Cancer treatments such as chemotherapy and radiotherapy are widely used to treat primary and metastatic cancers. Epidemiological studies have demonstrated that these types of treatment can effectively and successfully extend the lifespan of cancer patients, but they are also associated with various neurological complications such as cognitive deficits, seizures, and emotional problems (Taphoorn and Klein, 2004; Yang and Moon, 2013; Son et al., 2015b). In particular, emerging clinical evidence has revealed that there is a higher incidence of cognitive deficits among various types of cancer patients with peripheral tumors, including breast cancer, colorectal cancer, lymphoma, and brain tumors, following cancer therapy compared with healthy control subjects (Taphoorn and Klein, 2004; Dietrich et al., 2008; Pereira Dias et al., 2014). Thus, of the neurological complications, cognitive impairments are considered to be the major side effect because humans have highly developed cognitive abilities, and deficits in this area may ultimately lead to dementia and a diminished quality of life. Several brain regions, including the hippocampus, prefrontal cortex, amygdala, striatum, and several parts of the parietal lobe, are involved in cognitive processing (Pasupathy and Miller, 2005; Yang and Moon, 2013). In particular, the hippocampus is a limbic structure that is essential for working memory, spatial memory, the consolidation of new information into long-term memories, and the acquisition and retrieval of stored memories. As a result, studies investigating cancer treatment-induced cognitive impairments typically assess hippocampal dysfunction. Thus, the present article reviews the possible mechanisms underlying hippocampus-related cognitive impairments following chemotherapy and radiotherapy.
Effects of cancer therapy on neurogenesis and synaptic plasticity in the hippocampus: The process of generating new neurons in the hippocampus, or hippocampal neurogenesis, is putatively regarded as a principle target of chemotherapy- and radiotherapy-induced side effects, particularly in relation to cognitive deficits. Following chemotherapy or radiotherapy, experimental animals exhibit significant reductions in neurogenesis and cell proliferation in the dentate gyrus of the hippocampus in conjunction with memory impairments across diverse behavioral paradigms (Yang et al., 2011, 2012a). Reductions in neurogenesis and diminished memory capabilities have also been observed following relatively low doses of ionizing radiation (e.g., 2 Gy of γ-ray and 0.8 Gy of neutrons) or cyclophosphamide treatment (40 mg/kg, intraperitoneal (i.p.)), but these changes are reversible over time (Kim et al., 2008; Yang et al., 2010a, b, 2012a). However, exposure to relatively high doses of γ-ray (10 Gy) irradiation or methotrexate (MTX; 40 mg/kg, i.p.) treatment results in long-lasting alterations of neurogenesis and memory function (Yang et al., 2011; Son et al., 2014, 2015a).
Irradiation or MTX may also alter synaptic plasticity-related signals, which support memory function, for approximately 14 days after treatment (Yang et al., 2012a; Son et al., 2015a). Synaptic plasticity, which is closely linked with hippocampal neurogenesis, varies at different cellular levels and under diverse conditions, affecting processes such as dendritic growth, axonal sprouting, synaptic remodeling, and the creation of new synapses (Mesulam, 1999; Yang and Moon, 2013). Therefore, it appears that changes in synaptic plasticity-related signals, including cAMP-response element binding protein (CREB), extracellular signal-regulated kinase (ERK) 1/2, and calcium/calmodulin-dependent protein kinases II (CAMKII), in the hippocampus following MTX treatment or 10 Gy γ-ray irradiation affect neuronal survival, plasticity-related cellular remodeling, and memory (Yang et al., 2012a; Son et al., 2015a). Based on this evidence, recent studies have focused on various factors that might influence neurogenesis and synaptic plasticity in the hippocampus to better understand the mechanisms underlying the memory impairments that are associated with acute and chronic cancer therapy.
Effects of cancer therapy on neurotrophic factors: Neurotrophic factors play important roles in brain development, adult neurogenesis, emotional regulation (i.e., depression and anxiety), and learning and memory. Of these factors, brain-derived neurotrophic factor (BDNF) is well-known to be involved in cell survival, adult neurogenesis, and neuroplasticity. Although this evidence indicates that BDNF plays a crucial role in learning and memory processes, its effects on memory remain controversial. BDNF-deficient animal models exhibit defective learning and memory, whereas chronic overexpression of BDNF also results in learning deficits (Cunha et al., 2009).
Previous studies conducted by our research group have found that various levels of BDNF are associated with memory impairments following radiotherapy or chemotherapy. For example, 10 Gy γ-ray irradiation significantly reduces mRNA and protein levels of BDNF at 1 month post-irradiation. Furthermore, the downregulation of common BDNF levels and diverse exon variants coincide with the reduced phosphorylation of CREB (Son et al., 2014, 2015a), and this reduction in CREB/BDNF signaling after cranial radiation can potentially lead to impairments in cognitive functioning (Son et al., 2015a). Conversely, MTX significantly increases BDNF levels 7–14 days post-treatment, which suggests that this increase may contribute to the remodeling of hippocampal dendritic spines and influence changes in the activity of plasticity-related proteins during the late phases of treatment (Yang et al., 2012a). Therefore, it is feasible that altered levels of neurotrophic factors following cancer therapy may play a significant role in the proliferation of neural precursor cells in the hippocampus as well as the activity of synaptic plasticity-related signals. Furthermore, these alterations may affect hippocampal functions, including memory abilities.
Effects of cancer therapy on glial cells: Microglia and astrocytes are important modulators of homeostasis in the brain microenvironment via the secretion of BDNF and glial cell line-derived neurotrophic factor (GDNF) and the regulation of brain immune responses, such as the secretion of cytokines. Changes in the brain microenvironment, which reflect levels of microglia and astrocytes, and the extent of glial cell-secreted inflammatory cytokines are considered to be causal factors underlying cancer therapy-induced memory impairments as well as other neurological complications, including depression. Additionally, neuroinflammation contributes to cognitive impairments via interactions between neurons and glial cells that could facilitate either neuronal regeneration or damage. Thus, the identification of a patient's inflammatory status after cancer therapy would aid in determining the related mechanisms underlying memory impairment.
High-dose irradiation and the chemotherapeutic agents used in cancer therapies, such as doxorubicin, 5-fluorouracil, and paclitaxel, also induce inflammatory responses. MTX-treated mice showed an inflammatory response that was reflected in an increase of Iba1-positive microglia and an upregulation of cyclooxygenase (COX)-2 and inducible nitric oxide synthase (iNOS) levels on 1 day post-treatment. Moreover, this inflammatory response was intensified when a peripheral tumor was present. The activated microglial response following MTX treatment contributes to the upregulation of pro-inflammatory enzymes (COX-2 and iNOS), the inhibition of neurogenesis, and subsequent cognitive deficits (Yang et al., 2012b).
A recent study from our group found that 10 Gy cranial irradiation did not alter the numbers of microglia or inflammatory cytokines (interleukin (IL)-1β, IL-6, and interferon (IFN)-γ) in the hippocampus during the early and intermediate phases of treatment (1–8 days post-irradiation). However, the irradiation still appeared to reduce neurogenesis and lead to memory dysfunction. Based on these findings, the present authors assumed that the inflammatory status of a patient during the early post-irradiation phases is not correlated with the inhibition of neurogenesis or the presence of memory impairments (Son et al., 2014). However, during the chronic post-irradiation phase (1–3 months), there was a significant reduction in the number of microglia in treated animals compared with age-matched sham controls, and this was concomitant with the downregulation of BDNF and GDNF, but not of inflammatory cytokines, in the hippocampus (Son et al., 2014). In that study, the reduced levels of microglia did not reflect the inflammatory status of the patients during the chronic post-irradiation phase, but they may have affected neurotrophic factors such as BDNF and GDNF. Thus, the reduction in microglia influenced hippocampal neurogenesis, synaptic plasticity, and, ultimately, hippocampal function. Although microglia in the brain act differently during the early and late phases of radiotherapy and chemotherapy, the abnormal regulation of glial cells, either in a positive or negative direction, may alter the brain microenvironment and contribute to changes in synaptic plasticity-related signals and neurogenesis in the hippocampus. Thus, we suggest that the immune status of a patient following cancer treatment may affect diverse mechanisms associated with cognitive impairments, such as neurogenesis, synaptic plasticity, and neurotrophic factors.
Effects of cancer on immune status: In some clinical cases, the immune systems of cancer patients undergoing cancer therapy, or even those who are operating under normal conditions, can become hyperactivated, and there is a subsequent increase in the levels of cytokines derived from tumor cells (Culig, 2011; Gilbert and Slingerland, 2013). Thus, to confirm the influence of cancer itself on inflammatory status, memory function, and related signaling, our research group used specific cancer cell lines to create tumor-bearing mice and then inoculated identical strain-derived cancer cell lines within each strain. Female BALB/c mice were inoculated with a BALB/c strain-derived colon carcinoma cell line (CT26) and female C3H/HeN mice were inoculated with a C3H/HeN strain-derived breast cancer cell line (FM3A) (Yang et al., 2012b, 2014). In these tumor-bearing mouse models, the peripheral tumors induced the upregulation of pro-inflammatory enzymes, such as COX-2 and iNOS, and of various inflammatory cytokines, IL-6 and tumor necrosis factor (TNF-α), in the hippocampus as well as serum IL-6. Additionally, the tumor-bearing mice exhibited reductions in hippocampal neurogenesis, which were detected with Ki-67 and DCX, in conjunction with deficits in object-recognition memory and passive-avoidance memory. Thus, upregulated inflammatory responses in the brains of tumor-bearing mice may negatively affect hippocampal neurogenesis and memory function.
In conclusion: The aforementioned preclinical data are consistent with the findings of clinical studies (Taphoorn and Klein, 2004; Miller et al., 2008; Pereira Dias et al., 2014) regarding the following conclusions: (1) following cancer therapy, cancer patients experience cognitive impairments during their survived period; (2) there are reduced levels of BDNF and neurogenesis in patients exhibiting memory deficits; and (3) there is an increased inflammatory response in cancer patients that is concomitant with behavioral symptoms.
Current research has demonstrated that cancer therapy results in the alteration of a patient's immune status, causes changes in levels of neurotrophic factors, and negatively affects synaptic plasticity-related signals and neurogenesis in the hippocampus. Consequently, cancer treatments that induce microenvironmental imbalances and changes in hippocampal functioning may lead to cognitive impairments in surviving cancer patients who were treated with chemotherapy or radiotherapy (Figure 1). Although a majority of studies assessing the effects of cancer therapy have investigated the cognitive abilities of patients, there has been a recent increase in preclinical and clinical interest regarding the manifestation of depression. Even though the influence of cancer therapies on the prevalence of mood disorders has received less research attention than their effects on cognition, the present article may further the understanding of the development of depression and other neuropsychological deficits following cancer therapy.
Figure 1.
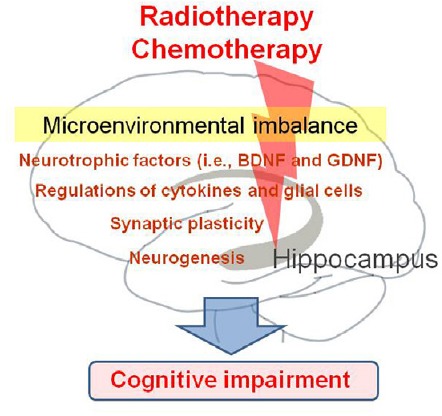
Schematic overview illustrating the putative mechanisms underlying the development of cognitive impairment following cancer therapy.
This work was supported by Wonkwang University in 2015.
References
- 1.Culig Z. Cytokine disbalance in common human cancers. Biochim Biophys Acta. 2011;1813:308–314. doi: 10.1016/j.bbamcr.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 2.Cunha C, Angelucci A, D’Antoni A, Dobrossy MD, Dunnett SB, Berardi N, Brambilla R. Brain-derived neurotrophic factor (BDNF) overexpression in the forebrain results in learning and memory impairments. Neurobiol Dis. 2009;33:358–368. doi: 10.1016/j.nbd.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Dietrich J, Monje M, Wefel J, Meyers C. Clinical patterns and biological correlates of cognitive dysfunction associated with cancer therapy. Oncologist. 2008;13:1285–1295. doi: 10.1634/theoncologist.2008-0130. [DOI] [PubMed] [Google Scholar]
- 4.Gilbert CA, Slingerland JM. Cytokines, obesity, and cancer: new insights on mechanisms linking obesity to cancer risk and progression. Annu Rev Med. 2013;64:45–57. doi: 10.1146/annurev-med-121211-091527. [DOI] [PubMed] [Google Scholar]
- 5.Kim JS, Lee HJ, Kim JC, Kang SS, Bae CS, Shin T, Jin JK, Kim SH, Wang H, Moon C. Transient impairment of hippocampus-dependent learning and memory in relatively low-dose of acute radiation syndrome is associated with inhibition of hippocampal neurogenesis. J Radiat Res. 2008;49:517–526. doi: 10.1269/jrr.08020. [DOI] [PubMed] [Google Scholar]
- 6.Mesulam MM. Neuroplasticity failure in Alzheimer's disease: bridging the gap between plaques and tangles. Neuron. 1999;24:521–529. doi: 10.1016/s0896-6273(00)81109-5. [DOI] [PubMed] [Google Scholar]
- 7.Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol. 2008;26:971–982. doi: 10.1200/JCO.2007.10.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pasupathy A, Miller EK. Different time courses of learning-related activity in the prefrontal cortex and striatum. Nature. 2005;433:873–876. doi: 10.1038/nature03287. [DOI] [PubMed] [Google Scholar]
- 9.Pereira Dias G, Hollywood R, Bevilaqua MC, da Luz AC, Hindges R, Nardi AE, Thuret S. Consequences of cancer treatments on adult hippocampal neurogenesis: implications for cognitive function and depressive symptoms. Neuro Oncol. 2014;16:476–492. doi: 10.1093/neuonc/not321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Son Y, Yang M, Wang H, Moon C. Hippocampal dysfunctions caused by cranial irradiation: A review of the experimental evidence. Brain Behav Immun 45C. 2015b:287–296. doi: 10.1016/j.bbi.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Son Y, Yang M, Kim JS, Kim J, Kim SH, Kim JC, Shin T, Wang H, Jo SK, Jung U, Moon C. Hippocampal dysfunction during the chronic phase following a single exposure to cranial irradiation. Exp Neurol. 2014;254:134–144. doi: 10.1016/j.expneurol.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 12.Son Y, Yang M, Kang S, Lee S, Kim J, Kim J, Park S, Kim JS, Jo SK, Jung U, Shin T, Kim SH, Wang H, Moon C. Cranial irradiation regulates CREB-BDNF signaling and variant BDNF transcript levels in the mouse hippocampus. Neurobiol Learn Mem. 2015a;121:12–19. doi: 10.1016/j.nlm.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Taphoorn MJ, Klein M. Cognitive deficits in adult patients with brain tumours. Lancet Neurol. 2004;3:159–168. doi: 10.1016/S1474-4422(04)00680-5. [DOI] [PubMed] [Google Scholar]
- 14.Yang M, Moon C. Neurotoxicity of cancer chemotherapy. Neural Regen Res. 2013;8:1606–1614. doi: 10.3969/j.issn.1673-5374.2013.17.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang M, Kim H, Kim J, Kim SH, Kim JC, Bae CS, Kim JS, Shin T, Moon C. Fast neutron irradiation deteriorates hippocampus-related memory ability in adult mice. J Vet Sci. 2012a;13:1–6. doi: 10.4142/jvs.2012.13.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang M, Kim JS, Kim J, Jang S, Kim SH, Kim JC, Shin T, Wang H, Moon C. Acute treatment with methotrexate induces hippocampal dysfunction in a mouse model of breast cancer. Brain Res Bull. 2012b;89:50–56. doi: 10.1016/j.brainresbull.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 17.Yang M, Kim J, Kim JS, Kim SH, Kim JC, Kang MJ, Jung U, Shin T, Wang H, Moon C. Hippocampal dysfunctions in tumor-bearing mice. Brain Behav Immun. 2014;36:147–155. doi: 10.1016/j.bbi.2013.10.022. [DOI] [PubMed] [Google Scholar]
- 18.Yang M, Kim JS, Kim J, Kim SH, Kim JC, Kim J, Wang H, Shin T, Moon C. Neurotoxicity of methotrexate to hippocampal cells in vivo and in vitro. Biochem Pharmacol. 2011;82:72–80. doi: 10.1016/j.bcp.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 19.Yang M, Kim JS, Song MS, Kim JC, Shin T, Lee SS, Kim SH, Moon C. Dose-response and relative biological effectiveness of fast neutrons: induction of apoptosis and inhibition of neurogenesis in the hippocampus of adult mice. Int J Radiat Biol. 2010a;86:476–485. doi: 10.3109/09553001003667990. [DOI] [PubMed] [Google Scholar]
- 20.Yang M, Kim JS, Song MS, Kim SH, Kang SS, Bae CS, Kim JC, Wang H, Shin T, Moon C. Cyclophosphamide impairs hippocampus-dependent learning and memory in adult mice: Possible involvement of hippocampal neurogenesis in chemotherapy-induced memory deficits. Neurobiol Learn Mem. 2010b;93:487–494. doi: 10.1016/j.nlm.2010.01.006. [DOI] [PubMed] [Google Scholar]


