Traumatic brain injury (TBI) poses a significant socioeconomic burden in the world. The long lasting consequences in cognitive impairments are often underreported and its mechanisms are unclear. In this perspective, cholinergic dysfunction and therapeutic strategy targeting this will be reviewed. Novel agents that can target specific subtype of acetylcholine receptors have been developed over the recent years and are at various stages of development, which include AR-R 17779, GTS-21, SSR-180711A, AR-R17779, and PNU-282987. A detailed review on this topic has been previously published (Shin and Dixon, 2015).
Cholinergic system is regarded as an important modulator of cognitive function. It has an important role in learning, memory formation, and attention. Thus, in pathologic neurodegenerative diseases such as Alzheimer's disease (AD), loss of cholinergic functions are believed to be an important contributor to cognitive deficits. Similarly, TBI induces dysregulation of the cholinergic system, and this is believed to be one of the significant underlying mechanisms of cognitive deficits (Zafonte et al., 2012; Shin and Dixon, 2015). With recent advancements in pharmacological science, novel agents that target specific receptor subtypes of the cholinergic system have been developed (Sun et al., 2013; Barbier et al., 2015; Dineley et al., 2015). Specifically, α7 nicotinic acetylcholine receptors (nAChRs) have been shown to have a major role in both the neuronal injury as well as cognitive dysfunction after TBI. Agents that target these specific receptors are promising potential future targets in both animal studies and clinical trials.
Acetylcholine transduces signals by muscarinic and nicotinic acetylcholine receptors. Whereas muscarinic receptors are G-protein coupled receptors, nicotinic receptors are ligand gated ion channels composed of five subunits. Binding of a ligand will induce conformational change to an open state, allowing an outflux of K+, and influx of Na+ and Ca2+ to a minor extent. Various subunits of nAChRs have been characterized over the years. They form heteromeric combinations of α2–10 and α2–4 subunits and α7 homopentamers. As previously reviewed (Dineley et al., 2015), α4β2 subtype is a major nAChR subtype in the brain, whereas α3β4 is a major subtype found in the peripheral nervous system. Specifically, α4β2 and α7 nAChRs in the basal forebrain acetylcholine neurotransmission have major roles in cognitive performance.
Nicotinic receptors are found widely throughout the brain, and their contribution to cognitive function has been tested in several important regions. Specifically, nAChRs in the hippocampus are involved in the regulation of working memory: blockade of nAChRs in ventral or dorsal hippocampus leads to decreased performance in radial-arm maze task (Bancroft and Levin, 2000; Levin et al., 2002). Infusions of nAChR antagonists to habenula (Sanders et al., 2010) or brainstem (Cannady et al., 2009) also lead to memory deficits. As shown in Figure 1, activation of nAChR leads to complex intracellular signaling pathways leading to transcription of genes important for memory formation. The activation of nAChRs also indirectly enhances dopaminergic signaling, as nAChR activation on dopaminergic neurons would lead to dopamine release in the frontal cortex or striatum (Shin et al., 2012) which are also important components of cognitive function. Recovery from dopaminergic deficit after TBI can be indirectly enhanced by nAChR activation as previously shown (Shin et al., 2012).
Figure 1.
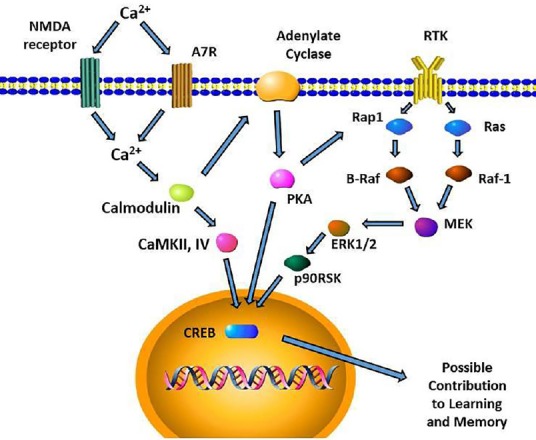
Postsynaptic functions of α7 nAChR in the hippocampus.
Intracellular signaling cascades leading to CREB activation for long term memory formation are depicted. Presynaptic glutamate release as well as α7 nAChR activation will increase Ca2+ influx. This increase in Ca2+ leads to activation of ERK, CaMKII/IV, and PKA which all leads to CREB activity. Though activation of ERK can be triggered by the activity of growth factor receptor tyrosine kinase (RTK), PKA can activate ERK via RAP1. Intracellular Ca2+ can also activate protein kinase C (PKC) which in turn activates Ras/Raf-1 cascade. PKA activity is also known to activate Raf-1 in SH-SY5Y cells. The activation of α7 nAChR subsequently may contribute to enhancement of learning and memory, among numerous other biochemical pathways that modulates it.
α7 nAChR: α7 nicotinic acetylcholine receptors; CaMKII/IV: calmodulin regulated kinases II/IV; CREB: cAMP response element-binding protein; ERK: extracellular signal-regulated kinase; MEK: Methyl ethyl ketone; NMDA: N-methyl-D-aspartate; PKA: protein kinase A; RAF: rapidly accelerated fibrosarcoma; Rap1: Ras-proximate-1; p90RSK: p90 ribosomal S6 kinase.
Recent studies have focused on the role of each nAChR subtype in cognitive function. The α7 receptors are of particular interest due to many studies showing their important contribution to learning and memory. These α7 receptors are found in the central nervous system as well as peripheral organs and immune cells. They have been found in various cell types such as astroglia, microglia, oligodendricytes, and endothelial cells. Their distribution in the hippocampus as well as high permeability to calcium are consistent with the fact that animal studies show their important role in enhancing cognitive performance (Shin and Dixon, 2015). Activation of α7 receptors also modulates production of inflammatory cytokines (Han et al., 2014).
In vitro studies have shown that cell lines expressing α7 receptors have activation of signal transduction pathways important for learning and memory. Activation of α7 receptors induces the activation of extracellular signal-regulated kinase ½ (ERK ½) (Dickinson et al., 2008). ERK is activated by phosphorylation, leading to the activation of its downstream kinase p90 ribosomal S6 kinase (p90RSK) (Figure 1). This subsequently leads to activation of cAMP response element-binding protein (CREB), which modulates the transcription of downstream genes that formulate learning and memory. The high permeability of calcium by α7 receptors can also lead to calcium regulated cascades which activate calmodulin and calmodulin regulated kinases II and IV (CaMKII, CaMKIV) leading to the activation of CREB. This enhancement of learning and memory has been demonstrated for AR-R 17779, an α7 receptor agonist as previously reviewed (Shin and Dixon, 2015). Other α7 receptor activating agents such as GTS-21, SSR-180711A, AR-R17779, and PNU-282987 have been developed and will be useful future candidates in TBI research as previously reviewed (Shin and Dixon, 2015). Animal models of TBI using controlled cortical impact (Dixon et al., 1991), fluid percussion injury (McIntosh et al., 1987), and weight drop model (Marmarou et al., 1994) are well characterized methods to study the effects of these agents in the setting of TBI. These models often used reliable and reproducible tests of working memory such as Morris Water Maze and tests of motor function such as balance walking, beam walking, and rotarod test (Hamm, 2001). Future studies for these new agents using various models of injury are warranted to characterize the contribution of each nAChR subtypes.
Compared to other subtypes of nAChRs, α7 subunits are unique entities in the setting of TBI. In TBI rats studied using quantitative autoradiography, there is a reduction of α7 receptor binding at wide range of areas, whereas α3 or α4 subtypes showed lower magnitude of reduction and in fewer regions (Verbois et al., 2002). Asides from their role in cognitive function, α7 nAChRs are recently noted as possible targets that can lead to neuroprotective effects in the setting of various acute injuries. Several studies using agents that activate α7 nAChRs show improvement in memory and survival of neurons, as well as reduction of inflammatory response to injury (Shin and Dixon, 2015). Microglia expresses α7 nAChRs, and activation of these receptors leads to reduction of inflammatory cytokine release. The neuroprotective effect of α7 receptor activation was also shown in the peripheral nervous system. In the setting of sciatic nerve crush injury, activation of α7 nAChR by PNU-282987 lead to decreased TNF-α level and increased axonal regeneration (Wang et al., 2015).
Another group of agents, known as positive allosteric modulators (PAM) for nAChRs, have been developed in the recent years. Unlike agonists that directly bind to and activate the receptors, PAMs enhance the amplitude of response or increase the duration of activity when there is a preexisting cholinergic signaling. A newly developed PAM agent, PNU-120596 was shown to protect against ischemic brain injury and improve motor function (Sun et al., 2013; Shin and Dixon, 2015). In subarachnoid hemorrhage model of rats, PNU-282987 improves motor function by reducing inflammation and neuronal loss whereas α4β2 agonist RJR-2403 does not have this effect. However, this neuroprotective effect may be injury specific and cell type specific, as SH-SY5Y cells and rat hippocampal cultures provided with PNU-120596 had decreased viability due to overloading of intracellular Ca2+ leading to cell death (Guerra-Alvarez et al., 2015).
Another major agent of interest is CDP-Choline, otherwise known as the effect of Citicholine. It has both α7 nAChRs agonist effect as well as the effect of enhancing neuronal membrane synthesis. When taken as dietary intake, it is metabolized to cytidine and choline before resynthesizing into the CDP-choline in liver and other tissues. However, direct injection of this agent into the local neural tissue can activate α7 nAChRs. It also reduces apoptosis in AD models of animals and improves cognitive performance in schizophrenia, such as working memory, verbal learning, verbal memory, and executive function (Knott et al., 2015). These neuroprotective effects may lead one to expect therapeutic benefit when applied in TBI. Disappointingly, COBRIT, a multicenter, randomized, double-blind, place-controlled trial showed no beneficial effect of this agent (Zafonte et al., 2012). This lack of improvement in cognitive function was attributed to the possibility that different levels of injury severity may have different responses to this agent. However, it should also be noted that this agent was taken enterally, and the degree of direct activation of α7 nAChRs in the central nervous system by this route of administration is unclear. Clinical trials of α7 enhancing agents DMXB-A, UCI-4083, and TC-5619 are ongoing in Schizophrenia patients and showed some degree of success in improving cognitive outcome (Freedman, 2014). However, these agents have not been used in human TBI trials. Among the many agents mentioned in this article, clinical trials for some of these agents in TBI will likely take place in the near future.
Asides the agents previously mentioned, pharmacological research still continues in developing various new drugs that are α7 nAChR specific. These even more novel ligands that are α7 receptor specific are currently under research investigation to be used as therapeutic agents or radioligands. Examples are a radioligand [18F]NS10743 (Teodoro et al., 2014) and α7 nAChR partial agonist Encenicline (Barbier et al., 2015) which are currently at development stages. Further validation on dosing as well as their applicability in TBI must be clarified in the next few years to come. However, with such variety of α7 targeting agents already available for animal studies, some of these agents may become key players in the long awaited pharmacological treatment regimen for TBI.
Although there are many beneficial effects of nAChR activation, the possibility of using nAChR modulating agents must be approached with caution. Prior studies have shown various side effects of nAChR activation. High doses of nicotine administration can increase symptoms of anxiety and depression (Newhouse et al., 1988) as well as increased heart rate and blood pressure. This occurs via nonspecific activation of nAChRs throughout the body, since nAChRs are found widely in autonomic nervous system as well as adrenal medulla. Activation of adrenal medulla leads to increase in corticosterone, epinephrine, and norepinephrine levels in blood (Cryer et al., 1976). Higher doses can even induce convulsions (Okamoto et al., 1992) and can be lethal (Okamoto et al., 1994). The synthetic nAChR agonists discussed in this article were designed to have reduced side effect profiles, with less effect on cardiovascular parameters. Commonly studied nAChR agonists in neurodegenerative disease and injury models are central nervous system specific, such as mecamylamine (Shytle et al., 2002).
Moreover, there is a limitation of looking at the functional deficits after TBI only from the perspective of nicotinic receptor dysfunction. The mechanism of TBI is much more complex, involving dysfunction of other neurotransmitters, as well as oxidative stress and inflammation. Dopamine synthesis and release deficit has been characterized after TBI (Shin et al., 2011), as well as alterations in the postsynaptic regulators of dopamine neurotransmission (Bales et al., 2009, 2010, 2011). Therapeutic effects of enhancing other neurotransmitter systems such as the serotonin system, have also been shown to be beneficial in animal models (Kline et al., 2012; Yelleswarapu et al., 2012). Also, therapeutic strategies targeting oxidative stress, such as antioxidant glutathione administration, reduce cell death and inflammation in animals (Roth et al., 2014) and improve post TBI symptoms (Hoffer et al., 2013). In the midst of complex interplay of various pathological cascades, targeting only α7 nAChRs may not be effective. Combination therapies that target multiple pathological mechanisms of TBI have recently gained interest (Margulies et al., 2015), and the use of α7 nAChR activating agent in combination with other novel agents targeting multiple pathways may be the most effective future treatment regimen for TBI.
References
- 1.Bales JW, Wagner AK, Kline AE, Dixon CE. Persistent cognitive dysfunction after traumatic brain injury: a dopamine hypothesis. Neurosci Biobehav Rev. 2009;33:981–1003. doi: 10.1016/j.neubiorev.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bales JW, Ma X, Yan HQ, Jenkins LW, Dixon CE. Expression of protein phosphatase 2B (calcineurin) subunit A isoforms in rat hippocampus after traumatic brain injury. J Neurotrauma. 2010;27:109–120. doi: 10.1089/neu.2009.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bales JW, Yan HQ, Ma X, Li Y, Samarasinghe R, Dixon CE. The dopamine and cAMP regulated phosphoprotein, 32 kDa (DARPP-32) signaling pathway: a novel therapeutic target in traumatic brain injury. Exp Neurol. 2011;229:300–307. doi: 10.1016/j.expneurol.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bancroft A, Levin ED. Ventral hippocampal alpha4beta2 nicotinic receptors and chronic nicotine effects on memory. Neuropharmacology. 2000;39:2770–2778. doi: 10.1016/s0028-3908(00)00099-x. [DOI] [PubMed] [Google Scholar]
- 5.Barbier AJ, Hilhorst M, Vliet AV, Snyder P, Palfreyman MG, Gawryl M, Dgetluck N, Massaro M, Tiessen R, Timmerman W, Hilt DC. Pharmacodynamics, pharmacokinetics, safety, and tolerability of encenicline, a selective α7 nicotinic receptor partial agonist, in single ascending-dose and bioavailability studies. Clin Ther. 2015;37:311–324. doi: 10.1016/j.clinthera.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 6.Cannady R, Weir R, Wee B, Gotschlich E, Kolia N, Lau E, Brotherton J, Levin ED. Nicotinic antagonist effects in the mediodorsal thalamic nucleus: regional heterogeneity of nicotinic receptor involvement in cognitive function. Biochem Pharmacol. 2009;78:788–794. doi: 10.1016/j.bcp.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 7.Cryer PE, Haymond MW, Santiago JV, Shah SD. Norepinephrine and epinephrine release and adrenergic mediation of smoking-associated hemodynamic and metabolic events. N Engl J Med. 1976;295:573–577. doi: 10.1056/NEJM197609092951101. [DOI] [PubMed] [Google Scholar]
- 8.Dickinson JA, Kew JN, Wonnacott S. Presynaptic alpha 7- and beta 2-containing nicotinic acetylcholine receptors modulate excitatory amino acid release from rat prefrontal cortex nerve terminals via distinct cellular mechanisms. Mol Pharmacol. 2008;74:348–359. doi: 10.1124/mol.108.046623. [DOI] [PubMed] [Google Scholar]
- 9.Dineley KT, Pandya AA, Yakel JL. Nicotinic ACh receptors as therapeutic targets in CNS disorders. Trends Pharmacol Sci. 2015;36:96–108. doi: 10.1016/j.tips.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dixon CE, Clifton GL, Lighthall JW, Yaghmai AA, Hayes RL. A controlled cortical impact model of traumatic brain injury in the rat. J Neurosci Method. 1991;39:253–262. doi: 10.1016/0165-0270(91)90104-8. [DOI] [PubMed] [Google Scholar]
- 11.Freedman R. Alpha7-nicotinic acetylcholine receptor agonists for cognitive enhancement in schizophrenia. Ann Rev Med. 2014;65:245–261. doi: 10.1146/annurev-med-092112-142937. [DOI] [PubMed] [Google Scholar]
- 12.Guerra-Alvarez M, Moreno-Ortega AJ, Navarro E, Fernandez-Morales JC, Egea J, Lopez MG, Cano-Abad MF. Positive allosteric modulation of alpha-7 nicotinic receptors promotes cell death by inducing Ca release from the endoplasmic reticulum. J Neurochem. 2015;133:309–319. doi: 10.1111/jnc.13049. [DOI] [PubMed] [Google Scholar]
- 13.Hamm RJ. Neurobehavioral assessment of outcome following traumatic brain injury in rats: an evaluation of selected measures. J Neurotrauma. 2001;18:1207–1216. doi: 10.1089/089771501317095241. [DOI] [PubMed] [Google Scholar]
- 14.Han Z, Li L, Wang L, Degos V, Maze M, Su H. Alpha-7 nicotinic acetylcholine receptor agonist treatment reduces neuroinflammation, oxidative stress, and brain injury in mice with ischemic stroke and bone fracture. J Neurochem. 2014;131:498–508. doi: 10.1111/jnc.12817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffer ME, Balaban C, Slade MD, Tsao JW, Hoffer B. Amelioration of acute sequelae of blast induced mild traumatic brain injury by N-acetyl cysteine: a double-blind, placebo controlled study. PLoS One. 2013;8:e54163. doi: 10.1371/journal.pone.0054163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kline AE, Olsen AS, Sozda CN, Hoffman AN, Cheng JP. Evaluation of a combined treatment paradigm consisting of environmental enrichment and the 5-HT1A receptor agonist buspirone after experimental traumatic brain injury. J Neurotrauma. 2012;29:1960–1969. doi: 10.1089/neu.2012.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knott V, de la Salle S, Choueiry J, Impey D, Smith D, Smith M, Beaudry E, Saghir S, Ilivitsky V, Labelle A. Neurocognitive effects of acute choline supplementation in low medium and high performer healthy volunteers. Pharmacol Biochem Behav. 2015;131:119–129. doi: 10.1016/j.pbb.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Levin ED, Bradley A, Addy N, Sigurani N. Hippocampal alpha 7 and alpha 4 beta 2 nicotinic receptors and working memory. Neuroscience. 2002;109:757–765. doi: 10.1016/s0306-4522(01)00538-3. [DOI] [PubMed] [Google Scholar]
- 19.Margulies SS, Anderson GD, Atif F, Badaut J, Clark RS, Empey P, Guseva M, Hoane MR, Huh JW, Pauly JR, Raghupathi R, Scheff S, Stein D, Tang H, Hicks M. Combination therapies for traumatic brain injury: retrospective considerations. J Neurotrauma. 2015 doi: 10.1089/neu.2014.3855. doi:10.1089/neu.2014.3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marmarou A, Foda MA, van den Brink W, Campbell J, Kita H, Demetriadou K. A new model of diffuse brain injury in rats. Part I: Pathophysiology and biomechanics. J Neurosurg. 1994;80:291–300. doi: 10.3171/jns.1994.80.2.0291. [DOI] [PubMed] [Google Scholar]
- 21.McIntosh TK, Noble L, Andrews B, Faden AI. Traumatic brain injury in the rat: characterization of a midline fluid-percussion model. Cent Nerv Syst Trauma. 1987;4:119–134. doi: 10.1089/cns.1987.4.119. [DOI] [PubMed] [Google Scholar]
- 22.Newhouse PA, Sunderland T, Tariot PN, Blumhardt CL, Weingartner H, Mellow A, Murphy DL. Intravenous nicotine in Alzheimer's disease: a pilot study. Psychopharmacology. 1988;95:171–175. doi: 10.1007/BF00174504. [DOI] [PubMed] [Google Scholar]
- 23.Okamoto M, Kita T, Okuda H, Tanaka T, Nakashima T. Effects of acute administration of nicotine on convulsive movements and blood levels of corticosterone in old rats. Jpn J Pharmacol. 1992;60:381–384. doi: 10.1254/jjp.60.381. [DOI] [PubMed] [Google Scholar]
- 24.Okamoto M, Kita T, Okuda H, Tanaka T, Nakashima T. Effects of aging on acute toxicity of nicotine in rats. Pharmacol Toxicol. 1994;75:1–6. doi: 10.1111/j.1600-0773.1994.tb00316.x. [DOI] [PubMed] [Google Scholar]
- 25.Roth TL, Nayak D, Atanasijevic T, Koretsky AP, Latour LL, McGavern DB. Transcranial amelioration of inflammation and cell death after brain injury. Nature. 2014;505:223–228. doi: 10.1038/nature12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanders D, Simkiss D, Braddy D, Baccus S, Morton T, Cannady R, Weaver N, Rose JE, Levin ED. Nicotinic receptors in the habenula: importance for memory. Neuroscience. 2010;166:386–390. doi: 10.1016/j.neuroscience.2009.12.035. [DOI] [PubMed] [Google Scholar]
- 27.Shin SS, Dixon CE. Alterations in cholinergic pathways and therapeutic strategies targeting cholinergic system after traumatic brain injury. J Neurotrauma. 2015 doi: 10.1089/neu.2014.3445. doi:10.1089/neu.2014.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shin SS, Bray ER, Dixon CE. Effects of nicotine administration on striatal dopamine signaling after traumatic brain injury in rats. J Neurotrauma. 2012;29:843–850. doi: 10.1089/neu.2011.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shin SS, Bray ER, Zhang CQ, Dixon CE. Traumatic brain injury reduces striatal tyrosine hydroxylase activity and potassium-evoked dopamine release in rats. Brain Res. 2011;1369:208–215. doi: 10.1016/j.brainres.2010.10.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shytle RD, Silver AA, Lukas RJ, Newman MB, Sheehan DV, Sanberg PR. Nicotinic acetylcholine receptors as targets for antidepressants. Mol Psychiatry. 2002;7:525–535. doi: 10.1038/sj.mp.4001035. [DOI] [PubMed] [Google Scholar]
- 31.Sun F, Jin K, Uteshev VV. A type-II positive allosteric modulator of alpha7 nAChRs reduces brain injury and improves neurological function after focal cerebral ischemia in rats. PLoS One. 2013;8:e73581. doi: 10.1371/journal.pone.0073581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teodoro R, Wenzel B, Oh-Nishi A, Fischer S, Peters D, Suhara T, Deuther-Conrad W, Brust P. A high-yield automated radiosynthesis of the alpha-7 nicotinic receptor radioligand [F]NS10743. Appl Radiat Isot 95C. 2014:76–84. doi: 10.1016/j.apradiso.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 33.Verbois SL, Scheff SW, Pauly JR. Time-dependent changes in rat brain cholinergic receptor expression after experimental brain injury. J Neurotrauma. 2002;19:1569–1585. doi: 10.1089/089771502762300238. [DOI] [PubMed] [Google Scholar]
- 34.Wang D, Wang X, Geng S, Bi Z. Axonal regeneration in early stages of sciatic nerve crush injury is enhanced by alpha7nAChR in rats. Mol Biol Rep. 2015;42:603–609. doi: 10.1007/s11033-014-3805-2. [DOI] [PubMed] [Google Scholar]
- 35.Yelleswarapu NK, Tay JK, Fryer WM, Shah MA, Garcia AN, Cheng JP, Kline AE. Elucidating the role of 5-HT(1A) and 5-HT(7) receptors on 8-OH-DPAT-induced behavioral recovery after experimental traumatic brain injury. Neurosci Lett. 2012;515:153–156. doi: 10.1016/j.neulet.2012.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zafonte RD, Bagiella E, Ansel BM, Novack TA, Friedewald WT, Hesdorffer DC, Timmons SD, Jallo J, Eisenberg H, Hart T, Ricker JH, Diaz-Arrastia R, Merchant RE, Temkin NR, Melton S, Dikmen SS. Effect of citicoline on functional and cognitive status among patients with traumatic brain injury: Citicoline Brain Injury Treatment Trial (COBRIT) JAMA. 2012;308:1993–2000. doi: 10.1001/jama.2012.13256. [DOI] [PubMed] [Google Scholar]


