Abstract
For the diagnosis of prostate cancer trans-rectal prostate biopsy (TRPB) is used commonly, the procedure is associated with infective complications. There is evidence that antibiotics (ABx) decrease infective events after TRPB, but different regimens are used. To systematically review different regimens of prophylactic oral ABx in TRPB. MEDLINE, EMBASE, clinical trials site, and Cochrane library were searched, experts were consulted for relevant studies. Randomized clinical trials conducted in the last 20 years, which investigated the different oral antibiotic regimens in TRPB, and compared their efficacy to reduce infectious complications were analyzed. Primary outcomes were bacteriuria, urinary tract infection (UTI), fever, bacteremia, and sepsis. Secondary outcomes were the hospitalization rate and the prevalence of ABx-resistant bacteria. Nine trials were eligible with 3012 patients. ABx prevented bacteriuria (3.5% vs. 9.88%), UTI (4.46% vs. 9.75%), and hospitalization (0.21% vs. 2.13%) significantly in comparison with placebo or no treatment. No significant difference was found in all the outcomes of the review between the single dose regimen and the 3 days. The single dose regimen was as effective as the multiple doses except in bacteriuria (6.75% vs. 3.25%), and the prevalence of ABx-resistant bacteria (1.57% vs. 0.27%). Quinolones reduced only UTI significantly in comparison with other ABx (chloramphenicol, trimethoprim-sulfamethoxazol). It is essential to prescribe prophylactic ABx in TRPB. No conclusive evidence could be claimed about the superiority of the multiple or the 3 days regimens to the single dose regimen. Unexpectedly, ABx-resistant bacteria were identified more often in the single dose cohorts.
Keywords: Antibiotics, fever, infection, trans-rectal prostate biopsy
INTRODUCTION
Prostate cancer is the most common cancer among males in the USA.[1] In the UK, 134 new cases were found in every 100,000 males.[2,3] To diagnose prostate cancer, a prostate biopsy should be performed. Trans-rectal prostate biopsy (TRPB) is one of the most commonly used urological procedures and the most common to diagnose prostate cancer.[3,4,5,6] TRPB is associated with many complications, these are usually self-limiting rarely fatal[7] and mortal.[8] Many studies showed that prophylactic antibiotics (ABx) in TRPB decrease infectious events.[9,10,11] Prevalence of ABx-resistant bacteria has increased in the last decade, this increase has led to more infectious complications and hospitalization rate.[12] In Kuwait, the incident of septicemia after trans-rectal ultrasound (TRUS) guided biopsy of prostate increased between 2001 and 2005 due to quinolone-resistant Escherichia coli in a study conducted by Kehinde et al. 2013, it was found that the addition of intravenous amikacin to quinolone decreased the number of septicemic cases.[13] In Australia, a study conducted to estimate the prevalence of ABx-resistant bacteria among hospitalized septic patients after TRUS biopsy showed that 41% of septic patients had multi-drug-resistant organisms.[14]
Prophylactic ABx for TRPB is still an issue of debate, different regimens were recommended and studied. In a survey conducted by Davis et al. about preparations for TRPB, 80% of responders provided different techniques and preparations, but the majority of them prescribed prophylactic ABx and used enema.[15]
Oral prophylactic ABx in TRPB are not inferior to parenteral route, in a study conducted by Roach et al., patients who received oral ciprofloxacin had significantly lower rates of bacteremia compared to patients who received intravenous gentamicin.[16] Cam et al. showed no difference in morbidity between oral and systematic ABx.[17] In a Canadian randomized controlled trial (RCT) published in 2004, there was no statistical nor clinical difference between 1-day and 3-day regimen among patients who underwent TRUS guided prostate biopsy.[18] In a prospective randomized study with 236 patients enrolled, 1-day course was associated with low events of febrile infective complication, and there was no difference between the two regimens in terms of elevation of white blood cells and C-reactive protein after TRPB.[19] The EAU 2015 guideline for urological infections states that 1-day course or even a single dose of prophylactic ABx is adequate in low risk patients.
Therefore, I decided to review recent RCTs about oral prophylactic ABx in TRPB. To my knowledge, this is the first systematic review focused solely on oral prophylactic ABx in TRPB.
Objectives/aims
To evaluate and review randomized prospective trials performed in English, during the last 20 years (1995–2015) about prophylactic oral ABx which compared different types of ABx, and different duration of therapy in TRPB, to prevent postbiopsy infectious complications (bacteriuria, urinary tract infection [UTI], fever, sepsis, and bacteremia), hospitalization, and prevalence of ABx-resistant organisms.
Registration
This systematic review was registered with PROSPERO, registration number: RD42015016906.
METHOD OF SEARCH/DESIGN
I searched MEDLINE, EMBASE, clinical trials site (www.clinicaltrials.gov), and Cochrane library, experts were consulted about the latest relevant trials to my search and review.
Searched keywords included (antibiotics before prostate biopsy randomized clinical trial), (prophylactic antibiotics prostate biopsy randomized comparative trial), and (prostate biopsy antibiotic prophylaxis).
Overall 324 studies were found: 67 results for the first keywords, 14 results for the second keywords, and 243 results for the third keywords. Last date of the search was 13th February 2015.
Selection criteria
RCTs with the following interventions were included:
Oral prophylactic ABx versus placebo/no treatment
Oral prophylactic ABx versus another oral ABx
Combination therapy of two different classes of ABx, e.g., combining Ciprofloxacin with metronidazol versus combination therapy.
And compared dosage, frequency of administration, length of therapy, in reducing infective complications postbiopsy, hospitalization, and ABx-resistant bacteria were eligible.
RCTs which did not exclude patients with the following conditions before the intervention were not eligible: (1) UTI, or urinary symptoms, or positive urine culture/bacteriuria, and (2) urinary catheter, or stent.
Definitions
Short course of antibiotic(s): Antibiotic(s) given as a single dose, or for 1-day
Long course of antibiotic(s): Antibiotic(s) given for 2–3 days.
RESULTS
Eligibility was decided by myself after careful reading and screening of the relevant papers. Thirty-three studies were screened, and full text assessment for eligibility was carried out, data were extracted by myself. After studying the result of my search, 3 authors were contacted through email to give details about their exclusion criteria in their reports (Stacy Loeb, Robert Sabbagh, and Bosquet Sanz), one reply from one author was received.
Twenty-one Studies were relevant to the review. Eleven studies were excluded from this review, these studies are Sabbagh et al. 2004,[18] Bosquet Sanz 2006,[20], Mari 2007,[21] Loeb et al. 2012,[22] Chan et al. 2012,[23] and Petteffi 2002[24] due to insufficient exclusion criteria in these studies. Shigemura et al. 2005[19] were excluded due to inappropriate randomization, Yang et al. 2001[25] and Tekdogan et al. 2006[26] were excluded due to language barrier. Aus et al. 1996[3] were excluded due to very long scheme of ABx (7 days), Cam et al.'s 2008[17] study was excluded because it examined the intramuscular route, Argyropoulos et al. 2007 were excluded because I could not include it in any section of my review [Flow Chart 1].
Flow Chart 1.
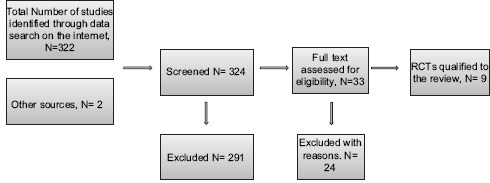
Studies selection process
Qualified studies for this review were: Linden-Castro et al. 2014,[27] Bateni et al. 2013,[28] Briffaux et al. 2008,[29] Yamamoto et al. 2008,[30] Schaeffer et al. 2007,[31] Argyropoulos et al. 2007,[32] Tobias-Machado et al. 2003,[33] Aron et al. 2000,[10] Isen et al. 1999,[34] Kapoor et al. 1998.[9]
Data on the following outcomes were extracted and evaluated carefully:
Primary outcomes assessed in this review
Bacteriuria–midstream urine sample with at least 100,000 CFU/ml or more of bacteria, in men.
UTI – infection of the urinary tract with dysuria, frequency, and suprapubic pain.[35]
Fever (high grade or low grade).
Bacteremia (bacteria in blood culture).
Sepsis – clinical syndrome caused by bacteremia associated with tachypnea (>20 breaths/min), fever or hypothermia, tachycardia (>90 beats/min), and white blood cells count >12000 cells/mm3 or <4000 cells/mm3 or >10% immature (band) forms.
Secondary outcomes
Hospitalization rate
Prevalence of antibiotic-resistant bacteria.
The total population of the studies included in this review is 3012 patients. Geographical distribution of the population reviewed included Mexico, Iran, France, Japan, Brazil, Canada, Italy, Spain, USA, Turkey, and India. Two studies were multicenter (Kapoor 1998, Schaeffer 2007), five studies lost patients due to various reasons, these trials are: Kapoor 1998, Schaeffer 2007, Bateni 2013, Castro 2014, Briffaux 2008 [Table 1].
Table 1.
Population of the review

Details of the selected studies
Overall nine RCTs were eligible for this systematic review (Bateni et al. 2013, Briffaux et al. 2008, Yamamoto et al. 2008, Schaeffer et al. 2007, Tobias-Machado et al. 2003, Aron et al. 2000, and Isen et al. 1999, Kapoor et al. 1998) as mentioned above, with a total of 3012 patients.
Two studies contained three arms, one study contained four arms, and six studies had two arms.
One study investigated placebo versus 1 dose of ciprofloxacin 500 mg (Kapoor 1998), one study investigated no treatment versus single dose of ofloxacin 400 mg versus single dose of trimethoprim-sulfamethoxazol (TMP-SMX) (Isen 1999), Aron 2000 investigated placebo versus single dose of combination therapy (ciprofloxacin 500 mg plus Tinidazol 600 mg) versus 3 days of the same combination therapy.
Tobias-Machado 2003 studied single dose of ciprofloxacin 500 mg versus 3 days of the same antibiotic versus 3 days of chloramphenicol 600 mg versus 3 days of norfloxacin 400 mg. Schaeffer 2007 investigated 1-day of ciprofloxacin 1000 mg extended release versus. Three days of the same antibiotic.
Briffaux 2008 studied single dose of ciprofloxacin 500 mg versus 3 days of the same medication. Yamamoto 2008 studied 2 days of tusofloxacin 300 mg versus levofloxacin 200 mg with the same duration of therapy. Bateni 2013 examined single dose of combination therapy (ciprofloxacin 500 mg plus two tablets of metronidazole 250 mg) against 3 days of the same combination, and Castro 2014 studied single dose of levofloxacin 500 mg versus 3 days regimen.
Five studies examined ciprofloxacin, three studies contained another type of quinolone, and one study examined ciprofloxacin versus another type of quinolone.
Four studies contained other ABx than quinolones, one study had TMP-SMX, another one contained chloramphnicol 600 mg, and two studies contained nitro-imidazols (metronidazol 250 mg, tinidazol 600 mg).
The enema was used in five studies, three studies did not use it, and in one study nothing was mentioned about enema, so it was not clear if it was used or not. All included studies reported the number of samples taken during the biopsy or the mean number of cores, except one study (Aron 2000). The size of the needle used during the procedure was mentioned in five studies out of the selected nine. Utilization of TRUS guided biopsy was reported in six studies, one study utilized digitally guided TRPB, and in two studies it was not clear which technique was used.
Follow-up visits were mentioned in all nine RCTs, which ranged from 48 h to 3 weeks.
Symptoms were assessed by a questionnaire in two studies (Isen 1999, Briffaux 2008), International Prostate Symptoms Score was used in Bateni 2013.
Patients mean age was mentioned in eight studies, the mean age of the total cohort of these eight studies was 67.3 years.
DATA SYNTHESIS
Section 1 – oral antibiotics versus placebo/no treatment
In this section, data were extracted from three studies (Kapoor 1998, Isen 1999, Aron 2000) with a total number of patients being 898.
The summary of the findings is shown in [Chart 1].
Chart 1.
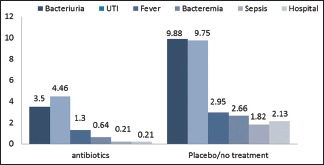
Outcomes of the review in antibiotics versus placebo/no treatment, in percentage (%)
Bacteriuria
Bacteriuria was examined in two studies (Kapoor 1998, Isen 1999), with 667 patients. Placebo/no treatment arms had 25 incidents out of 253 patients (9.88%). While in patients who received ABx, the incident of bacteriuria was 11/314 patients (3.5%). There was a remarkable difference in favor of ABx usage. Relative risk: 0.3764, 95% confidence interval (CI): 0.1886–0.7510, P = 0.0056.
Urinary tract infection
UTI events were recorded in all three studies. Patients in the placebo/no treatment arms had 32 events out of 328 patients (9.75%). Patients assigned to receive ABx in all three studies had overall 21 UTI events out of 470 investigated patients (4.46%). A significant difference was found and favored the usage of ABx. Relative risk: 0.4812, 95% CI: 0.2823–0.8202, P = 0.0072.
Fever
Information about fever can be found in two studies (Kapoor 1998, Aron 2000) out of the three, with a cohort number of 788. In placebo/no treatment arms 9 out of 305 (2.95%) had fever shortly after the biopsy. In ABx arms collected data from the two studies indicated that 5 out of 383 (1.30%) patients had fever. There was no remarkable difference between the groups. Relative risk: 0.4496, 95% CI: 0.1522–1.3280, P = 0.1480.
Bacteremia
In this section of our review only Aron 2000 reported this outcome, with 231 patients, 2 patients out of 75 patients in the placebo arm had this complication, overall 2.66% of patients who received placebo had bacteremia.
In the ABx arms, 1 patient had bacteremia (1/156, 0.64%), and he was found in the group who received 3 day course of prophylactic ABx, no significant difference was found. Relative risk: 0.2452, 95% CI: 0.0226–2.6628, P = 0.2480.
Sepsis
Data on sepsis can be collected from two studies (Kapoor 1998, Aron 2000), with a total of 788 patients. Six patients who did not receive antibiotic had septic incident out of 305 (1.96%). In the ABx arms only one patient out of 383 patients had this complication (0.26%). There was no significant difference. Relative risk: 0.1350, 95% CI: 0.0163–1.1153, P = 0.0631.
Hospitalization rate
Hospitalization occurred anytime during the follow-up was reported in Isen 1999 and Kapoor 1998 with 667 patients.
In the placebo/no treatment arms, 7 patients were hospitalized. The overall incident is 7/328 (2.13%). On the contrary, the ABx arms of the three studies had only one case of hospitalization 1/470 (0.21%). A significant difference was found in favor of ABx. Relative risk: 0.1151, 95% CI: 0.0143–0.9295, P = 0.0425.
Antibiotics-resistance
This outcome was reported in Aron 2000, no resistant bacteria to ciprofloxacin was detected.
Section 2 – single dose versus 3 days regimen in trans-rectal prostate biopsy
In this section, studies which investigated 1 dose regimen and compared it to 3 days were reviewed. Five studies were included in this part of the systematic review, Aron 2000, Tobias-Machado 2003, Schaeffer 2007, Briffaux 2008, and Castro 2014 with overall 1922 patients.
Bacteriuria
This event was reported in Schaeffer 2007 and Briffaux 2008 with 819 patients. There were 17 events in the single dose arm (4.87%), and 10 events in the 3 day regimen cohort (2.85%). No significant difference was found. Relative risk: 1.7552, 95% CI: 0.8153–3.7782, P = 0.1504.
Urinary tract infection
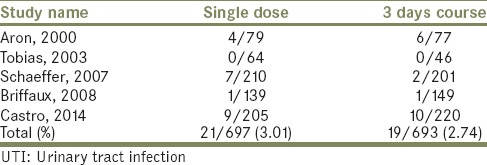
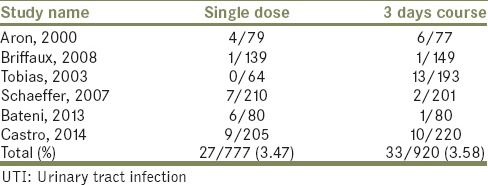
This outcome was reported in all of the reviewed studies. Twenty-one patients had this complication after TRPB in the single dose arms out of 697 patients. In the 3 days course regimen, 19 patients suffered from UTI out of 693 patients. No significant difference was found [Table 2]. Relative risk: 1.0989, 95% CI: 0.5961–2.0257, P = 0.7624.
Table 2.
UTI events in the single dose versus 3 days course
Fever
This outcome was reported in Aron 2000, Tobias 2003, and Castro 2014 with 1103 patients. Data about fever were unclear in Schaeffer 2007. Thirteen patients in each single dose arm and the 3 days course arm had this event, the difference was extremely tiny. Relative risk: 0.9861, 95% CI: 0.4636–2.0975, P = 0.9711.
Bacteremia
Data about this complication can be extracted from three studies (Aron 2000, Tobias 2003, Schaeffer 2007) with 985 patients. One case of bacteremia was reported in the single dose regimen and in the 3 days. Almost no difference was found. Relative risk: 0.9178, 95% CI: 0.0576–14.6147, P = 0.9516.
Sepsis
Aron 2000 reported one case of this outcome in the 3 days regimen group (1/77, 1.29%, and 0/79). In Castro 2014, 2 patients had this complication in the single dose cohort, while no case was reported in the 3 days regimen cohort. Overall 2 reported in the single dose cohort (2/284, 0.70%), and 1 case in the 3 days regimen cohort (1/297, 0.33%), no remarkable difference was found. Relative risk: 1.0458, 95% CI: 0.0657–16.6405, P = 0.9747.
Hospitalization
Data about this end point can be found in Tobias 2003 and Castro 2014 with 872 patients. Castro 2014 stated that patients with febrile UTI were hospitalized, so in this section we added the number of patients who had febrile UTI episodes from Castro's study. While in Tobias 2003, noone was hospitalized from groups 1 and 2. Overall 9 out of 269 (3.34%) in the single dose cohort, and 10 patients out of 266 (3.75%) in the 3 day regimen cohort were hospitalized. No significant difference was noticed. Relative risk: 0.8900, 95% CI: 0.3675–2.1552, P = 0.7961.
Antibiotics-resistant bacteria
Data about this outcome was reported in four studies with 1665 patients. In Aron 2000, no incident of ABx-resistance was found. In schaeffer 2007, 5 isolates were resistant to ciprofloxacin (4 E. coli, 1 Staphylococcus aureus) in the single dose group (5/210, 2.38%), in the 3 days group, no event was reported. In Briffaux 2008 reported three resistant isolates (Enterococcus faecalis, Morganella morgana, and E. coli), one in group 1, and two in group 2, all were resistant to ciprofloxacin (1/139, 0.71%, 2/149, and 1.34%). No case of this complication was reported in Castro 2014. No significant difference was shown. Relative risk: 3.0664, 95% CI: 0.6212–15.1358, P = 0.1690 [Chart 2].
Chart 2.
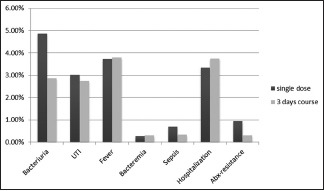
Outcomes of the review in single dose cohort versus 3 days, in percentage (%)
Section 3 – single dose versus multiple dose regimen in trans-rectal prostate biopsy
In this section, 1 reviewed studies which compared patients who received a single dose of ABx, with those who received multiple doses. Six studies were included (Aron 2000, Tobias-Machado 2003, Schaeffer 2007, Briffaux 2008, Bateni 2013, and Castro 2014) with overall 2102 patients. Summary of this section can be found in [Chart 3].
Chart 3.
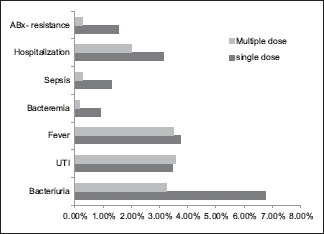
Outcomes of the review in single dose versus multiple dose regimen
Bacteriuria
This outcome can be found in three studies (Schaeffer 2007, Briffaux 2008, Bateni 2013) with 999 patients. Combining the results of the three studies shows that 29 patients in the single dose groups had this incident out of 430 (29/429, 6.75%), while in the multiple dose group 14 events occurred out of 430 patients (14/430, 3.25%), multiple dose regimen was more effective to reduce this outcome. Relative risk: 2.0081, 95% CI: 1.0755–3.7493, P = 0.0286.
Urinary tract infection
Data on UTI events can be collected from the six studies, there was no difference between the two groups. Table 3 illustrates the results of this outcome, no significant difference was found. Relative risk: 0.9698, 95% CI: 0.5883–1.5988, P = 0.9043.
Table 3.
UTI events in the single dose versus multiple dose course
Fever
Information about fever can be collected from five studies (Aron 2000, Tobias 2003, Schaeffer 2007, Bateni 2013, and Castro 2014) with 1680 patients. There was no significant difference between the two groups. Relative risk: 1.0631, 95% CI: 0.5573–2.0277, P = 0.8527.
Bacteremia
Findings of this outcome can be found in four studies (Aron 2000, Tobias 2003, Schaeffer 2007, and Bateni 2013) with a total of 1165 patients. No extreme difference was found. Relative risk: 6.3014, 95% CI: 0.7389–53.7408, P = 0.0923.
Sepsis
Data about this outcome were collected from three studies (Aron 2000, Bateni 2013, and Castro 2014) with 1026 patients. Over all 5 cases in the single dose cohort had sepsis out of 364 patients (5/364, 1.37%), while in the multiple dose cohort 1 case was reported (1/377, 0.26%), no significant difference was found. Relative risk: 5.1220, 95% CI: 0.6013–43.6329, P = 0.1350.
Hospitalization
Data about this outcome can be extracted from three studies (Castro 2014, Bateni 2013, and Tobias 2003) with overall 1052 patients. 11 out of 349 were hospitalized in the single dose division (11/349, 3.15%). On the other hand, 11 hospital admissions occurred out of 493 patients (2.02%) in the multiple dose cohort, there was no remarkable difference. Relative risk: 1.4000, 95% CI: 0.6137–3.1938, P = 0.4239.
Antibiotics-resistant bacteria
Data about the prevalence of ABx-resistant bacteria can be found in five studies (Castro 2014, Bateni 2013, Briffaux 2008, Schaeffer 2007, and Aron 2000) with 1845 patients. There was a remarkable difference between the two groups in favor of the multiple dose regimen. Relative risk: 5.5380, 95% CI: 1.2318–24.8978, P = 0.0256.
Section 4 – single dose of combination therapy (quinolone and nitro imidazole) versus multiple dose of combination therapy (quinolone and nitro imidazole)
In this section, 1 reviewed studies which examined single dose of ABx combining ciprofloxacin with other nitroimidazole (metronidazole or tinidazol) and compared it to multiple doses course of the same combination. Two studies addressed this issue, Bateni 2013, and Aron 2000 with overall 411 patients randomized to different arms. Summary of this section can be found in [Chart 4].
Chart 4.

Outcomes of the review in single dose versus multiple doses of combination therapy, in percentage (%)
Bacteriuria
Data were found only in Bateni 2013 with 180 patients. In the single dose group 12 patients out of 80 had this outcome (12/80, 15%), while in the multiple dose regimen, 4 patients out of 80 had it (4/80, 5%), there was no significant difference. Relative risk: 2.73, 95% CI: 0.91–8.16, P = 0.0706.
Urinary tract infection
Information about this end point can be extracted from both studies, Aron 2000 and Bateni 2013. In Aron et al. 2000, 4 patients in the single dose group had UTI events out of 79 (4/79, 5.06%). In the 3 days group of the same study 6 patients out of 77 had it (6/77, 7.79%). In Bateni et al. 2013, 6 patients out of 80 in the single dose group, had UTI events without fever (6/80, 7.5%). While in the multiple dose group, 1 patient out of 80 had it (1/80, 1.25%), there was no significant difference. Relative risk: 1.3863, 95% CI: 0.5406–3.5550, P = 0.4966.
Fever
This outcome was reported in both studies. In Aron 2000, 2 patients in each group had fever (2/79, 2.53%, 2/77, and 2.59%). On the other hand, Bateni reported 3 cases of feverish patients out of 80 in the single dose arm, while no patients had this event in the multiple doses (3/80, 3.75%). There was no remarkable difference. Relative risk: 2.42, 95% CI: 0.4771–12.3124, P = 0.2857.
Bacteremia
This outcome was reported in both reviewed studies. In Aron 2000, only one patient in the multiple dose group had bacteremia (1/77, 1.29%), while in the single dose group, no one had this outcome. In Bateni 2013, 3 feverish patients had positive blood cultures, these patients were randomized to receive a single dose of combination therapy (3/80, 3.75%). To the contrary, no patients in multiple dose group had this event. No remarkable difference was seen. Relative risk: 2.9259, 95% CI: 0.3076–27.8321, P = 0.3502.
Sepsis
This outcome was reported in both studies. In Aron 2000, one patient in the multiple dose group had a fever with bacteremia (sepsis) out of 77 (1/77, 1.29%), while in the single dose division no one had this complication. In Bateni 2013, the author mentioned that the three patients with fever from the single dose arm had E. coli in their blood culture (3/80, 3.75%), while the multiple dose arm did not have any event, no huge difference was found. Relative risk: 2.9259, 95% CI: 0.3076–27.8321, P = 0.3502.
Hospitalization
Information about this outcome can be found in Bateni 2013. Two of the three feverish patients from the single dose group had to be admitted to the hospital (2/80, 2.5%), no one was admitted from the multiple dose arm, there was no remarkable difference between the two groups. P = 0.3038.
Antibiotics-resistant bacteria
Both studies reported the results of this outcome. In Aron 2000, all isolates were susceptible to ciprofloxacin. In Bateni 2013, five patients (2 with UTI, 3 with fever) in the single dose arm had ciprofloxacin resistant isolates (4 E. coli, 1 Pseudomonas spp.) out of 80 patients (5/80, 6.25%), while in the cohort who received multiple doses of combination therapy, they did not report any antibiotic-resistant organism, no significant difference was recognized. P = 0.1053.
Section 5 – quinolones versus other antibiotics
In this section, data can be found in two studies, Isen 1999 and Tobias-Machado 2003 with 367 patients. A summary of this section is shown in [Chart 5].
Chart 5.

Outcomes of this review in patients who received prophylactic quinolones versus patients who received other prophylactic antibiotics (chloramphenicol, trimethoprim-sulfamethoxazole), in percentage (%)
Bacteriuria
Data about this end point can be found in Isen 1999 with 110 patients. Three of 45 patients who received TMP-SMX had positive cultures, 2 E. coli, and 1 E. faecalis, while in the ofloxacin arm 2 patients out of 42 had positive cultures, one with E. coli and the other with Staphylococcus coagulase, no remarkable difference was found. Relative risk: 0.72, 95% CI: 0.12–4.15, P = 0.72
Urinary tract infection
This end point can be extracted from both studies. In Tobias's study, nine events occurred in the chloramphenicol's arm (9/71, 12.6%) and four UTI events in quinolones arms (4/186, 2.15%). While in Isen et al., 2 incidents were reported in the Ofloxacin's arm and 3 incidents in the TMP-SMX arm. Overall 6 events occurred in patients who received quinolones out of 228 (6/228, 2.63%), while 12 events occured out of 116 patients who received other classes of ABx (12/116, 10.34%), there was a significant difference and the results showed that quinolones are better in preventing UTI. Relative risk: 0.27, 95% CI: 0.10–0.71, P = 0.0079.
Fever
Data about fever can be found only in Tobias 2003, with 257 patients. In quinolones arms, 7 patients had fever out of 186 (7/186, 3.76%). On the other hand, chloramphenicol's arm had 3 feverish patients out of 71 (3/71, 4.22%), no remarkable difference was found. Relative risk: 0.77, 95% CI: 0.19–3.00, P = 0.7075.
Bacteremia
Information about this end point can be found in Tobias 2003. One patient in the Chloramphenicol's arm had bacteremia (1/71, 1.40%), and one had it in the quinolones arm. The isolate was Staphylococcus epidermidis in the quinolones arm (1/186, 0.53%), no remarkable difference was found. Relative risk: 0.38, 95% CI: 0.024–6.07, P = 0.4977.
Sepsis
Was not reported in any study.
Hospitalization
This outcome was reported in both studies, In quinolones arms no patient was admitted to hospital, while one patient in the chloramphenicol arm was hospitalized due to bacteremia, and no one in the TMX arm had this event (1/116, 0.86%), no significant difference was shown. P = 0.2795.
Antibiotics-resistant bacteria
Was not reported.
DISCUSSION
Regimens of prophylactic ABx is still an issue of debate in urology. Several authors tried to investigate durations of therapy and other techniques to lower infectious complications after TRPB. Ghafoori et al. found that rectal cleansing with povidone-iodine can reduce infective complications after the biopsy,[36] while using disposable needle was not successful in diminishing it.[37] Studies about prebiopsy enema gave conflicting results.[38,39] Other studies gave a promising future for targeted prophylactic ABx.[40,41] This systematic review strictly focused on oral prophylactic ABx, and contained five sections.
The first section of this review which investigated prophylactic ABx versus placebo or no treatment, results showed that ABx decreased all the infectious outcomes, especially bacteriuria (3.5% vs. 9.88%) and UTI (4.46% vs. 9.75%). Hospitalization was reduced in the ABx cohort which is an economic advantage of the ABx usage, in addition reducing hospitalization rate would lead to a lower rate of hospital-acquired infections.
Zani et al.[42] published a review and meta-analysis which showed that ABx reduced all infectious complication (bacteriuria, bacteremia, fever, and UTI) and hospitalization more than placebo/no treatment.
Similarly, Bootsma et al.[11] found that ABx reduce postbiopsy bacteriuria, but they did not find a strong evidence that ABx reduce fever, bacteremia, and symptomatic UTI.
In the second section, results show that there is no significant difference between the two different regimens in all the outcomes of the review. In fact, the single dose regimen prevented more fever, bacteremia, and hospitalization than the 3 days regimen.
Loeb et al.[43] findings support these results. No significant advantage was found by using ABx for more than 1-day to reduce infectious complications in Loeb's review, but they included in their review RCTs without exclusion criteria,[18,24] an RCT with inappropriate randomization.[19]
In the third section, single dose regimen versus the multiple doses were reviewed. The finding of the review showed that there is no significant difference between the single dose regimen and the multiple dose in UTI, fever, bacteremia, and hospitalization rate, while bacteriuria events and the prevalence of Abx-resistant bacteria were significantly decreased in the multiple dose cohort.
Zani et al.[42] found that there is no significant difference between multiple dose regimen and the single dose, except in bacteriuria in the benefit of the multiple dose therapy. Their results also showed that the multiple dose regimen had lower rates of fever, UTI, sepsis, and hospitalization, which is in agreement with the results of my review.
Some studies proposed that recent hospitalization, and diabetes mellitus increase the risk of hospitalization postbiopsy.[12,44] In the second section of my review, the rate of hospitalization was 3.34% in the single dose cohort, 3.75% in the 3 days cohort. Zani et al. found that 1.65% of patients who received 1-day regimen were hospitalized while none was hospitalized in the 3 days regimen.
In the third section, the results were almost identical to the second section with 3.15% of patients hospitalized in the single dose group, and 2.02% in the multiple doses.
In the fourth section of the review, single dose regimen of combination therapy was compared to the multiple doses. The results indicated that the multiple dose regimen is more effective in preventing all the infective outcomes of the review, hospitalization rate, and the prevalence of ABx-resistant bacteria (0% vs. 3.14%), but the difference was not significant.
In a study conducted by Adibi et al.,[45] it was shown that combination therapy was cost-effective in preventing hospitalization.
In the fifth section, oral quinolones were compared with other oral classes of ABx (chloramphenicol, TMP-SMX). There was no significant difference between quinolones and other classes of ABx except in UTI (2.36 vs. 10.34%).
In a meta-analysis conducted by Yang et al.,[46]12 studies were included with 1987 patients, they found that quinolones, co-quinolone plus nitroimidazole, and co-TMP-SMX are similarly effective to reduce bacteriuria, bacteremia, and middle degree fever.
The prevalence of ABx-resistant bacteria has increased in recent years.[47] Previous quinolones consumption was associated with extended-spectrum β-lactamase-producing E. coli,[48] targeted ABx therapy is getting popularity, but further studies are required to investigate the best tool for the detection of resistant E. coli before the biopsy.[49] In a randomized European study, the highest bacterial resistance was against TMP-SMX, and Amoxicillin.[22] In this review Abx-resistant bacteria was diagnosed more often in the short course regimens (0.94% vs. 0.30%, 1.54% vs. 0.27%, and 3.14% vs. 0%). Moreover, the difference was significant in the third section (1.54% vs. 0.27%) in favor of the long course regimen.
The cause of this result is unknown, all the included studies in the third section excluded patients who received ABx during the last 1–2 weeks before the biopsy, except one study,[10] but no incident of bacterial resistance was reported in this study. This interesting and surprising result needs more studies to confirm it.
PRACTICAL IMPACT OF THE REVIEW
There is no doubt now that ABx decrease infective complications. The length of ABx therapy will depend on the patient's risk factors and the urologist in charge, but in healthy individuals or in individuals with minimal risk factors it is appropriate to prescribe single dose or 1-day regimen. Other ABx classes than quinolones could be used as a prophylaxis in TRPB.
CONCLUSION
The review confirms that prescribing prophylactic ABx in TRPB is an adequate practice. There is undoubtful evidence that single dose regimen is equally effective as the multiple and the 3 days dose to prevent infective outcomes of the review. Quinolones decreased only UTI events more efficiently than other classes of ABx. Surprisingly, ABx-resistant bacteria was detected more often in the short course cohorts, more studies should be carried out to confirm this finding.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
This piece of work was undertaken as part of the Edinburgh specialist surgical qualification. Special thanks to Dr. E O. Kehinde for supervising the work, and to Dr. T. Lam for providing insights into medical statistics. The review was submitted in June 2015 to the Royal College of Surgeons Edinburgh.
REFERENCES
- 1.Centre for disease control and prevention. Cdc.gov. CDC - Cancer - Statistics By Demographic -Cancer among Men. 2015:N.p.. Web; 7 May, 2015. [Google Scholar]
- 2.Cancerresearchuk.org. Prostate Cancer Incidence Statistics: Cancer Research UK. 2015:N.p.. Web; 7 May, 2015. [Google Scholar]
- 3.Aus G, Ahlgren G, Bergdahl S, Hugosson J. Infection after transrectal core biopsies of the prostate – Risk factors and antibiotic prophylaxis. Br J Urol. 1996;77:851–5. doi: 10.1046/j.1464-410x.1996.01014.x. [DOI] [PubMed] [Google Scholar]
- 4.Mowatt G, Scotland G, Boachie C, Cruickshank M, Ford JA, Fraser C, et al. The diagnostic accuracy and cost-effectiveness of magnetic resonance spectroscopy and enhanced magnetic resonance imaging techniques in aiding the localisation of prostate abnormalities for biopsy: A systematic review and economic evaluation. Health Technol Assess. 2013;17:vii–xix. 1–281. doi: 10.3310/hta17200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Cancer Society. Prostate Cancer Prevention and Early Detection. Available from: http://www.cancer.org/acs/groups/cid/documents/webcontent/003182-pdf.pdf .
- 6.Patel AR, Jones JS. Optimal biopsy strategies for the diagnosis and staging of prostate cancer. Curr Opin Urol. 2009;19:232–7. doi: 10.1097/mou.0b013e328329a33e. [DOI] [PubMed] [Google Scholar]
- 7.Berger AP, Gozzi C, Steiner H, Frauscher F, Varkarakis J, Rogatsch H, et al. Complication rate of transrectal ultrasound guided prostate biopsy: A comparison among 3 protocols with 6, 10 and 15 cores. J Urol. 2004;171:1478–80. doi: 10.1097/01.ju.0000116449.01186.f7. [DOI] [PubMed] [Google Scholar]
- 8.Challacombe B, Dasgupta P, Patel U, Amoroso P, Kirby R. Recognizing and managing the complications of prostate biopsy. BJU Int. 2011;108:1233–4. doi: 10.1111/j.1464-410X.2011.10621.x. [DOI] [PubMed] [Google Scholar]
- 9.Kapoor DA, Klimberg IW, Malek GH, Wegenke JD, Cox CE, Patterson AL, et al. Single-dose oral ciprofloxacin versus placebo for prophylaxis during transrectal prostate biopsy. Urology. 1998;52:552–8. doi: 10.1016/s0090-4295(98)00296-9. [DOI] [PubMed] [Google Scholar]
- 10.Aron M, Rajeev TP, Gupta NP. Antibiotic prophylaxis for transrectal needle biopsy of the prostate: A randomized controlled study. BJU Int. 2000;85:682–5. doi: 10.1046/j.1464-410x.2000.00576.x. [DOI] [PubMed] [Google Scholar]
- 11.Bootsma AM, Laguna Pes MP, Geerlings SE, Goossens A. Antibiotic prophylaxis in urologic procedures: A systematic review. Eur Urol. 2008;54:1270–86. doi: 10.1016/j.eururo.2008.03.033. [DOI] [PubMed] [Google Scholar]
- 12.Loeb S, Carter HB, Berndt SI, Ricker W, Schaeffer EM. Complications after prostate biopsy: Data from SEER-Medicare. J Urol. 2011;186:1830–4. doi: 10.1016/j.juro.2011.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kehinde EO, Al-Maghrebi M, Sheikh M, Anim JT. Combined ciprofloxacin and amikacin prophylaxis in the prevention of septicemia after transrectal ultrasound guided biopsy of the prostate. J Urol. 2013;189:911–5. doi: 10.1016/j.juro.2012.08.237. [DOI] [PubMed] [Google Scholar]
- 14.Leahy OR, O'Reilly M, Dyer DR, Phillips D, Grummet JP. Transrectal ultrasound-guided biopsy sepsis and the rise in carbapenem antibiotic use. ANZ J Surg. 2014;30 doi: 10.1111/ans.12933. doi: 10.1111/ans.12933. [DOI] [PubMed] [Google Scholar]
- 15.Davis M, Sofer M, Kim SS, Soloway MS. The procedure of transrectal ultrasound guided biopsy of the prostate: A survey of patient preparation and biopsy technique. J Urol. 2002;167(2 Pt 1):566–70. doi: 10.1016/S0022-5347(01)69087-6. [DOI] [PubMed] [Google Scholar]
- 16.Roach MB, Figueroa TE, McBride D, George WJ, Neal DE., Jr Ciprofloxacin versus gentamicin in prophylaxis against bacteremia in transrectal prostate needle biopsy. Urology. 1991;38:84–7. doi: 10.1016/0090-4295(91)80024-2. [DOI] [PubMed] [Google Scholar]
- 17.Cam K, Kayikci A, Akman Y, Erol A. Prospective assessment of the efficacy of single dose versus traditional 3-day antimicrobial prophylaxis in 12-core transrectal prostate biopsy. Int J Urol. 2008;15:997–1001. doi: 10.1111/j.1442-2042.2008.02147.x. [DOI] [PubMed] [Google Scholar]
- 18.Sabbagh R, McCormack M, Péloquin F, Faucher R, Perreault JP, Perrotte P, et al. A prospective randomized trial of 1-day versus 3-day antibiotic prophylaxis for transrectal ultrasound guided prostate biopsy. Can J Urol. 2004;11:2216–9. [PubMed] [Google Scholar]
- 19.Shigemura K, Tanaka K, Yasuda M, Ishihara S, Muratani T, Deguchi T, et al. Efficacy of 1-day prophylaxis medication with fluoroquinolone for prostate biopsy. World J Urol. 2005;23:356–60. doi: 10.1007/s00345-005-0024-4. [DOI] [PubMed] [Google Scholar]
- 20.Bosquet Sanz M, Gimeno Argente V, Arlandis Guzmán S, Bonillo García MA, Trassierra Villa M, Jiménez Cruz JF. Comparative study between tobramicin and tobramicin plus ciprofloxacin in transrectal prostate biopsy prophylaxis. Actas Urol Esp. 2006;30:866–70. doi: 10.1016/s0210-4806(06)73552-5. [DOI] [PubMed] [Google Scholar]
- 21.Mari M. Single dose versus 5-day course of oral prulifloxacin in antimicrobial prophylaxis for transrectal prostate biopsy. Minerva Urol Nefrol. 2007;59:1–10. [PubMed] [Google Scholar]
- 22.Loeb S, van den Heuvel S, Zhu X, Bangma CH, Schröder FH, Roobol MJ. Infectious complications and hospital admissions after prostate biopsy in a European randomized trial. Eur Urol. 2012;61:1110–4. doi: 10.1016/j.eururo.2011.12.058. [DOI] [PubMed] [Google Scholar]
- 23.Chan ES, Lo KL, Ng CF, Hou SM, Yip SK. Randomized controlled trial of antibiotic prophylaxis regimens for transrectal ultrasound-guided prostate biopsy. Chin Med J (Engl) 2012;125:2432–5. [PubMed] [Google Scholar]
- 24.Petteffi L, Toniazzo GP, Sander GB, Stein AC, Koff WJ. Efficiency of short and long term antimicrobial therapy in transrectal ultrasound-guided prostate biopsies. Int Braz J Urol. 2002;28:526–32. [PubMed] [Google Scholar]
- 25.Yang L, Hu J, Wei H, Wang L, Zhong H. Clinical significance of antibiotic prophylaxis for transrectal prostate biopsy. Zhonghua Wai Ke Za Zhi. 2001;39:940–2. [PubMed] [Google Scholar]
- 26.Tekdogan U, Tuncel A, Eroglu M, Unsal A, Atan A, Balbay MD. The efficiency of prophylactic antibiotic treatment in patients without risk factor who underwent transrectal. Turk J Urology. 2006;32:261–7. [Google Scholar]
- 27.Linden-Castro E, Pelayo-Nieto M, Alias-Melgar A, Carreño-de la Rosa F. Single dose of Levofloxacin Versus Three Dosages for Prophylaxis in Prostate Biopsy. International Scholarly Research Notices. 2014:1–4. doi: 10.1155/2014/875670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heidari Bateni Z, Shahrokh H, Salimi H, Safari H, Tabatabai M, Saedi D. Single-dose versus multiple-dose ciprofloxacin plus metronidazole prophylaxis in transrectal ultrasound-guided biopsy of the prostate: A randomized controlled trial. Acta Med Iran. 2014;52:664–70. [PubMed] [Google Scholar]
- 29.Briffaux R, Coloby P, Bruyere F, Ouaki F, Pires C, Doré B, et al. Single pre-operative dose randomised against 3-day antibiotic prophylaxis for transrectal ultrasound guided prostate biopsy: More is not better. Eur Urol Suppl. 2008;7:272. [Google Scholar]
- 30.Yamamoto S, Ishitoya S, Segawa T, Kamoto T, Okumura K, Ogawa O. Antibiotic prophylaxis for transrectal prostate biopsy: A prospective randomized study of tosufloxacin versus levofloxacin. Int J Urol. 2008;15:604–6. doi: 10.1111/j.1442-2042.2008.02056.x. [DOI] [PubMed] [Google Scholar]
- 31.Schaeffer AJ, Montorsi F, Scattoni V, Perroncel R, Song J, Haverstock DC, et al. Comparison of a 3-day with a 1-day regimen of an extended-release formulation of ciprofloxacin as antimicrobial prophylaxis for patients undergoing transrectal needle biopsy of the prostate. BJU Int. 2007;100:51–7. doi: 10.1111/j.1464-410X.2007.06848.x. [DOI] [PubMed] [Google Scholar]
- 32.Argyropoulos AN, Doumas K, Farmakis A, Liakatas I, Gkialas I, Lykourinas M. Time of administration of a single dose of oral levofloxacin and its effect in infectious complications from transrectal prostate biopsy. Int Urol Nephrol. 2007;39:897–903. doi: 10.1007/s11255-006-9112-7. [DOI] [PubMed] [Google Scholar]
- 33.Tobias-Machado M, Corrêa TD, De Barros EL, Wroclawski ER. Antibiotic prophylaxis in prostate biopsy. A comparative randomized clinical assay between ciprofloxacin, norfloxacin and chloramphenicol. Int Braz J Urol. 2003;29:313–9. doi: 10.1590/s1677-55382003000400005. [DOI] [PubMed] [Google Scholar]
- 34.Isen K, Kupeli B, Sinik Z, Sozen S, Bozkirli I. Journal search results – Cite this for me. Int Urol Nephrol. 1999;31:491–5. doi: 10.1023/a:1007115312039. [DOI] [PubMed] [Google Scholar]
- 35.Nicolle LE, Bradley S, Colgan R, Rice JC, Schaeffer A, Hooton TM. Infectious Diseases Society of America; American Society of Nephrology; American Geriatric Society. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis. 2005;40:643–54. doi: 10.1086/427507. [DOI] [PubMed] [Google Scholar]
- 36.Ghafoori M, Shakiba M, Seifmanesh H, Hoseini K. Decrease in infection rate following use of povidone-iodine during transrectal ultrasound guided biopsy of the prostate: A double blind randomized clinical trial. Iran J Radiol. 2012;9:67–70. doi: 10.5812/iranjradiol.7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gurbuz C, Canat L, Atis G, Caskurlu T. Reducing infectious complications after transrectal prostate needle biopsy using a disposable needle guide: Is it possible? Int Braz J Urol. 2011;37:79–84. doi: 10.1590/s1677-55382011000100010. [DOI] [PubMed] [Google Scholar]
- 38.Kam SC, Choi SM, Yoon S, Choi JH, Lee SH, Hwa JS, et al. Complications of transrectal ultrasound-guided prostate biopsy: Impact of prebiopsy enema. Korean J Urol. 2014;55:732–6. doi: 10.4111/kju.2014.55.11.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carey JM, Korman HJ. Transrectal ultrasound guided biopsy of the prostate. Do enemas decrease clinically significant complications? J Urol. 2001;166:82–5. [PubMed] [Google Scholar]
- 40.Taylor AK, Zembower TR, Nadler RB, Scheetz MH, Cashy JP, Bowen D, et al. Targeted antimicrobial prophylaxis using rectal swab cultures in men undergoing transrectal ultrasound guided prostate biopsy is associated with reduced incidence of postoperative infectious complications and cost of care. J Urol. 2012;187:1275–9. doi: 10.1016/j.juro.2011.11.115. [DOI] [PubMed] [Google Scholar]
- 41.Duplessis CA, Bavaro M, Simons MP, Marguet C, Santomauro M, Auge B, et al. Rectal cultures before transrectal ultrasound-guided prostate biopsy reduce post-prostatic biopsy infection rates. Urology. 2012;79:556–61. doi: 10.1016/j.urology.2011.09.057. [DOI] [PubMed] [Google Scholar]
- 42.Zani EL, Clark OA, Rodrigues Netto N., Jr Antibiotic prophylaxis for transrectal prostate biopsy. Cochrane Database Syst Rev. 2011;5:CD006576. doi: 10.1002/14651858.CD006576.pub2. doi: 10.1002/14651858.CD006576.pub2. [DOI] [PubMed] [Google Scholar]
- 43.Loeb S, Vellekoop A, Ahmed HU, Catto J, Emberton M, Nam R, et al. Systematic review of complications of prostate biopsy. Eur Urol. 2013;64:876–92. doi: 10.1016/j.eururo.2013.05.049. [DOI] [PubMed] [Google Scholar]
- 44.Carignan A, Roussy JF, Lapointe V, Valiquette L, Sabbagh R, Pépin J. Increasing risk of infectious complications after transrectal ultrasound-guided prostate biopsies: Time to reassess antimicrobial prophylaxis? Eur Urol. 2012;62:453–9. doi: 10.1016/j.eururo.2012.04.044. [DOI] [PubMed] [Google Scholar]
- 45.Adibi M, Pearle MS, Lotan Y. Cost-effectiveness of standard vs intensive antibiotic regimens for transrectal ultrasonography (TRUS)-guided prostate biopsy prophylaxis. BJU Int. 2012;110(2 Pt 2):E86–91. doi: 10.1111/j.1464-410X.2011.10768.x. [DOI] [PubMed] [Google Scholar]
- 46.Yang M, Zhao X, Wu Z, Xiao N, Lu C. Meta-analysis of antibiotic prophylaxis use in transrectal prostatic biopsy. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2009;34:115–23. [PubMed] [Google Scholar]
- 47.Feliciano J, Teper E, Ferrandino M, Macchia RJ, Blank W, Grunberger I, et al. The incidence of fluoroquinolone resistant infections after prostate biopsy – Are fluoroquinolones still effective prophylaxis? J Urol. 2008;179:952–5. doi: 10.1016/j.juro.2007.10.071. [DOI] [PubMed] [Google Scholar]
- 48.Tumbarello M, Trecarichi EM, Bassetti M, De Rosa FG, Spanu T, Di Meco E, et al. Identifying patients harboring extended-spectrum-beta-lactamase-producing Enterobacteriaceae on hospital admission: Derivation and validation of a scoring system. Antimicrob Agents Chemother. 2011;55:3485–90. doi: 10.1128/AAC.00009-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Williamson DA, Barrett LK, Rogers BA, Freeman JT, Hadway P, Paterson DL. Infectious complications following transrectal ultrasound-guided prostate biopsy: New challenges in the era of multidrug-resistant Escherichia coli. Clin Infect Dis. 2013;57:267–74. doi: 10.1093/cid/cit193. [DOI] [PubMed] [Google Scholar]


