Abstract
Context:
Partial nephrectomy is becoming the standard of care in management of small renal tumors and excision of the peritumor fat is recommended for accurate staging. During the surgery, the overlying fat may be excised for accurate visualization of margins or maybe inadvertently left behind when performing a partial nephrectomy in an obese patient. We investigated the prevalence of fat involvement in these patients.
Aims:
The aim was to document the prevalence of peritumor fat involvement discovered after partial nephrectomy performed for radiologic T1 renal cancer.
Settings and Design:
Between 2005 and 2011, 107 partial nephrectomy procedures were performed for radiologic T1 disease.
Statistical Analysis:
All analyses were performed using SAS 9.2.
Subjects and Methods:
Patients were classified as: Group A (n = 88 patients), patients with stage T1a (tumor size ≤4 cm) and Group B (n = 24 patients) patients with stage T1b (tumor size 4-7 cm).
Results:
The overall prevalence of peritumor fat involvement was 1.86% (n = 2). The two patients had tumor ≤4 cm in size of the papillary subtype and were followed for 61 and 57 months, respectively. Both were living and without recurrence. Patient demographics and tumor characteristics did not differ between the two groups except, Fuhrman Grades 3 and 4 were statistically more prevalent in Group B (<0.01). Tumor grade, clear cell type cancer and stage T1b did not correlate with peritumor fat involvement in the study population.
Conclusions:
Our study revealed a low prevalence of peritumor fat involvement in radiologic pT1 renal cancer; however, peritumor fat removal is still recommended.
Keywords: Kidney neoplasms, minimally invasive, nephrectomy, obesity, surgical procedures
INTRODUCTION
Renal cell carcinoma (RCC) is one of the leading causes of malignant tumors of the kidney with an increase in incidence over the past several decades.[1] The progression from total nephrectomy to partial nephrectomy for certain stages of RCC was to address the large number of patients at-risk for chronic kidney disease due to total nephrectomy. Multiple studies have shown that treatment with partial nephrectomy was either equivalent or improved for 5- or 10-year survival compared to total nephrectomy.[2,3] As such, treatment using partial nephrectomy is widely used to treat T1a tumors of the kidney (≤4 cm) and is a viable surgical option for T1b tumors as well (4-7 cm).[4] Traditionally, when performing a partial nephrectomy, excision of the overlying tumor fat with the tumor mass is recommended for accurate staging.[5] Occasionally, during the surgery, the overlying fat may need to be excised off the tumor for accurate visualization of the margins of resection and then be submitted separately. Furthermore, some of that peritumor fat may inadvertently be left behind in the patient, especially when performing a partial nephrectomy in an obese patient. Currently, there are no radiologic means to accurately identify those patients with T1 tumors that may have peritumor fat involvement.[1,5] As such, for patients undergoing partial nephrectomy for T1 disease there may be a potential risk of fat remaining after surgery.
Our primary objective of this study was to document the prevalence of peritumor fat involvement in radiologic T1 disease which, to the best of our knowledge, the prevalence of has not been reported before. We also aimed to identify potential predictors of peritumor fat involvement in radiologic pT1 renal cancer. We retrospectively assessed the prevalence of peritumor fat involvement in our patients who had partial nephrectomy for radiologic T1 disease in an attempt to document that prevalence.
SUBJECTS AND METHODS
Between 2005 and 2011, 107 partial nephrectomy procedures were performed at our academic institution. All procedures were performed by fellowship trained urologic oncologists.
Surgical method
Partial nephrectomy was performed either open (number %), laparoscopic (number %), and robotic (number %). Cold ischemia was utilized in open partial nephrectomy patients while warm ischemia was utilized in both laparoscopic and robotic approaches. Excision of the tumor with overlying perinephric fat was attempted in all cases taking adequate safety margin. In open cases, selective control of bleeding vessels in the tumor bed was performed using 3-0/4-0 absorbable suture on RB-1 needle. The edges of nephrotomy were approximated using 2-0 absorbable suture on SH needle (Ethicon, Bridgewater, NJ, USA). Frequently bolsters or gel-foam cut into small pieces were insinuated in the tumor bed cavity to assist in hemostasis and prevent tearing through the renal parenchyma when tying the outer renorrhaphy sutures. In the case of laparoscopic and robotic partial nephrectomy, inner renorrhaphy was performed in a continuous running suture manner using a 3-0 absorbable suture on RB-1 needle cut into 5-6 inches with a Lapra-TY® (Ethicon Inc., Somerville, NJ, US) at the end and using the sliding clip technique. The outer renorraphy was performed using 2-0 absorbable suture in the same manner.
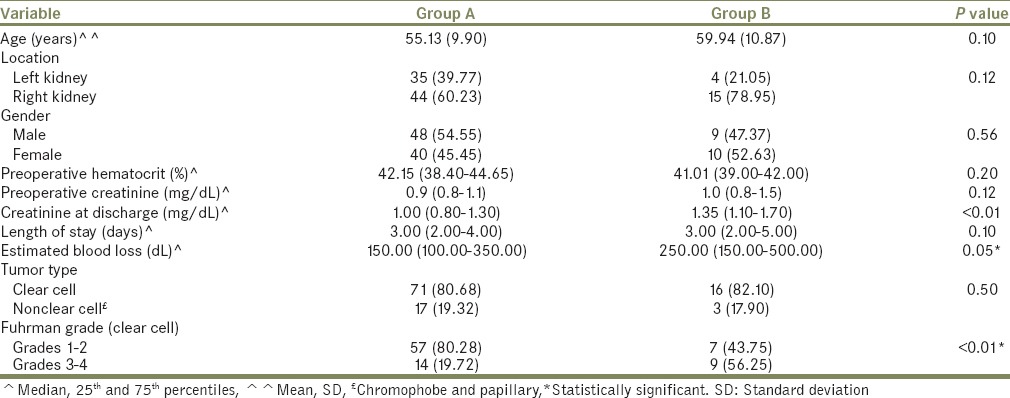
There were 49 open, 37 laparoscopic (pure/hand assisted) and 21 robotic partial nephrectomy procedures. These patients were classified into two groups. Group A (n = 88) with radiologic T1a tumors (≤4 cm) and Group B (n = 19) with radiologic T1b tumors (4-7 cm). Patient demographics are illustrated in Table 1.
Table 1.
Patient demographics
Inclusion criteria
Tumors that are renal cell cancer and on preoperative computed tomography (CT) scan were radiologically ≤7 cm.
Exclusion criteria
Tumors on preoperative CT scan or on postoperative pathology report were >7 cm (T2). Furthermore, patients underwent partial nephrectomy and postoperative pathology revealed benign renal tumors as oncocytoma, metastasis from a nonrenal primary or rare renal tumors as leiomyosarcoma were excluded from the study.
The distribution of types of renal cancer and Fuhrman grading for the clear cell type among the two groups are illustrated in Table 1. We classified the renal cancer into clear and nonclear (papillary and chromophobe).
Statistical analysis
All analyses were performed using SAS 9.2 (Cary, NC, USA). Participants were classified into two groups. The 1st group included patients who had stage T1a (tumors sized at <4 cm) while the 2nd group had stage T1b (tumors sized 4 cm to <7 cm). A comparison of the categorical variables such as tumor location and pathology across the two groups was made by the Chi-square test. Fisher's exact test was performed to compare mortality percentage between the two study groups.
RESULTS
Overall, only two patients (1.86%) had peritumor fat involvement. Both were in Group A (≤4 cm). One patient had a 2-cm solid enhancing exophytic left renal mass on preoperative CT scan. He underwent a laparoscopic partial nephrectomy. The procedure was uneventful. Postoperative pathology revealed a 1.7-cm Type 1 papillary renal cell cancer with negative margins and transcapsular extension of the tumor into peritumor adipose tissue. Sixty-one months after his surgery, the patient is alive with no sign of tumor recurrence. The second patient had a 3.2-cm, Bosniac IV cyst. He underwent uneventful robotic partial nephrectomy. Postoperative pathology revealed Type 2 papillary RCC 3 cm in size with negative margins and involving the peritumor adipose tissue. Fifty-seven months since his surgery, the patient is alive and with no sign of tumor recurrence.
There was no difference between the two groups regarding patient demographics or tumor characteristics except for Fuhrman grade. The lower Fuhrman Grade (1-2) prevailed in Group A and the higher Fuhrman Grade (3-4) prevailed in Group B (P < 0.01) [Table 1].
Recurrence
Median follow-up was 50 months (range: 1-95). Overall 6 (5.06%) patients developed recurrence. Five developed distant recurrence (metastasis). Of these five patients, three died as a result of the distant recurrence and two still alive on systemic biologic therapy. One patient with a single kidney developed local recurrence 4-year after his initial partial procedure and was treated by radical nephrectomy. He is currently alive on regular hemodialysis.
Complications
One intraoperative complication was noted. A pleural injury in a patient underwent open partial nephrectomy and was successfully repaired. Postoperative complications were encountered in 9 (8.41%) patients. Using the Clavien-Dindo classification of surgical complications,[6] we had a Grade 1 complication (blood transfusion) in one patient. Five Grade 2 complications (two developed ischemic heart events treated medically, two developed postoperative pneumonia treated with antibiotics and one developed atelectasis managed conservatively). Two Grade 3 complications (two developed urine leak managed by retrograde stent).
DISCUSSION
Partial nephrectomy for radiologic T1 disease is a widely accepted treatment modality. It was our purpose to document the prevalence of peritumor fat involvement in the patients undergoing this treatment modality for T1a and T1b stages.[7] We observed that preoperative radiologic findings that may suggest peritumor fat involvement include peritumor fat stranding and that is nonspecific or distinct soft tissue density in the perinephric space and that is rare. The overall accuracy of both CT and MRI in detection of perinephric fat involvement is low indicating this extension is usually microscopic.[8] We have noticed that following a partial nephrectomy for stage 1 renal cancer, it is very rare to encounter a peritumor fat invasion in the pathology report and consequently we decided to look into our partial nephrectomy database for that prevalence. In particular that occasionally in difficult partial nephrectomy in an obese patient, the fat needs to be stripped of the tumor and it is not uncommon some of the peritumor fat may be left behind. This underscores the importance of reports like the present one attempting to identify the true prevalence of peritumor fat involvement.
We sought to assess for an impact of tumor size, type of cancer and grade as predictors of peritumor fat involvement. However, the two patients with peritumor fat involvement were in Group A (≤4 cm), both had papillary RCC. It has been suggested that the multicentric nature of papillary RCC may cause difficulty in the interpretation of surgical margins as positive.[9] There was no statistically significant difference in the mean tumor size, distribution of the various types of kidney cancer between Groups A and B [Table 1]. Though the higher Fuhrman Grade (3-4) prevailed in Group B (≥4 cm), none of these patients developed peritumor fat invasion. Our study is limited by its retrospective nature and small sample size. However, our study broadens our understanding of RCC by reporting the prevalence of peritumor fat invasion in patients undergoing partial nephrectomy for radiologic T1 disease. This prevalence has not been previously reported and was found to be small.
CONCLUSIONS
Removing peritumor fat during partial nephrectomy is recommended and justified. However, our study revealed a low prevalence of peritumor fat involvement in radiologic pT1 renal cancer.
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.Kim C, Choi HJ, Cho KS. Diagnostic performance of multidetector computed tomography in the evaluation of perinephric fat invasion in renal cell carcinoma patients. J Comput Assist Tomogr. 2014;38:268–73. doi: 10.1097/RCT.0b013e3182aa672a. [DOI] [PubMed] [Google Scholar]
- 2.Antonelli A, Ficarra V, Bertini R, Carini M, Carmignani G, Corti S, et al. Elective partial nephrectomy is equivalent to radical nephrectomy in patients with clinical T1 renal cell carcinoma: Results of a retrospective, comparative, multi-institutional study. BJU Int. 2012;109:1013–8. doi: 10.1111/j.1464-410X.2011.10431.x. [DOI] [PubMed] [Google Scholar]
- 3.Van Poppel H, Da Pozzo L, Albrecht W, Matveev V, Bono A, Borkowski A, et al. A prospective, randomised EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol. 2011;59:543–52. doi: 10.1016/j.eururo.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 4.Novick AC. CSBAea. American Urological Association Education and Research. 2009. [Last cited on 2013 Apr 24]. Available from: http://www.auanet.org/content/media/renalmass09.pdf .
- 5.Kenny PA, Wotkowicz C, Libertino JA. Contemporary open surgery of the kidney. In: Wein AJ, editor. Campbell's Urology. 10th ed. Philadelphia: Saunders; 2012. pp. 1554–627. [Google Scholar]
- 6.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joniau S, Vander Eeckt K, Van Poppel H. The indications for partial nephrectomy in the treatment of renal cell carcinoma. Nat Clin Pract Urol. 2006;3:198–205. doi: 10.1038/ncpuro0458. [DOI] [PubMed] [Google Scholar]
- 8.Campbell SC, Lane BR. Malignant Renal Tumors. In: Wein AJ, editor. Campbell's Urology. 10th ed. Philadelphia: Saunders; 2012. pp. 1413–1505. [Google Scholar]
- 9.Laryngakis NA, Guzzo TJ. Tumor enucleation for small renal masses. Curr Opin Urol. 2012;22:365–71. doi: 10.1097/MOU.0b013e3283551f84. [DOI] [PubMed] [Google Scholar]


