Abstract
Objective:
The objective was to review the impact of transperineal biopsy (TPB) at our institution by assessing rates of cancer detection/grading, treatment outcomes and complications.
Patients and Methods:
A retrospective review of TPBs between 2009 and 2013 was performed. Variables included reason for TPB, age, prostate-specific antigen, previous histology, TPB histology, and management outcomes.
Results:
In total, 110 patients underwent 111 TPBs at our institution. On average, 22 cores were taken from each procedure. Disease-upgrade occurred in 37.5% of active surveillance patients, 35% of patients with previous negative transrectal ultrasound, and 58.8% in patients undergoing TPB for other reasons. Of these patients, anterior and/or transition zones were involved in 66%, 79%, and 80%, respectively. Involvement in anterior and/or transition zones only occurred in 40%, 37%, and 10%, respectively. About 77% of patients with disease-upgrading underwent treatment with curative intent. Complications included a 6.3% rate of acute urinary retention and 2.7% of clot retention, with no episodes of urosepsis.
Conclusions:
Transperineal biopsy at our institution showed a high rate of disease-upgrading, with a large proportion involving anterior and transition zones. A significant amount of patients went on to receive curative treatment. TPB is a valuable diagnostic procedure with minimal risk of developing urosepsis. We believe TBP should be offered as an option for all repeat prostate biopsies and considered as an option for initial prostate biopsy.
Keywords: Biopsy, complications, prostate, prostatic neoplasms, sepsis, transperineal
INTRODUCTION
Prostate biopsies have developed significantly since their first introduction by Ferguson as a finger-guided transperineal aspirate over 80 years ago.[1] However, the currently widely accepted transrectal ultrasound (TRUS)-guided biopsy still suffers from poor sensitivity [2] and has a reported false-negative incidence of up to 23%.[3] TRUS biopsies are also associated with a risk of infection of up to 5% despite standard prophylactic oral antibiotics.[4,5,6] Other complications of TRUS biopsy include acute urinary retention and clot retention.
Consequently, there has been a recent renewed interest in prostate biopsy through the transperineal approach. The transperineal biopsy (TPB) with a brachytherapy template grid provides systematic sampling of the entire prostate including anterior and transition zones-areas which have been shown to harbor 25–55% of cancer.[7,8] Recent studies have also shown disease-upgrading or new cancer detection in 26–36% of patients undergoing TPB compared with previous TRUS biopsies.[9,10]
Furthermore, sepsis rates of TPB have been shown to be negligible in multiple studies.[10,11,12,13,14,15] In addition, TPB is a suitable alternative for patients who have had previous radiotherapy or surgery to the rectum, whereby TRUS may lead to an increased risk of complications such as fistula. TPB is also used in patients with risk factors for sepsis, such as recent overseas travel or prior quinolone use.
Our institution, a tertiary referral center, introduced routine transperineal template biopsies in 2009. We aim to review the impact of TPB at our institution by assessing rates of cancer detection and disease-upgrading, involvement of anterior and/or transition zones, overall treatment outcomes, as well as complication rates.
PATIENTS AND METHODS
Transperineal biopsies at our institution are performed by both consultant Urologists as well as Urology trainees under supervision. These are performed as day cases in the operating theatre with patients under general anesthesia in the exaggerated dorsal lithotomy position. A biplanar TRUS probe is used mounted on a stabilizer and stepper with a brachytherapy template grid. After the prostate volume is calculated, an 18-gauge biopsy needle is directed through the template grid to obtain biopsies under direct ultrasound guidance. Approximately, 20 cores are taken from the bilateral anterior base, transition zone, posterior base, anterior mid, posterior mid, anterior apex, and posterior apex.
Patients are assessed preoperatively, and a sterile urine microscopy and culture are ensured. They are administered an intravenous dose of cefazolin. Patients are all followed up at the outpatient clinic 2 weeks following the procedure.
Data were collected through a retrospective chart review performed for all patients who underwent TPB in 2009–2011. From June 2011, a prospective database was collected for all TPB patients. Variables collected in the database include age, prostate-specific antigen (PSA), previous histological diagnosis where relevant, indication for TPB, TPB histology, ongoing management plan, and complications.
Ethics approval was obtained for use and collection of these data for research purposes at our Institution's Human Research and Ethics Committee.
RESULTS
Patient demographics
Between September 2009 and March 2013, 110 consecutive patients underwent 111 TPBs at our institution. One patient underwent TPB initially for a previous negative TRUS biopsy and again for active surveillance. No patients were excluded from the analysis.
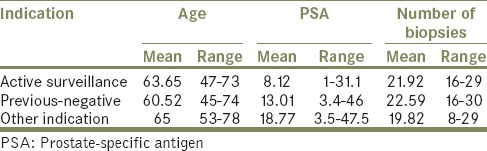
About 36% (n = 40) of all biopsies were for active surveillance, 49% (n = 54) for previous negative biopsies and 15% (n = 17) for other indications such as colorectal disease, previous brachytherapy or significantly higher risk of sepsis. Patients who had previous negative biopsies also had their original TRUS biopsy at our institute. A breakdown of age, pre-TPB PSA, as well as the number of biopsies taken per procedure for these respective groups, can be found in Table 1.
Table 1.
Age, PSA, and number of biopsies
Cancer detection
Disease-upgrade from Gleason 6 cancer occurred in 37.5% (15 out of 40 cases) in Active Surveillance and new diagnoses of cancer were made in 35% (19 out of 53 cases) in patients with previous negative TRUS biopsies and 58.8% (10 out of 17 cases) in patients undergoing TPB for other reasons. This is shown graphically in Figure 1. There was a 35% negative biopsy rate in patients undergoing active surveillance (14 out of 40 cases).
Figure 1.
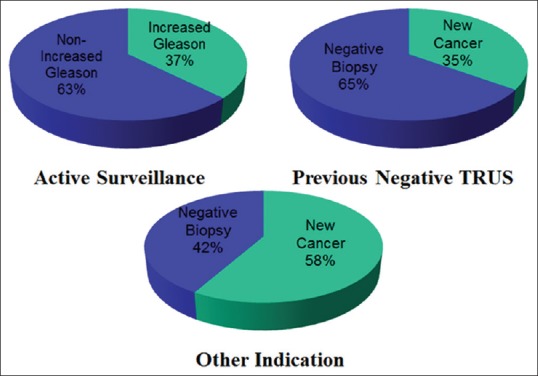
Disease-upgrade and new cancer diagnoses
In patients with new diagnoses of cancer, clinically significant cancers (Gleason score ≥7) were diagnosed in 74% (14 out of 19) in patients with previous-negative TRUS biopsies and 100% (10 out of 10) in patients undergoing TPB for other reasons.
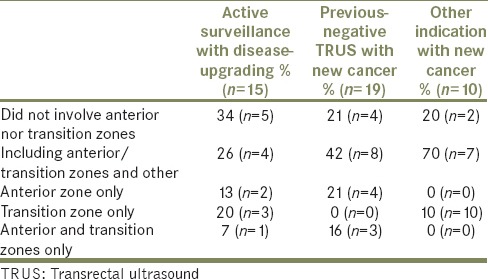
Of these patients who had disease-upgrading or new cancer diagnosis as a result of TPB, 66% had involvement of anterior and/or transition zones in the active surveillance group, 79% in the previous-negative TRUS group and 80% in the other indication group [Table 2].
Table 2.
Location of disease-upgrading and new cancer
Impact on management
Totally, 44 patients out of our cohort of 110 patients had a new cancer diagnosis or disease-upgrading as a result of TPB of the prostate. Of these 44 patients, 80% (n = 35) underwent treatment with a curative intent and 20% (n = 9) went on to have active surveillance. 50% (n = 22) of patients underwent radical prostatectomy following their TPB, 25% (n = 11) underwent external beam radiotherapy and 5% (n = 2) underwent seed brachytherapy. This is displayed graphically in Figure 2.
Figure 2.
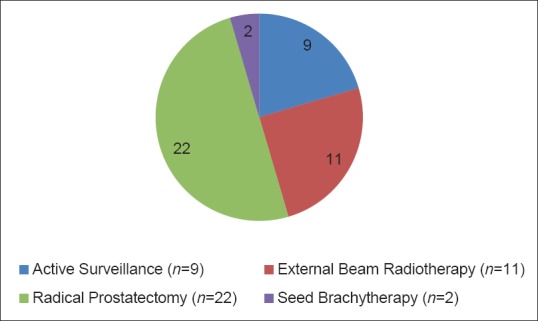
Management of patients with disease-upgrading/new cancer
Complications
In 111 patients, 6.3% (n = 7) were complicated by acute urinary retention and 2.7% (n = 3) experienced clot retention. There were no episodes of urosepsis from our cohort.
DISCUSSION
Transperineal biopsy has seen a renewed interest in recent years owing to the increased detection of cancers particularly in the anterior and transitional zones, as well as a negligible risk of sepsis.[9,10,11,12,13,14,15]
Transperineal biopsy at our institution was associated with a 37% disease-upgrading in patients undergoing active surveillance and a 35% new diagnosis rate in patients with previously negative TRUS biopsies. Of these, a considerable majority of patients had a new diagnosis of clinically significant prostate cancer. Our results were incorporated into a larger study by Grummet et al.[15] with a total 245 patients, with comparable overall results. In this combined cohort, 30% of patients had disease-upgrading in patients undergoing active surveillance and 39% of new cancer diagnosis in remaining patients.
Our results are comparable to other published data series. Mabjeesh et al.[9] reported a rate of 26.1% of new diagnoses in patients with at least two previous-negative TRUS biopsies in patients from Israel. In another Australian series, Symons et al.[10] reported a 35.6% rate of new diagnoses in previous-negative TRUS biopsies in 136 patients. In this same study, 74% of patients with an overall positive biopsy had a Gleason score of ≥7.
We found a 35% negative biopsy rate for patients undergoing active surveillance. There are minimal specific data on negative biopsy rates in TPB, with most of the literature focused on disease-upgrading in active surveillance. Ayres et al.,[16] reported a negative biopsy rate of 15% in their cohort of 101 patients. Though this rate is lower, their disease-upgrading of 34% is consistent with ours. Our negative biopsy rate is consistent with those of TRUS biopsies reported in the literature between 21% and 52%.[17] A comparison between results published from PRIAS [18] thus far, with their 37% negative biopsy rate and 21.4% Gleason score upgrading, reveals a similar negative biopsy rate but a higher disease-upgrading rate in our cohort.
We detected cancers involving anterior or transitional zones in 75% (n = 33) of patients who had predominantly clinically significant new cancer diagnoses or disease-upgrading. Furthermore, 52% (n = 23) involved only transition or anterior zones. This is consistent with the literature suggesting these areas include 25–55% of disease [7,8] as well as specific studies showing that tumors subsequently identified upon repeat transperineal prostate biopsy are most frequently found in the anterior prostate up to a rate of 94.1%.[9,19,20,21,22] These anterior prostate cancers have been named “prostate evasive anterior tumors” or PEATS by Lawrentschuk et al. who have advocated the use of MRI to detect them and for these tumors to be biopsied via a Transperineal approach.[23]
Transperineal biopsy performed at our institution led to no episodes of Urinary sepsis. This finding reiterates multiple studies indicating a negligible risk of sepsis associated with TPB.[10,11,12,13,14,15] This is particularly of importance when considering the increasing rise of multi-resistant bacteria including extended-spectrum beta-lactamase and quinolone-resistant bacteria, which are now commonly found in the rectal flora.[24] Even more concerning, there are now reports of carbapenem-resistant enterobacter in the UK as well as Australia.[25,26]
Despite the multiple benefits that TPB has to offer, there are also several drawbacks that have prevented more widespread use. TPB routinely requires a general anesthetic, although nerve block techniques and local anesthesia have been reported.[27,28] There is also a learning curve for Urologists who are not familiar with the procedure. It is a more labor intensive and coupled with the need for specialized equipment including a brachytherapy grid, stabilizer, stepper, and a bi-plane transducer, the procedure itself is more costly and a significant drain on time and resources. However, the cost of sepsis and multi-resistant organisms associated with the ongoing use of TRUS biopsy must also be considered.
Our acute urinary retention rate of 6.3% (n = 7) is within an accepted rate. The largest series of TP biopsy reported a rate of 6.7% of 3,000 patients with only 56% taking an alpha-blocker.[12] The rates of urinary retention between TPB and TRUS are comparable.[29] Although there is no evidence of alpha-blockers reducing the rate of acute urinary retention in TPB specifically, there is some scarce evidence to suggest it may help in TRUS biopsies. Bozlu et al. demonstrated in a small, nonblinded randomized controlled trial a reduction in acute urinary retention from 9% to 3% in patients without alpha-blocker compared with those with alpha-blocker, respectively.[30] As a result of our analysis, patients undergoing TPB at our institution are now prescribed a 10-day course of tamsulosin 400 μg daily, starting 3 days prior to the procedure.
We recognize the limitations of this study. First, this is the study was performed at a single institution with a relatively small cohort of 110 patients. Furthermore, data for the first 20 months of this 3.5-year cohort were collected retrospectively. However, our results were collected during the early stages of TPB and correlate well with other studies in the literature. In addition, our paper illustrates the significant impact that TPB has made at our institution.
Transperineal biopsy at our institution between 2009 and 2013 was associated with a 37% rate of disease-upgrading in patients undergoing active surveillance and a 35% rate of new cancers detected in patients with previous-negative TRUS biopsies. These rates are comparable with the literature. As a result of TPB at our institution, this significant proportion of patients have subsequently undergone treatment with curative intent for clinically significant prostate cancer. With the combination of high cancer detection and negligible risks of sepsis, we believe that TPB should be offered for all repeat prostate biopsies and considered for initial prostate biopsy, especially in patients with a higher risk of developing sepsis from TRUS biopsy.
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.Ferguson R. Prostatic neoplasms: Their diagnosis by needle puncture and aspiration. Am J Surg. 1930;9:507. [Google Scholar]
- 2.Patel AR, Jones JS. Optimal biopsy strategies for the diagnosis and staging of prostate cancer. Curr Opin Urol. 2009;19:232–7. doi: 10.1097/mou.0b013e328329a33e. [DOI] [PubMed] [Google Scholar]
- 3.Rabbani F, Stroumbakis N, Kava BR, Cookson MS, Fair WR. Incidence and clinical significance of false-negative sextant prostate biopsies. J Urol. 1998;159:1247–50. [PubMed] [Google Scholar]
- 4.Simsir A, Kismali E, Mammadov R, Gunaydin G, Cal C. Is it possible to predict sepsis, the most serious complication in prostate biopsy? Urol Int. 2010;84:395–9. doi: 10.1159/000296290. [DOI] [PubMed] [Google Scholar]
- 5.Taylor S, Margolick J, Abughosh Z, Goldenberg SL, Lange D, Bowie WR, et al. Ciprofloxacin resistance in the faecal carriage of patients undergoing transrectal ultrasound guided prostate biopsy. BJU Int. 2013;111:946–53. doi: 10.1111/j.1464-410X.2012.11637.x. [DOI] [PubMed] [Google Scholar]
- 6.Patel U, Dasgupta P, Amoroso P, Challacombe B, Pilcher J, Kirby R. Infection after transrectal ultrasonography-guided prostate biopsy: Increased relative risks after recent international travel or antibiotic use. BJU Int. 2012;109:1781–5. doi: 10.1111/j.1464-410X.2011.10561.x. [DOI] [PubMed] [Google Scholar]
- 7.McNeal JE, Bostwick DG. Anatomy of the prostate: Implication for disease. In: Bostwick DG, editor. Pathology of the Prostate. New York: Churchill-Livingstone; 1990. pp. 1–4. [Google Scholar]
- 8.Chen ME, Johnston DA, Tang K, Babaian RJ, Troncoso P. Detailed mapping of prostate carcinoma foci: Biopsy strategy implications. Cancer. 2000;89:1800–9. doi: 10.1002/1097-0142(20001015)89:8<1800::aid-cncr21>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 9.Mabjeesh NJ, Lidawi G, Chen J, German L, Matzkin H. High detection rate of significant prostate tumours in anterior zones using transperineal ultrasound-guided template saturation biopsy. BJU Int. 2012;110:993–7. doi: 10.1111/j.1464-410X.2012.10972.x. [DOI] [PubMed] [Google Scholar]
- 10.Symons JL, Huo A, Yuen CL, Haynes AM, Matthews J, Sutherland RL, et al. Outcomes of transperineal template-guided prostate biopsy in 409 patients. BJU Int. 2013;112:585–93. doi: 10.1111/j.1464-410X.2012.11657.x. [DOI] [PubMed] [Google Scholar]
- 11.Moran BJ, Braccioforte MH. Stereotactic transperineal prostate biopsy. Urology. 2009;73:386–8. doi: 10.1016/j.urology.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 12.Pepe P, Aragona F. Morbidity after transperineal prostate biopsy in 3000 patients undergoing 12 vs 18 vs more than 24 needle cores. Urology. 2013;81:1142–6. doi: 10.1016/j.urology.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki M, Kawakami S, Asano T, Masuda H, Saito K, Koga F, et al. Safety of transperineal 14-core systematic prostate biopsy in diabetic men. Int J Urol. 2009;16:930–5. doi: 10.1111/j.1442-2042.2009.02386.x. [DOI] [PubMed] [Google Scholar]
- 14.Galfano A, Novara G, Iafrate M, Cosentino M, Cavalleri S, Artibani W, et al. Prostate biopsy: The transperineal approach. EAU EBU Update Ser. 2007;5:241–9. [Google Scholar]
- 15.Grummet JP, Weerakoon M, Huang S, Lawrentschuk N, Frydenberg M, Moon DA, et al. Sepsis and ‘superbugs’: Should we favour the transperineal over the transrectal approach for prostate biopsy? BJU Int. 2014;114:384–8. doi: 10.1111/bju.12536. [DOI] [PubMed] [Google Scholar]
- 16.Ayres BE, Montgomery BS, Barber NJ, Pereira N, Langley SE, Denham P, et al. The role of transperineal template prostate biopsies in restaging men with prostate cancer managed by active surveillance. BJU Int. 2012;109:1170–6. doi: 10.1111/j.1464-410X.2011.10480.x. [DOI] [PubMed] [Google Scholar]
- 17.Wong LM, Alibhai SM, Trottier G, Timilshina N, Van der Kwast T, Zlotta A, et al. A negative confirmatory biopsy among men on active surveillance for prostate cancer does not protect them from histologic grade progression. Eur Urol. 2014;66:406–13. doi: 10.1016/j.eururo.2013.04.038. [DOI] [PubMed] [Google Scholar]
- 18.Bul M, Zhu X, Valdagni R, Pickles T, Kakehi Y, Rannikko A, et al. Active surveillance for low-risk prostate cancer worldwide: The PRIAS study. Eur Urol. 2013;63:597–603. doi: 10.1016/j.eururo.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Kawakami S, Kihara K, Fujii Y, Masuda H, Kobayashi T, Kageyama Y. Transrectal ultrasound-guided transperineal 14-core systematic biopsy detects apico-anterior cancer foci of T1c prostate cancer. Int J Urol. 2004;11:613–8. doi: 10.1111/j.1442-2042.2004.00863.x. [DOI] [PubMed] [Google Scholar]
- 20.Furuno T, Demura T, Kaneta T, Gotoda H, Muraoka S, Sato T, et al. Difference of cancer core distribution between first and repeat biopsy: In patients diagnosed by extensive transperineal ultrasound guided template prostate biopsy. Prostate. 2004;58:76–81. doi: 10.1002/pros.10298. [DOI] [PubMed] [Google Scholar]
- 21.Satoh T, Matsumoto K, Fujita T, Tabata K, Okusa H, Tsuboi T, et al. Cancer core distribution in patients diagnosed by extended transperineal prostate biopsy. Urology. 2005;66:114–8. doi: 10.1016/j.urology.2005.01.051. [DOI] [PubMed] [Google Scholar]
- 22.Gershman B, Zietman AL, Feldman AS, McDougal WS. Transperineal template-guided prostate biopsy for patients with persistently elevated PSA and multiple prior negative biopsies. Urol Oncol. 2013;31:1093–7. doi: 10.1016/j.urolonc.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Lawrentschuk N, Haider MA, Daljeet N, Evans A, Toi A, Finelli A, et al. 'Prostatic evasive anterior tumours': The role of magnetic resonance imaging. BJU Int. 2010;105:1231–6. doi: 10.1111/j.1464-410X.2009.08938.x. [DOI] [PubMed] [Google Scholar]
- 24.Williamson DA, Barrett LK, Rogers BA, Freeman JT, Hadway P, Paterson DL. Infectious complications following transrectal ultrasound-guided prostate biopsy: New challenges in the era of multidrug-resistant Escherichia coli. Clin Infect Dis. 2013;57:267–74. doi: 10.1093/cid/cit193. [DOI] [PubMed] [Google Scholar]
- 25.Kotsanas D, Wijesooriya WR, Korman TM, Gillespie EE, Wright L, Snook K, et al. “Down the drain”: Carbapenem-resistant bacteria in intensive care unit patients and handwashing sinks. Med J Aust. 2013;18(198):267–9. doi: 10.5694/mja12.11757. [DOI] [PubMed] [Google Scholar]
- 26.Drew RJ, Turton JF, Hill RL, Livermore DM, Woodford N, Paulus S, et al. Emergence of carbapenem-resistant Enterobacteriaceae in a UK paediatric hospital. J Hosp Infect. 2013;84:300–4. doi: 10.1016/j.jhin.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Miller J, Perumalla C, Heap G. Complications of transrectal versus transperineal prostate biopsy. ANZ J Surg. 2005;75:48–50. doi: 10.1111/j.1445-2197.2005.03284.x. [DOI] [PubMed] [Google Scholar]
- 28.Abdollah F, Novara G, Briganti A, Scattoni V, Raber M, Roscigno M, et al. Trans-rectal versus trans-perineal saturation rebiopsy of the prostate: Is there a difference in cancer detection rate? Urology. 2011;77:921–5. doi: 10.1016/j.urology.2010.08.048. [DOI] [PubMed] [Google Scholar]
- 29.Shen PF, Zhu YC, Wei WR, Li YZ, Yang J, Li YT, et al. The results of transperineal versus transrectal prostate biopsy: A systematic review and meta-analysis. Asian J Androl. 2012;14:310–5. doi: 10.1038/aja.2011.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bozlu M, Ulusoy E, Doruk E, Cayan S, Canpolat B, Schellhammer PF, et al. Voiding impairment after prostate biopsy: Does tamsulosin treatment before biopsy decrease this morbidity? Urology. 2003;62:1050–3. doi: 10.1016/j.urology.2003.07.006. [DOI] [PubMed] [Google Scholar]


