Peripheral motor and sensory neuropathies are diseases with different etiologies emerging from genetic disorders, diabetes, infection or inflammation, paraneoplastic damage or intoxications including alcohol abuse (Martyn and Hughes, 1997). Hereditary neuropathies, most of which are subsumed under the hypernym Charcot-Marie Tooth (CMT) disease, are clinically heterogeneous: about 50 affected gene loci encoding about 30 genes have been so far identified to be involved in the pathogenesis of different CMTs (Niemann et al., 2006). Depending on structural and electrophysiological features CMTs are divided into axonal and demyelinating forms. Peripheral myelin protein 22 (PMP22), myelin protein zero (MPZ) and connexin 32 (GJB1) are the most frequently affected genes in human demyelinating neuropathies and a variety of animal models have been designed to mimic these human disorders (Bouhy and Timmerman, 2013). These models rely on the deletion, overexpression or mutation of the gene relevant for a human neuropathy and usually clinical symptoms occur early in life and the course of disease is progressive. Recently, Sanz-Moreno et al. (2014) published the partial deletion of the transcription factor Myc interacting zinc finger protein 1 (Miz1) in Schwann cells of mice and observed a late onset demyelinating neuropathy with a spontaneous clinical remission, introducing a unique new model to study peripheral nerve regeneration.
Miz1 was originally discovered in a yeast two hybrid screen searching for Myc binding partners (Herkert and Eilers, 2010). Beside 13 zinc fingers in the mid and C-terminal part of the protein, Miz1 possesses a Poxvirus and zinc finger domain (POZ) domain at the N-terminus. One main function of this domain lies in the di- and tetramerisation of Miz1 which is necessary to confer a proper binding of this transcription factor to DNA. In addition, the POZ domain can bind different other proteins like TopBP1, HCF1 or HectH9, which modify the function of Miz1. However, the interaction with proteins is not restricted to the POZ domain, e.g., Myc binds to a sequence between the 12th and 13th zinc fingers, and binding sites for other proteins like p300, Smad3/4 or 14-3-3′ are scattered over the mid and C-terminal part of Miz1. Binding of Myc, Bcl2 or Gfi inhibits Miz1 activated transcription which has been extensively analysed for cdkn1a (encoding p21cip) or cdkn2b (encoding p15ink4), linking Miz1 to the regulation of the cell cycle and to senescence. Miz1 usually binds to the initiation region of a gene and based on CHIP sequencing data, recent publications identified about 250 Miz1 binding sites in neuronal cells, as well as a Miz1 binding consensus sequence (Wolf et al., 2013). Thus, there is a plethora of genes which are potentially regulated, but it is not known whether the occupation of a Miz1 binding site is cell type and context dependent.
Miz1 is essential in early embryonic development since a constitutive knockout of Miz1 is lethal at embryonic day 7.5 (E7.5). For this reason, Miz1 function has been analysed in a conditional mouse model where exons 3 and 4, encoding the POZ domain are flanked by lox sites and cells expressing the Cre recombinase possess only a truncated form of Miz1 (Miz1ΔPOZ animals) (Gebhardt et al., 2007). In this model, the Miz1 POZ domain has been deleted in keratinocytes, B- and T-cells, lung epithelial cells, mammary gland cells, neuronal cells and recently in Schwann cells, and the affected tissues exhibited complex phenotypes (Sanz-Moreno et al., 2014).
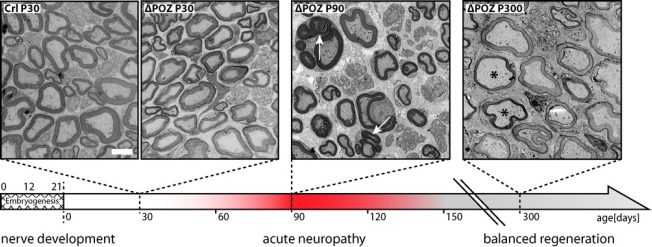
In case of Schwann cells, where the Miz1 POZ domain deletion is induced around E12 when Cre recombinase is expressed under the desert hedgehog promotor, peripheral nervous tissue develops normally. In 30 day old Miz1ΔPOZ animals, the number of nerve fibers and their myelination is undistinguishable from wildtype mice (Figure 1), although Miz1 is usually expressed in Schwann cells at least from day 10 postpartum (P10) (Sanz-Moreno et al., 2014). Whether Miz1 fulfills other functions which have no impact on the structural features of developing nerve fibers, or whether there are other transcription factors which can compensate for the truncated Miz1 is unknown. In line with the intact morphology, no motor disabilities are observed until P90. However, 90 day old Miz1ΔPOZ animals develop a palsy of their hindlimbs leading e.g., to brush marks in a footprint assay and a reduction in grip strength of their forelimbs (Sanz-Moreno et al., 2014). These clinical symptoms are associated with a severe dys- and demyelination of sciatic nerve fibers (Figure 1). Pathological hallmarks are extensive outfoldings of the myelin sheet, tomacula, degradation of myelin by autophagy and by macrophages, and axons which are partly or completely devoid of myelin, but still surrounded by Schwann cells.
Figure 1.
Time course of the developing neuropathy with subsequent remission of clinical symptoms in Miz1ΔPOZ animals.
The expression of the Cre recombinase starts at developmental day 12 under the desert hedgehog promotor. On day 30 postpartum, when the postnatal development of the peripheral nervous system is completed, no structural lesions are observed in the sciatic nerve of Miz1ΔPOZ animals. Between day 90 and day 120, an acute neuropathy with clinical symptoms of motor disabilities is observed. Outfoldings (arrows) of the myelin sheet are morphological hallmarks at this stage. Miz1ΔPOZ animals older than 120 days show a remission of the clinical symptoms and there is no further acute episode, at least up to one year. In older animals outfoldings are rare and the morphological hallmarks are hypomyelinated (scale bar) or demyelinated nerve fibers. Scale bar: 3 μm.
These morphological changes are accompanied by the repression of genes encoding structural proteins in differentiated Schwann cells, like myelin protein zero (Mpz), periaxin (Prx) or myelin basic protein (Mbp). Simultaneously, the expression of genes indicative for reprogramming of Schwann cells to a less differentiated state, like Krox24, Cyclin D1 or Jun, are upregulated (Svaren and Meijer, 2008). These changes of gene expression are similar to that being observed in Wallerian degeneration or other neuropathy models.
Unexpectedly, in Miz1ΔPOZ mice the clinical symptoms vanish after 4 weeks. Only meticulous analysis reveals minor persisting motor disabilities of the hindlimbs, while the grip strength of the forelimbs is normal. Morphologically, outfoldings are rare now, most fibers are hypomyelinated or still demyelinated. Animals were observed up to one year of age, but there was no additional clinical episode, indicating that the spontaneous regeneration, although incomplete, leads to a stable and balanced state without obvious clinical symptoms (Sanz-Moreno et al., 2014).
Also, neuregulin 1 (Nrg1) type I is strongly upregulated in nerve tissue from Miz1ΔPOZ animals. Nrg1 type I has been described as a Schwann cell autonomous growth factor in regenerating nerves. The postulated model is based on observations after nerve injury, indicating that Nrg1 type III, usually expressed by the axon, suppresses the expression of Nrg1 typ I in Schwann cells. Following the injury of an axon, Schwann cells detach from the axon, Nrg1 type III concentration decreases locally and Schwann cells answer with an elevation of Nrg1 type I production, which in turn leads to a paracrine or autocrine stimulation of Schwann cell differentiation and thus to nerve regeneration (Stassart et al., 2013). In Miz1ΔPOZ animals, we neither observe an increase of unphosphorylated neurofilament-H, an axonal degeneration marker, nor a change of succinate dehydrogenase pattern in calf muscles of up to 240 day old animals, indicating that no sprouting takes place. Also, preliminary data show that the number of axons per area is not changed in 300 day old Miz1ΔPOZ mice (control: 61.4 ± 8.4 vs. Miz1ΔPOZ: 74.8 ± 11.2; n = 3 for each group; P = 0.379). Finally, we do not see degenerated axons by electron microscopy. Thus, in Miz1ΔPOZ mice Nrg1 type I is upregulated in nerve tissue without obvious axonal damage, but the observed de- and dysmyelination could already impair the reciprocal interaction between nerve and Schwann cell. Especially in older Miz1ΔPOZ mice demyelination is often accompanied by a complete detachment of Schwann cells from axons, leaving empty basement membrane foldings behind. This could contribute to an increased Nrg1 type I expression (see below) which we still observe in older animals (unpublished data). Alternatively, the repression of Nrg1 type I could be dependent directly on Miz1. Whether the upregulation of Nrg1 type I gene expression is Schwann cell autonomous or a result of an altered axon/Schwann cell interaction or both remains to be elucidated. In any case, as Nrg1 type I has been shown to reverse Schwann cell dedifferentiation and improve the etiopathology in a PMP22 neuropathy mouse model (Fledrich et al., 2014), elevated concentrations of Nrg1 type I could support the clinical remission in Miz1ΔPOZ mice.
Gene repression by a Myc/Miz1 complex has been extensively analyzed for Cdkn1a encoding the cyclin dependent kinase inhibitor p21cip. In an in vivo model of skin papilloma it was shown that deletion of the Miz1 POZ domain in keratinocytes leads to an increase of p21cip, most likely due to the absence of the repressive complex. This came along with a reduction in tumor growth, which was rescued in mice lacking Cdkn1a (Hönnemann et al., 2012). In Schwann cells, p21cip is upregulated during injury and it has been shown that this cyclin dependent kinase inhibitor is mandatory for a proper regulation of Schwann cell proliferation during nerve regeneration, both by activating cyclin D and by inhibiting cell cycle progression and probably promoting differentiation (Atanasoski et al., 2006). Deletion of the Miz1 POZ domain in Schwann cells also creates a higher level of p21cip in this cell type in 90 day old animals (Sanz-Moreno et al., 2014). In addition, we revealed for the first time that two senescence markers, H3K9me3 and the senescence associated (SA) galactosidase, are increased (Sanz-Moreno et al., 2014). It is not clear whether the elevation of senescence markers in the nerve tissue of Miz1ΔPOZ mice is directly mediated via p21cip. However, it has been shown that p21cip can participate in the establishment of a senescence phenotype (Romanov et al., 2012). The relation between p21cip, H3K9me3 and SA-galactosidase and their impact on nerve regeneration has to be uncovered and experiments are under progress to analyse the Miz1ΔPOZ Schwann cell phenotype on a Cdkn1a null background.
As Miz1 is involved in a variety of pathways and is associated with different cellular functions like cell cycle progression, cell adhesion, autophagy or vesicular transport, phenotypes induced by the deletion of the Miz1 POZ domain are complex and can be the result of an overlap between different primary dysfunctions. The late onset of the observed de- and dysmyelinating phenotype suggests that in the absence of functional Miz1 some proteins accumulate until they exceed a certain threshold where a transdifferentiation of the Schwann cell is initiated. Such dosage dependent pathologies have been described for PMP22 or MPZ (Niemann et al., 2006). However, while other animal models mimicking this situation are progressive, Miz1ΔPOZ animals pass through one acute clinical episode, followed by a partial regeneration or regenerative processes leading to a permanently stable and clinically silent situation. As outlined above, there is some evidence that an elevated expression of Cdkn1a and Nrg1 are part of the mechanistic scenario triggering the regeneration in Miz1ΔPOZ Schwann cells. Understanding the dynamic of this balanced stage with ongoing degenerating and regenerating processes will potentially help to uncover regeneration pathways which can be utilized as starting points on new therapeutic strategies for peripheral neuropathies.
This work was supported by Deutsche Forschungsgemeinschaft (DFG grant EL125/6-1).
References
- 1.Atanasoski S, Boller D, De Ventura L, Koegel H, Boentert M, Young P, Werner S, Suter U. Cell cycle inhibitors p21 and p16 are required for the regulation of Schwann cell proliferation. Glia. 2006;53:147–157. doi: 10.1002/glia.20263. [DOI] [PubMed] [Google Scholar]
- 2.Bouhy D, Timmerman V. Animal models and therapeutic prospects for Charcot-Marie-Tooth disease. Ann Neurol. 2013;74:391–396. doi: 10.1002/ana.23987. [DOI] [PubMed] [Google Scholar]
- 3.Fledrich R, Stassart RM, Klink A, Rasch LM, Prukop T, Haag L, Czesnik D, Kungl T, Abdelaal TAM, Keric N, Stadelmann C, Brück W, Nave KA, Sereda MW. Soluble neuregulin-1 modulates disease pathogenesis in rodent models of Charcot-Marie-Tooth disease 1A. Nat Med. 2014;20:1055–1061. doi: 10.1038/nm.3664. [DOI] [PubMed] [Google Scholar]
- 4.Gebhardt A, Kosan C, Herkert B, Möröy T, Lutz W, Eilers M, Elsässer HP. Miz1 is required for hair follicle structure and hair morphogenesis. J Cell Sci. 2007;120:2586–2593. doi: 10.1242/jcs.007104. [DOI] [PubMed] [Google Scholar]
- 5.Herkert B, Eilers M. Transcriptional repression: the dark side of myc. Genes Cancer. 2010;1:580–586. doi: 10.1177/1947601910379012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hönnemann J, Sanz-Moreno A, Wolf E, Eilers M, Elsässer HP. Miz1 is a critical repressor of cdkn1a during skin tumorigenesis. PLoS One. 2012;7:e34885. doi: 10.1371/journal.pone.0034885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martyn CN, Hughes RA. Epidemiology of peripheral neuropathy. J Neurol Neurosurg Psychiatry. 1997;62:310–318. doi: 10.1136/jnnp.62.4.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niemann A, Berger P, Suter U. Pathomechanisms of mutant proteins in Charcot-Marie-Tooth disease. Neuromolecular Med. 2006;8:217–242. doi: 10.1385/nmm:8:1-2:217. [DOI] [PubMed] [Google Scholar]
- 9.Romanov VS, Pospelov VA, Pospelova TV. Cyclin-dependent kinase inhibitor p21(Waf1): contemporary view on its role in senescence and oncogenesis. Biochemistry. 2012;77:575–584. doi: 10.1134/S000629791206003X. [DOI] [PubMed] [Google Scholar]
- 10.Sanz-Moreno A, Fuhrman D, Zankel A, Reingruber H, Kern L, Meijer D, Niemann A, Elsässer HP. Late onset neuropathy with spontaneous clinical remission in mice lacking the POZ domain of the transcription factor Myc-interacting-zinc-finger-protein 1 (Miz1) in Schwann cells. J Biol Chem. 2014 doi: 10.1074/jbc.A114.605931. doi: 10.1074/jbc.M114.605931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stassart RM, Fledrich R, Velanac V, Brinkmann BG, Schwab MH, Meijer D, Sereda MW, Nave KA. A role for Schwann cell-derived neuregulin-1 in remyelination. Nat Neurosci. 2013;16:48–54. doi: 10.1038/nn.3281. [DOI] [PubMed] [Google Scholar]
- 12.Svaren J, Meijer D. The molecular machinery of myelin gene transcription in Schwann cells. Glia. 2008;56:1541–1551. doi: 10.1002/glia.20767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolf E, Gebhardt A, Kawauchi D, Walz S, von Eyss B, Wagner N, Renninger C, Krohne G, Asan E, Roussel MF, Eilers M. Miz1 is required to maintain autophagic flux. Nat Commun. 2013;4:2535. doi: 10.1038/ncomms3535. [DOI] [PMC free article] [PubMed] [Google Scholar]