Scientists conclude that a combination of treatments involving rehabilitation, drug delivery, surgery and cell transplantation are necessary to achieve significant progress in regenerating the injured central nervous system (CNS). The benefits of pluripotent stem cells in neurodegenerative disorders are well recognised (Thompson and Bjorklund, 2015) and cell culture methods have advanced to condition and enrich cells of therapeutic interest, thereby optimising their assimilation into diseased CNS regions. Radial glial cells are a unique cell type that act as both cell progenitors and scaffolds during development, orchestrating many brain and spinal cord formation events. In light of new developments elucidating their precursor and regenerative capacities, we will place a spot light on the transplantation potentials of embryonic stem (ES) cell or cell line derived radial glia and endogenous radial glial cell populations in re-establishing neural connectivity and restoring cell populations following neurodegeneration.
Radial glial cells mediate neural connectivity during development: The proliferating CNS exists as a population of multipotent neural stem cells and progenitor cells arranged as polarized neuroepithelium which ultimately produces all neurons, glia and oligodendrocytes in the adult CNS. The earliest glial progeny of the neuroepithelium are termed radial glial cells, a secondary class of neural progenitor which gives rise to the majority of brain neurons and astrocytes. Radial glia and their subtypes are found throughout the developing CNS and in neurogenic niches in the adult (Barry et al., 2014). They possess an apical - basal polarity which creates the architectural framework for the laminar patterning of migrating neurons (Rakic, 1972). This morphology also facilitates their interaction with axons along a number of axes providing boundaries, conduits and sorting structures in the brain and spinal cord (Norris and Kalil, 1991; Barry et al., 2013). These proliferative and boundary forming functions are especially significant when understanding the developmental defects affecting motor and sensory systems in vertebrates and present multiple therapeutic opportunities for radial glial cell transplantation into the degenerating or injured CNS. Moreover, the roles of radial glia in regenerative neuroscience in amphibians and fish are becoming more widely studied, where it appears they effectively safeguard the mature brain and spinal cord from permanent injury by restoring entire CNS compartments (Becker and Becker, 2015).
Transplantation of ES cells and cell line derived radial glial cells: A major obstacle to functional recovery after mammalian CNS degeneration is the near inability of damaged or dead neurons and glial cells to regenerate and restore synaptic connectivity. However, in recent decade a clearer understanding of the trophic factors, drug treatments, transplantation vectors and tissue scaffolds that promote the survival of neurons and glial cells have benefitted the outcomes of cell replacement therapies. In particular, cell transplantation strategies aim to compensate for CNS defects using endogenous embryonic neurons, grafts of neural tissue or conditioned immortalized neural precursors that may or may not be genetically modified. Recently, increasing attention has been placed on the benefits of transplanting both differentiated and/or pluripotent ES cells. ES cells proliferate extensively (Thomson et al., 1998; Gage, 2000), yet their allogenic transplantation often results in tumour formation. This limitation has resulted in greater interest being placed on pre-differentiated ES cells that are less tumorigenic (Batista et al., 2014). For example, the transplantation of dopaminergic neurons in Parkinson's disease (Shin et al., 2014; Han et al., 2015) and motor neurons in amyotrophic lateral sclerosis (Coatti et al., 2015) have yielded significant functional recovery in humans.
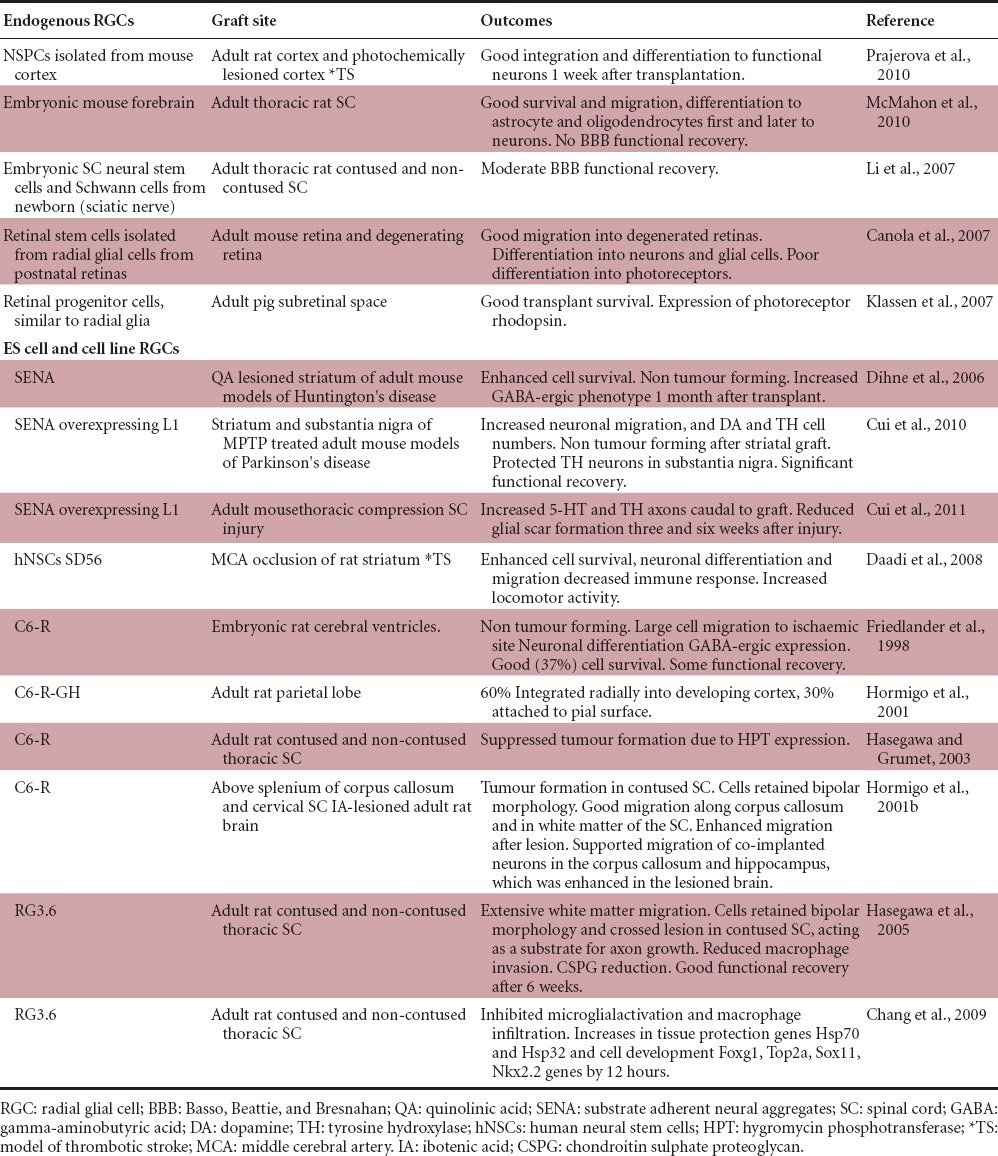
Generating primary radial glial cell cultures generally requires their co-culture with neurons or other brain extracts (Hunter and Hatten, 1995), making them difficult to study in isolation or to prepare pure populations for transplantation. However, cell line derived collections of radial glia and neurons, termed substrate adherent neural aggregates (SENAs), have proven to be an excellent source of neuronally-fated cells and have been successfully transplanted into mouse models of Huntington's disease (Dihne et al., 2006), Parkinson's disease (Cui et al., 2010) and spinal cord injury (Cui et al., 2011), without tumour formation. The majority of cells in these aggregates initially show similarities to in vivo cortical radial glial cells, including nestin expression and a propensity to differentiate to neurons in culture (Dihne et al., 2006). However, their identity as true radial glial cells that recapitulate their in vivo counterparts is debatable. Notwithstanding the phenotypic consistency of SENAs, an isolated human neural stem cell line termed SD56, expressing vimentin, nestin and 3CB2 (markers of early appearing radial glia (Prada et al., 1995)) showed extensive migration without tumorigenesis around a striatal ischaemic lesion in the rat, significantly improving the independent use of the stroke impaired forelimb (Daadi et al., 2008).
Perhaps a more accurate paradigm of radial glial cell transplantation is the C6 glioma derived radial glia-like cell line C6-R. C6-R cells show a bipolar morphology in vitro and support neuronal migration, while expressing markers typical of in vivo radial glia including vimentin, nestin, glial fibrillary acidic protein (GFAP) and RC1 (Friedlander et al., 1998). These cells integrate well into the developing forebrain and are capable of adopting the typical radial glial apical - basal polarity without forming tumours over time. They also infiltrated into the adult forebrain and spinal cord white matter after implantation, where they supported the migration of co-implanted primary neurons in the healthy and lesioned cortex, corpus callosum and hippocampus (Hormigo et al., 2001a, b). While their potential in brain injury is clear, C6-R cells formed tumours when implanted into the contused spinal cord (Hasegawa and Grumet, 2003), rendering them impractical in spinal cord injury. However, a similar clone RG3.6 produces radial glial cells that express brain lipid binding protein (BLBP), glutamate aspartate transporter and vimentin, which are markers of mature radial glia in the brain and spinal cord, are bipolar and migrate extensively through the spinal cord white matter without forming tumours after transplantation (Hasegawa et al., 2005; Chang et al., 2009). When transplanted into the contused rat spinal cord, RG3.6 cells localized extensively above and below the injury epicentre and decreased the appearance of macrophages and chondroitin sulfate proteoglycans, thus limiting inflammation. Indeed, axon growth across the lesion was enhanced, leading to increased Basso Beattie Bresnahan scores of greater than two points within the first week following the injury (Hasegawa et al., 2005). Moreover, Chang et al. (2009) showed that transplanted RG3.6 cells upregulate various protective factors in the host including anti-apoptotic Hsp70 mRNA as well as the neurogenic and cell lineage factors Foxg1, Top2a, Nkx2.2 and Sox11, a factor critical for neurite growth and survival. Together these studies show that both stem cell derived radial glia and aggregates of radial glia and neurons repopulate and offer neurotrophic support at various CNS lesion sites (Table 1).
Table 1.
Radial glial cell transplantation strategies and outcomes
The transplantation and manipulation of radial glia-like cells such as pre-differentiated astrocytes is also effective in improving outcomes following spinal cord injury by dorsolateral funiculus transection of the adult rat cervical spinal cord (Davies et al., 2011). In this study, human glial precursor cells were differentiated into GFAP-expressing astrocytes using either bone morphogenic protein (BMP) or ciliary neurotropic factor (CNTF). Interestingly, cells differentiated with BMP expressed brain-derived neurotrophic factor, connexin-43 and glutamate transporter-1, which are proteins expressed in vivo in astrocytes. Furthermore, rats transplanted with astrocytes differentiated with BMP, but not undifferentiated precursors or CNDF-derived astrocytes, exhibited elevated numbers of neurons at the injury site and significantly improved functional recovery via the grid walk test. Moreover, the recent rapid progression in our understanding of cell reprogramming technologies has placed induced pluripotent stem (iPS) cells as a viable alternative to ES cells as the future of human cell therapy, thereby bypassing ethical and transplantation rejection issues (Thompson and Bjorklund, 2015). Indeed, a recent report described the production of human radial glial cells from human pluripotent stem cells that performed similar lineage and patterning roles as in vivo after ventricular transplantation (Duan et al., 2015). This represents an exciting advance, yet at the time of writing the regenerative potentials of iPS-derived radial glial cells in trauma conditions such as spinal cord injury or stroke had not been reported.
Transplantation of endogenous radial glial cells: The most widely understood use of endogenous radial glia in a clinical context is in spinal cord injury, which likely underlies their in vivo axon guidance capacities. Simply stated, as upper motor neuron cell bodies are located in the motor cortex it is more plausible to regenerate axons at the lesion site before they die. Radial glial cells have been demonstrated to reappear in response to injury, at least in the spinal cord. Shibuya et al. (2003) reported 3CB2-expressing radial glial cells in both grey and white matter regions near an adult thoracic spinal cord compression lesion site 1 week after injury. Their processes became radialized after 4 weeks, resembling their embryonic morphology. In addition, Nomura et al. (2010) showed that endogenous radial glial cells have the potential to differentiate and migrate across the loci of a complete adult spinal cord transection site promoting the movement of axons through a chitosan channel, acting as a neural bridge. Likewise, White et al. (2010) demonstrated that at 3 days post mid-thoracic contusion injury cells expressing BLBP, but not GFAP, were present at the injury epicentre, some of which also expressed nestin. As these cells were no longer found at 7 days post injury, it is possible that the endogenous BLBP-expressing population differentiated into mature astrocytes between 3 and 7 days after contusion, and may be manipulated in order to facilitate repair. While transplantation of neurotrophic cells remains perhaps the most viable strategy for many neurodegenerative diseases, improving the local microenvironment by introducing neurotrophic factors to the lesioned areas also promotes synaptogenesis and native cell proliferation. Interestingly, treatment of the contused spinal cord with transforming growth factor-α (TGF-α) resulted in a shift in astrocyte phenotype from hypertrophied and interdigitated to an elongated shape reminiscent of radial glia (White et al., 2011). Furthermore, in vitro experiments showed that dorsal root ganglion cells co-cultured with TGF-α treated astrocytes exhibited neurite outgrowth capabilities similar to those cultured on laminin, while fetal bovine serum-treated astrocytes had significantly shorter neurites than those grown on laminin. In addition, fibroblast growth factor (FGF) has demonstrated much therapeutic promise after injury in a variety of CNS regions. Goldshmit et al. (2014) showed that FGF2 mediated a reduction in reactive astrocyte invasion to the glial scar and an up-regulation of pro-regenerative glial precursor cells, likely radial glia, in the grey and white matter, which accompanied significant motor recovery in the mouse. FGF2 is currently being trialled in humans with cervical spinal cord injury.
While difficult to purify and maintain in culture, endogenous populations of early appearing neuronal stem/progenitor cells (NSPCs) can be preserved in the short term and have been homotopically transplanted into the neocortex, hippocampus, olfactory bulb and striatum, where they preserve their lineage fates (Carletti et al., 2004). Furthermore, when heterotopically transplanted they respond to their local microenvironment by showing site specific integration and differentiation (Gaillard et al., 2003; Kallur et al., 2006). However, transplanted progenitors become more fate restricted as development proceeds (Brock et al., 1998; Pinaudeau et al., 2000). These temporal, lineage-restriction characteristics of neural progenitors have been exploited as rat cortical NSPCs were grown as neurospheres, expressing markers typical of radial glia including BLBP and GFAP, and then transplanted to the lesioned adult cortex in a model of thrombotic stroke, where they primarily differentiated to functional neurons 1 week after implantation and replaced lost cells (Prajerova et al., 2010). Furthermore, multipotent radial glial cells have been transplanted in the contused spinal cord of adult rats following isolation and co-culture with Schwann cells from the developing spinal cord (Li et al., 2007) and forebrain (McMahon et al., 2010) (Table 1). Both migration and integration was observed in these cases and transplanted radial glia differentiated into neurons, oligodendrocytes and astrocytes (McMahon et al., 2010).
A further application for radial glial cells lies in retinal degeneration as cell transplantation is rapidly becoming a viable strategy for treating neuro-retinal diseases including retinitis pigmentosa and age-related macular degeneration. A subtype of radial glial cell, the Müller glial cell, has been assigned some limited regenerative capacities in the mammalian retina following neurotoxic insult (Ooto et al., 2004; Jayaram et al., 2014), an attribute greatly magnified in fish (Yurco and Cameron, 2005). Moreover, the survival and integration of transplanted retinal stem cells into normal and diseased retinas in mice and pigs has been reported and these cells expressed markers typical of radial glial cells including RC2, Pax6 and Mash1 (Canola et al., 2007) and nestin, vimentin and LewisX (Klassen et al., 2007). Many of these cells migrated and differentiated into layer-specific retinal cells, while Klassen et al. (2007) demonstrated their potential to become subretinal photoreceptors cells. Moreover, purified Müller glia express protein similarities with dopaminergic (DA) neurons, including tyrosine hydrolase, L-DOPA decarboxlyase, the nuclear receptor-related factor 1 and DA associated transporter expression (Stutz et al., 2014). Their subsequent transplantation into the striatum of hemi-Parkinson's disease mice resulted in increased DA and 3,4-dihydroxyphenylacetic acid expression when compared to the contralateral brain, which significantly enhanced motor functions (Stutz et al., 2014).
In a rapidly advancing field, biomaterial-based transplantation platforms such as hydrogels and nanofibre scaffolds are enhancing engraftment by allowing multiple cell matrixes to be implanted, thereby replacing both the cells lost due to injury and the neurotrophic populations necessary to enrich them and modulate immune responses at the injury site (Tam et al., 2014). For example, we are currently growing radial glial cell-rich cultures isolated from the embryonic spinal cord on specialised biopolymers and aim to apply these to spinal cord injury loci to recreate the supportive embryonic CNS microenvironment (unpublished).
Closing remarks: It is clear that intricate networks of radial glial cells or their progeny form scaffolds that segregate/guide growing axons, while contributing to both gliogenesis and neurogenesis during development. Although significant strides have been made to elucidate their roles and regenerative potentials in some injury paradigms, they seem as yet to be an untapped resource to promote recovery in multiple neurological conditions. Recent reports describing the ability of radial glial cells to re-differentiate at injury loci, and offer neurotrophic support to surviving cells in both amphibians and mammals, will ensure that attention will continually be placed on radial glia and their derivatives. By combining this research with technological developments in neural tissue engineering to support the growth and transplantation of CNS progenitors, we are confident that radial glial cells and in particular ES cell derivatives such as RG3.6 cells will play significant roles in advancing cell replacement and regeneration therapies.
References
- 1.Barry DS, Pakan JM, McDermott KW. Radial glial cells: key organisers in CNS development. Int J Biochem Cell Biol. 2014;46:76–79. doi: 10.1016/j.biocel.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 2.Barry DS, Pakan JM, O’Keeffe GW, McDermott KW. The spatial and temporal arrangement of the radial glial scaffold suggests a role in axon tract formation in the developing spinal cord. J Anat. 2013;222:203–213. doi: 10.1111/joa.12006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Batista CE, Mariano ED, Marie SK, Teixeira MJ, Morgalla M, Tatagiba M, Li J, Lepski G. Stem cells in neurology--current perspectives. Arq Neuropsiquiatr. 2014;72:457–465. doi: 10.1590/0004-282x20140045. [DOI] [PubMed] [Google Scholar]
- 4.Becker CG, Becker T. Neuronal regeneration from ependymo-radial glial cells: cook, little pot, cook! Dev Cell. 2015;32:516–527. doi: 10.1016/j.devcel.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Canola K, Angenieux B, Tekaya M, Quiambao A, Naash MI, Munier FL, Schorderet DF, Arsenijevic Y. Retinal stem cells transplanted into models of late stages of retinitis pigmentosa preferentially adopt a glial or a retinal ganglion cell fate. Invest Ophthalmol Vis Sci. 2007;48:446–454. doi: 10.1167/iovs.06-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang YW, Goff LA, Li H, Kane-Goldsmith N, Tzatzalos E, Hart RP, Young W, Grumet M. Rapid induction of genes associated with tissue protection and neural development in contused adult spinal cord after radial glial cell transplantation. J Neurotrauma. 2009;26:979–993. doi: 10.1089/neu.2008.0762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coatti GC, Beccari MS, Olavio TR, Mitne-Neto M, Okamoto OK, Zatz M. Stem cells for amyotrophic lateral sclerosis modeling and therapy: myth or fact? Cytometry A. 2015;87:197–211. doi: 10.1002/cyto.a.22630. [DOI] [PubMed] [Google Scholar]
- 8.Cui YF, Xu JC, Hargus G, Jakovcevski I, Schachner M, Bernreuther C. Embryonic stem cell-derived L1 overexpressing neural aggregates enhance recovery after spinal cord injury in mice. PLoS One. 2011;6:e17126. doi: 10.1371/journal.pone.0017126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cui YF, Hargus G, Xu JC, Schmid JS, Shen YQ, Glatzel M, Schachner M, Bernreuther C. Embryonic stem cell-derived L1 overexpressing neural aggregates enhance recovery in Parkinsonian mice. Brain. 2010;133:189–204. doi: 10.1093/brain/awp290. [DOI] [PubMed] [Google Scholar]
- 10.Daadi MM, Maag AL, Steinberg GK. Adherent self-renewable human embryonic stem cell-derived neural stem cell line: functional engraftment in experimental stroke model. PLoS One. 2008;3:e1644. doi: 10.1371/journal.pone.0001644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies SJ, Shih CH, Noble M, Mayer-Proschel M, Davies JE, Proschel C. Transplantation of specific human astrocytes promotes functional recovery after spinal cord injury. PLoS One. 2011;6:e17328. doi: 10.1371/journal.pone.0017328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dihne M, Bernreuther C, Hagel C, Wesche KO, Schachner M. Embryonic stem cell-derived neuronally committed precursor cells with reduced teratoma formation after transplantation into the lesioned adult mouse brain. Stem Cells. 2006;24:1458–1466. doi: 10.1634/stemcells.2005-0413. [DOI] [PubMed] [Google Scholar]
- 13.Duan L, Peng CY, Pan L, Kessler JA. Human pluripotent stem cell-derived radial glia recapitulate developmental events and provide real-time access to cortical neurons and astrocytes. Stem Cells Transl Med. 2015;4:437–447. doi: 10.5966/sctm.2014-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedlander DR, Brittis PA, Sakurai T, Shif B, Wirchansky W, Fishell G, Grumet M. Generation of a radial-like glial cell line. J Neurobiol. 1998;37:291–304. doi: 10.1002/(sici)1097-4695(19981105)37:2<291::aid-neu8>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 15.Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 16.Goldshmit Y, Frisca F, Pinto AR, Pebay A, Tang JK, Siegel AL, Kaslin J, Currie PD. Fgf2 improves functional recovery-decreasing gliosis and increasing radial glia and neural progenitor cells after spinal cord injury. Brain Behav. 2014;4:187–200. doi: 10.1002/brb3.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han F, Wang W, Chen B, Chen C, Li S, Lu X, Duan J, Zhang Y, Zhang YA, Guo W, Li G. Human induced pluripotent stem cell-derived neurons improve motor asymmetry in a 6-hydroxydopamine-induced rat model of Parkinson's disease. Cytotherapy. 2015;17:665–679. doi: 10.1016/j.jcyt.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Hasegawa K, Grumet M. Trauma-induced tumorigenesis of cells implanted into the rat spinal cord. J Neurosurg. 2003;98:1065–1071. doi: 10.3171/jns.2003.98.5.1065. [DOI] [PubMed] [Google Scholar]
- 19.Hasegawa K, Chang YW, Li H, Berlin Y, Ikeda O, Kane-Goldsmith N, Grumet M. Embryonic radial glia bridge spinal cord lesions and promote functional recovery following spinal cord injury. Exp Neurol. 2005;193:394–410. doi: 10.1016/j.expneurol.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 20.Hormigo A, Friedlander DR, Brittis PA, Zagzag D, Grumet M. Reduced tumorigenicity of rat glioma cells in the brain when mediated by hygromycin phosphotransferase. J Neurosurg. 2001a;94:596–604. doi: 10.3171/jns.2001.94.4.0596. [DOI] [PubMed] [Google Scholar]
- 21.Hormigo A, McCarthy M, Nothias JM, Hasegawa K, Huang W, Friedlander DR, Fischer I, Fishell G, Grumet M. Radial glial cell line C6-R integrates preferentially in adult white matter and facilitates migration of coimplanted neurons in vivo. Exp Neurol. 2001b;168:310–322. doi: 10.1006/exnr.2000.7620. [DOI] [PubMed] [Google Scholar]
- 22.Hunter KE, Hatten ME. Radial glial cell transformation to astrocytes is bidirectional: regulation by a diffusible factor in embryonic forebrain. Proc Natl Acad Sci U S A. 1995;92:2061–2065. doi: 10.1073/pnas.92.6.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klassen H, Kiilgaard JF, Zahir T, Ziaeian B, Kirov I, Scherfig E, Warfvinge K, Young MJ. Progenitor cells from the porcine neural retina express photoreceptor markers after transplantation to the subretinal space of allorecipients. Stem Cells. 2007;25:1222–1230. doi: 10.1634/stemcells.2006-0541. [DOI] [PubMed] [Google Scholar]
- 24.Li J, Sun CR, Zhang H, Tsang KS, Li JH, Zhang SD, An YH. Induction of functional recovery by co-transplantation of neural stem cells and Schwann cells in a rat spinal cord contusion injury model. Biomed Environ Sci. 2007;20:242–249. [PubMed] [Google Scholar]
- 25.McMahon SS, Albermann S, Rooney GE, Shaw G, Garcia Y, Sweeney E, Hynes J, Dockery P, O’Brien T, Windebank AJ, Allsopp TE, Barry FP. Engraftment, migration and differentiation of neural stem cells in the rat spinal cord following contusion injury. Cytotherapy. 2010;12:313–325. doi: 10.3109/14653241003695018. [DOI] [PubMed] [Google Scholar]
- 26.Nomura H, Kim H, Mothe A, Zahir T, Kulbatski I, Morshead CM, Shoichet MS, Tator CH. Endogenous radial glial cells support regenerating axons after spinal cord transection. Neuroreport. 2010;21:871–876. doi: 10.1097/WNR.0b013e32833d9695. [DOI] [PubMed] [Google Scholar]
- 27.Norris CR, Kalil K. Guidance of callosal axons by radial glia in the developing cerebral cortex. J Neurosci. 1991;11:3481–3492. doi: 10.1523/JNEUROSCI.11-11-03481.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ooto S, Akagi T, Kageyama R, Akita J, Mandai M, Honda Y, Takahashi M. Potential for neural regeneration after neurotoxic injury in the adult mammalian retina. Proc Natl Acad Sci U S A. 2004;101:13654–13659. doi: 10.1073/pnas.0402129101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prada FA, Dorado ME, Quesada A, Prada C, Schwarz U, de la Rosa EJ. Early expression of a novel radial glia antigen in the chick embryo. Glia. 1995;15:389–400. doi: 10.1002/glia.440150404. [DOI] [PubMed] [Google Scholar]
- 30.Rakic P. Mode of cell migration to the superficial layers of fetal monkey neocortex. J Comp Neurol. 1972;145:61–83. doi: 10.1002/cne.901450105. [DOI] [PubMed] [Google Scholar]
- 31.Shibuya S, Miyamoto O, Itano T, Mori S, Norimatsu H. Temporal progressive antigen expression in radial glia after contusive spinal cord injury in adult rats. Glia. 2003;42:172–183. doi: 10.1002/glia.10203. [DOI] [PubMed] [Google Scholar]
- 32.Shin ES, Hwang O, Hwang YS, Suh JK, Chun YI, Jeon SR. Enhanced efficacy of human brain-derived neural stem cells by transplantation of cell aggregates in a rat model of Parkinson's disease. J Korean Neurosurg Soc. 2014;56:383–389. doi: 10.3340/jkns.2014.56.5.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thompson LH, Bjorklund A. Reconstruction of brain circuitry by neural transplants generated from pluripotent stem cells. Neurobiol Dis. 2015;79:28–40. doi: 10.1016/j.nbd.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 34.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 35.White RE, McTigue DM, Jakeman LB. Regional heterogeneity in astrocyte responses following contusive spinal cord injury in mice. J Comp Neurol. 2010;518:1370–1390. doi: 10.1002/cne.22282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.White RE, Rao M, Gensel JC, McTigue DM, Kaspar BK, Jakeman LB. Transforming growth factor alpha transforms astrocytes to a growth-supportive phenotype after spinal cord injury. J Neurosci. 2011;31:15173–15187. doi: 10.1523/JNEUROSCI.3441-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yurco P, Cameron DA. Responses of Muller glia to retinal injury in adult zebrafish. Vision Res. 2005;45:991–1002. doi: 10.1016/j.visres.2004.10.022. [DOI] [PubMed] [Google Scholar]


