Similarly to other adult tissues, a hierarchical structure has been established for the brain, where the differentiated cell types (neurons, oligodendrocytes and astrocytes) are generated from primary progenitor cells, known as type B astrocytes or neural stem cells (NSC), through one or multiple stages of amplification (transient amplifying cells) that generate precursor cells (iPC) with more restricted potential nIPCs (neural), aIPCs (astrocytes), oIPC (oligodendrocytes) (Kriegstein and Alvarez-Buylla, 2009). In the adult rodent brain, NSCs and iPCs are mainly restricted to the niches on the subventricular zone (SVZ) of the lateral ventricles (Doetsch et al., 1999; Kriegstein and Alvarez-Buylla, 2009). This hierarchical structure of the brain is critical for the understanding of brain development and adult neurogenesis, to develop new strategies for brain repair, or for the understanding of brain tumor development.
Neurogenesis in the adult brain is well documented, in particular for maintaining the homeostasis of the olfactory bulb, where a continuous supply of neuroblasts, migrating from their SVZ niche to the olfactory bulb, through the rostral migratory stream (RMS). The migrating neuroblasts are required for the continuous generation of periglomerular interneurons in the olfactory bulb (Doetsch et al., 1999). However, recent data obtained by us and other laboratories demonstrate that the migration of iPCs is not restricted to the olfactory bulb. For this purpose, we took advantage of two monoclonal antibodies (mAbs) generated against mouse neurospheres (Del Valle et al., 2010) that identify surface antigens on neuroblasts (Nilo2) (Elvira et al., 2012), and NSC (type B astrocytes) in the mouse adult brain as well as radial glia in the mouse embryo (Nilo1) (Elvira et al., 2015). These antibodies were coupled to magnetic nanoglicoparticles to in vivo identify by magnetic resonance imaging (MRI) neuroblasts or NSCs in their niches, and track their migration in response to brain damage. These experiments allowed us to demonstrate in vivo that both neuroblasts and NSC are mobilized from their SVZ niches to the brain damage site, where they migrate in a fast and orderly way. In less than 3 hours from the insult, the first migrating neuroblasts and NSC start to reach the damage site. Migration of Neuroblasts and NSGs seem to be a generalized trait in response to brain damage, since it occurs during growth of a tumor, but also in response to a cryolesion, focal demyelinization, or a mechanical lesion (Figure 1) (Elvira et al., 2012, 2015). Neuroblasts and NSCs migrate to the brain damage site with similar kinetics, suggesting that both cell types respond to “damage signals” of still unknown nature, where chemoattractants or growth factors may play a role.
Figure 1.
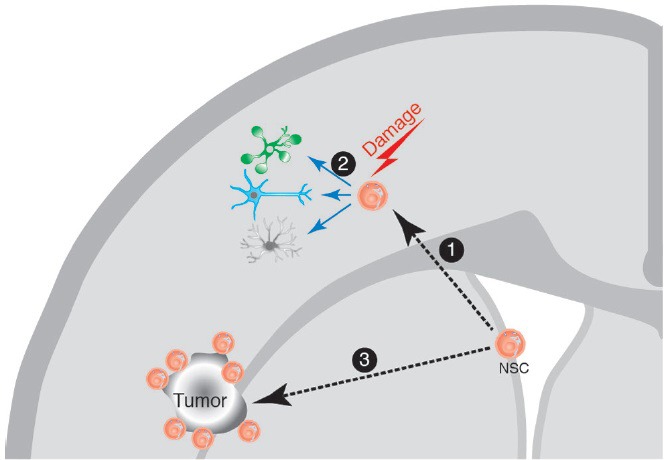
Neural stem cell (NSC) response to brain damage.
The NSC in the adult brain are localized in their niche at the brain subventricular zone (SVZ). In response to a brain damage insult, the NSCs migrate ① towards the damage site, where they can proliferate and generate differentiated cell types (neurons, astrocytes and glia) ②, on what seems to be a generalized response mechanism to damage. Similarly, during the growth of a brain tumor, the NSC migrate towards the tumor site ③, and can be visualized surrounding the tumor, although in this case there is no evidence for repair mechanisms. The NSC are not the only cells migrating in response to brain damage, since there is also evidence for the migration of neuroblasts towards the damage sites, although not depicted in the cartoon for clarity.
MRI has been previously used to monitor migration of either in vitro labeled cells that were then deposited on a recipient mouse, or endogenous neural cells after in situ endocytosis of particles (Shapiro et al., 2006; Panizzo et al., 2009; Sumner et al., 2009; Nieman et al., 2010; Vreys et al., 2010). Thus, these experiments were unable to monitor an endogenous particular progenitor subpopulation migrating in the brain. We could achieve this goal with the use of mAbs specifically identifying surface antigens on neuroblasts (Nilo2) or NSC (Nilo1). Having established the migration of NSC and neuroblasts towards a brain damage site, their physiological role at the damage site remains to be unraveled. It is enlightening, however, that i) after NSC and neuroblast migration in response to a brain puncture with a stereotaxic needle, most of the cells filling the needle track do not express NSC or neuroblast markers; and ii) in response to a focal demyelinated lesion induced by lysolecithin, remyelination could be confirmed by the presence of O4+ or myelin+ cells. Thus, NSC and neuroblasts at the damage site trigger the migration of differentiated cells towards the brain damage site, or, most likely, these cells differentiate in situ to generate the appropriate cell types, to repair the damage. Although we cannot formally distinguish between these possibilities, it is clear that the models and tools described, together with in vivo labeling of proliferating cells might allow to establish the cellular mechanism(s) involved in damage repair. In addition, migration of NSC or neuroblasts in vivo could be used as a biomarker to identify the focal sites on the brain affected by epileptic seizures or neurodegenerative processes such as Parkinson's or Alzheimer's disease.
NSC and neuroblats also migrate towards a tumor growth site; it is conceivable that this happens in response to the same or similar “damage signal(s)” as that in the other damage models. During tumor growth, there is no evidence of tissue repair, this may be due to the rapid growth of the tumors. Interestingly, however, the fast migration of NSCs and neuroblasts towards the tumor site could be used to precisely identify the tumor site, even before contrast agents such as gadolinium can traverse the blood-brain barrier. The most appealing finding, however, using these antibodies is the discovery that Nilo1 mAb in addition to identify mouse radial glia on the developing brain and NSC on the adult mouse, also identifies a subpopulation of cells on human gliomas. Although it is likely that Nilo1 mAb identifies the cancer stem cell population (CSC) in human gliomas, this remains to be formally demonstrated. If this is the case it might represent the first opportunity of generating an antibody against neural CSC. In this regard, the presence of CSC was first demonstrated in vivo in glioblastomas, intestinal tumors, and skin cancer, using genetic tracing of the cells (Baker, 2012). The glioblastoma experiments further demonstrated that standard chemotherapy treatments induced tumor shrinkage, but the tumors quickly returned. If chemotherapy was administered at the same time that the CSC were suppressed using a genetic trick, the tumors shrank back into “residual vestiges” that did not resemble glioblastomas (Chen et al., 2012). Thus, these data draw attention to the relevance of treatments using the neural CSC as a target, as it would be the case for Nilo1-derived drugs.
In conclusion, the use of mAbs recognizing surface markers on either NSC or neuroblasts open up a series of possibilities (Figure 2), for example, they allow to purify these cells from cell suspensions, identify them in their niches, or demonstrate in vivo their migration towards damage sites. In addition allows envisaging the possibility of identifying the focal sites on epileptic brains after a seizure, or on initial stages of Parkinson's or Alzheimer's diseases by the migration of NSCs towards the damage sites. Finally, if the mAb anti-NSC recognizes the CSC in human gliomas, it might turn out as the first therapeutic drug targeting neural CSC.
Figure 2.
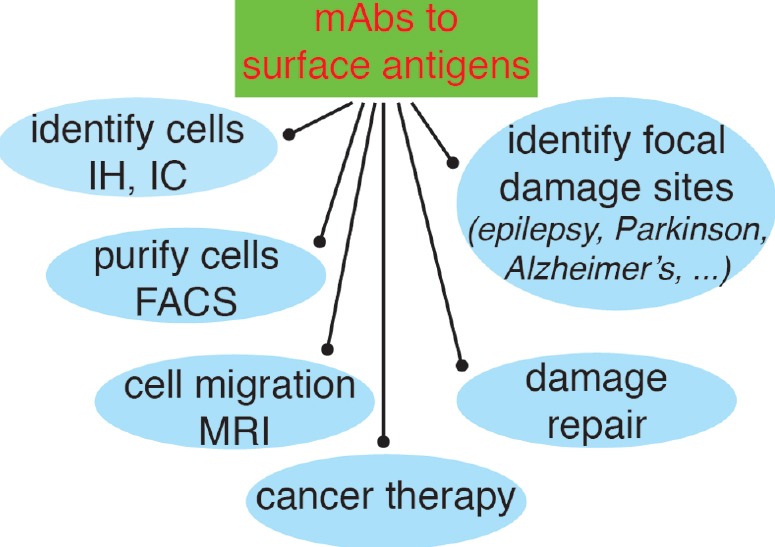
Possibilities opened-up by the use of mAbs recognizing surface antigens in neural stem cells (NSC) and neuroblasts.
The main advantage of using mAbs identifying surface receptors in NSC and neuroblasts is that they can be used experimentally on live cells, or even in vivo, in addition to immunohistochemistry (IH) and immunocytology (IC), to identify the cells bearing these antigens and purify them (or deplete that population) by fluorescence-activated cell sorting (FACS); to use them in vivo, after coupling to magnetic nanoparticles, to identify cell niches or to analyze cell migration; to analyze the repair mechanisms in response to a damage insult; to identify focal damage sites on epileptic seizures or on neurodegenerative diseases such as Parkinson's or Alzheimer's disease; and finally, if they identify the appropriate antigens on the neural cancer stem cells as therapeutic tools for cancer therapy.
References
- 1.Baker M. Cancer stem cells tracked. Nature. 2012;488:13–14. doi: 10.1038/488013a. [DOI] [PubMed] [Google Scholar]
- 2.Chen J, Li Y, Yu TS, McKay RM, Burns DK, Kernie SG, Parada LF. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488:522–526. doi: 10.1038/nature11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Del Valle I, Elvira G, Garcia-Benzaquen L, Armesilla-Diaz A, Kremer L, Garcia-Sanz JA, Martinez S, Silva A. Characterization of novel monoclonal antibodies able to identify neurogenic niches and arrest neurosphere proliferation and differentiation. Neuroscience. 2010;169:1473–1485. doi: 10.1016/j.neuroscience.2010.04.058. [DOI] [PubMed] [Google Scholar]
- 4.Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 5.Elvira G, Garcia I, Benito M, Gallo J, Desco M, Penades S, Garcia-Sanz JA, Silva A. Live imaging of mouse endogenous neural progenitors migrating in response to an induced tumor. PLoS One. 2012;7:e44466. doi: 10.1371/journal.pone.0044466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elvira G, Garcia I, Gallo J, Benito M, Montesinos P, Holgado-Martin E, Ayuso-Sacido A, Penades S, Desco M, Silva A, Garcia-Sanz JA. Detection of mouse endogenous type B astrocytes migrating towards brain lesions. Stem Cell Res. 2015;14:114–129. doi: 10.1016/j.scr.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nieman BJ, Shyu JY, Rodriguez JJ, Garcia AD, Joyner AL, Turnbull DH. In vivo MRI of neural cell migration dynamics in the mouse brain. NeuroImage. 2010;50:456–464. doi: 10.1016/j.neuroimage.2009.12.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Panizzo RA, Kyrtatos PG, Price AN, Gadian DG, Ferretti P, Lythgoe MF. In vivo magnetic resonance imaging of endogenous neuroblasts labelled with a ferumoxide-polycation complex. NeuroImage. 2009;44:1239–1246. doi: 10.1016/j.neuroimage.2008.10.062. [DOI] [PubMed] [Google Scholar]
- 10.Shapiro EM, Gonzalez-Perez O, Manuel Garcia-Verdugo J, Alvarez-Buylla A, Koretsky AP. Magnetic resonance imaging of the migration of neuronal precursors generated in the adult rodent brain. NeuroImage. 2006;32:1150–1157. doi: 10.1016/j.neuroimage.2006.04.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sumner JP, Shapiro EM, Maric D, Conroy R, Koretsky AP. In vivo labeling of adult neural progenitors for MRI with micron sized particles of iron oxide: quantification of labeled cell phenotype. NeuroImage. 2009;44:671–678. doi: 10.1016/j.neuroimage.2008.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vreys R, Vande Velde G, Krylychkina O, Vellema M, Verhoye M, Timmermans JP, Baekelandt V, Van der Linden A. MRI visualization of endogenous neural progenitor cell migration along the RMS in the adult mouse brain: validation of various MPIO labeling strategies. NeuroImage. 2010;49:2094–2103. doi: 10.1016/j.neuroimage.2009.10.034. [DOI] [PubMed] [Google Scholar]


