Abstract
Despite the capacity of Schwann cells to support peripheral nerve regeneration, functional recovery after nerve injuries is frequently poor, especially for proximal injuries that require regenerating axons to grow over long distances to reinnervate distal targets. Nerve transfers, where small fascicles from an adjacent intact nerve are coapted to the nerve stump of a nearby denervated muscle, allow for functional return but at the expense of reduced numbers of innervating nerves. A 1-hour period of 20 Hz electrical nerve stimulation via electrodes proximal to an injury site accelerates axon outgrowth to hasten target reinnervation in rats and humans, even after delayed surgery. A novel strategy of enticing donor axons from an otherwise intact nerve to grow through small nerve grafts (cross-bridges) into a denervated nerve stump, promotes improved axon regeneration after delayed nerve repair. The efficacy of this technique has been demonstrated in a rat model and is now in clinical use in patients undergoing cross-face nerve grafting for facial paralysis. In conclusion, brief electrical stimulation, combined with the surgical technique of promoting the regeneration of some donor axons to ‘protect’ chronically denervated Schwann cells, improves nerve regeneration and, in turn, functional outcomes in the management of peripheral nerve injuries.
Keywords: peripheral nerve injury, nerve repair, nerve regeneration, Schwann cells, electrical nerve stimulation, axon regeneration
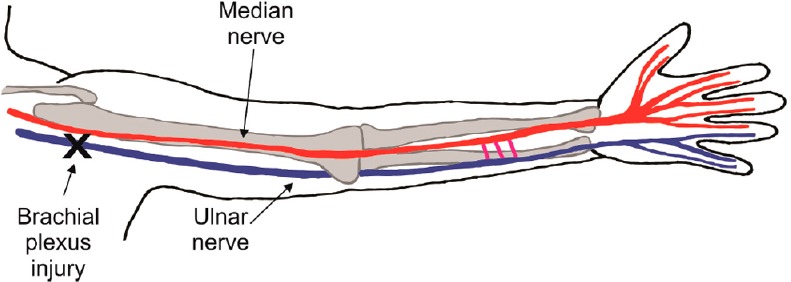
The glial Schwann cells normally myelinate the larger caliber axons but they undergo a transformation to their growth supportive state after peripheral nerve injuries (Gordon, 2015). Schwann cells are essential for nerve regeneration and contrast with the analogous oligodendrocytes which myelinate central axons but do not support their regeneration. Yet, despite the capacity of the Schwann cells to support nerve regeneration, functional recovery after nerve injuries is frequently poor, especially for proximal injuries that necessitate that the regenerating axons grow over long distances to reinnervate denervated targets. Adult brachial plexus injury (Figure 1) is often sustained in motorcycle and snowmobile accidents and is generally regarded as being ‘too far’ from the denervated muscles and sense organs of the hand for nerve regeneration to restore useful function. Indeed, a generally held misconception is that chronically denervated muscles undergo irreversible atrophy and are replaced by fat, making reinnervation of these targets impossible (Gordon, 2015).
Figure 1.
Brachial plexus injuries in adults.
Injured nerves must regenerate over long distances to reinnervate targets such as denervated muscle and sensory targets in the hand. At a regeneration rate of 1mm/day in humans, as much as an 800 day delay may be incurred for axons to regenerate over a distance of a metre from the injury site to the denervated targets in the hand. Reinnervation of the hand almost never occurs even after surgical nerve repair in these situations.
With the maximal regeneration rate being ~1 mm/day in humans, two or more years would pass before regenerating axons from the brachial plexus might reach the denervated targets in the hand (Figure 1). We developed an experimental rat model of delayed nerve repair where the period of time during which injured neurons remain without target connections was protracted in a state of chronic axotomy and, where the time of chronic denervation of the Schwann cells in the denervated distal nerve stump was prolonged independently (Fu and Gordon, 1995a, b). We demonstrated that the prolonged axotomy in of itself, progressively reduced the regenerative capacity of neurons to 33% of normal within 4–6 months and that prolonged Schwann cell denervation reduced the capacity even further to ~5–10% of normal within the same time period (Fu and Gordon, 1995a, b). Of particular interest was the finding that, even after periods of a year or more, the axons that did manage to regenerate their axons did reinnervate chronically denervated muscle fibers, each axon supplying up to 3 times as many muscle fibers as each axon normally does. This capacity for motor unit enlargement equaled the capacity of regenerating axons that reinnervate denervated targets within a short space of time. Nonetheless, the fact that the muscle fibers did not regain their normal dimensions argued that the satellite muscle cells that provide nuclei to the multinucleated skeletal muscle fibers in proportion to their size may become depleted after the excessive denervation atrophy after prolonged periods of time.
A variety of surgical strategies are currently practiced for complex nerve injuries such as brachial plexus injuries. Particularly in the circumstances where the spinal roots are avulsed with death of the motoneurons and dorsal root ganglion sensory neurons whose cell bodies are so close to the site of the injury and/or suffer the excessive traction on their axons, the surgeons must rely on the capacity of surviving neurons to regenerate their axons and to reinnervate denervated targets. On the basis of the presumed deterioration of chronically denervated targets, contemporary strategies often rely on transferring small fascicles from an intact nerve to the distal nerve stumps of nearby denervated muscles. Such nerve transfers include the transfer of an ulnar nerve fascicle to the biceps muscle and one median nerve fascicle to the brachialis muscle in the upper arm (Oberlin II transfer or “double fascicular transfer”) (Mackinnon et al., 2005). The concept of the nerve transfer is to sacrifice the function of a redundant nerve and/or donor muscle to revive function in the recipient nerve and muscle that will undergo reinnervation. For example, with the Oberlin II transfer, redundant nerve fascicles to the flexor digitorum superficialis and other muscles are used to innervate biceps or brachialis muscle but grip strength is unchanged (presumably because of the ability of motor nerves to supply enlarged motor units) (Ray et al., 2011; Ladak and Spinner, 2013). The donor nerve is a healthy one and the target nerve stumps are directly coapted to avoid nerve grafting with a tension free suture and consequent limited donor site morbidity. These techniques allow for return of function of the elbow flexor muscles and can be very successful in cases where sufficient innervation of the hand is available for use. The return of function is generally reasonable but it must be remembered that as few as 20% of the normal number of motoneurons supplying a muscle is sufficient to reinnervate all the muscle fibers. This is the result of the 5-fold capacity of human nerves to expand their peripheral field of innervation (Gordon et al., 1993). This is at the expense of return of normal capacity for graded control of muscle contraction.
Recently, we have reported that brief electrical stimulation of the proximal nerve stump of a transected nerve is effective in accelerating the outgrowth of axons across the lesion site (Al-Majed et al., 2000; Brushart et al., 2002) and in turn, leading to accelerated reinnervation of denervated targets, even after delayed nerve repair (Elzinga et al., 2015). The efficacy of brief electrical stimulation of 20 Hz for 1-hour, even after delayed nerve repair, was demonstrated in human patients after carpal tunnel release surgery (Gordon et al., 2010).
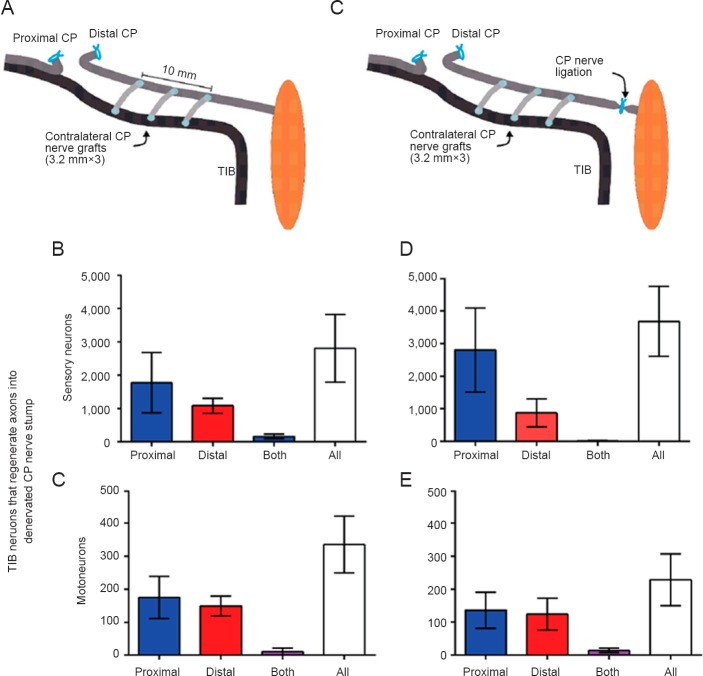
More recently, we investigated a novel strategy in which donor axons are enticed to grow through small nerve grafts (“cross-bridges”) into a denervated nerve stump as a means of ‘protecting’ the resident denervated Schwann cells until such time as the regenerating axons from a more distant proximal site ‘reach’ the chronically denervated Schwann cells. In a rat model we used two end-to-side neurorrhaphies that do not require the transection of any nerve (Gordon et al., 2015). Viterbo et al. (1994) pioneered the end-to-side technique but, in contrast to their assertion that small windows cut into the epi- or perineurium were unnecessary, we found that the number of donor tibial motor and sensory neurons that grew across the bridges into the recipient denervated common peroneal nerve stump depended on the size of the window that was opened (Gordon et al., 2015). First we determined whether donor neurons sprouted their axons through the bridges and/or were damaged and regenerated their axons into the recipient denervated distal nerve stump. This was done three months after the sterile insertion of the bridges by applying fluorogold and rubyred dyes for one hour to either the tibial and common peroneal nerves distal to the bridges. We found that very few tibial neurons had sprouted axons from the donor nerve, the vast majority of the nerves being sufficiently injured that they regenerated across the bridges. Of particular interest and of enormous importance for the application of this technique of creating side-to-side bridges from a donor nerve into a recipient denervated nerve stump, is that, with retrograde labeling techniques described in the Figure 2 legend, motor and sensory neurons regenerated their axons equally well either side of the bridges (Figure 2). Indeed, it did not matter whether or not the distal nerve stump was in continuity with denervated targets because the same pattern of nerve growth either side of the bridges was observed whether or not the distal stump was isolated from the denervated targets by a ligature (Figure 2). In our experimental rat model, we coapted the proximal and distal nerve stumps of the injured common peroneal nerve after a delay of three months during which the distal nerve stump either was or was not ‘protected’ by the donor tibial nerves (Figure 3). We found that all the motoneurons regenerated their axons after the protection whilst only a third had regenerated their axons in the absence of cross-bridges (Figure 3A). Similarly there was a three-fold increase in the numbers of sensory neurons that regenerated their axons (Figure 3B). These findings were particularly exciting given that the regenerating common peroneal axons proceeded through the distal nerve stump whose endoneurial tubes were partially occupied with donor tibial axons, which were myelinated sheaths and presumably were functional.
Figure 2.
Experimental rat model of securing 3 side-to-side 3.2 mm long autologous common peroneal nerve cross-bridges (dissected from the contralateral hindlimb) between 500 μm perineurial windows cut into a donor intact tibial (TIB) nerve and a recipient denervated common peroneal (CP) distal nerve stump.
The sterile surgery was performed under halothane anesthesia. Either the CP nerve stump remained in continuity with denervated flexor musculature in 12 rats (A) or the continuity was interrupted in 6 rats by cutting and ligating the nerve to eliminate possible retrograde neurotrophic influences from the denervated targets (C). Three months later, retrograde dyes, rubyred and fluorogold, were each applied to the CP nerve stump 5 mm either side of the cross-bridges for one hour, again under halothane anesthesia and using sterile technique. A week later, the dorsal root ganglia and spinal cord were removed to enumerate the backlabelled neurons that had regenerated their axons in transverse sections of the spinal cord and dorsal root ganglia. Irrespective of whether the distal nerve stump (A) remained in continuity with the denervated muscles and sensory targets or (C) the continuity was interrupted by cutting and ligating the nerve to eliminate possible retrograde neurotrophic influence(s) from the denervated targets, equal numbers of TIB (B, D) sensory and (C, E) motor neurons regenerated axons across the bridges and grew into the recipient denervated CP nerve stump either side of the cross-bridges. Very few grew in both directions, the axons growing either proximal or distal to the bridges within the recipient CP nerve stump. The data are expressed as the mean ± SE.
Figure 3.
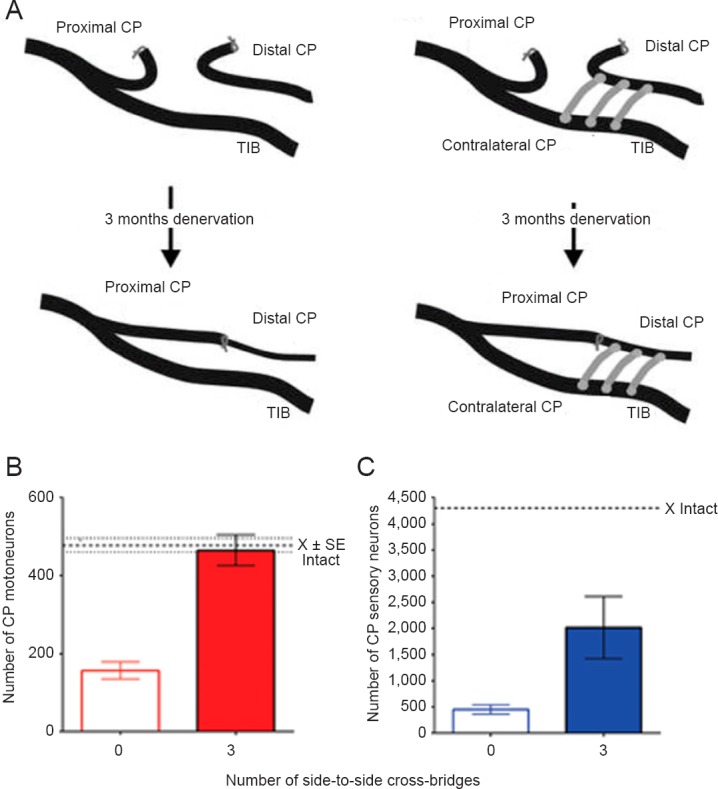
Regeneration of the axons of common peroneal (CP) motoneurons and sensory neurons was compared after delayed surgical coaptation of the proximal and distal nerve stumps (nerve repair) under halothane anesthesia and using aseptic technique when no (0) and 3 autologous CP cross-bridges were placed between the donor tibial (TIB) nerve and the recipient denervated CP nerve stump 3 months prior to the surgical repair.
(A) The number of CP motoneurons (B) and sensory neurons (C) that regenerated their axons through chronically CP denervated nerve stumps was increased by a factor of 3 by ‘protection’ of the nerve stumps with TIB axons that regenerated through the cross-bridges into the recipient denervated CP nerve stump. The heavy dotted lines in B and C represent the mean number of CP neurons that project axons into the intact CP nerve. The light dotted lines in B show the SE of the mean number of motoneurons that normally send axons into the intact CP nerve. The data are expressed as the mean ± SE, the stars representing a significant increase with 3 bridges as compared to 0 bridges (P < 0.01) (n = 6 for each of the bars in the histograms plotted in B and C).
Whilst the molecular mechanism of the protection has not been determined, it is clear that the denervated Schwann cells that would undergo progressive atrophy and many of which would culminate in death, survived with the protection. Moreover, the common peroneal axons that regenerated into the ‘protected’ distal nerve stumps clearly were supported by the indwelling Schwann cells. One plausible explanation that we provided was that the donor nerves released agents that might include mitogens such as cAMP and neuregulin which, in turn, could sustain growth supportive Schwann cells. These Schwann cells in turn, supported the regeneration of axons after delayed nerve repair. Under conditions of the growth of regenerating axons over distance and time, the capacity of donor axons to sustain Schwann cells that remain denervated could conceivably promote long-distance nerve regeneration, and in turn, functional recovery. It must also be noted, importantly that the donor axons reinnervate denervated targets with the result that these targets would also be sustained until the regenerating axons make their way back to these targets.
We know that there is some preferential motor and sensory nerve regeneration into denervated distal nerve targets that is associated with differential expression of neurotrophic factors in the Schwann cells of motor and sensory nerves (Hoke et al., 2006). However, regenerating motor nerves randomly reinnervate target muscles, many inappropriately with consequent synkinesis (Thomas et al., 1987). This remains a challenge but should regenerating axons be directed towards functional synergists, functional return is more likely.
Translation of the side-to-side bridge technique from animal to man must take into account the differences in morphology of human and animal nerves. The human nerves contrast with those of the animal nerves in their high content of connective tissue between the fascicles. Differences in the number of fascicles between nerves and where epi- and perineurial windows should be located to promote the growth of sufficient donor axons into the denervated fascicles will have to be determined. With the rapidly increasing ability of the advancing imaging techniques that are becoming available, these issues are likely to be resolved in the not too distant future. Presently, the technique of ‘protection’ is being translated from laboratory findings in the rat for the cross-face grafting that is performed to innervate the mimetic muscles of a free muscle transplant for emotional facial reanimation (Placheta et al., 2015). In the rat model, the sensory occipital nerves were coapted end-to-side to the 30 mm long cross-face common peroneal nerve graft that connected the proximal stump of the transected right buccal branch of the facial nerve and the distal stumps of the transected left buccal and marginal mandibular branches (Figure 4A). Not only were there more motoneurons that regenerated their axons (Figure 4B) and a corresponding greater number of axons (Figure 4C) through and distal to the cross-face nerve grafts which were ‘protected’ by the few occipital nerves that grew into the common peroneal nerve graft but the rats were able to move their whiskers significantly better. The latter movements were analysed from 3–5 minute video recordings, determining frequency, amplitude, and angular velocity and acceleration. In our human patients, 9–12 months pass during which time the facial nerve regenerates through the cross-face graft before the distal end of the graft is sutured into a muscle transplant. The long passage of time is likely to compromise the nerve regeneration through the nerve graft. In order to combat chronic denervation of the Schwann cells within the cross-face sural nerve graft, we now transfer a redundant branch of a regional sensory nerve end-to-end into the end of the graft. Thereby we recapitulate the neurophysiologic “Schwann cell protection” design of the animal study while translating it into humans, taking the anatomic differences into account.
Figure 4.
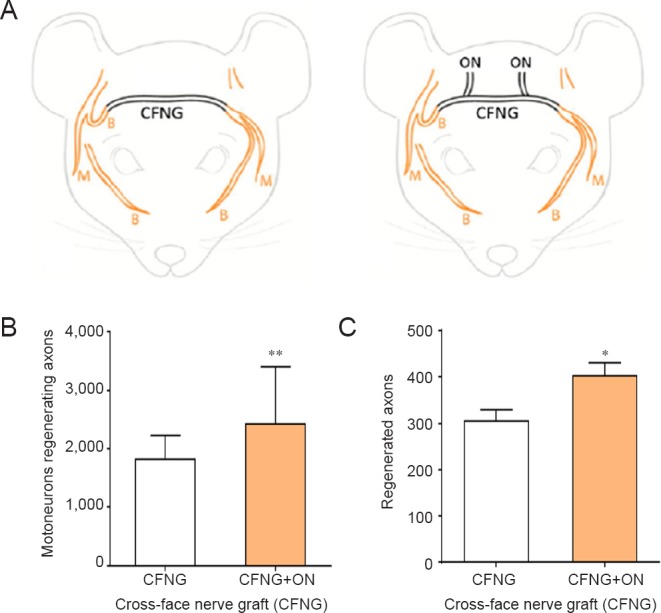
Insertion of 2 sensory neurons in an end-to-side fashion into a denervated nerve stump improves the regeneration of axons through the nerve stump.
(A) Under halothane anesthesia and using aseptic technique, sensory occipital nerves were coapted end-to-side to the 30 mm long cross-face common peroneal nerve graft (CFNG) that connected the proximal stump of the transected right buccal branch of the facial nerve and the distal stumps of the transected left buccal and marginal mandibular branches. The motoneurons that regenerated their axons into the two left branches were backlabelled by exposing the two branches 10 mm distal to the CFNG to fluorogold and rubyred retrograde dyes for one hour, 16 weeks after the first surgery. (B) The number of motoneurons regenerating their axons across the CFNG was significantly increased after ‘protection’ of the chronically denervated distal facial nerve stump by the ingrowth of sensory neurons from the two sensory occipital nerves (ON) that were sutured to the side of the CFNG. (C) Regenerated axons enumerated from cross-sections of the buccal and marginal mandibular branches (taken ~11 mm distal to the CFNG), were also significantly increased in number when compared to the number of axons that regenerated through the unprotected distal stump of the facial nerve. The data are expressed as the mean ± SE, the stars representing a significant increase when sensory occipital nerves were coapted end-to-side (n = 12 for each of the CFNG and CFNG + ON groups; *P < 0.05; **P < 0.01).
In conclusion, application of a 1-hour period of electrical stimulation combined with the surgical technique of contributing donor axons to ‘protect’ chronically denervated Schwann cells is likely to make an important contribution to improving nerve regeneration and functional outcomes in surgical management of peripheral nerve injuries.
References
- 1.Al-Majed AA, Neumann CM, Brushart TM, Gordon T. Brief electrical stimulation promotes the speed and accuracy of motor axonal regeneration. J Neurosci. 2000;20:2602–2608. doi: 10.1523/JNEUROSCI.20-07-02602.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brushart TM, Hoffman PN, Royall RM, Murinson BB, Witzel C, Gordon T. Electrical stimulation promotes motoneuron regeneration without increasing its speed or conditioning the neuron. J Neurosci. 2002;22:6631–6638. doi: 10.1523/JNEUROSCI.22-15-06631.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elzinga K, Tyreman N, Ladak A, Savaryn B, Olson J, Gordon T. Brief electrical stimulation improves nerve regeneration after delayed repair in Sprague Dawley rats. Exp Neurol. 2015;269:142–153. doi: 10.1016/j.expneurol.2015.03.022. [DOI] [PubMed] [Google Scholar]
- 4.Fu SY, Gordon T. Contributing factors to poor functional recovery after delayed nerve repair: prolonged axotomy. J Neurosci. 1995a;15:3876–3885. doi: 10.1523/JNEUROSCI.15-05-03876.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fu SY, Gordon T. Contributing factors to poor functional recovery after delayed nerve repair: prolonged denervation. J Neurosci. 1995b;15:3886–3895. doi: 10.1523/JNEUROSCI.15-05-03886.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gordon T, Yang JF, Ayer K, Stein RB, Tyreman N. Recovery potential of muscle after partial denervation: a comparison between rats and humans. Brain Res Bull. 1993;30:477–482. doi: 10.1016/0361-9230(93)90281-f. [DOI] [PubMed] [Google Scholar]
- 7.Gordon T, Amirjani N, Edwards DC, Chan KM. Brief post-surgical electrical stimulation accelerates axon regeneration and muscle reinnervation without affecting the functional measures in carpal tunnel syndrome patients. Exp Neurol. 2010;223:192–202. doi: 10.1016/j.expneurol.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 8.Gordon T. Nerves and Nerve Injuries. New York: Elservier; 2015. The biology, limits, and promotion of peripheral nerve regeneration in rats and human; pp. 903–1019. [Google Scholar]
- 9.Gordon T, Hendry M, Lafontaine CA, Cartar H, Zhang JJ, Borschel GH. Nerve cross-bridging to enhance nerve regeneration in a rat model of delayed nerve repair. PLoS One. 2015;10:e0127397. doi: 10.1371/journal.pone.0127397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoke A, Redett R, Hameed H, Jari R, Zhou C, Li ZB, Griffin JW, Brushart TM. Schwann cells express motor and sensory phenotypes that regulate axon regeneration. J Neurosci. 2006;26:9646–9655. doi: 10.1523/JNEUROSCI.1620-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ladak A, Spinner RJ. Double fascicular nerve transfer for elbow flexion: is 2 better than 1? Neurosurgery. 2013;72:1055–1056. doi: 10.1227/01.neu.0000430600.51158.3e. [DOI] [PubMed] [Google Scholar]
- 12.Mackinnon SE, Novak CB, Myckatyn TM, Tung TH. Results of reinnervation of the biceps and brachialis muscles with a double fascicular transfer for elbow flexion. J Hand Surg Am. 2005;30:978–985. doi: 10.1016/j.jhsa.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 13.Placheta E, Wood MD, Lafontaine C, Liu EH, Hendry JM, Angelov DN, Frey M, Gordon T, Borschel GH. Enhancement of facial nerve motoneuron regeneration through cross-face nerve grafts by adding end-to-side sensory axons. Plast Reconstr Surg. 2015;135:460–471. doi: 10.1097/PRS.0000000000000893. [DOI] [PubMed] [Google Scholar]
- 14.Ray WZ, Pet MA, Yee A, Mackinnon SE. Double fascicular nerve transfer to the biceps and brachialis muscles after brachial plexus injury: clinical outcomes in a series of 29 cases. J Neurosurg. 2011;114:1520–1528. doi: 10.3171/2011.1.JNS10810. [DOI] [PubMed] [Google Scholar]
- 15.Thomas CK, Stein RB, Gordon T, Lee RG, Elleker MG. Patterns of reinnervation and motor unit recruitment in human hand muscles after complete ulnar and median nerve section and resuture. J Neurol Neurosurg Psychiatry. 1987;50:259–268. doi: 10.1136/jnnp.50.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Viterbo F, Trindade JC, Hoshino K, Mazzoni A. Two end-to-side neurorrhaphies and nerve graft with removal of the epineural sheath: experimental study in rats. Br J Plast Surg. 1994;47:75–80. doi: 10.1016/0007-1226(94)90162-7. [DOI] [PubMed] [Google Scholar]