species. Hence, the involvement of in vivo NO-releasing action of OA was unclear in our previous study. It was necessary to study the antihypertensive activity of OA giving more attention to the endothelial NO system.
Pharmacological long-term blockade of NO synthesis by the chronic administration of Nω-nitro-L-arginine methyl ester (L-NAME), an inhibitor of nitric oxide synthase (NOS), induces systemic arterial hypertension, vascular structural change, and renal dysfunction.[11,12,13,14] Considering this, we have planned to evaluate antihypertensive activity of OA in vivo in L-NAME hypertensive rats wherein NO is completely blocked which will allow exploring possibility of involvement of NO-releasing action of OA in preventing hypertension.
MATERIALS AND METHODS
Isolation of oleanolic acid
Oleanolic acid was isolated from the cuticular wax of Viscum articulatum and purity was confirmed by high performance liquid chromatography (HPLC) as described previously.[2,6] The purity of the OA was confirmed by HPLC analysis for which OA was dissolved in methanol. An Exsil oxide dispersion-strengthened column (250 cm × 4 mm, 5 μm particle size) was used in the isocratic mode with acetonitrile: Water (85:15 v/v) as the solvent system. The flow rate and column temperature were maintained at 0.3 mL/min and 30°C, respectively, and OA was analyzed at 215 nm using ultraviolet detector.
Experimental animals
Male Wistar rats weighing 280–320 g were procured from Bharat Serum, Thane (Maharashtra, India). The animals were allocated to treatment groups at random. They were placed in polypropylene cages with paddy husk for bedding. The animals were maintained on a 12 h light and dark cycle, at 24°C ± 2°C, fed ad libitum with a standard pellet diet and provided free access to water. All the experimental procedures and protocols used in the study were reviewed and approved by the Institutional Animal Ethical Committee, which is registered under the Committee for the Purpose of Control and Supervision on Experiments on Animals, India (registration no. RCPIPER/IAEC/2009-10/02).
Reagents and chemicals
Nω-nitro-L-arginine methyl ester (Fluka analytical), Griess reagent (Fluka analytical), and vanadium (III) chloride (Aldrich chemistry) were procured from Sigma-Aldrich Chemie, Germany. L-arginine was purchased from Himedia, India. Standard diagnostic kits for creatinine estimation were purchased from Span Diagnostic Pvt., Ltd., India. All the other reagents and solvents used were of analytical grade.
Experimental design
Prior to experimentation, the animals were trained and acclimatized to the condition of restrainer (used for noninvasive blood pleasure [BP] measurement) and metabolic cages for 10 days. After the completion of training, those rats that did not struggle or display hyperactivity while being restrained were selected for the experiment. The animals were allowed to rest for 2 days before starting the experiment, during which noninvasive systolic blood pressure (SBP) and heart rate (HR) measurements were made on them.
The rats (N = 34) were randomly divided into two groups of eight rats and three groups of six rats. After baseline data were collected, the toxicant (L-NAME) and drugs were administered to the rats for 4 weeks as indicated below.
To produce a long-term hypertension, L-NAME was dissolved in drinking water at a concentration of 300 mg/L. The fluid intake in each rat was approximately 150 mL/kg/day, and so the L-NAME dose was ∼ 40 mg/kg/day. L-NAME was administration for 4 weeks to all groups except to the first group (N = 6) which served as the normal control (NORM-CON) and received distilled water for 4 weeks. The second group (L-NAME) served as a negative control (N = 8) and received L-NAME alone.[15] The drugs were administered orally at the volume corresponding to 1 mL/100 g body weight (BW). The third (L-ARG; N = 6) and fourth (ENALAPRIL; N = 6) groups were treated with L-arginine (100 mg/kg/day) and enalapril (15 mg/kg/day) for 4 weeks, respectively.[16,17] The fifth (OA60) group (N = 8) was treated with OA (60 mg/kg/day) for 4 weeks.[2,3]
The SBP, HR, and BW were monitored weekly for four consecutive weeks. All precautions were taken during the entire experiment to avoid any stress on the animals due to the experimental procedures (viz. urine collection, drug dosing, etc.), or otherwise measurement of the SBP and HR would be affected. However, the SBP and HR were measured first followed by other experimental procedures/estimations.
At the end of the experiment, invasive direct BP measurement by cannulation of the carotid artery was conducted followed by blood collection through the cardiac puncture. Serum was separated by centrifugation and was used to estimate the creatinine and nitrate/nitrite (NOx) concentrations.
The animals were then sacrificed and the heart, aorta, and kidney were excised free of fats and connective tissue for hypertrophy and histopathological evaluation. The heart weight (HW), left ventricular weight along with septum (LVW), right ventricular weight, and kidney weight (KW) were measured. The data were expressed as organ weigh (in milligrams) per 100 g BW. The left ventricle, aorta, and kidney were fixed in 10% neutral buffered formalin solution for histopathological evaluation.
Noninvasive blood pressure and heart rate measurement
The SBP and HR were measured on weeks 1, 2, 3, and 4 using a noninvasive tail-cuff system (AD Instrument PowerLab Data Acquisition System 8/30, Australia). Rats were allowed to stabilize in restrainers on a heating plate (39–40°C) for approximately 35 min. To ensure an adequate flow of blood through the tail veins, the temperature in the restrainer was maintained at approximately 40°C. Several SBP measurements were recorded for each rat, and the mean of four values among which the difference was not >10 mmHg was accepted as the SBP.[18,19]
Invasive blood pressure measurement
At the end of the experiment, after noninvasive blood pressure measurement, the rats were anesthetized with urethane (1.2 g/kg, i. p.) and the trachea was cannulated by polyethylene tube (PE200). The invasive (direct) blood pressure was measured by cannulation of the carotid artery by a polyethylene catheter (PE50) filled with heparinized saline connected to physiological pressure transducer (SP844) of PowerLab Data acquisition system 8/30. The mean arterial blood pressure (MABP) was recorded.
Serum nitrate/nitrite
The serum NOx concentration was determined by the spectrophotometric method described by Miranda et al., using Griess reagent as the color reagent and vanadium (III) as the reducing agent.[20] Briefly, Griess reagent (100 μL) and a deproteinized plasma samples (100 μL) were added to a 96-well plate in duplicate. 100 μL of vanadium chloride of concentration 8 mg/mL in 1 M HCl was added to each well, and the absorbance was measured at 540 nm using a microplate reader (Biotek, USA).
Urine volume, urine sodium, and urine potassium
Each group of rats was maintained in a separate metabolic cage for quantitative urine collections over 24 h. In view of the complete 24 h urine collection, the rats were provided with free access to water during the procedure. Urine samples were collected after SBP measurements on day 0, 14, and 28 for determination of the urine volume (Uv) and the levels of urine sodium (UNa) and urine potassium (UK). UNa and UK were determined by flame photometry.
Serum creatinine
On the day of sacrifice, serum concentration of creatinine was determined spectrophotometrically using commercially available standard kits (Span Diagnostic Pvt., Ltd., India).
Statistical analysis
The data were expressed as the mean ± standard error of the mean. The results were analyzed using one-way and two-way analysis of variance (ANOVA), followed by Bonferroni as a post ANOVA test using GraphPad Prism version 4.00 (GraphPad Software, Inc., USA). A value of p < 0.05 was considered significant.
RESULTS
Effects of different treatments on systolic blood pressure, mean arterial blood pressure, heart rate, and body weight
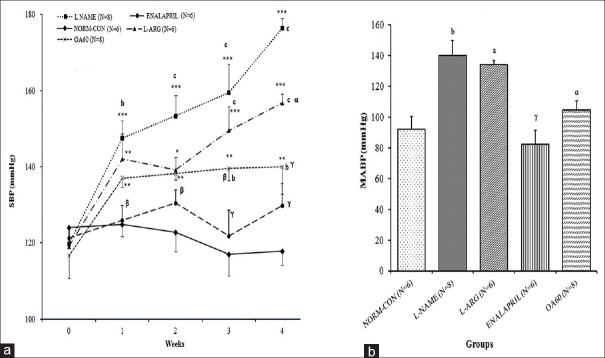
Figure 1a and b shows the time course effects of different treatments on the SBP of rats and day 28 MABP from different groups, respectively. At the beginning of the experiment, the baseline SBP values did not differ among the different experimental groups. SBP of the L-NAME group was raised by 47.3% after L-NAME administration for 28 days (p < 0.001 vs. day 0) which was significantly different from NORM-CON rats (p < 0.001). Similarly, MABP of rats of L-NAME group after invasive BP measurement was significantly increased from that of NORM-CON (p < 0.001). On day 28, the SBP and MABP of the rats in the ENALAPRIL (p < 0.001) and OA60 (p < 0.001 and p < 0.05, respectively) groups were lower than that of those in the L-NAME group. An increase in the SBP and MABP due to NO inhibition was not prevented significantly in the L-ARG group.
Figure 1.
Effect of different treatments on blood pressure: (A) Time course of systolic blood pressure (SBP in mmHg) measured using the tail cuff method; (B) Mean arterial blood pressure (MABP in mmHg) on Day 28 measured by invasive carotid artery cannulation. Data expressed as mean ± SEM. N= number of rats **p < 0.01, ***p < 0.001 compared with basal SBP (at week 0) (within the group comparison) using one-way ANOVA followed by Bonferroni as a post-ANOVA test. ap < 0.05, bp < 0.01, cp < 0.001 compared with NORM-CON; αp < 0.05, βp < 0.01, γp < 0.001 compared with L-NAME control (inter-group comparison) using two-way (for SBP) and one way ANOVA (for MABP on Day 28) followed by Bonferroni as a post-ANOVA test
At the end of the first week, the decrease in HR in L-NAME (p < 0.001 vs. NORM-CON) was not sustained over the entire experimental period [Figure 2]. At the end of the study, none of the treatments has significantly affected the HR and BW [Figure 2].
Figure 2.
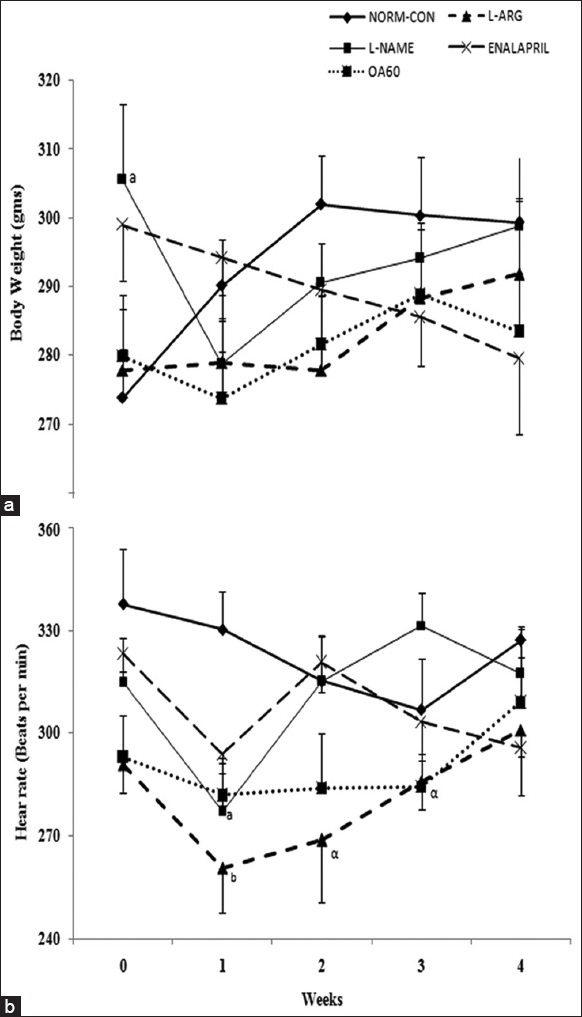
Time-course effects different treatments on the heart rate and body weight. Data expressed as mean ± standard error. N = Number of rats, ap < 0.05, bp < 0.01 compared with normal control; αp <0.05 compared with Nω-Nitro-L-Arginine Methyl Ester control using two-way followed by Bonferroni as a postanalysis of variance test
Serum nitrate/nitrite concentration
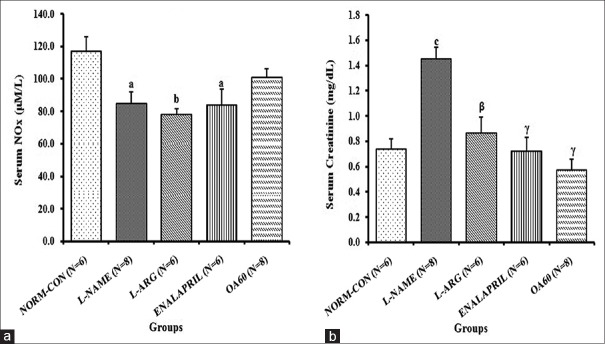
The serum NOx was significantly reduced in L-NAME (84.7 ± 7.3 μm, p < 0.05), as compared to the NORM-CON (116.6 ± 9.1 μm) [Figure 3a]. However, we did not observe significant recovery in NOx levels in any of the treatment groups. However in OA60, the NOx concentration was found 100.8 ± 5.5 μm which was not significantly different from NORM-CON.
Figure 3.
Effects of chronic treatments on the serum concentration of (a) nitrate/nitrite and (b) creatinine on day 28. Data expressed as mean ± standard error. N = Number of rats ap <0.05, bp <0.01, cp <0.001 compared with normal control; βp <0.01, γp <0.001 compared with Nω-Nitro-L-Arginine Methyl Ester control using one-way analysis of variance followed by Bonferroni as a postanalysis of variance test
Urine volume, urine sodium, urine potassium, and serum creatinine levels
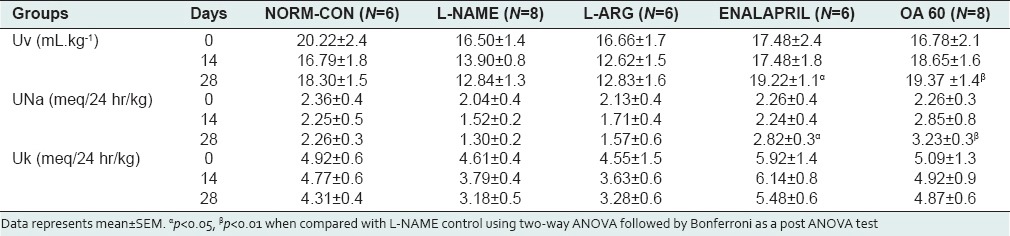
Urinary parameters are described in Table 1. Chronic treatment with L-NAME reduced the Uv up to 21.3% with reduced UNa (14.1%) and UK (32.2%) compared with the day 0. Treatment with enalapril and OA produced a rise in Uv (up to 18.2% and 26.4%, respectively) when compared with day 0. UNa was raised in the OA60 group (p < 0.05 vs. L-NAME control). There was no significant effect of any treatment on UK.
Table 1.
Time-course effects of different treatments on urinary parameters
Serum creatinine was increased significantly (p < 0.001) in the L-NAME group (1.5 ± 0.1 mg/dL). Treatment with l-arginine, enalapril, and OA for four successive weeks resulted in a significant prevention of the increase in serum creatinine level due to L-NAME administration [Figure 3b].
Cardiac mass index and kidney weight
None of the treatments affected the LVW, HW, and KW, except enalapril in which the LVW (p < 0.01 vs. L-NAME) and HW (p < 0.01) were found to be decreased [Figure 4].
Figure 4.
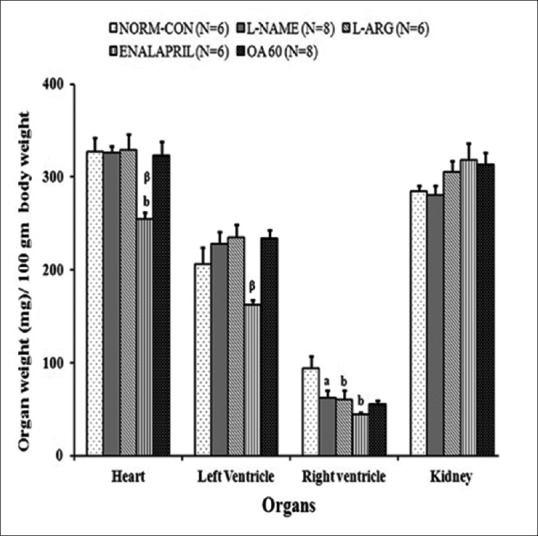
Effect of enalapril, L-arginine, and oleanolic acid on (a) organ hypertrophy in Nω-Nitro-L-Arginine Methyl Ester hypertensive rats at the end of 4 weeks. Data represent mean ± standard error ap < 0.05, bp < 0.01 when compared with normal control; αp <0.05, βp <0.01 compared with Nω-Nitro-L-Arginine Methyl Ester control using one-way analysis of variance followed by Bonferroni as a postanalysis of variance test
Histological analysis
Chronic oral administration of L-NAME over 4 weeks caused morphological abnormalities such as moderate diffuse myocardial degeneration and necrosis with inflammatory infiltration in heart muscles [Figure 5(i)] while cystic dilation of tubules, atrophy of the tubular epithelium and fewer glomeruli with mild degree increase in glomerular filtration space in the kidney [Figure 5(ii)]. These abnormalities were partially prevented by treatment with OA. There was no abnormality detected in the thoracic aorta in any of the group [Figure 5(iii) c].
Figure 5.
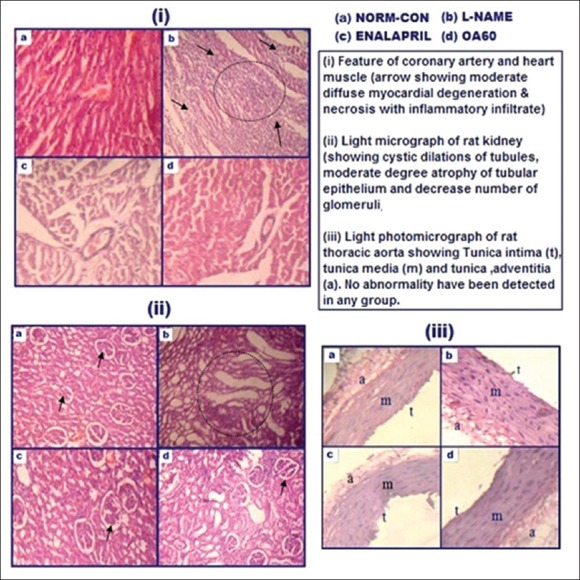
Light photomicrographs of rat (i) heart (ii) kidney and (iii) thoracic aorta in different experimental groups
DISCUSSION
The present study is prompted by our previous study demonstrating antihypertensive activity of OA in glucocorticoid-induced hypertension with the restoration of NO level. The OA restoration was may be attributed to the antioxidant or NO-releasing action of OA.[2] Accordingly, a study of OA in NO-dependent hypertension like L-NAME induced hypertension would prove to be useful in exploring the involvement of NO-releasing action of OA in preventing hypertension. In the present study, the antihypertensive activity of OA was explored in L-NAME-induced hypertension in rats in view of the possibility of involvement of NO-releasing action of OA in preventing hypertension.
In accordance with the existing literature, we observed and confirmed that chronic treatment with the arginine analog, L-NAME, results in arterial hypertension and renal dysfunctioning.[11,13] NO is synthesized by NOS, present in vascular endothelial cells from l-arginine.[21] In present study, inability of l-arginine treatment to reverse increased BP also signified the complete inhibition of NOS by L-NAME. In our study, raised SBP and MABP following chronic treatment with L-NAME were associated with decrease in the serum NOx and renal dysfunctioning. Increase in SBP and MABP due to L-NAME was delayed by co-administration of OA. However, OA failed to prevent decrease in serum NOx. Several parameters like vascular study showing ach induced vasorelaxation, NOx determination in serum, plasma, urine, tissues etc., using the Griess method and tissue NOS activity are used to study endothelial dysfunction and altered NO production.[20,22,23,24,25] Like most researchers, we considered that it is appropriate to determine the NOx level using the “Griess reagent in biological fluid method” as a potential indicator of NO production.[15,19,26] The decrease in NOx level was not influenced significantly by any of the treatments, indicating the absence of any effect on NO inhibition or NO release and involvement of other mechanisms for their action. OA produced a small, though nonsignificant, increase in the NOx level, and this did not signify an action of OA in preventing NO inhibition. This indicates that OA may not have any effect at the endothelial level that is responsible for its antihypertensive action against L-NAME-induced hypertension. The antihypertensive effect may instead be due to its other effects like nephro-protection and diuretic activities; which were also explored in the present study.
None of the treatments affected the HR, which is in accordance with the findings of previous studies.[27] Along with the NO inhibition, enhanced oxidative stress is also associated with NO-deficient hypertension. This enhanced oxidative stress plays a vital role in the pathogenesis of vascular tissue angiotensin-converting enzyme activation, salt sensitivity, and modulation of renal hemodynamic and excretory functions.[28,29,30] We observed an increase in the serum creatinine level upon administration of L-NAME, reflecting kidney dysfunctioning. This was confirmed by the decrease in Uv, UNa and UK and by histopathological studies showing cystic dilations of tubules and atrophy of the tubular epithelium. The increase in Uv and UNa in OA60 and enalapril treated group along with a reduction in the severity of lesions such as cystic dilations of tubules and atrophy of the tubular epithelium in the kidney (from histopathological examinations) indicated the diuresis and nephro-protective actions of OA and enalapril. In addition, the antioxidant action of OA may also be responsible for these effects.[3]
There was no effect of L-NAME administration on the cardiac mass index, in contrast with the findings of previous studies which showed a significant rise in the cardiac mass index resulting from oxidative stress and pressure overload in rats.[11,12,13,14,16,31] In accordance with previous studies, a significant decrease in the LVW in enalapril-treated rats was may be due to the decrease in LV collagen concentration following enalapril treatment.[32,33] These results indicated that the antihypertensive activity of OA in the present study was due to its diuretic and nephroprotective activity. In addition, antioxidant activity of OA may also be contributing for effects of OA in L-NAME model.[3]
As we have estimated only NOx estimation, which is the indirect measure of NO level, the potential effects of OA toward increasing NO availability cannot be ruled out. Moreover along with NOx estimation, NOS expression study may provide major insight about the possibility of NO-releasing activity of OA. Further exploring the antihypertensive activity of OA in spontaneous hypertensive rats (animal model of essential hypertension) and evaluating more mechanistic parameters as mentioned above will provide a better insight of OA action. While performing this, attention needs to be given to pharmacokinetics of OA considering its lipophilicity.
CONCLUSION
Our results showed that OA prevents progression of hypertension in rats produced by chronic administration of L-NAME, which may be due to its diuretic, nephroprotective, and antioxidant effects.
Footnotes
Source of Support: The authors would like to thank Dr. Sanjay J. Surana, Principal, RCPIPER, Shirpur (India) for providing necessary facilities for conducting the experiments
Conflicts of Interest: None declared.
REFERENCES
- 1.Liu J. Oleanolic acid and ursolic acid: Research perspectives. J Ethnopharmacol. 2005;100:92–4. doi: 10.1016/j.jep.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 2.Bachhav SS, Patil SD, Bhutada MS, Surana SJ. Oleanolic acid prevents glucocorticoid-induced hypertension in rats. Phytother Res. 2011;25:1435–9. doi: 10.1002/ptr.3431. [DOI] [PubMed] [Google Scholar]
- 3.Somova LO, Nadar A, Rammanan P, Shode FO. Cardiovascular, antihyperlipidemic and antioxidant effects of oleanolic and ursolic acids in experimental hypertension. Phytomedicine. 2003;10:115–21. doi: 10.1078/094471103321659807. [DOI] [PubMed] [Google Scholar]
- 4.Kim KA, Lee JS, Park HJ, Kim JW, Kim CJ, Shim IS, et al. Inhibition of cytochrome P450 activities by oleanolic acid and ursolic acid in human liver microsomes. Life Sci. 2004;74:2769–79. doi: 10.1016/j.lfs.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 5.Mengoni F, Lichtner M, Battinelli L, Marzi M, Mastroianni CM, Vullo V, et al. In vitro anti-HIV activity of oleanolic acid on infected human mononuclear cells. Planta Med. 2002;68:111–4. doi: 10.1055/s-2002-20256. [DOI] [PubMed] [Google Scholar]
- 6.Patil CR, Jadhav RB, Singh PK, Mundada S, Patil PR. Protective effect of oleanolic acid on gentamicin induced nephrotoxicity in rats. Phytother Res. 2010;24:33–7. doi: 10.1002/ptr.2861. [DOI] [PubMed] [Google Scholar]
- 7.Teodoro T, Zhang L, Alexander T, Yue J, Vranic M, Volchuk A. Oleanolic acid enhances insulin secretion in pancreatic beta-cells. FEBS Lett. 2008;582:1375–80. doi: 10.1016/j.febslet.2008.03.026. [DOI] [PubMed] [Google Scholar]
- 8.Liu J, Liu Y, Mao Q, Klaassen CD. The effects of 10 triterpenoid compounds on experimental liver injury in mice. Fundam Appl Toxicol. 1994;22:34–40. doi: 10.1006/faat.1994.1005. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez-Rodriguez R, Stankevicius E, Herrera MD, Ostergaard L, Andersen MR, Ruiz-Gutierrez V, et al. Oleanolic acid induces relaxation and calcium-independent release of endothelium-derived nitric oxide. Br J Pharmacol. 2008;155:535–46. doi: 10.1038/bjp.2008.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodríguez-Rodríguez R, Herrera MD, Perona JS, Ruiz-Gutiérrez V. Potential vasorelaxant effects of oleanolic acid and erythrodiol, two triterpenoids contained in ‘orujo’ olive oil, on rat aorta. Br J Nutr. 2004;92:635–42. doi: 10.1079/bjn20041231. [DOI] [PubMed] [Google Scholar]
- 11.Ribeiro MO, Antunes E, de Nucci G, Lovisolo SM, Zatz R. Chronic inhibition of nitric oxide synthesis. A new model of arterial hypertension. Hypertension. 1992;20:298–303. doi: 10.1161/01.hyp.20.3.298. [DOI] [PubMed] [Google Scholar]
- 12.Baylis C, Mitruka B, Deng A. Chronic blockade of nitric oxide synthesis in the rat produces systemic hypertension and glomerular damage. J Clin Invest. 1992;90:278–81. doi: 10.1172/JCI115849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Devlin AM, Brosnan MJ, Graham D, Morton JJ, McPhaden AR, McIntyre M, et al. Vascular smooth muscle cell polyploidy and cardiomyocyte hypertrophy due to chronic NOS inhibition in vivo. Am J Physiol. 1998;274:H52–9. doi: 10.1152/ajpheart.1998.274.1.H52. [DOI] [PubMed] [Google Scholar]
- 14.Vardi N, Ozturk F, Fadillioúlu E, Otlu A, Yaúmurca M. Histological changes in the rat thoracic aorta after chronic nitric oxide synthase inhibition. Turk J Med Sci. 2003;33:141–7. [Google Scholar]
- 15.Rossoni G, Manfredi B, De Gennaro Colonna V, Berti M, Guazzi M, Berti F. Sildenafil reduces L-NAME-induced severe hypertension and worsening of myocardial ischaemia-reperfusion damage in the rat. Br J Pharmacol. 2007;150:567–76. doi: 10.1038/sj.bjp.0707131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguelefack-Mbuyo PE, Nguelefack TB, Dongmo AB, Afkir S, Azebaze AG, Dimo T, et al. Anti-hypertensive effects of the methanol/methylene chloride stem bark extract of Mammea africana in l-NAME-induced hypertensive rats. J Ethnopharmacol. 2008;117:446–50. doi: 10.1016/j.jep.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 17.Afkir S, Nguelefack TB, Aziz M, Zoheir J, Cuisinaud G, Bnouham M, et al. Arbutus unedo prevents cardiovascular and morphological alterations in L-NAME-induced hypertensive rats Part I: Cardiovascular and renal hemodynamic effects of Arbutus unedo in L-NAME-induced hypertensive rats. J Ethnopharmacol. 2008;116:288–95. doi: 10.1016/j.jep.2007.11.029. [DOI] [PubMed] [Google Scholar]
- 18.Bachhav SS, Bhutada MS, Patil SD, Baser B, Chaudhari KB. Effect of Viscum articulatum Burm. (Loranthaceae) in Nω-nitro-L-arginine methyl ester induced hypertension and renal dysfunction. J Ethnopharmacol. 2012;142:467–73. doi: 10.1016/j.jep.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 19.Miao Y, Zhang Y, Lim PS, Kanjanapan Y, Mori TA, Croft KD, et al. Folic acid prevents and partially reverses glucocorticoid-induced hypertension in the rat. Am J Hypertens. 2007;20:304–10. doi: 10.1016/j.amjhyper.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Miranda KM, Espey MG, Wink DA. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 2001;5:62–71. doi: 10.1006/niox.2000.0319. [DOI] [PubMed] [Google Scholar]
- 21.Palmer RM, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333:664–6. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- 22.O’sullivan SE, Randall MD, Gardiner SM. The in vitro and in vivo cardiovascular effects of Delta9-tetrahydrocannabinol in rats made hypertensive by chronic inhibition of nitric-oxide synthase. J Pharmacol Exp Ther. 2007;321:663–72. doi: 10.1124/jpet.106.116566. [DOI] [PubMed] [Google Scholar]
- 23.Kang DG, Sohn EJ, Lee YM, Lee AS, Han JH, Kim TY, et al. Effects of bulbus Fritillaria water extract on blood pressure and renal functions in the L-NAME-induced hypertensive rats. J Ethnopharmacol. 2004;91:51–6. doi: 10.1016/j.jep.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 24.Bernátová I, Pechánová O, Simko F. Effect of captopril in L-NAME-induced hypertension on the rat myocardium, aorta, brain and kidney. Exp Physiol. 1999;84:1095–105. [PubMed] [Google Scholar]
- 25.Bernátová I, Pechánová O, Babál P, Kyselá S, Stvrtina S, Andriantsitohaina R. Wine polyphenols improve cardiovascular remodeling and vascular function in NO-deficient hypertension. Am J Physiol Heart Circ Physiol. 2002;282:H942–8. doi: 10.1152/ajpheart.00724.2001. [DOI] [PubMed] [Google Scholar]
- 26.Mondo CK, Yang WS, Su JZ, Huang TG. Atorvastatin prevented and reversed dexamethasone-induced hypertension in the rat. Clin Exp Hypertens. 2006;28:499–509. doi: 10.1080/10641960600798713. [DOI] [PubMed] [Google Scholar]
- 27.Nguelefack TB, Mekhfi H, Dongmo AB, Dimo T, Watcho P, Zoheir J, et al. Hypertensive effects of oral administration of the aqueous extract of Solanum torvum fruits in L-NAME treated rats: Evidence from in vivo and in vitro studies. J Ethnopharmacol. 2009;124:592–9. doi: 10.1016/j.jep.2009.04.057. [DOI] [PubMed] [Google Scholar]
- 28.Usui M, Egashira K, Kitamoto S, Koyanagi M, Katoh M, Kataoka C, et al. Pathogenic role of oxidative stress in vascular angiotensin-converting enzyme activation in long-term blockade of nitric oxide synthesis in rats. Hypertension. 1999;34:546–51. doi: 10.1161/01.hyp.34.4.546. [DOI] [PubMed] [Google Scholar]
- 29.Kopkan L, Majid DS. Superoxide contributes to development of salt sensitivity and hypertension induced by nitric oxide deficiency. Hypertension. 2005;46:1026–31. doi: 10.1161/01.HYP.0000174989.39003.58. [DOI] [PubMed] [Google Scholar]
- 30.Kopkan L, Majid DS. Enhanced superoxide activity modulates renal function in NO-deficient hypertensive rats. Hypertension. 2006;47:568–72. doi: 10.1161/01.HYP.0000200027.34925.93. [DOI] [PubMed] [Google Scholar]
- 31.Arnal JF, Warin L, Michel JB. Determinants of aortic cyclic guanosine monophosphate in hypertension induced by chronic inhibition of nitric oxide synthase. J Clin Invest. 1992;90:647–52. doi: 10.1172/JCI115906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rizzoni D, Porteri E, Piccoli A, Castellano M, Bettoni G, Muiesan ML, et al. Effects of losartan and enalapril on small artery structure in hypertensive rats. Hypertension. 1998;32:305–10. doi: 10.1161/01.hyp.32.2.305. [DOI] [PubMed] [Google Scholar]
- 33.Susic D, Varagic J, Frohlich ED. Pharmacologic agents on cardiovascular mass, coronary dynamics and collagen in aged spontaneously hypertensive rats. J Hypertens. 1999;17:1209–15. doi: 10.1097/00004872-199917080-00022. [DOI] [PubMed] [Google Scholar]