Abstract
Background:
Prostate cancer is a major health problem throughout the developed world. Tumor grade is one of the most important prognostic factors of prostate cancer. At present, adequate prognostic markers for prostate cancer progression are still lacking, in spite of intensive investigation. Accordingly, we studied the role of immunohistochemical (IHC) expression of p53 and Ki-67 as a prognostic factor in carcinoma prostate and correlated their expression with Gleason's grade.
Materials and Methods:
In this prospective study, a total of 60 cases including 50 cases of prostate carcinoma and 10 of benign prostatic hyperplasia (BPH) were taken. Tumor grade was determined according to Gleason's grading system. p53 and Ki-67 expressions were determined by IHC staining. The obtained results were analyzed and evaluated using Spearman's statistical test (SPSS version 20).
Results:
In BPH, p53 was expressed in only 2 of 10 (20%) cases while in carcinoma it was expressed in 38 of 50 (76%) cases. Ki-67 was expressed in only 1 of 10 (10%) BPH cases while in carcinoma it was expressed in 32 of 50 (64%) cases. In present study, 1 of 4 (25%) well differentiated, 23 of 31 (74.19%) moderately differentiated and 14 of 15 (93.33%) poorly differentiated tumors revealed p53 immunopositivity and a statistically significant correlation was observed between p53 expression and increased Gleason's grade (P = 0.038). All 4 (100%) cases of well-differentiated carcinoma were negative for Ki-67 expression. Nineteen of 31 (61.29%) moderately differentiated and 13 of 15 (86.66%) poorly differentiated tumors were positive for Ki-67 and a statistically significant correlation was observed between Ki-67 positivity and increased Gleason's grade (P = 0.002).
Conclusions:
Both p53 and Ki-67 were significantly up-regulated in malignant lesions as compared to benign lesions and a strong relationship with the Gleason's grading was noticed, therefore, we propose that these markers can be applied along with other prostate cancer prognostic factors.
Keywords: Gleason's grade, Ki-67 (MIB-1), p53, prostate cancer
INTRODUCTION
Prostate cancer is the second most frequently diagnosed cancer and the fifth leading cause of cancer death in males.[1] Incidence increases from 20% in men in their fifties to approximately 70% in men between the age of 70 and 80 years.[2] Prostate cancer is not only significant for its lethality but also for the extremely high morbidity associated with it.
Nowadays, more patients are diagnosed at earlier stages, due to increased availability of prostatic-specific antigen (PSA) measurement and other diagnostic methods. With delay in diagnosis of the low-grade tumor, the quality or length of patient's life is not significantly changed, but a high-grade tumor in a young person might spread quickly and lead to the patient's death within 2 years. The absence of prognostic information has also led to significant “overtreatment” of patients who would otherwise require only conservative management.
Therefore, much research has been dedicated to identifying prognostic factors that distinguish indolent versus aggressive forms of prostate cancer.[3]
Prognostic factors are divided into clinical and biological groups. Clinical factors are obtained using blood tests, radiological and microscopic evaluation of biopsies. Biological factors are other categories of prognostic factors.[4] Grade and stage, the traditional prognostic markers, are useful, but for individual patients, it is difficult to predict the outcome. With recent advances in molecular biology, the concept of oncogenes, tumor suppressor genes has dominated basic science research of tumorigenesis. Evaluation of these genes and their protein products may provide new prognostic markers, with p53 and Ki-67 gaining special attention.[5]
In some studies, the incidence of p53 has been associated with higher grades of prostatic tumors and worse prognosis of the disease.[6] Although another studies revealed different results, nuclear staining for p53 was positive in at least a subset of prostatic cancers and there is still a discrepancy in the frequency of p53 mutations in carcinoma prostate and on its prognostic role.[4,7] Ki-67 index is higher in carcinoma than hyperplasia and still higher in metastatic than nonmetastatic cases, thus an increased Ki-67 index may indicate a poor prognosis of disease.[4] However, its role as an independent prognostic marker among patients with prostate carcinoma is still controversial.
Considering the proven correlation between Gleason's grading and prognosis of prostate cancer, along with proposing of p53 (tumor suppressor protein) and Ki-67 (cell proliferation marker) as prognostic factors; this study was performed to study the frequency of these markers expression in prostatic cancer and their probable relation with Gleason's grading.
MATERIALS AND METHODS
Case selection
The present study was conducted in Department of Pathology Pt. B.D. Sharma Post Graduate Institute of Medical Sciences, Rohtak during the period from 2011 to 2014. In this prospective study, a total of 60 cases including 50 cases of prostate carcinoma and 10 of benign nodular hyperplasia were taken. The tissue samples obtained during transurethral electro resection, enucleation or needle biopsies were considered. Inadequate biopsies and cases with marked inflammation were excluded. Brief clinical data were noted from case records, which included age, presenting symptoms, per rectal and ultrasound findings, serum PSA levels and clinical diagnosis.
Morphological evaluation
Prostate fragments were fixed in 10% formalin, paraffin- embedded, sectioned and standard H and E stained sections were studied under light microscope and classified into benign and malignant lesions. Carcinoma cases were histologically graded according to Gleason's grading system, and Gleason's score was noted (well differentiated 2–4, Moderately differentiated 5–7, poorly differentiated 8–10).[8,9] Associated prostatic tissue changes like tumor invasion, prostatic intraepithelial neoplasia (PIN), prostatitis and others if any, were also analyzed. Special stains like van Gieson, Periodic acid-Schiff, Masson's trichrome and reticulin were employed whenever required for histopathological diagnosis.
Immunohistochemical analysis
Immunohistochemical (IHC) profile of the tumor was assessed by subjecting one section each from a representative block to p53 and Ki-67 immunostain. IHC was performed on 4 μm thick sections from 10% formalin-fixed paraffin-embedded specimens, according to the streptoavidin-biotin immunoperoxidase technique (Dako-cytomation). Multiple slides were evaluated, and ideal section was used IHC staining. Positive and negative control were run simultaneously. Strong brown nuclear immunoreactivity was considered as positive staining.
The immunoquantification was performed using percentage of tumor cells that react with the antibody. Each slide was evaluated at × 40 magnification in order to find areas with maximum positive cells. Then these areas were examined at × 400 magnification and the percentage of positive cells to total cells was calculated. At least 500 cells were counted, and only the cells that were definitely positive for the desired marker were considered.
A semiquantitative scoring system was employed to assess the level of p53 reactivity: 0 - was assigned when no staining was observed, 1 - when < 10% of tumor cell nuclei were reactive, 2 - when more than 10%, but < 33% of the nuclei stained, and 3 - if more than 33% of nuclei were positive.[10]
The tumors were divided into five groups regarding the percentage of Ki-67 positive cells. Cases in which the percentage of stained cells was ≤2% were considered negative. Cases with Ki-67 index of ≤25% were considered 1+, 26–50% as 2+, 51–75% as 3+ and 76–100% as 4+.[4]
Statistical analysis
The results of the study were statistically analyzed using the Statistical Package for the Social Sciences (SPSS) version 20 (IBM Corp. SPSS statistics, in Armonk NY) for windows. Data were expressed as mean ± SD for quantitative variables, numbers, and percentage. Comparison between multiple groups was made using student t-test, Chi-square test and Anova test whichever was appropriate. The correlation between Gleason's grade and IHC expression was analyzed using the Spearman's correlation test with an accompanying P value. A value of P < 0.05 was taken as significant and P < 0.01 was taken as highly significant.
RESULTS
A total of 60 cases were taken including 10 cases of benign prostatic hyperplasia (BPH) and 50 cases of carcinoma prostate. Patients of BPH were in the age group of 35–85 years with a mean age of 63.30 ± 14.08 years. Patients of carcinoma prostate were in the age group of 41–90 years with a mean age of 69.98 ± 44 years.
Carcinoma prostate patients were categorized according to Gleason's score (combined Gleason's grade). Gleason's score of 6 was the commonest pattern observed in 14 (28%) cases, followed by Gleason's score of 7 in 13 (26%) cases and Gleason's score of 8 in 10 (20%) cases. Gleason's score of 4, 5, 9 were seen in 4 (8%) cases each and Gleason's score of 10 was seen in only one (2%) case. In our study, Gleason's score of 4 was observed as the minimum Gleason's score as needle biopsy was the predominant surgical specimen. Based on tumor differentiation 4 cases (8%) were well differentiated with a score of 2–4 while 31 (62%) were moderately differentiated with a score of 5–7 and 15 cases (30%) were poorly differentiated with a score of 8–10.
p53 immunoreactivity
In BPH, p53 was expressed in only 2 of 10 (20%) cases while in carcinoma it was expressed in 38 of 50 (76%) cases. There was statistically significant difference in expression of p53 between cases of BPH and carcinoma prostate, indicated by P value of 0.001.
In prostatic carcinoma, three out of 4 (75%) well- differentiated tumors showed absence of positivity while 1 (25%) case showed grade 1 positivity [Figure 1a]. Eight of 31 (25.81%) moderately differentiated tumors revealed absence of positivity while 23 cases (74.19%) revealed strong nuclear positivity, including 13 (41.94%) cases with grade 3 positivity [Figure 2a], followed by 6 (19.35%) cases with grade 2 positivity and 4 (12.90%) cases with grade 1 positivity. Fourteen out of 15 (93.33%) poorly differentiated tumors revealed strong nuclear positivity, including 7 (46.67%) cases with grade 3 positivity [Figure 3a], followed by 4 (26.66%) cases with grade 2 positivity and 3 (20%) case with grade 1 positivity. Only one (6.67%) case among poorly differentiated tumors showed absence of positivity [Table 1].
Figure 1.
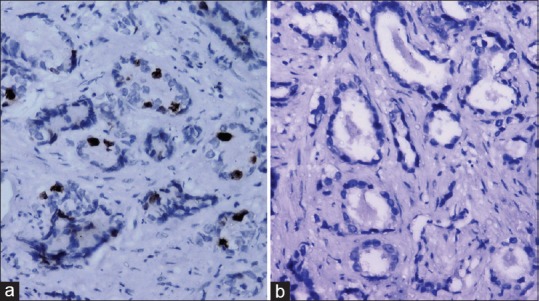
(a) Well differentiated prostatic carcinoma showing p53 positivity (grade 1) (immunohistochemical [IHC], ×200) and (b) absence of Ki-67 expression (IHC, ×200)
Figure 2.
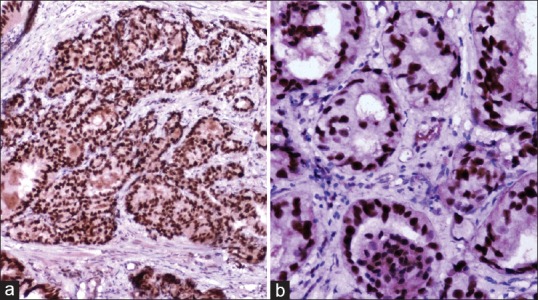
Moderately differentiated prostatic carcinoma showing p53 positivity (grade 3) (immunohistochemical [IHC], ×200) (b) Ki-67 positivity (index 3+) (IHC, ×200)
Figure 3.
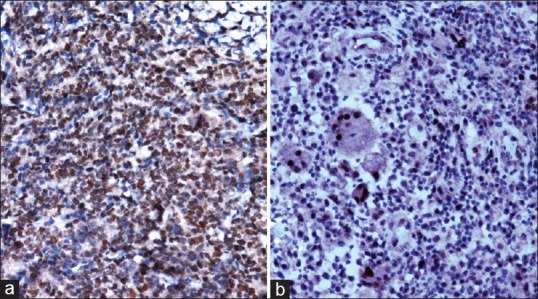
Poorly differentiated prostatic carcinoma showing p53 positivity (grade 3) (immunohistochemical [IHC], ×200) and (b) Ki-67 positivity (index 3+) (IHC, ×200)
Table 1.
Frequency of the p53 expression in relation to tumor differentiation and Gleason's grade
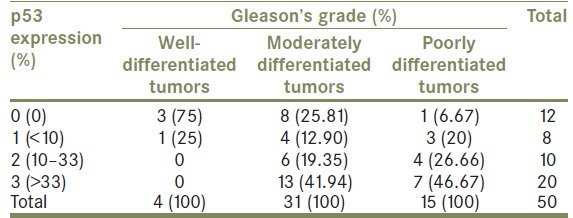
As a result, statistically significant correlation was observed between p53 expression and Gleason's grade of prostatic carcinoma, indicated by P value of 0.038.
Ki-67 immunoreactivity
Ki-67 was expressed in only 1 of 10 (10%) BPH cases while in carcinoma it was expressed in 32 of 50 (64%) cases. There was statistically significant difference in expression of Ki-67 between BPH and carcinoma prostate, indicated by P value of 0.003.
Ki-67 expression was negative (index < 2%) in all 4 (100%) well differentiated tumors [Figure 1b]. Twelve out of 31 (38.71%) moderately differentiated tumors were negative while 19 (61.29%) cases were positive, including 10 (32.26%) cases with 1+ positivity followed by 8 (25.81%) cases with 2+ positivity and only 1 (3.22%) case showed 3+ positivity [Figure 2b]. Thirteen out of 15 (86.67%) poorly differentiated tumors were positive, including 5 (33.33%) cases each with 1+ and 2+ positivity, followed by 3 (20%) cases with 3+ positivity [Figure 3b] and only 2 (13.33%) cases were negative. No case showed 4+ positivity [Table 2].
Table 2.
Frequency of the Ki-67 labeling index in relation to tumor differentiation and Gleason's grade
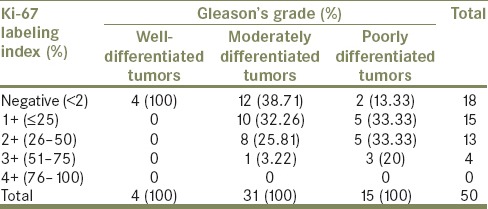
Consequently, a statistically significant correlation was observed between Ki-67 positivity and Gleason's grade of prostatic carcinoma, indicated by P value of 0.002.
Among 10 cases of BPH, 2 cases (20%) revealed positive p53 expression, of which one showed grade 1 positivity and other showed grade 3 positivity. For Ki-67, 1 case (10%) showed 2+ positivity.
In addition, Low-grade PIN was seen associated with one case of BPH, which showed grade 3 positivity with p53 and 2+ positivity with Ki-67. Two cases of high-grade PIN (HGPIN) were seen associated with moderately differentiated carcinoma. Among two cases of HGPIN, one showed grade 3 positivity, and other showed grade 1 positivity with p53 while 2+ and negative expression with Ki-67 respectively.
DISCUSSION
In the present study, p53 expression was significantly up-regulated in prostatic carcinoma (76%) as compared with benign prostatic tissue (20%), (P = 0.001). These findings are in agreement with Jiang et al.[11] in which positive staining rates of p53 protein were 51.1% and 10% respectively in patients with carcinoma and BPH (P < 0.05), whereas Sasor et al.[12] and Petrescu et al.[10] revealed lack of p53 immunoreactivity in BPH.
We observed 2 cases (3.33%) of HGPIN associated with prostatic carcinoma, which revealed grade 3 and grade 1 positivity each for p53 immunostain. Petrescu et al.[10] also observed occasional p53 reactivity in HGPIN cells adjacent to areas harboring tumor. They suggested that the presence of p53 overexpression in PIN tissue raises the question as to whether the occurrence of p53 mutations in prostatic carcinoma is an early event. With the passage of time, some of these basal cells might sustain further somatic mutations that allow progression to malignancy, whereas Sasor et al.[12] observed no immunopositivity of p53 protein in this group considering p53 immunoreactivity as a rare and late event in prostate cancer. These findings need to be evaluated further since number of cases with PIN were too small to be conclusive.
In our study, 38 of 50 (76%) cases of carcinoma revealed strong nuclear positivity with p53 immunostain. There have been widely ranging reports of the incidence of p53 immunoexpression ranging from 4% to 79%.[13,14] Most of the variations are attributed to methodological differences in tissue sampling, scoring, and the antibody clone used.
In present study, 1 of 4 (25%) well differentiated, 23 of 31 (74.19%) moderately differentiated and 14 of 15 (93.33%) poorly differentiated tumors revealed p53 immunopositivity and a statistically significant correlation was observed between p53 expression and increased Gleason's grade (P = 0.038). This is in concordance with many studies.[8,11,15,16] Concluding it as an important independent prognostic factor, inversely associated with patient survival. However, in few studies,[4,12] p53 though found to be elevated in prostatic carcinoma but no significant correlation was found with Gleason's grade. This can be justified by the fact that not all cases of p53 positive patients are detected by IHC staining methods. Indeed, formalin fixation reduces expression of p53.
Ki-67 expression was also significantly up-regulated in prostate cancer (64%) as compared with BPH (10%), (P = 0.003). This finding is in agreement with Nikoleishvili et al.[17] and Rashed et al.[18] who also found that this marker is highly expressed in prostate cancer as compared with BPH, (P = 0.019) and (P = 0.023) respectively. Among two cases of HGPIN, one showed 2 + positivity, and other was negative for Ki-67 immunostain. Similar changes were observed by Sasor et al.[12] in which only 3 out of 10 HGPIN exhibited Ki-67 positivity.
In the study by Madani et al.,[4] Ki-67 was negative in all 3 (100%) well-differentiated tumors. Totally, 13 of 21 (61.90%) moderately differentiated tumors and 22 of 25 (88%) poorly differentiated tumors were positive for Ki-67. In our study also, all 4 (100%) cases of well-differentiated carcinoma were negative for Ki-67 expression. Totally, 19 of 31 (61.29%) moderately differentiated and 13 of 15 (86.66%) poorly differentiated tumors were positive for Ki-67. Similar changes were observed by Rashed et al.[18] These observations indicate that greatest proliferative indices are noted in poorly differentiated tumors concluding that Ki-67 index increases in aggressive and high-grade prostatic carcinoma.
The present study revealed a statistically significant correlation between Ki-67 positivity and increased Gleason's grade (P = 0.002) and this is in concordance with studies by Feneley et al.[19] (P < 0.001), Madani et al.[4] (P = 0.001) and Rashed et al.[18] (P = 0.02) which concluded that Ki-67 can be used as prognostic factor for prostate cancer, whereas Thompson et al.[20] found Ki-67 expression to be elevated in cancer as compared to BPH, but no significant correlation was observed between tumor grade and Ki-67 staining, but still concluded that Ki-67 staining may allow identification of tumors with a high rate of cell growth and may present development of prognostic factor.
Further, the Ki-67 score in cancers positive for p53 was greater than that found in cancers negative for p53, and a statistically significant correlation was observed between p53 and Ki-67 expression, (P < 0.05). Our findings are similar to that observed by Kim et al.[21] and Thompson et al.,[20] whereas Sasor et al.[12] observed a significant positive correlation between expression of Ki-67 and p53 protein, only in low-grade prostatic carcinoma.
CONCLUSION
From the present study, it can be concluded that frequency of expression of both p53, a tumor suppressor protein, and Ki-67, a cell proliferation marker is significantly up-regulated in malignant lesions as compared to benign lesions.
Since most cases of prostate cancer are diagnosed microscopically before metastatic spread and among these, few cases have rapid and life-threatening outcome. Therefore, if indolent versus aggressive forms of prostate cancer can be differentiated from each other, we can help patients remarkably. In the current study, p53 and Ki-67 markers were shown to have a strong relationship with increased Gleason's grade, which has an important relationship with the prognosis of prostate cancer. Therefore, we propose that these markers can be applied along with other prostate cancer prognostic factors. However, further studies on larger samples are required to elucidate their role in the identification of premalignant lesions.
It is likely that p53 and Ki-67 positive tumors detected at biopsy display aggressive biologic features, hence these might be an independent prognostic indicators among metastatic risk cases. Further prospective clinical studies including long-term follow-up and molecular genetic analysis need to be undertaken to understand the biology of these IHC markers and to assess their prognostic significance in patients with prostate carcinoma.
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012: Estimated cancer incidence, mortality and prevalence worldwide in 2012. Lyon, France: International Agency for Research on Cancer; 2013. [Last accessed on 2014 Jul 20]. Available from: http://globocan.iarc.fr.[Internet] [Google Scholar]
- 2.Epstein JI. The lower urinary tract and male genital system. In: Kumar V, Abbas AK, Fausto N, editors. Robbins and Cotran Pathologic Basis of Disease. 8th ed. Philadelphia: Saunders; 2010. pp. 993–1002. [Google Scholar]
- 3.Shen MM, Abate-Shen C. Molecular genetics of prostate cancer: New prospects for old challenges. Genes Dev. 2010;24:1967–2000. doi: 10.1101/gad.1965810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Madani SH, Ameli S, Khazaei S, Kanani M, Izadi B. Frequency of Ki-67 (MIB-1) and P53 expressions among patients with prostate cancer. Indian J Pathol Microbiol. 2011;54:688–91. doi: 10.4103/0377-4929.91492. [DOI] [PubMed] [Google Scholar]
- 5.Moul JW, Gaddipati J, Srivastava S. Molecular biology of prostate cancer: Oncogenes and tumor suppressor genes. In: Dawson NA, Vogelzang NJ, Srivastava S, editors. Current Clinical Oncology: Prostate Cancer. New York: Wiley-Liss; 1994. pp. 19–46. [Google Scholar]
- 6.Aprikian AG, Cordon-Cardo C, Fair WR, Zhang ZF, Bazinet M, Hamdy SM, et al. Neuroendocrine differentiation in metastatic prostatic adenocarcinoma. J Urol. 1994;151:914–9. doi: 10.1016/s0022-5347(17)35121-2. [DOI] [PubMed] [Google Scholar]
- 7.Bookstein R, MacGrogan D, Hilsenbeck SG, Sharkey F, Allred DC. p53 is mutated in a subset of advanced-stage prostate cancers. Cancer Res. 1993;53:3369–73. [PubMed] [Google Scholar]
- 8.Rosai J. Male reproduction system. In: Rosai J, editor. Rosai and Ackerman's Surgical Pathology. 10th ed. Missouri: Mosby; 2011. pp. 1287–312. [Google Scholar]
- 9.Humphrey PA. The prostate gland. In: Silverberg SG, editor. Principles and Practices of Surgical Pathology and Cytopathology. 4th ed. Philadelphia: Churchill Livingstone; 2006. pp. 1791–828. [Google Scholar]
- 10.Petrescu A, Mârzan L, Codreanu O, Niculescu L. Immunohistochemical detection of p53 protein as a prognostic indicator in prostate carcinoma. Rom J Morphol Embryol. 2006;47:143–6. [PubMed] [Google Scholar]
- 11.Jiang T, Jiang H, Song XS, Li XC, Li QL. P53 expression and its clinical significance in prostatic carcinoma. Zhonghua Nan Ke Xue. 2005;11:448–51. 454. [PubMed] [Google Scholar]
- 12.Sasor A, Wagrowska-Danilewicz M, Danilewicz M. Ki-67 antigen and P53 protein expression in benign and malignant prostatic lesions Immunohistochemical quantitative study. Pol J Pathol. 2000;51:31–6. [PubMed] [Google Scholar]
- 13.Van Veldhuizen PJ, Sadasivan R, Garcia F, Austenfeld MS, Stephens RL. Mutant p53 expression in prostate carcinoma. Prostate. 1993;22:23–30. doi: 10.1002/pros.2990220104. [DOI] [PubMed] [Google Scholar]
- 14.Voeller HJ, Sugars LY, Pretlow T, Gelmann EP. p53 oncogene mutations in human prostate cancer specimens. J Urol. 1994;151:492–5. doi: 10.1016/s0022-5347(17)35000-0. [DOI] [PubMed] [Google Scholar]
- 15.Bauer JJ, Sesterhenn IA, Mostofi KF, McLeod DG, Srivastava S, Moul JW. p53 nuclear protein expression is an independent prognostic marker in clinically localized prostate cancer patients undergoing radical prostatectomy. Cancer Res. 1995;1:1295–300. [PubMed] [Google Scholar]
- 16.Moul JW, Bettencourt MC, Sesterhenn IA, Mostofi FK, McLeod DG, Srivastava S, et al. Protein expression of p53, bcl-2, and KI-67 (MIB-1) as prognostic biomarkers in patients with surgically treated, clinically localized prostate cancer. Surgery. 1996;120:159–66. doi: 10.1016/s0039-6060(96)80283-2. [DOI] [PubMed] [Google Scholar]
- 17.Nikoleishvili D, Pertia A, Trsintsadze O, Gogokhia N, Managadze L, Chkhotua A. Expression of p27((Kip1)), cyclin D3 and Ki67 in BPH, prostate cancer and hormone-treated prostate cancer cells. Int Urol Nephrol. 2008;40:953–9. doi: 10.1007/s11255-008-9350-y. [DOI] [PubMed] [Google Scholar]
- 18.Rashed HE, Kateb MI, Ragab AA, Shaker SS. Evaluation of minimal prostate cancer in needle biopsy specimens using AMACR (p504s), p63 and Ki-67. Life Sci. 2012;9:12–21. [Google Scholar]
- 19.Feneley MR, Young MP, Chinyama C, Kirby RS, Parkinson MC. Ki-67 expression in early prostate cancer and associated pathological lesions. J Clin Pathol. 1996;49:741–8. doi: 10.1136/jcp.49.9.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thompson SJ, Mellon K, Charlton RG, Marsh C, Robinson M, Neal DE. P53 and Ki-67 immunoreactivity in human prostate cancer and benign hyperplasia. Br J Urol. 1992;69:609–13. doi: 10.1111/j.1464-410x.1992.tb15632.x. [DOI] [PubMed] [Google Scholar]
- 21.Kim DG, Kim KK, Lee KS. Expressions of p53, bcl-2 protein and Ki-67 labelling index and their relationships with prognostic factors in prostate cancer Korean. J Urol. 2001;42:828–33. [Google Scholar]


