Abstract
Purpose:
Oxidative stress has been implicated in the pathophysiology of glaucoma, cataract, and many degenerative diseases. The purpose of this study is to evaluate the systemic oxidative stress in black-African patients diagnosed with primary glaucoma or age-related cataract (ARC) and compare these indices to normal control patients and between the two conditions.
Methods:
This was a descriptive cross-sectional study of consecutive recruited subjects attending a tertiary care facility. One hundred adults were enrolled and sub-grouped into: Normal controls (n = 20), patients with primary glaucoma (n = 40), and patients with cataract (n = 40). The data were collected on patient demographics and clinical information. Ten milliliters of the venous blood was taken from each subject for the evaluation of serum biochemical indices of oxidative stress. Laboratory measurements of enzymatic and nonenzymic anti-oxidants, as well as lipid peroxidation, were conducted using established and validated spectrophotometric methods. The systemic oxidative stress was measured by the serum levels of anti-oxidant enzymes and lipid peroxidation, and compared between the groups and to a control group of patients.
Results:
Statistically, significantly reduced serum levels of glutathione, glutathione-S-transferase, superoxide dismutase, catalase, and ascorbic acid were found in the patients with glaucoma or cataract when compared with controls (P < 0.05 for all). Differences in serum lipid peroxidation levels across or between the groups were nonsignificant. Serum protein levels were significantly higher among the subjects with cataract or glaucoma than in controls.
Conclusion:
Our results concur with findings in Caucasian study cohorts. This indicates that in black-Africans, primary glaucoma, and ARC are associated with increased systemic oxidative stress. This supports the existing evidence on the role of oxidative stress in these ocular disorders and reinforces the rationale for the use of anti-oxidants in the management and possible prevention of these conditions.
Keywords: Anti-oxidants, Cataract, Glaucoma, Oxidative Stress
INTRODUCTION
Age-related cataract (ARC) and primary open-angle glaucoma (POAG) are important contributors to the global burden of blindness and visual impairment. ARC and POAG are, respectively, recognized as the leading cause of reversible and irreversible blindness in the most developing countries.1 Interventions that can modify the course of these conditions or offer benefits for their primary prevention will significantly impact the global public health. An enhanced understanding of the pathogenesis and molecular changes underlying these conditions will identify these interventions.
Among the several pathologic mechanisms that have been identified in ARC and POAG, oxidative stress is a relatively recent addition.2,3 Over the past three decades, there has been a gradual accumulation of evidence supporting the role of oxidative stress in these conditions.2,3,4,5 The implication of this growing body of evidence is that relevant modifications should be considered to the guidelines for therapy as well as the primary and secondary prevention and interventions for these conditions.
Oxidative stress results when the tissue or systemic levels of free radicals are high due to insufficient physiologic anti-oxidant defense.6,7 The free radicals are capable of causing damage to cell membranes or cell components (including nuclear and mitochondrial DNA). The free radicals are propagated by the reactive oxygen species such as superoxide ion (O2−), hydrogen peroxide (H2O2), and hydroxyl radical (OH−). These molecules are generated during the biochemical processes, most importantly, the respiratory electron transport chain. Functioning the enzymatic and nonenzymatic anti-oxidant systems is required for effective neutralization and prevention of tissue damage.6,7,8
Oxidative stress damages the DNA and cellular membrane pump systems, causing loss of epithelial cell viability and death by necrotic or apoptotic mechanisms. With respect to the effect of free radicals on the eye, early investigations had suggested that in the lens, the epithelial cell layer is the initial site of attacked by the free radicals, followed by the lens fibers leading to the cortical cataract.9 Evidence also suggests that the insidious progressive optic neuropathy that characterizes the POAG results from the death of retinal ganglion cells which may follow apoptosis.10 POAG was initially thought to be purely due to the raised intraocular pressure (IOP), but the growing evidence indicates that the loss of retinal ganglion cells could ensue from other causes including retinal ischemia, nutritional deficiencies, and oxidative stress.11,12
It is important to note that despite the high burden of blindness and eye diseases in sub-Saharan Africa, contributions from sub-Saharan Africa to this burgeoning evidence is rare. This study evaluates the systemic oxidative stress among the black-African patients with primary glaucoma or ARC at a tertiary healthcare facility in Lagos, Nigeria. Our objectives were to determine and compare the serum levels of enzymatic and nonenzymatic anti-oxidants, as well as lipid peroxidation parameters (and by inference the level of oxidative stress) in patients with either POAG or ARC and normal controls; and also between the two ocular conditions.
METHODS
This was a cross-sectional study of consecutively recruited participants. The study involved the interviews and clinical examinations, laboratory analysis of the blood samples for enzymatic and nonenzymatic anti-oxidants including lipid peroxidation. Each consenting participant underwent a clinical interview, ocular examination, and blood sample collection. Sociodemographic data, family history, and brief medical history were obtained from each patient. The study was conducted at the Lagos State Eye Institute. The center is situated within the Lagos State University Teaching Hospital, Ikeja, Lagos, Nigeria. The hospital is an academic tertiary care facility. The institute provides an outpatient and inpatient ophthalmic care services. The clinics are held every weekday with an average daily attendance of about 200 patients. The study was approved by the Research Ethics Committee.
One hundred adults were recruited in this study. The subjects were comprised patients with POAG (n = 40) (glaucoma group), patients with ARC (n = 40) (cataract group), and normal controls (n = 20) (control group). The controls were apparently healthy adults who had no other ocular pathology aside from refractive errors. Subjects in the control group also had normal findings on ocular examination (particularly for the physiologic lens, IOP, and central visual field), and no personal or family history of primary glaucoma. Subjects were recruited during clinic sessions at the institute.
Subjects and controls were required to be at least 40-year-old and willing to provide signed, informed consent prior to the recruitment. The patients with other forms of cataract aside from ARC were excluded. Also, patients who had undergone surgery for glaucoma or those with narrow-angle glaucoma were excluded. All potential participants who had used oral or topical anti-oxidant preparations within 8 weeks before the study began were excluded. We ensured that the conduct of the study conformed to the provisions of the Declaration of Helsinki (as revised in Edinburgh in the year 2000).13 This study was approved by the Institution's Research Ethics Committee. Signed, informed consent was obtained from each participant.
Sterile phlebotomy was performed to obtain 10 ml of venous blood from each subject. The blood samples were transferred into heparinized vacutainers. The Serum was extracted by centrifuging the heparinized whole blood in a Whisperfuge centrifuge (model 684, Westfalia Separator, GEA Group Aktiengesellschaft, Bochum, Germany) at 2500 revolutions for 10 min. The extracted serum was stored at − 83°C before analysis of the levels of ascorbic acid (Vitamin C), reduced glutathione (GSH), glutathione-S-transferase (GST), superoxide dimutase (SOD), catalase (CAT), protein, and thiobarbituric acid reactive substances (TBARS) - an established measure of lipid peroxidation using the malondialdehyde test, were conducted.
Serum analyses for the determination of anti-oxidant enzyme activity, ascorbic acid level, protein and lipid peroxidation were conducted using established and validated methods.14,15,16,17,18,19,20 Serum CAT activity was determined according to the method of Beers and Seizer14 by measuring the decrease in absorbance at 240 nm due to the decomposition of hydrogen peroxide in an ultraviolet spectrophotometer. The superoxide dismutase activity was determined by its ability to inhibit the auto-oxidation of epinephrine determined by the increase in absorbance at 480 nm as described by Sun and Zigman.15 GSH level in the serum was estimated according to the method described by Sedlak and Lindsay.16 GST was determined by the method developed by Habig et al.17 Serum TBARS level which was measured as the index of lipid peroxidation was determined using the method of Beuge and Aust.18 The serum protein was measured using the method described by Gornall et al.19 while serum ascorbic acid level was determined using the dinitrophenylhydrazine method developed by Roe and Kuether.20
The data analysis was performed using Graph Pad Prisms Software version 5 for windows (Graph Pad Software Inc. California, USA). Differences between the groups were analyzed for statistical significance with the Student's t-test, Wilcoxon-rank sum test, and Fisher's exact test. Differences across the three groups were evaluated using Kruskal–Wallis and Dunn's multiple comparison tests (post-hoc test). A P < 0.05 indicated as statistical significance.
RESULTS
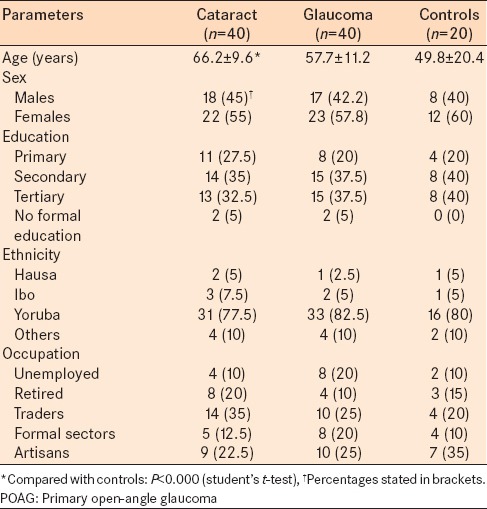
Sociodemographic data of the respondents are presented in Table 1. The patients with ARC were older than subjects in the other groups. The mean age of patients with cataract significantly differed from the mean age of controls (P < 0.05). The mean age of patients with glaucoma was not significantly different from the control group or the cataract group (P > 0.05). There was a slight female preponderance in each group (55–60%). This was most pronounced in the control group. Differences in the female-to-male composition of the groups were not statistically significant (P > 0.05 for all). Almost, all the subjects had a minimum of primary school education. The majority of the subjects (75%) were from the Yoruba ethnic group.
Table 1.
Sociodemographic characteristics of the POAG group, cataract group, and healthy control group
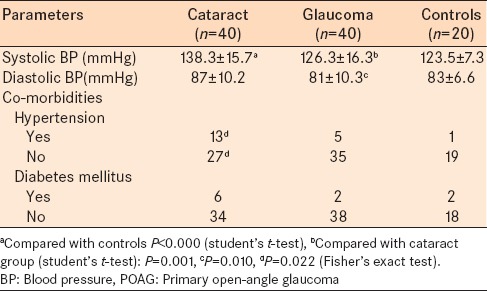
Comorbidities such as diabetes mellitus and hypertension were present in each group [Table 2]. There were no statistically significant differences (between groups) in the proportion of patients with diabetes. The proportion of patients with hypertension in the cataract group was significantly higher than among the control (P < 0.05). Also, the subjects with cataract had significantly higher mean systolic BP than the control and glaucoma groups (P < 0.05).
Table 2.
BP and co-morbidities of the POAG group, cataract group and healthy control group
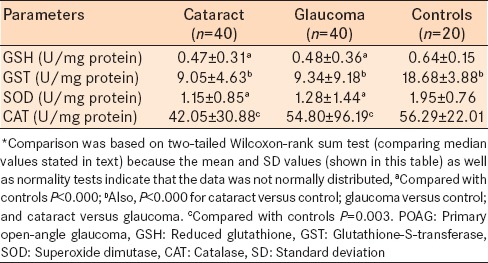
The serum levels of all enzymatic anti-oxidants (GSH, GST, SOD, and CAT) were statistically, significantly lower in subjects with cataract or glaucoma compared with the control group (P < 0.05 for all comparisons; Wilcoxon-rank sum test) [Table 3]. In the control group, the median serum levels (U/mg protein) of GSH, GST, SOD, and CAT were 0.60, 18.36, 2.05, and 58.90, respectively; the corresponding median values for the cataract and glaucoma groups were 0.39 and 0.39; 8.32 and 7.28; 0.91 and 0.83; and 33.89 and 31.08. The serum level of GST was statistically, significantly different between the cataract and glaucoma groups (P < 0.000) [Table 3]. There were no other statistical differences in the anti-oxidants levels between the glaucoma and cataract groups (P > 0.05 for all comparisons) [Table 3].
Table 3.
Serum levels of enzymatic anti-oxidants* of the POAG group, cataract group, and healthy control group
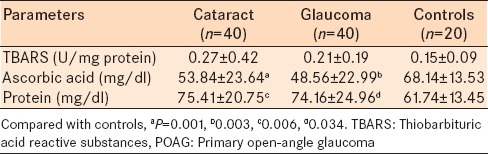
A slightly different trend was observed in the nonenzymatic anti-oxidant and the lipid peroxidation data [Table 4]. While the differences between the study groups in the level of TBARS were not statistically significant (P > 0.05 for all comparisons); the mean serum ascorbic acid (Vitamin C) levels was statistically, significantly higher in the control group compared to the glaucoma group (P = 0.003) or the cataract group (P = 0.001). Mean total serum protein level was statistically significantly lower in the control group (61.74 ± 13.45) compared to the cataract group (75.41 ± 20.75) and the glaucoma group (74.16 ± 24.96) (P < 0.05 for each comparison).
Table 4.
Serum levels of TBARS ascorbic acid and protein for the primary open angle glaucoma group, cataract group and healthy control group
DISCUSSION
We compared the systemic oxidative stress parameters in black-African patients having either ARC or primary glaucoma with normal controls. Our findings clearly indicated that compared with the controls, patients with either cataract or primary glaucoma had a significant reductions in serum levels of both enzymatic and nonenzymatic anti-oxidants. This implies that these conditions are associated with increased oxidative stress.
Our data corroborate the findings of previous studies of mostly Caucasian populations. The previous studies have used widely differing methods for measuring the anti-oxidant levels, all suggesting that POAG and ARC are associated with increased systemic oxidative stress.4,6,21,22,23,24
In the current study, a significant difference in age was observed between the patients with cataract and normal subjects. The importance of this finding hinges on available evidence suggesting that the oxidative stress increases with age and contributes to the aging process.21 The presence of ARC in relatively older individuals likely explains the observed difference. However, this may be inconsequential to our inferences because the despite having nonsignificant difference in age between the glaucoma and normal subjects, differences between the two groups in oxidative stress indices were similar to those observed between the controls and cataract subjects. Similarly, a statistically significant difference was observed in the prevalence of systemic hypertension among the cataract group compared with the control group. Again, this is likely a consequence of the age difference considering that ARC naturally selected for much older patients into the cataract group while the prevalence of arterial hypertension has been shown to increase with age in the most population studies.25 The relevance of this to our findings derives from a study by Zanon-Moreno et al. that reported a statistically significant difference in oxidative stress levels between the patients with hypertension and normotensive individuals.24 However, this may not be a significant confounder considering that despite a nonsignificant difference in the prevalence of hypertension between the glaucoma and control groups, the difference between their oxidative stress indices followed the similar trends as those observed between the control and cataract groups.
Our finding of a statistically significant reduction in the serum enzymatic anti-oxidants in subjects with ARC or POAG compared with controls concurs with the findings of previous studies of Caucasians.4,6,21,22,23,24,26
Cekic et al., reported a statistically significant difference in the serum levels of sulfhydryl groups - (an indirect measure of the level of reduced glutathione and the ability to replenish the oxidized glutathione) between patients with cortical cataract and age/sex matched controls. The mean values of total serum sulfhydryl groups were higher among the controls suggesting greater consumption of compounds donating the sulfhydryl groups (for the neutralization of free radicals) in the subjects with cataracts.4
Maurya et al.,23 reported the reduced serum levels of CAT (which CAT the breakdown of hydrogen peroxide into water and oxygen thus, preventing it from reacting with lipids) and superoxide dismutase (CAT the neutralization of superoxide radical) in subjects with senile cataracts. In addition, the investigators23 found further reductions in the levels of these enzymes in diabetic subjects with cataracts. Their23 findings supported the notion that diabetes increases the oxidative stress and predisposes to cataract formation.
With the exception of the serum levels of GST, the levels of other anti-oxidant enzymes in patients with ARC did not statistically differ from those in subjects with POAG. However, findings reported in a similar study by Ghanem et al., (in which oxidative stress parameters were analyzed in aqueous humor samples) demonstrated a statistically significant differences in the levels of anti-oxidant enzymes in the aqueous humor of patients with glaucoma and cataract.6 In their study Ghanem et al., found higher levels of SOD, glutathione peroxidase, and lipid peroxidation products in patients with POAG than in those with cataract.6 It was suggested that the higher levels of these parameters could suggest the increased need for the anti-oxidants as a consequence of the disease and not necessarily a cause.6
Glutathione-S-transferase catalyzes the conjugation of reduced glutathione via sulfhydryl groups to electrophilic centers on a wide variety of substrates. This is a vital for the detoxification of endogenous substances such as peroxidised lipids and exogenous compounds (xenobiotics).27 The reduction in GST levels may result from increased consumption of glutathione as a consequence of free radical generation and lipid peroxidation, reduced elaboration of the enzyme, or from immunologic factors.28 In this study, the higher mean level of GST was found among the patients with POAG than in those with cataract. This indicates the increased utilization of this enzyme in patients with cataract. However, this did not impact the difference in GSH levels between the two groups which remained statistically nonsignificant (P > 0.05, Dunn's test).
The serum levels of thiobarbituric acid reactive substances (an index of lipid peroxidation which was measured by the malondialdehyde test) did not differ significantly between the cataract and glaucoma groups. This finding concurs with that of Faschinger et al., who reported no statistically significant differences of TBARS in either the aqueous humor or in the serum of patients with POAG in comparison to those with cataracts or pseudoexfoliation glaucoma.21 However, this findings differs from Ghanem et al.'s study, who reported the higher TBARS levels in subjects with POAG than in subjects with cataract.6 It is possible that these differences may be a result of the difference in assay methods employed for the determination of TBARS levels. This difference could be explained by Li et al.,22 who reported no statistically significant differences in lipid peroxidation determined by malondialdehyde as well as 8-iso-PGF (2-alpha) between the subjects with and without early cataract. However, in the study by Li et al., serum levels of the isomers of 9- and 13-(Z, E)-hydroxyoctadecadienoic acid (another biomarker of lipid peroxidation) were significantly higher in subjects with early cataract as compared with those of noncataract subjects.22
We found the serum levels of ascorbic acid was statistically, significantly lower in patients with cataracts or glaucoma, compared with the controls. Ascorbic acid belongs to the class of compounds, classified as nonenzymatic anti-oxidants; along with albumin, GSH, bilirubin, tocopherol, and B-carotene. These compounds are important for the neutralization of reactive species derived from xenobiotics and termination of chain reactions that could ensue from the hydroxylation of lipids. This reduction of serum ascorbate levels in cataract and POAG suggests the increased utilization of ascorbate for the neutralization of adducts formed from the reaction between the reactive species and diverse biomolecules.
Conversely, the total serum protein was significantly higher among the patients with ARC or POAG than the controls in our study. The possible explanations for this finding include the increased albumin synthesis due to increased oxidative stress, or the possibility of underlying inflammatory processes associated with these conditions resulting in increased synthesis of inflammatory mediators. However, the serum albumin or specific inflammatory mediators were not measured in our study, and we can only speculate that this may account for the observed differences.
The limitations of this study include the absence of correlation with the clinical measures of visual function to address the direct clinical consequences of this data. Also, exclusion was not based on the current or recent use of eye drops. It has been suggested that the topical anti-glaucoma agents may increase the oxidative stress. However, this may not necessarily reflect in the systemic oxidative stress status, and it would have been ethically problematic to study only the glaucoma patients, who are not using eye drops. Resource constraints made it impossible for us to assess other parameters such as serum tocopherol (Vitamin E) levels or important parameters such as total anti-oxidant status or oxidative DNA damage. Future studies among this population will need to address these limitations and should be explore the inclusion of these parameters as well as the analysis of oxidative stress indices in aqueous humor samples. The latter is particularly important considering that recent evidence demonstrated the racial differences in oxygen levels in the human eye suggesting that this may reflect an important difference in oxidative metabolism in the cornea and lens as well as the systemic physiologic function.29
Our findings reinforce the available evidence supporting a role for oxidative stress in these ocular disorders. This lends a support for the incorporation of exogenous anti-oxidants in the treatment of glaucoma and cataract, especially at the early stages of these conditions. Exogenous anti-oxidants may help in modulating the oxidative stress and thus improve prognosis. A more versatile application of anti-oxidants could be in their use for the primary prevention of these ocular disorders.
CONCLUSION
The reduced levels of enzymatic and nonenzymatic anti-oxidants were found in the patients with ARC or POAG, compared with controls having neither of these conditions. By inference, this suggests the higher levels of systemic oxidative stress in these conditions. These findings suggest a potential role for anti-oxidants in the prevention and treatment of these conditions. Thus, the investigation for and development of ocular anti-oxidants, will be beneficial to ocular therapeutics on a global scale.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Global initiative for the elimination of avoidable blindness: The Global Picture. [Last accessed on 2012 Mar 02]. Available from: https://www.apps.who.int/inf-fs/en/fact213.htmlBlindness .
- 2.Beebe DC, Holekamp NM, Shui YB. Oxidative damage and the prevention of age-related cataracts. Ophthalmic Res. 2010;44:155–65. doi: 10.1159/000316481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calugaru D, Calugaru M. Tendencies in the etiopathogenesis of glaucoma – Present and future. Oftalmologia. 2008;52:10–22. [PubMed] [Google Scholar]
- 4.Cekic S, Zlatanovic G, Cvetkovic T, Petrovic B. Oxidative stress in cataractogenesis. Bosn J Basic Med Sci. 2010;10:265–9. doi: 10.17305/bjbms.2010.2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tezel G. Oxidative stress in glaucomatous neurodegeneration: Mechanisms and consequences. Prog Retin Eye Res. 2006;25:490–513. doi: 10.1016/j.preteyeres.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghanem AA, Arafa LF, El-Baz A. Oxidative stress markers in patients with primary open-angle glaucoma. Curr Eye Res. 2010;35:295–301. doi: 10.3109/02713680903548970. [DOI] [PubMed] [Google Scholar]
- 7.Vinson JA. Oxidative stress in cataracts. Pathophysiology. 2006;13:151–62. doi: 10.1016/j.pathophys.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Agte V, Tarwadi K. The importance of nutrition in the prevention of ocular disease with special reference to cataract. Ophthalmic Res. 2010;44:166–72. doi: 10.1159/000316477. [DOI] [PubMed] [Google Scholar]
- 9.Spector A, Garner WH. Hydrogen peroxide and human cataract. Exp Eye Res. 1981;33:673–81. doi: 10.1016/s0014-4835(81)80107-8. [DOI] [PubMed] [Google Scholar]
- 10.Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363:1711–20. doi: 10.1016/S0140-6736(04)16257-0. [DOI] [PubMed] [Google Scholar]
- 11.Van Buskirk EM, Cioffi GA. Glaucomatous optic neuropathy. Am J Ophthalmol. 1992;113:447–52. doi: 10.1016/s0002-9394(14)76171-9. [DOI] [PubMed] [Google Scholar]
- 12.Halpern DL, Grosskreutz CL. Glaucomatous optic neuropathy: Mechanisms of disease. Ophthalmol Clin North Am. 2002;15:61–8. doi: 10.1016/s0896-1549(01)00012-8. [DOI] [PubMed] [Google Scholar]
- 13.Declaration of Helsinki - Ethical Principles for Medical Research Involving Human Subjects. 52nd World Medical Assembly, Edinburgh. 2000 [Google Scholar]
- 14.Beers RF, Jr, Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952;195:133–40. [PubMed] [Google Scholar]
- 15.Sun M, Zigman S. An improved spectrophotometric assay for superoxide dismutase based on epinephrine autoxidation. Anal Biochem. 1978;90:81–9. doi: 10.1016/0003-2697(78)90010-6. [DOI] [PubMed] [Google Scholar]
- 16.Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 17.Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–9. [PubMed] [Google Scholar]
- 18.Beuge JA, Aust SD. The thiobarbituric acid assay. Methods Enzymol. 1978;52:306. [Google Scholar]
- 19.Gornall AG, Bardawill CJ, David MM. Determination of serum proteins by means of the biuret reaction. J Biol Chem. 1949;177:751–66. [PubMed] [Google Scholar]
- 20.Roe JH, Kuether CA. The determination of ascorbic acid in whole blood and urine through the 2,4-dinitrophenylhydrazine derivative of dehydroascorbic acid. J Biol Chem. 1943;147:399. [Google Scholar]
- 21.Faschinger C, Schmut O, Wachswender C, Mossböck G. Glaucoma and oxidative stress. Determination of malondialdehyde – A product of lipid peroxidation. Ophthalmologe. 2006;103:953–9. doi: 10.1007/s00347-006-1399-3. [DOI] [PubMed] [Google Scholar]
- 22.Li L, Duker JS, Yoshida Y, Niki E, Rasmussen H, Russell RM, et al. Oxidative stress and antioxidant status in older adults with early cataract. Eye (Lond) 2009;23:1464–8. doi: 10.1038/eye.2008.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maurya OP, Mohanty L, Bhaduri G, Chandra A. Role of anti-oxidant enzymes superoxide dismutase and catalase in the development of cataract: Study of serum levels in patients with senile and diabetic cataracts. J Indian Med Assoc. 2006;104:394–396. 7. [PubMed] [Google Scholar]
- 24.Zanon-Moreno V, Marco-Ventura P, Lleo-Perez A, Pons-Vazquez S, Garcia-Medina JJ, Vinuesa-Silva I, et al. Oxidative stress in primary open-angle glaucoma. J Glaucoma. 2008;17:263–8. doi: 10.1097/IJG.0b013e31815c3a7f. [DOI] [PubMed] [Google Scholar]
- 25.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: Analysis of worldwide data. Lancet. 2005;365:217–23. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 26.Sohal RS, Mockett RJ, Orr WC. Mechanisms of aging: An appraisal of the oxidative stress hypothesis. Free Radic Biol Med. 2002;33:575–86. doi: 10.1016/s0891-5849(02)00886-9. [DOI] [PubMed] [Google Scholar]
- 27.Khan MA, Tanja M, Zhang O, Cuen H. Antioxidant enzymes and cancer. Chin J Cancer Res. 2010;22:87–92. [Google Scholar]
- 28.Yang J, Tezel G, Patil RV, Romano C, Wax MB. Serum autoantibody against glutathione S-transferase in patients with glaucoma. Invest Ophthalmol Vis Sci. 2001;42:1273–6. [PubMed] [Google Scholar]
- 29.Siegfried CJ, Shui YB, Holekamp NM, Bai F, Beebe DC. Racial differences in ocular oxidative metabolism: Implications for ocular disease. Arch Ophthalmol. 2011;129:849–54. doi: 10.1001/archophthalmol.2011.169. [DOI] [PMC free article] [PubMed] [Google Scholar]


