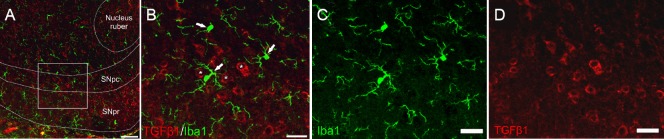
Parkinson's disease (PD) is characterized by the progressive loss of midbrain dopaminergic (mDA) neurons and a subsequent decrease in striatal dopamine levels, which cause the typical clinical motor symptoms such as muscle rigidity, bradykinesia and tremor. Although a subset of PD cases has been described to arise from inherited mutations of genes such as α-Synuclein or Lrkk2, the majority of PD cases develop spontaneously. Despite intensive research, the molecular mechanisms underlying degeneration of mDA neurons are only poorly understood. Interestingly, a common hallmark of virtually all PD cases is a neuroinflammatory response that is predominantly mediated by microglia – the resident immune cells of the central nervous system (CNS). In animal models for PD and in human PD cases, microglia have been shown to adopt an activated, reactive phenotype which is characterized by upregulation of pro-inflammatory genes and release of cytokines and/or chemokines that are believed to further threaten and stress mDA neurons and drive the progression of neurodegeneration (Hirsch and Hunot, 2009). Moreover, the current treatment strategy for PD patients using L-3,4-dihydroxyphenylalanine (L-DOPA) has been shown to provoke glia reactions (Bortolanza et al., 2014) and, thus might contribute to trigger microglia-mediated neuroinflammation and accelerate neurodegeneration. However, it has to be taken into consideration that cytokine release by microglia might also result in a protective stress response as recently discussed (Cebrian et al., 2015). Among the endogenous factors that are capable of regulating microglia activation states, transforming growth factor β1 (TGFβ1) has been shown to be one of the most potent factors in vivo (Butovsky et al., 2014) and in vitro (Spittau et al., 2013). Under physiological conditions, TGFβ1 is mainly expressed by neurons and to a lesser extent by glial fibrillary acidic protein-positive (GFAP+) astrocytes throughout the CNS. TGFβ1 mRNA has been detected in midbrain neurons, whereas TGFβ2 and TGFβ3 transcripts are hardly detectable in neurons of the ventral midbrain (Vincze et al., 2010). Figure 1 demonstrates that TGFβ1 immunoreactivity is detectable in midbrain neurons (indicated by white asterisks) but not in microglia (white arrows) which extend their processes towards TGFβ1-positive midbrain neurons and are located in close proximity to these neurons. This expression pattern suggests that neuron-derived TGFβ1 might be important to maintain microglia homeostasis under physiological conditions. Indeed, Butovsky et al. (2014) have reported that lack of TGFβ1 resulted in functional and morphological impairment of microglia. However, it has to be mentioned that the authors used TGFβ1-deficient mice, which were crossed to mice expressing TGFβ1 under the control of the interleukin 2 (IL2)-promoter. This approach prevents the lethal postnatal phenotype of TGFβ1-/- mice, which die due to a systemic inflammation mediated by T cells. It remains to be established whether neuron-derived TGFβ1 is essential to mediate microglia maintenance or whether peripheral effects of TGFβ1-deletion are responsible for the microglia phenotype observed by Butovsky et al. (2014).
Figure 1.
Transforming growth factor β1 (TGFβ1) is predominantly expressed in neurons in the midbrain.
50 μm vibratome sections from 12-week-old male C57BL/6 mice have been used for free-floating immunohistochemistry. (A) Overview image displaying the localization of substantia nigra pars compacta (SNpc), substantia nigra pars reticularis (SNpr) and nucleus ruber in the ventral midbrain. Rectangle marks the area displayed at high magnification images. Lines represent borders of SNpr, SNpc and nucleus ruber, respectively. Scale bar: 75 μm. (B) Microglia (Iba1+), as indicated by white arrows, show no TGFβ1 expression, whereas neurons (indicated by white asterisks) display a strong cytoplasmic immunoreactivity for TGFβ1. Single channel images for Iba1+ microglia (C) and TGFβ1 (D) confirm that neurons and not microglia are the primary source of TGFβ1 in the midbrain. Scale bars: 25 μm for B–D.
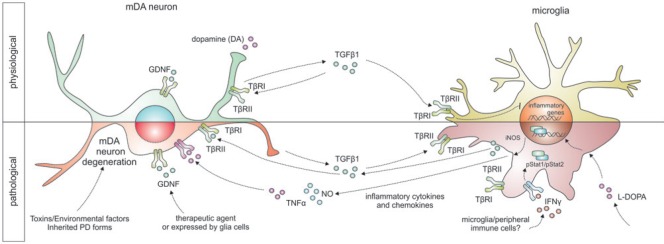
Under pathological conditions, such as ischemia, activated microglia have been shown to increase expression of TGFβ1 (Kiefer et al., 1995; Vincze et al., 2010), which is therefore referred to as a lesion-associated cytokine. Moreover, the expression of TGFβ receptors has further been observed to increase after ischemia in microglia, indicating an increased responsiveness of activated microglia towards TGFβ1 signals in the lesioned CNS (Pál et al., 2014). For the development and maintenance of mDA neurons, TGFβ1 seems to play essential roles. Roussa et al. (2009) have reviewed the effects of TGFβs for induction of the dopaminergic phenotype of midbrain neurons during embryonic development, where TGFβ cooperates with sonic hedgehog (SHH) to induce differentiation into functional tyrosine hydroxyase (TH)-positive neurons. Moreover, TGFβ has been reported to exert direct neurotrophic effects on mDA neurons after deprivation of classical neurotrophic support and after intoxication with 1-methyl-4-phenyl-pyridinium ion (MPP+), the active metabolite of the toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), which is a widely used toxin to selectively induce degeneration of dopaminergic neurons and, thus, mimic Parkinson's disease in rodents (Roussa et al., 2009). In the MPTP mouse model for PD, the survival promoting effects of glial cell-line-derived neurotrophic factor (GDNF), which is one of the most potent neurotrophic factors for mDA neurons in vivo and in vitro, were dependent on endogenous TGFβ. Application of TGFβ-neutralizing antibodies resulted in abrogation of neurotrophic effects on mDA neurons mediated by GDNF in this rodent mouse model for PD (Schober et al., 2007). Although neuroinflammation and microglia activation have not been addressed in this study, it might be possible that endogenous TGFβ is necessary to inhibit neuroinflammation as a prerequisite for GDNF-mediated neuroprotection. This hypothesis is further strengthened by the fact that GDNF itself is not able to inhibit activation of mouse microglia due to absence of c-RET expression, which is the essential GDNF signaling receptor (Zlotnik and Spittau, 2014). In rodent models for PD as well as in human PD cases, it remains to be established what actually triggers the activation of microglia to promote a neuroinflammatory response which further fuels degeneration of mDA neurons. An interesting candidate being involved in microglia activation in PD is the cytokine interferon-γ (IFNγ). IFNγ has been described to be upregulated in the blood plasma of PD patients and mice deficient for IFNγ displayed reduced microglia activation and decreased degeneration of mDA neurons after intoxication with MPTP. Expression of the IFNγ receptor in the midbrain seems to be restricted to microglia, indicating that IFNγ has no direct effect on neuron survival and that IFNγ-induced microglia activation is responsible for mDA neurodegeneration. Moreover, in the presence of microglia lacking the IFNγ receptor, mDA neurons are protected from microglia-dependent IFNγ-induced neurodegeneration (Mount et al., 2007). Our group has recently shown that TGFβ1 efficiently blocks microglia activation induced by IFNγ, which is characterised by the release of tumor necrosis factor α (TNFα) and nitric oxide (NO). TGFβ1 treatment abrogated IFNγ-mediated increase in TNFα and NO secretion by downregulation of genes involved in IFNγ signal transduction (Zhou et al., 2015). TNFα and NO are well known to exert neurotoxic effects in PD models (Block et al., 2007) and we further demonstrated that IFNγ, is not able to induce degeneration of mDA neurons in neuron-enriched cultures but sufficiently mediated neurotoxicity in the presence of microglia in neuron-glia cultures. Application of TGFβ1 was able to rescue mDA neurons from IFNγ-induced neurodegeneration in neuron-glia cultures (Zhou et al., 2015). These results, together with previous studies from our group, which clearly demonstrate that endogenous TGFβ1 promotes quiescence of microglia (Spittau et al., 2013) and that endogenous TGFβ signaling is necessary to induce alternative activation of microglia by interleukin-4 (IL4) underline the potential of TGFβ1 as a therapeutic agent to protect mDA neurons by regulating microglial activation states (Zhou et al., 2012). Figure 2 summarizes the effects of TGFβ1 on microglia and midbrain neurons under physiological and pathological conditions and further highlights possible interactions of TGFβ1 with factors such as GDNF and IFNγ.
Figure 2.
Schematic summary of TGFβ1-mediated effects under physiological and pathological (PD) conditions.
Whereas TGFβ1 expression is restricted to neurons under physiological conditions and is high likely to be involved in mediating microglial quiescence as well as neuronal survival, microglia increase TGFβ1 expression under pathological conditions. In this context, TGFβ1 exerts autocrine and paracrine effects by inhibiting microglia activation and promoting neuron survival. Crosstalks between different signalling pathways (e.g., GDNF, IFNγ and TGFβ1) are high likely to have impacts on mDA neuron survival as well as microglia reactivity, however, these interactions have been only partially understood and need to be further elucidated.
GDNF: Glial cell line-derived neurotrophic factor; IFNγ: interferon-γ; iNOS: inducible nitric oxide synthase; L-DOPA: L-3,4-dihydroxyphenylalanine; mDA: midbrain dopaminergic; NO: nitric oxide; PD: Parkinson's disease; TβR: transforming growth facter beta receptor; TGFβ1: transforming growth factor β1; TNFα: tumor necrosis factor α.
Several previous approaches to protect mDA neurons in PD models as well as in PD patients using infusion or overexpression of neurotrophic factors, such as GDNF, resulted in rather disappointing outcomes, which could at least in parts be due to the fact that most of the neurotrophic factors only exert direct protective effects without directly affecting microglia-mediated neuroinflammation. It has to be taken into consideration to design more effective future treatment approaches that involve combinations of direct neurotrophic factors and factors which aim to regulate microglia activation. According to the above mentioned functions and effects of TGFβ1 on microglia activation as well as TGFβ1-mediated neurotrophic effects on mDA neurons (Figure 2), a combination of GDNF and TGFβ1 could be a promising therapeutic approach to slow the progressive nature of mDA neuron degeneration and inhibit the accompanied microglia activation. However, the molecular mechanisms underlying TGFβ1-mediated regulation of microglia functions are only partially understood and further research is necessary to analyze the phenotypes of microglia induced by TGFβ1. Moreover, the complex mode of secretion, extracelular storage and activation of TGFβ1, which is initially released in a biologically inactive form, need to be further addressed before application as a therapeutic agent. Although TGFβ1 has a promising potential as a factor which might be applied to slow neurodegeneration and reduce neuroinflammation in animal models of PD and in human PD cases, at this time, several open issues on intra- and extracellular effects of TGFβ1 are of utmost interest and need to be elucidated in the future.
References
- 1.Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 2.Bortolanza M, Cavalcanti-Kiwiatkoski R, Padovan-Neto FE, da-Silvi CA, Mitkovski M, Raisman-Vozari R, Del-Bel E. Glial activation is associated with L-DOPA induced dyskinesia and blocked by a nitric oxide synthase inhibitor in a rat model of Parkinsons's disease. Neurobiol Dis 73C. 2014:377–387. doi: 10.1016/j.nbd.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 3.Butovsky O, Jedrychowski MP, Moore CS, Cialic R, Lanser AJ, Gabriely G, Koeglsperger T, Dake B, Wu PM, Doykan CE, Fanek Z, Liu L, Chen Z, Rothstein JD, Ransohoff RM, Gygi SP, Antel JP, Weiner HL. Identification of a unique TGF-β-dependent molecular and functional signature in microglia. Nat Neurosci. 2014;17:131–143. doi: 10.1038/nn.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cebrian C, Loike JD, Sulzer D. Neuroinflammation in Parkinson's disease animal models: a cell stress response or a step in neurodegeneration? Curr Top Behav Neurosci. 2015;22:237–270. doi: 10.1007/7854_2014_356. [DOI] [PubMed] [Google Scholar]
- 5.Hirsch EC, Hunot S. Neuroinflammation in Parkinson's disease: a target for neuroprotection? Lancet Neurol. 2009;8:382–397. doi: 10.1016/S1474-4422(09)70062-6. [DOI] [PubMed] [Google Scholar]
- 6.Kiefer R, Streit WJ, Toyka KV, Kreutzberg GW, Hartung HP. 1995 Transforming growth factor-beta 1: a lesion-associated cytokine of the nervous system. Int J Dev Neurosci. 13:331–339. doi: 10.1016/0736-5748(94)00074-d. [DOI] [PubMed] [Google Scholar]
- 7.Mount MP, Lira A, Grimes D, Smith PD, Faucher S, Slack R, Anisman H, Hayley S, Park DS. Involvement of interferon-gamma in microglial-mediated loss of dopaminergic neurons. J Neurosci. 2007;27:3328–3337. doi: 10.1523/JNEUROSCI.5321-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pál G, Lovas G, Dobolyi A. Induction of transforming growth factor beta receptors following focal ischemia in the rat brain. PLoS One. 2014;9:e106544. doi: 10.1371/journal.pone.0106544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roussa E, von Bohlen und Halbach O, Krieglstein K. TGF-beta in dopamine neuron development, maintenance and neuroprotection. Adv Exp Med Biol. 2009;651:81–90. doi: 10.1007/978-1-4419-0322-8_8. [DOI] [PubMed] [Google Scholar]
- 10.Schober A, Peterziel H, von Bartheld CS, Simon H, Krieglstein K, Unsicker K. GDNF applied to the MPTP-lesioned nigrostriatal system requires TGF-beta for its neuroprotective action. Neurobiol Dis. 2007;25:378–391. doi: 10.1016/j.nbd.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Spittau B, Wullkopf L, Zhou X, Rilka J, Pfeifer D, Krieglstein K. Endogenous transforming growth factor-beta promotes quiescence of primary microglia in vitro. Glia. 2013;61:287–300. doi: 10.1002/glia.22435. [DOI] [PubMed] [Google Scholar]
- 12.Vincze C, Pál G, Wappler EA, Szabó ER, Nagy ZG, Lovas G, Dobolyi A. Distribution of mRNAs encoding transforming growth factors-beta1, -2 and -3 in the intact rat brain and after experimentally induced focal ischemia. J Comp Neurol. 2010;518:3752–3770. doi: 10.1002/cne.22422. [DOI] [PubMed] [Google Scholar]
- 13.Zhou X, Spittau B, Krieglstein K. TGFβ signalling plays an important role in IL4-induced alternative activation of microglia. J Neuroinflammation. 2012;9:210. doi: 10.1186/1742-2094-9-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou X, Zöller T, Krieglstein K, Spittau B. TGFβ1 inhibits IFNγ-mediated microglia activation and protects mDA neurons from IFNγ-driven neurotoxicity. J Neurochem. 2015;134:125–134. doi: 10.1111/jnc.13111. [DOI] [PubMed] [Google Scholar]
- 15.Zlotnik A, Spittau B. GDNF fails to inhibit LPS-mediated activation of mouse microglia. J Neuroimmunol. 2014;270:22–28. doi: 10.1016/j.jneuroim.2014.03.006. [DOI] [PubMed] [Google Scholar]